Adsorption of Ammonium Nitrogen from Aqueous Solution on Chemically Activated Biochar Prepared from Sorghum Distillers Grain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Biochar
2.3. Ammonium Nitrogen Adsorption Experiments
2.4. Composition Analyses of Sorghum Distillers Grain
2.5. Characterization of Biochar
3. Results and Discussion
3.1. Characterization of Sorghum Distillers Grain
3.2. Adsorption of Ammonium Nitrogen on Biochar
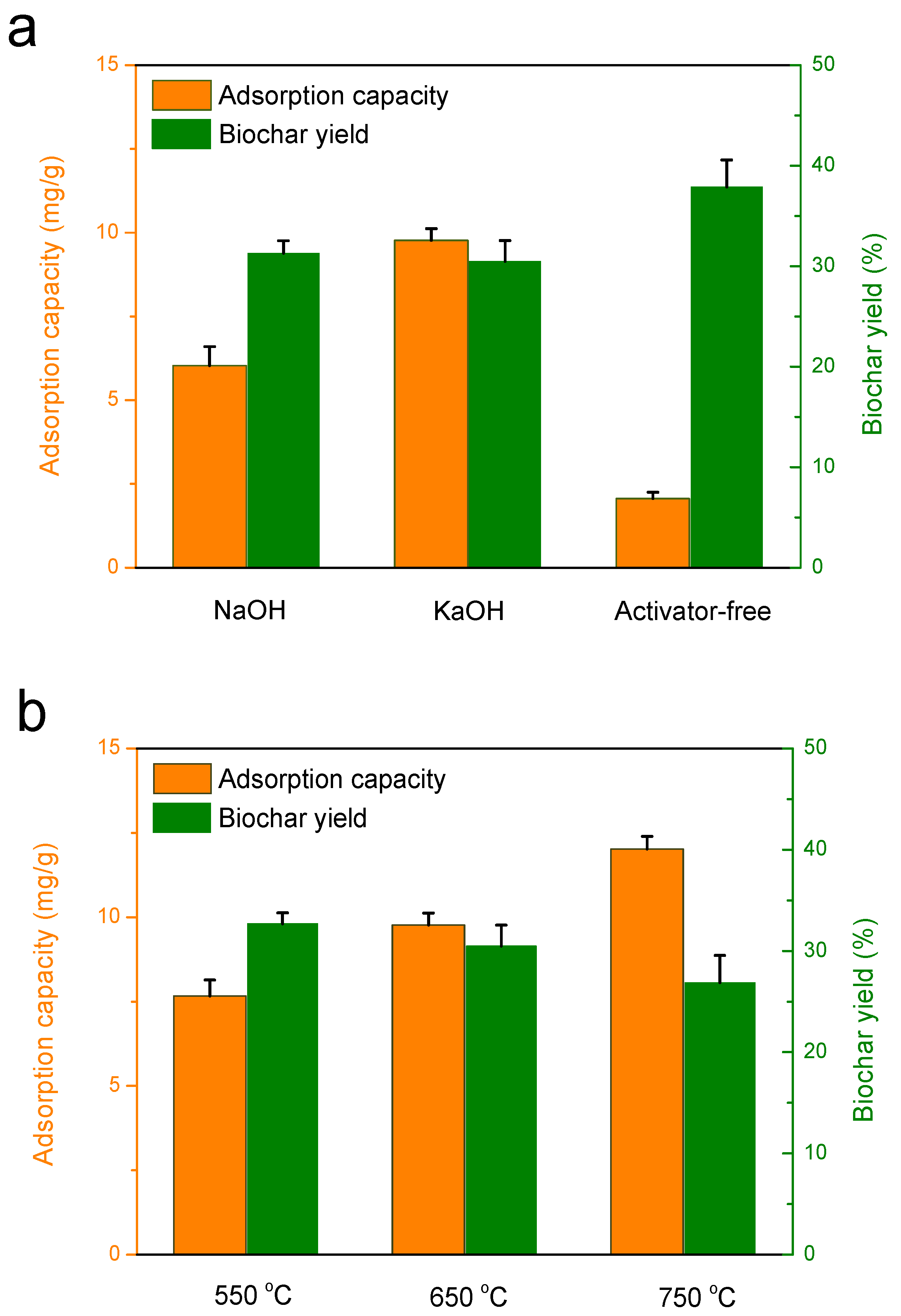
3.2.1. Effect of activation agents on the adsorption capacity of biochar
3.2.2. Effect of Activation Temperature on the Adsorption Capacity of Biochar
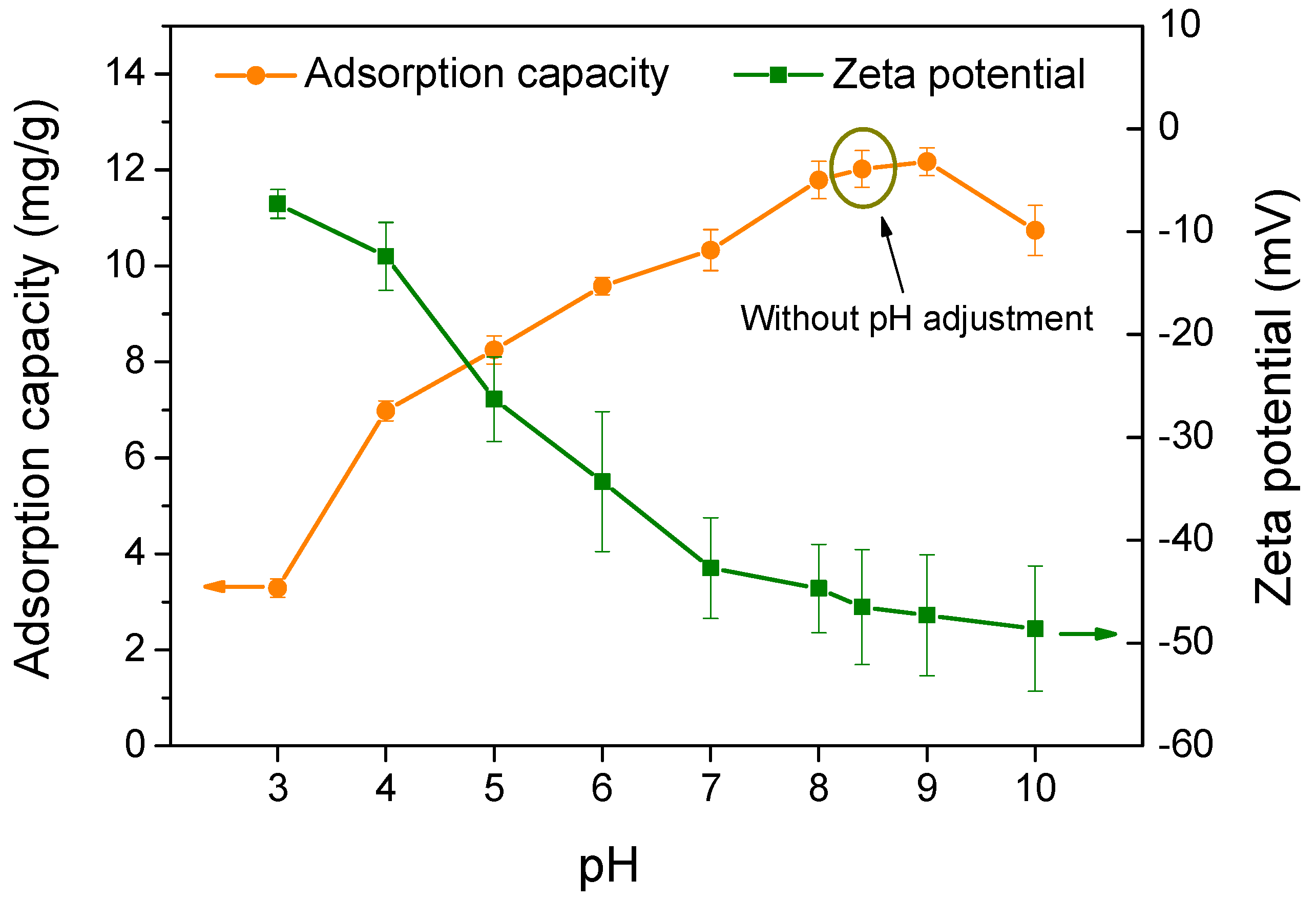
3.2.3. Effect of Adsorption pH on the Adsorption Capacity of Biochar
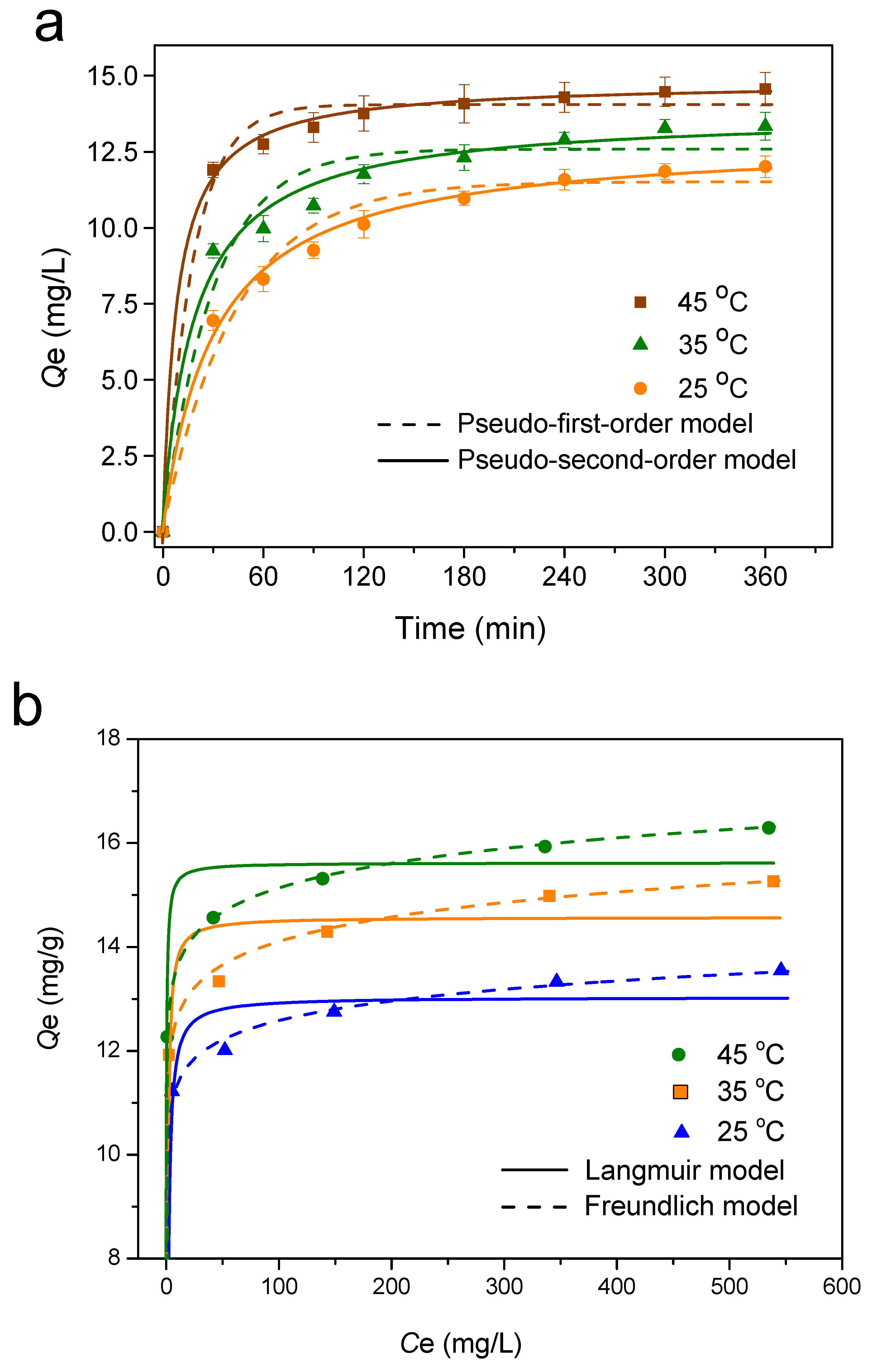
3.2.4. Adsorption Kinetics
3.2.5. Adsorption Isotherm
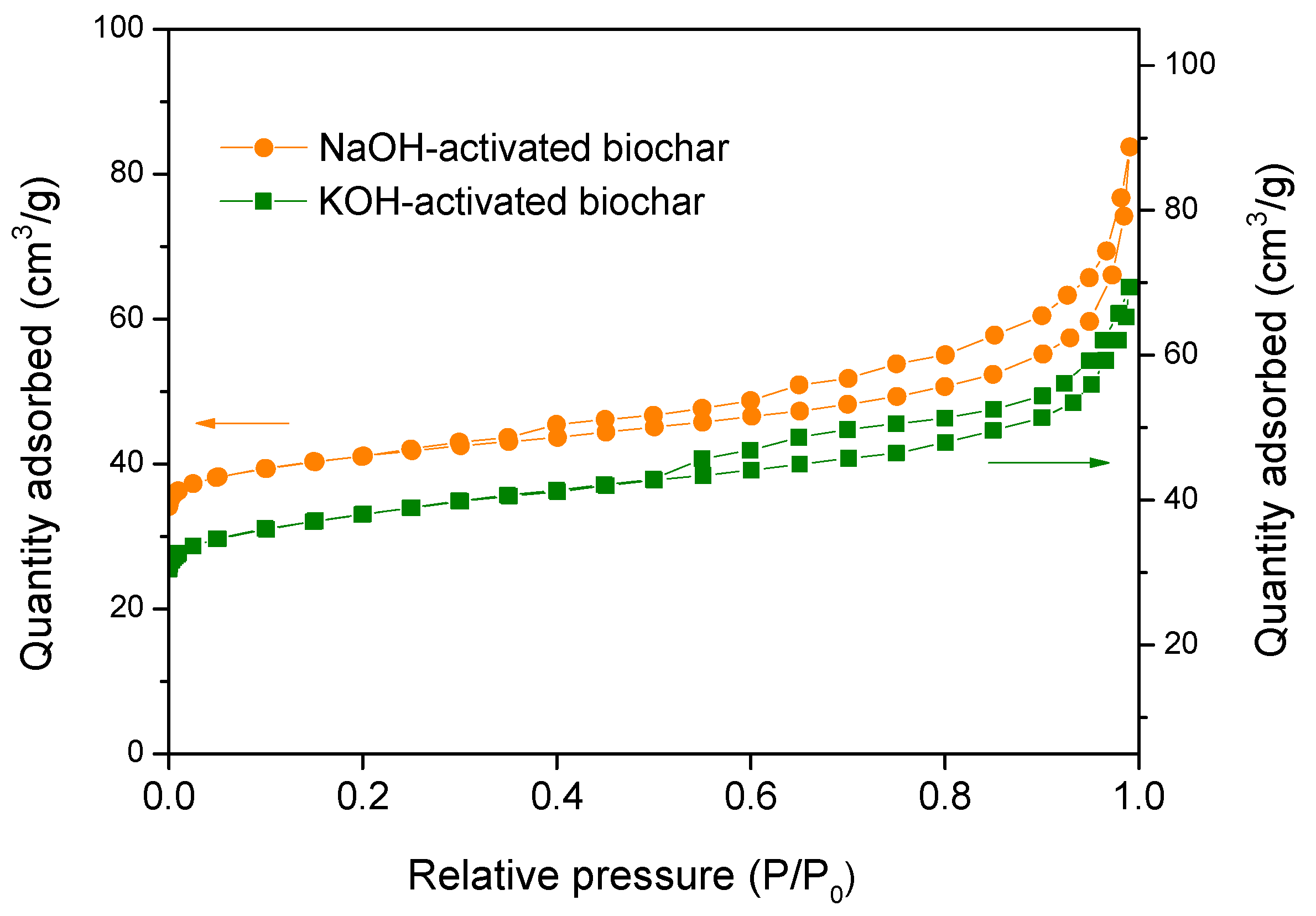
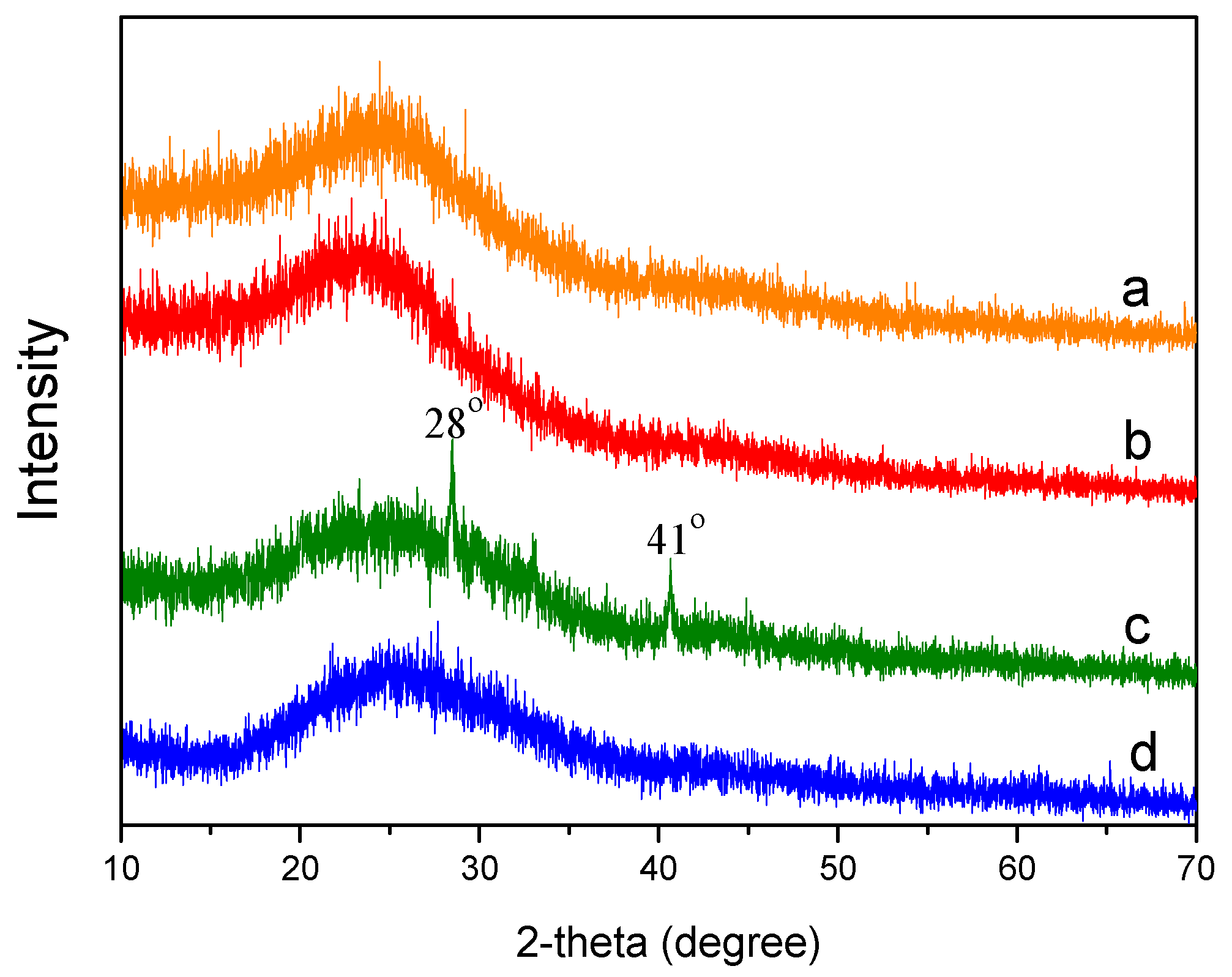
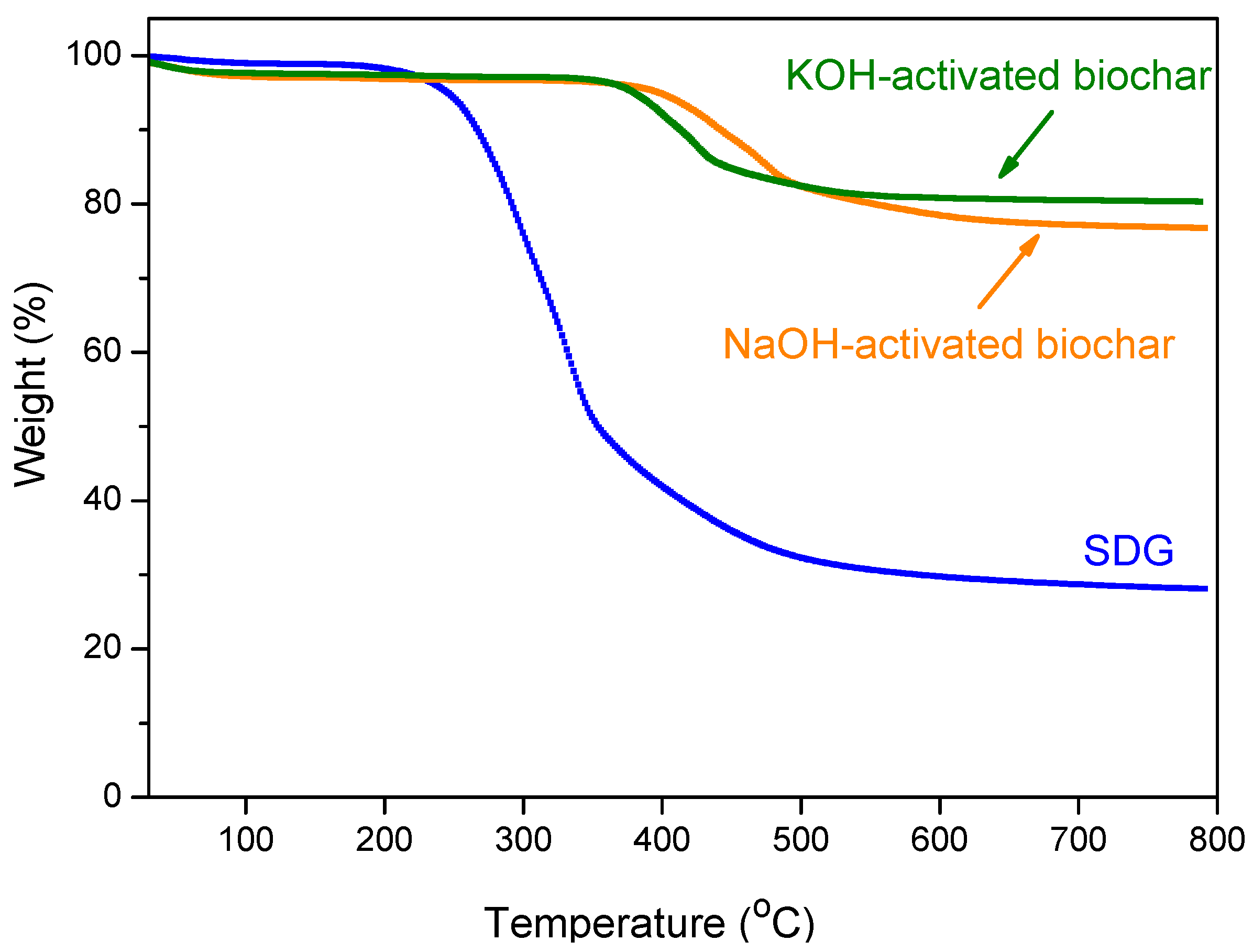
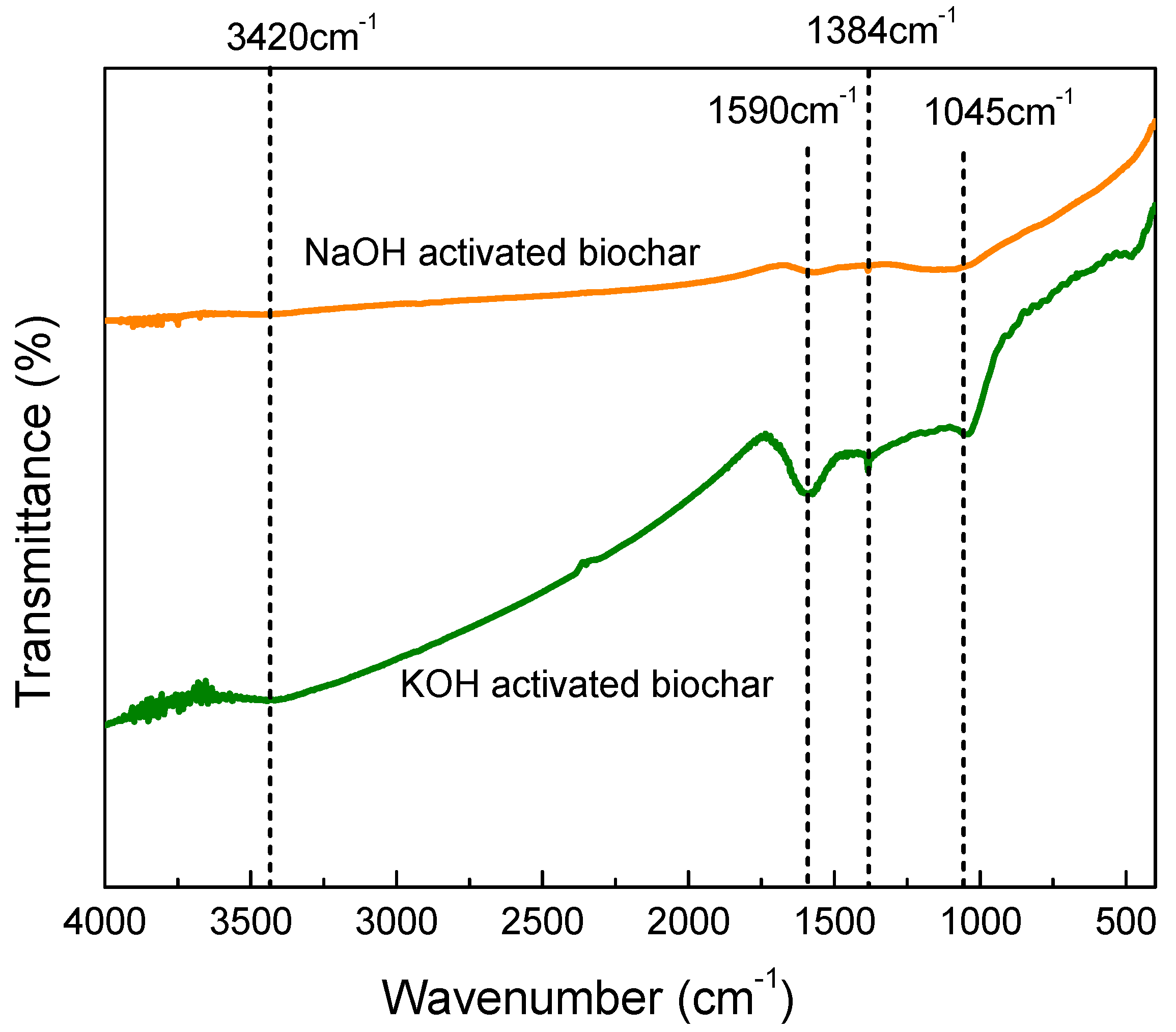
3.3. Characterization of the Biochar
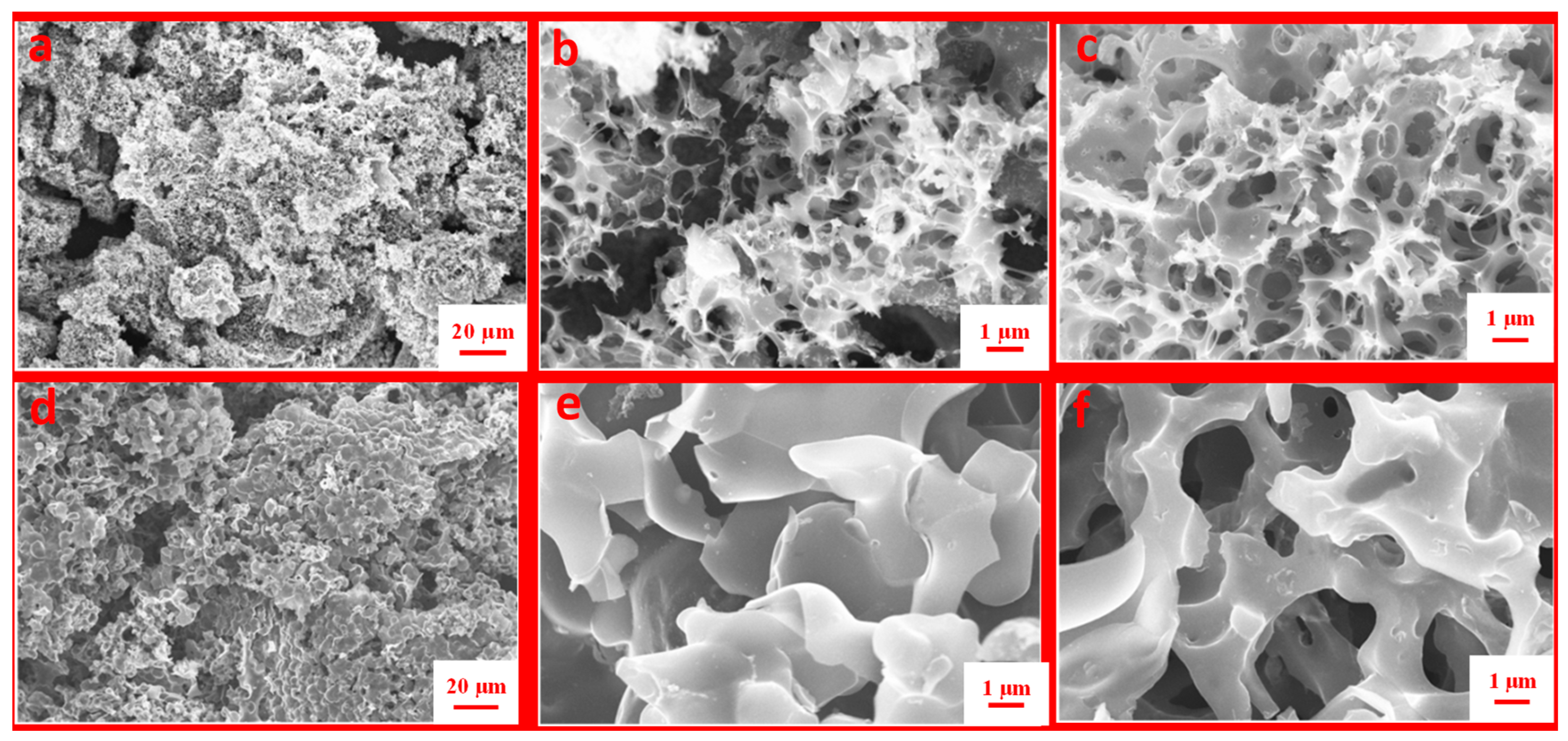
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. X-Ray Diffraction (XRD)
3.3.3. Thermogravimetry (TG)
3.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Cadar, O.; Miclean, M.; Cadar, S.; Tanaselia, C.; Senila, L.; Senila, M. Assessment of heavy metals in cows milk in Rodnei Mountains Area, Romania. Environ. Eng. Manag. J. 2015, 14, 2523–2528. [Google Scholar] [CrossRef]
