Adsorption and Desorption Mechanisms of Rare Earth Elements (REEs) by Layered Double Hydroxide (LDH) Modified with Chelating Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Adsorbent
2.1.1. Synthesis of Precursor L1
2.1.2. Synthesis of L2
2.2. Characterization of the Adsorbent
2.3. Adsorption Experiments Using L1 and L2 as Adsorbents
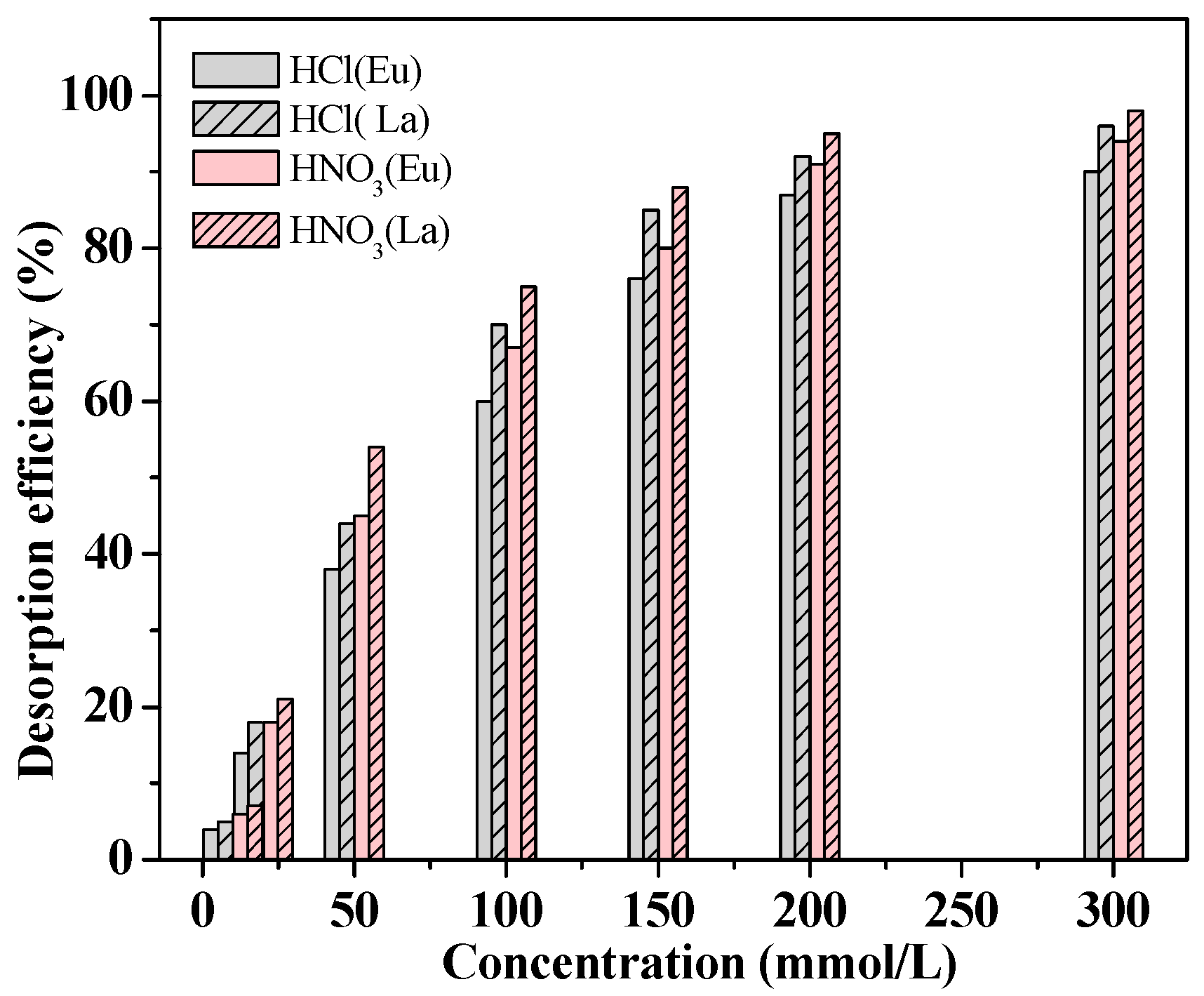
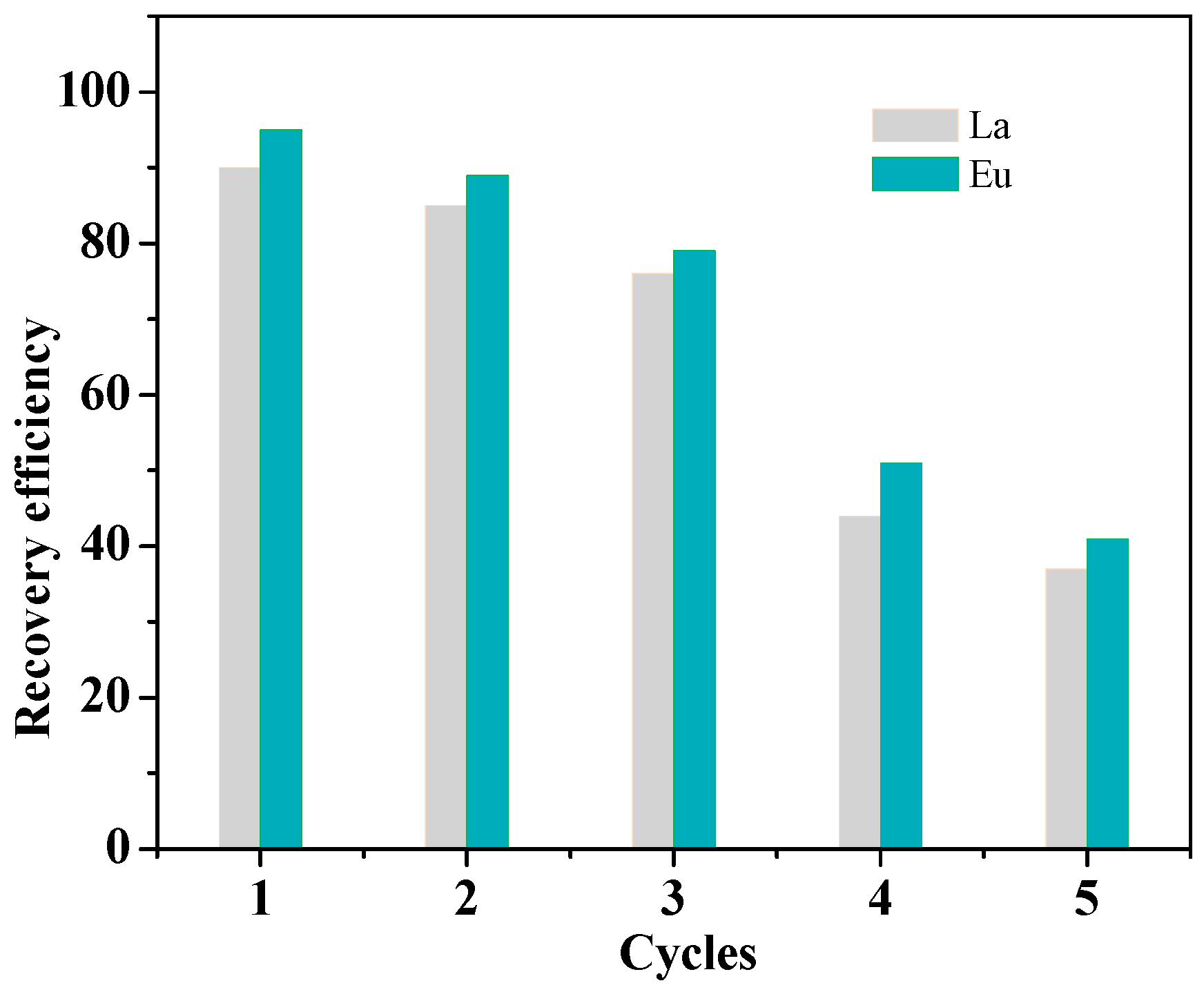
2.4. Desorption and Regeneration Experiments