- Hoaghia, M.A.; Cadar, O.; Hognogi, G.G.; Levei, E.; Moisa, C.; Roman, C. Quality and Human Health Risk Assessment of Metals and Nitrogen Compounds in Drinking Water from an Urban Area Near a Former Non-Ferrous Ore Smelter. Anal. Lett. 2019, 52, 1268–1281. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahdavianpour, M. The selective direct oxidation of ammonium in the contaminated water to nitrogen gas using the chemical-less VUV photochemical continuous-flow reactor. Chem. Eng. J. 2016, 295, 57–63. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.; Findlay, D.; Stainton, M.; Parker, B.; Paterson, M.; Beaty, K.; Lyng, M.; Kasian, S. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Wang, Y.F.; Lin, F.; Pang, W.Q. Ammonium exchange in aqueous solution using Chinese natural clinoptilolite and modified zeolite. J. Hazard. Mater. 2007, 142, 160–164. [Google Scholar] [CrossRef]
- Carrera, J.; Vicent, T.; Lafuente, J. Effect of influent COD/N ratio on biological nitrogen removal (BNR) from high-strength ammonium industrial wastewater. Process Biochem. 2004, 39, 2035–2041. [Google Scholar] [CrossRef]
- Thornton, A.; Pearce, P.; Parsons, S. Ammonium removal from digested sludge liquors using ion exchange. Water Res. 2007, 41, 433–439. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Shah, T.M.; Ramaswami, S.; Behrendt, J.; Otterpohl, R. Simultaneous removal of organics and ammonium-nitrogen from reverse osmosis concentrate of mature landfill leachate. J. Water Process Eng. 2017, 19, 126–132. [Google Scholar] [CrossRef]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: A review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Yu, Q.; Dong, X.; Li, H.; Ke, L.; Wang, Y.; Wang, H.; Zheng, Y.; Li, Q. Effectiveness and mechanisms of ammonium adsorption on Biochars derived from biogas residues. RSC Adv. 2016, 6, 88373–88381. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Q.; Chang, M.; Jang, J.; Sui, W.; Si, C.; Ni, Y. Novel Fe3O4@lignosulfonate/phenolic core-shell microspheres for highly efficient removal of cationic dyes from aqueous solution. Ind. Crops Prod. 2019, 127, 110–118. [Google Scholar] [CrossRef]
- Liu, J.; Su, Y.; Li, Q.; Yue, Q.; Gao, B. Preparation of wheat straw based superabsorbent resins and their applications as adsorbents for ammonium and phosphate removal. Bioresour. Technol. 2013, 143, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xie, Y.; Wang, A. Rapid and wide pH-independent ammonium-nitrogen removal using a composite hydrogel with three-dimensional networks. Chem. Eng. J. 2012, 179, 90–98. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q. Sustainable mechanisms of biochar derived from brewers’ spent grain and sewage sludge for ammonia–nitrogen capture. J. Clean. Prod. 2016, 112, 3927–3934. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Yang, S.; Wang, Q.; Feng, C.; Zhang, Z. Adsorption of high ammonium nitrogen from wastewater using a novel ceramic adsorbent and the evaluation of the ammonium-adsorbed-ceramic as fertilizer. J. Colloid Interface Sci. 2013, 393, 264–270. [Google Scholar] [CrossRef]
- Su, M.Y.; Tzeng, W.S.; Shyu, Y.T. An analysis of feasibility of bioethanol production from Taiwan sorghum liquor waste. Bioresour. Technol. 2010, 101, 6669–6675. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Z.; Xu, X.; Dai, M.; Guo, R. Characterization and ammonia adsorption of biochar prepared from distillers’ grains anaerobic digestion residue with different pyrolysis temperatures. J. Chem. Technol. Biotechnol. 2018, 93, 198–206. [Google Scholar] [CrossRef]
- Xia, D.; Tan, F.; Zhang, C.; Jiang, X.; Chen, Z.; Li, H.; Zheng, Y.; Li, Q.; Wang, Y. ZnCl2-activated biochar from biogas residue facilitates aqueous As (III) removal. Appl. Surf. Sci. 2016, 377, 361–369. [Google Scholar] [CrossRef]
- Zhu, Y.; Kolar, P.; Shah, S.B.; Cheng, J.J.; Lim, P.K. Avocado seed-derived activated carbon for mitigation of aqueous ammonium. Ind. Crops Prod. 2016, 92, 34–41. [Google Scholar] [CrossRef]
- Yusof, A.M.; Keat, L.K.; Ibrahim, Z.; Majid, Z.A.; Nizam, N.A. Kinetic and equilibrium studies of the removal of ammonium ions from aqueous solution by rice husk ash-synthesized zeolite Y and powdered and granulated forms of mordenite. J. Hazard. Mater. 2010, 174, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.N.; Calderon, J.; Temelli, F. Supercritical carbon dioxide extraction of corn distiller’s dried grains with solubles: Experiments and mathematical modeling. J. Agric. Food Chem. 2012, 60, 12482–12490. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Zhang, X.; Chen, X.; Yue, D.; Yin, Q.; Xiao, L.; Yang, L. Biochar from Alternanthera philoxeroides could remove Pb(II) efficiently. Bioresour. Technol. 2014, 171, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiao, A.; Wei, B.; Wang, Y.; Wu, C.; Jin, Z.; Tian, Y. Porous starch extracted from Chinese rice wine vinasse: Characterization and adsorption properties. Int. J. Biol. Macromol. 2013, 61, 156–159. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.E.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef]
- Brown, R.A.; Kercher, A.K.; Nguyen, T.H.; Nagle, D.C.; Ball, W.P. Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Org. Geochem. 2006, 37, 321–333. [Google Scholar] [CrossRef]
- Jing, Q.X.; Chai, L.Y.; Huang, X.D.; Tang, C.J.; Guo, H.; Wang, W. Behavior of ammonium adsorption by clay mineral halloysite. Trans. Nonferrous Met. Soc. China 2017, 27, 1627–1635. [Google Scholar] [CrossRef]