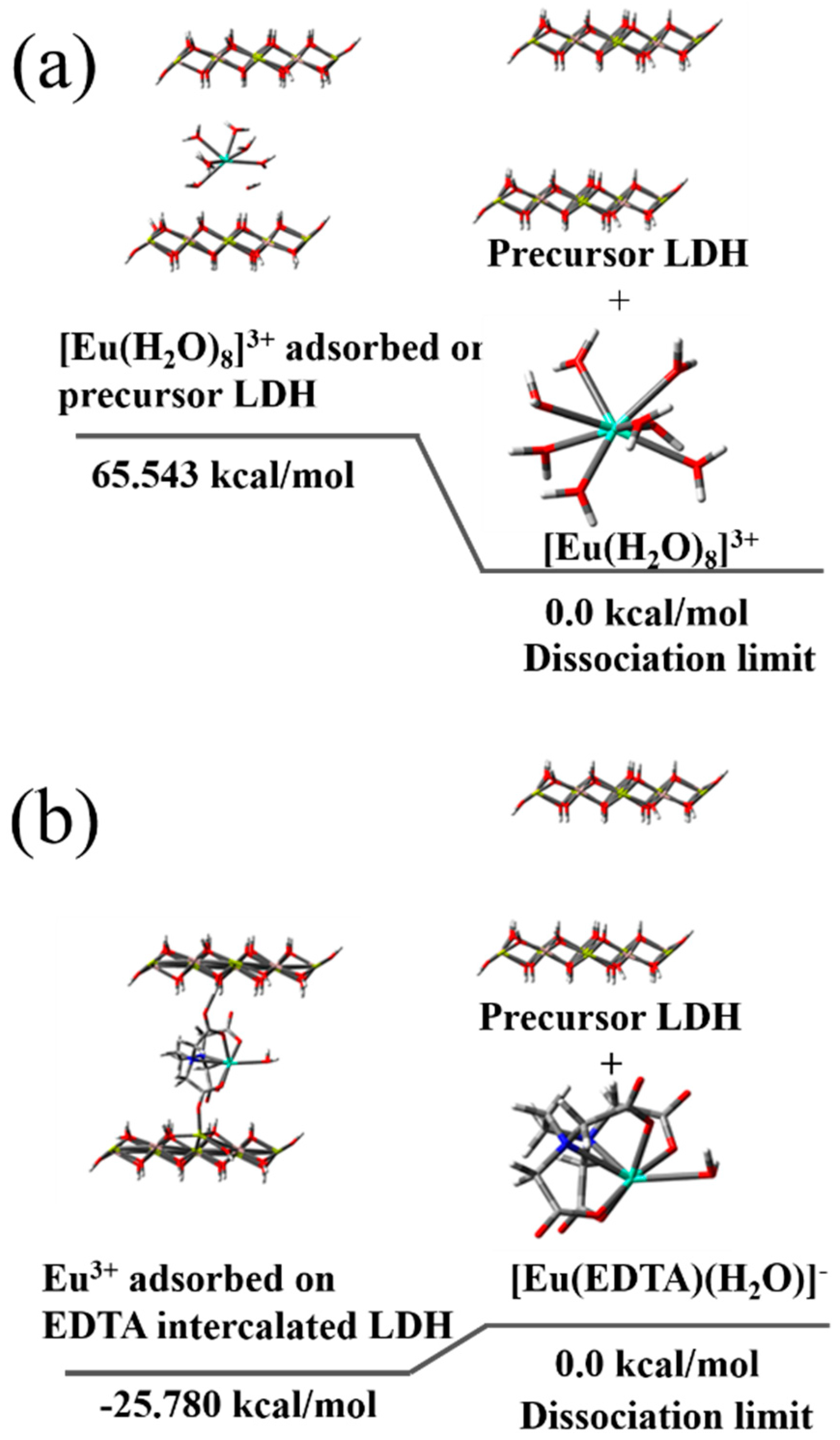
2.5. Quantum Chemistry Calculation
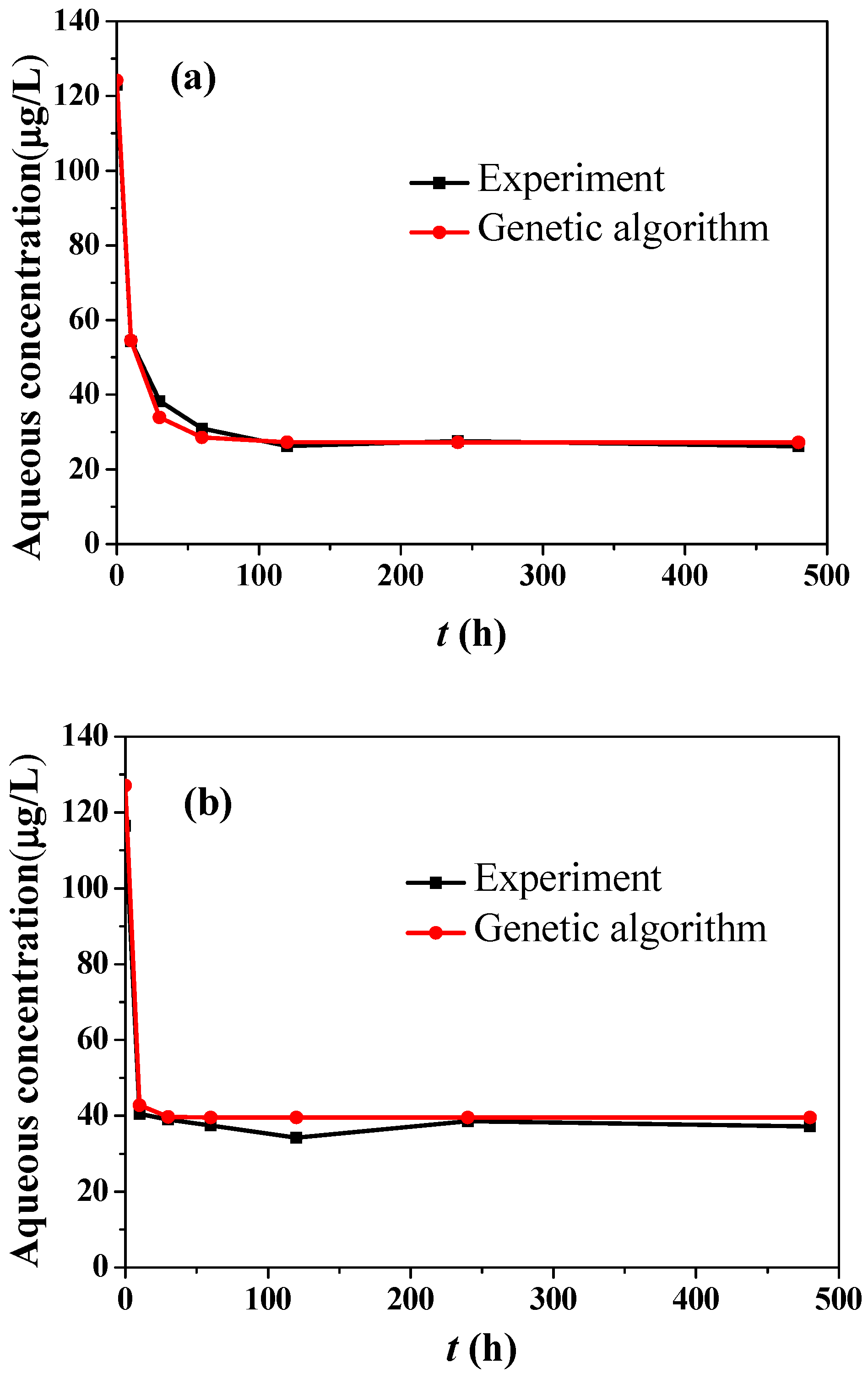