- Vu, T.M.; Trinh, V.T.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lehmann, J.; Hanley, K.; Hestrin, R.; Enders, A. Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 2015, 138, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.C.S.; Teodoro, F.S.; Mageste, A.B.; Gil, L.F.; de Freitas, R.P.; Gurgel, L.V.A. Application of a new carboxylate-functionalized sugarcane bagasse for adsorptive removal of crystal violet from aqueous solution: Kinetic, equilibrium and thermodynamic studies. Ind. Crops Prod. 2015, 65, 521–534. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.; Chen, G. Fast removal and recovery of Cr (VI) using surface-modified jacobsite (MnFe2O4) nanoparticles. Langmuir 2005, 21, 11173–11179. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, X.; Luo, X.; Zhang, C.; Zhu, H. A modified lignin adsorbent for the removal of 2, 4, 6-trinitrotoluene. Chem. Eng. J. 2011, 168, 1055–1063. [Google Scholar] [CrossRef]
- Başar, C.A. Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J. Hazard. Mater. 2006, 135, 232–241. [Google Scholar] [CrossRef]
- Tseng, R.L.; Wu, F.C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant. J. Hazard. Mater. 2008, 155, 277–287. [Google Scholar] [CrossRef]
- Huang, X.; Gao, N.Y.; Zhang, Q.L. Thermodynamics and kinetics of cadmium adsorption onto oxidized granular activated carbon. J. Environ. Sci. 2007, 19, 1287–1292. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Sadraei, R.; Paganini, C.M.; Calza, P.; Magnacca, G. An Easy Synthesis for Preparing Bio-Based Hybrid Adsorbent Useful for Fast Adsorption of Polar Pollutants. Nanomaterials 2019, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Sadraei, R.; Murphy, R.S.; Laurenti, E.; Magnacca, G. Characterization Methodology To Evaluate the Activity of Supported Soybean Peroxidase. Ind. Eng. Chem. Res. 2019, 58, 19082–19089. [Google Scholar] [CrossRef]
- Bansode, R.R.; Losso, J.N.; Marshall, W.E.; Rao, R.M.; Portier, R.J. Pecan shell-based granular activated carbon for treatment of chemical oxygen demand (COD) in municipal wastewater. Bioresour. Technol. 2004, 94, 129–135. [Google Scholar] [CrossRef]
- Tang, Y.B.; Liu, Q.; Chen, F.Y. Preparation and characterization of activated carbon from waste ramulus mori. Chem. Eng. J. 2012, 203, 19–24. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, H.; Liu, G.; Qiao, J.; Wang, J.; Lu, H.; Yang, L.; Wu, Y. Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms. Bioresour. Technol. 2012, 125, 138–144. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef]
| T (°C) | Qe (mg/g) | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|---|
| k1 (1/min) | Qe,cal (mg/g) | R2 | k2 (g/(mg·min)) | Qe,cal (mg/g) | R2 | ||
| 25 | 12.01 | 0.0232 | 11.44 | 0.9688 | 0.0026 | 12.86 | 0.9942 |
| 35 | 13.34 | 0.0338 | 12.54 | 0.9543 | 0.0040 | 13.72 | 0.9875 |
| 45 | 14.56 | 0.0580 | 14.01 | 0.9876 | 0.0086 | 14.72 | 0.9985 |
| T (°C) | Langmuir Model | Freundlich Model | Van ’t Hoff | ||||||