2.6. Calculation of Adsorption and Desorption Rates
3. Results and Discussion
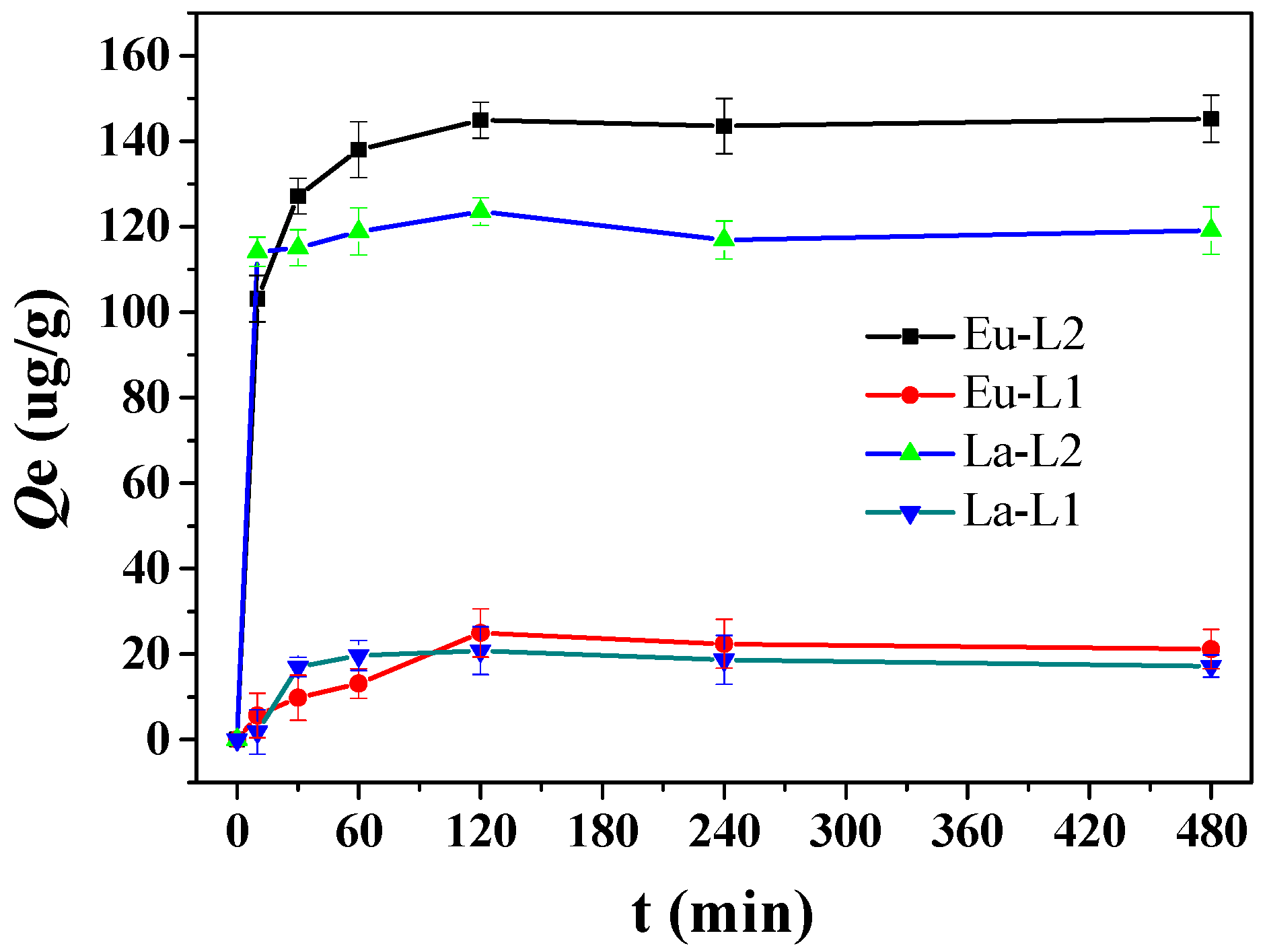