|---|---|---|---|---|---|---|---|---|---|
| kL (L/mg) | Qm (mg/g) | R2 | n | kF (mg/g) | R2 | ΔG0 (kJ/mol) | ΔS0 (J/(K·mol)) | ΔH0 (kJ/mol) | |
| 25 | 1.143 | 13.0 | 0.9899 | 23.8 | 10.4 | 0.9993 | −13.82 | 589.36 | 40.47 |
| 35 | 1.891 | 14.5 | 0.9862 | 21.5 | 11.4 | 0.9994 | −13.59 | ||
| 45 | 4.000 | 15.6 | 0.9900 | 22.6 | 12.3 | 0.9999 | −15.00 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, D.; Lu, C.; Pang, T.; Wang, Y.; Wang, G. Adsorption of Ammonium Nitrogen from Aqueous Solution on Chemically Activated Biochar Prepared from Sorghum Distillers Grain. Appl. Sci. 2019, 9, 5249. https://doi.org/10.3390/app9235249
Hsu D, Lu C, Pang T, Wang Y, Wang G. Adsorption of Ammonium Nitrogen from Aqueous Solution on Chemically Activated Biochar Prepared from Sorghum Distillers Grain. Applied Sciences. 2019; 9(23):5249. https://doi.org/10.3390/app9235249
Chicago/Turabian StyleHsu, Derlin, Changyi Lu, Tairan Pang, Yuanpeng Wang, and Guanhua Wang. 2019. "Adsorption of Ammonium Nitrogen from Aqueous Solution on Chemically Activated Biochar Prepared from Sorghum Distillers Grain" Applied Sciences 9, no. 23: 5249. https://doi.org/10.3390/app9235249
APA StyleHsu, D., Lu, C., Pang, T., Wang, Y., & Wang, G. (2019). Adsorption of Ammonium Nitrogen from Aqueous Solution on Chemically Activated Biochar Prepared from Sorghum Distillers Grain. Applied Sciences, 9(23), 5249. https://doi.org/10.3390/app9235249