3.1. Adsorption Experiment
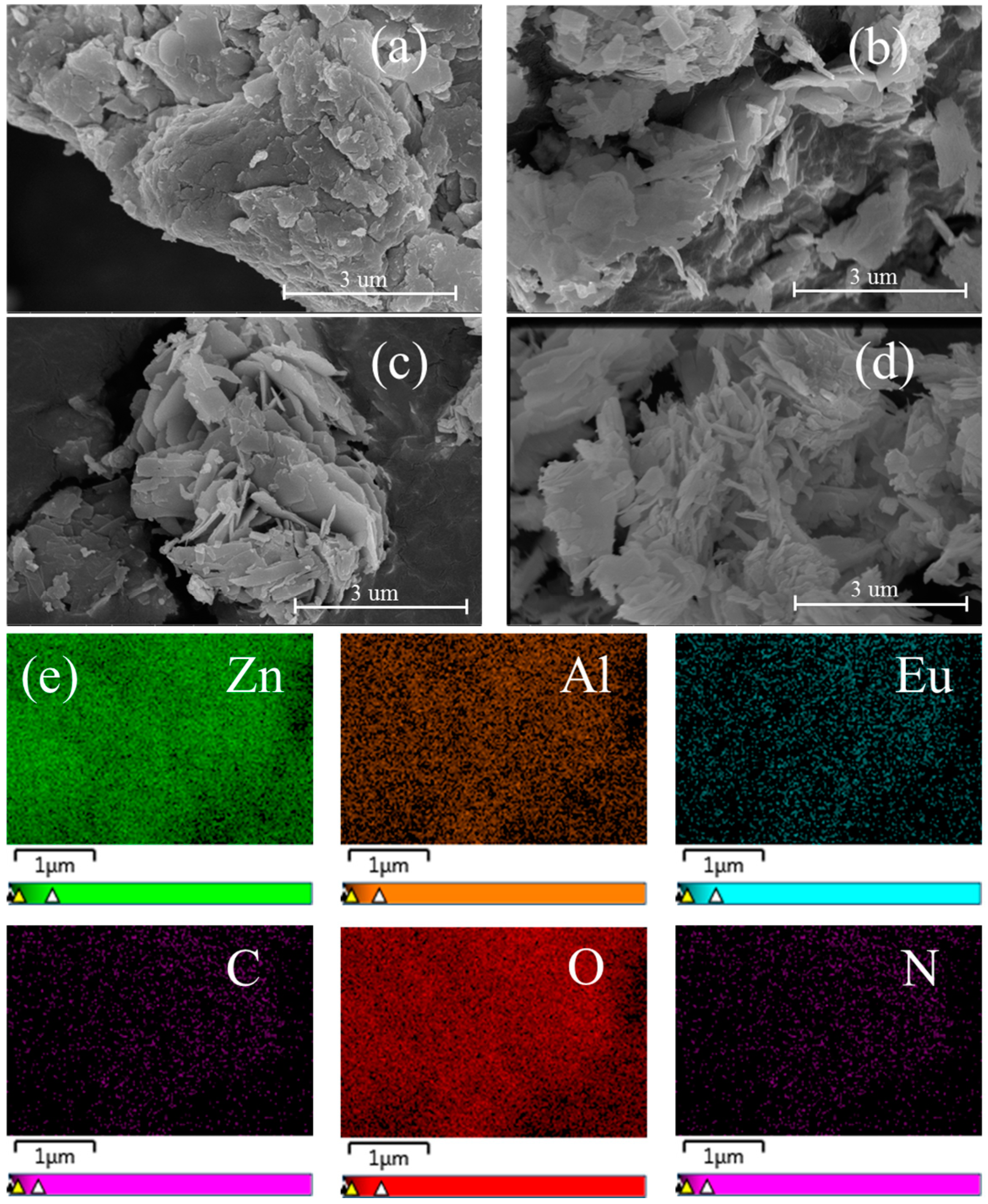
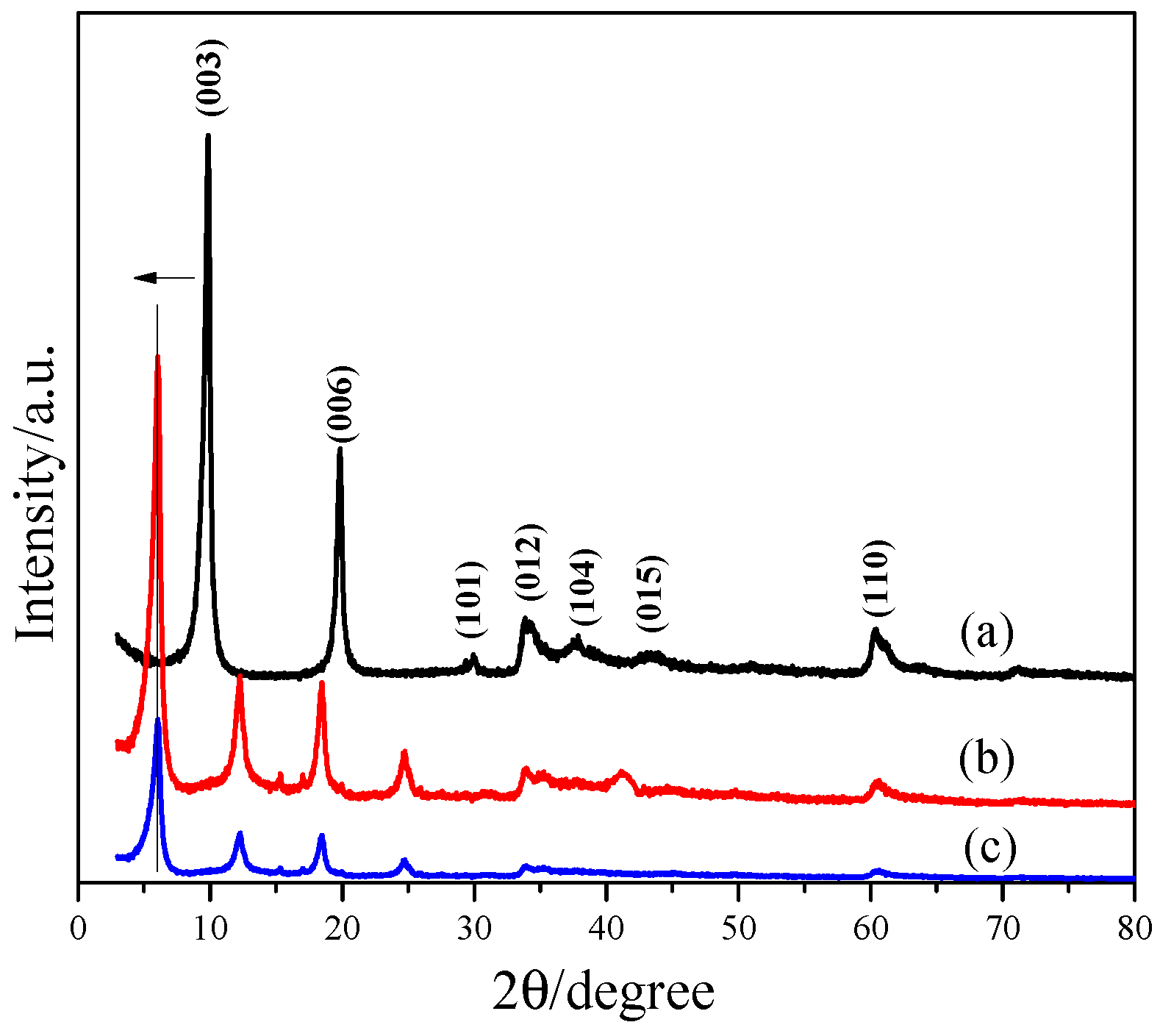
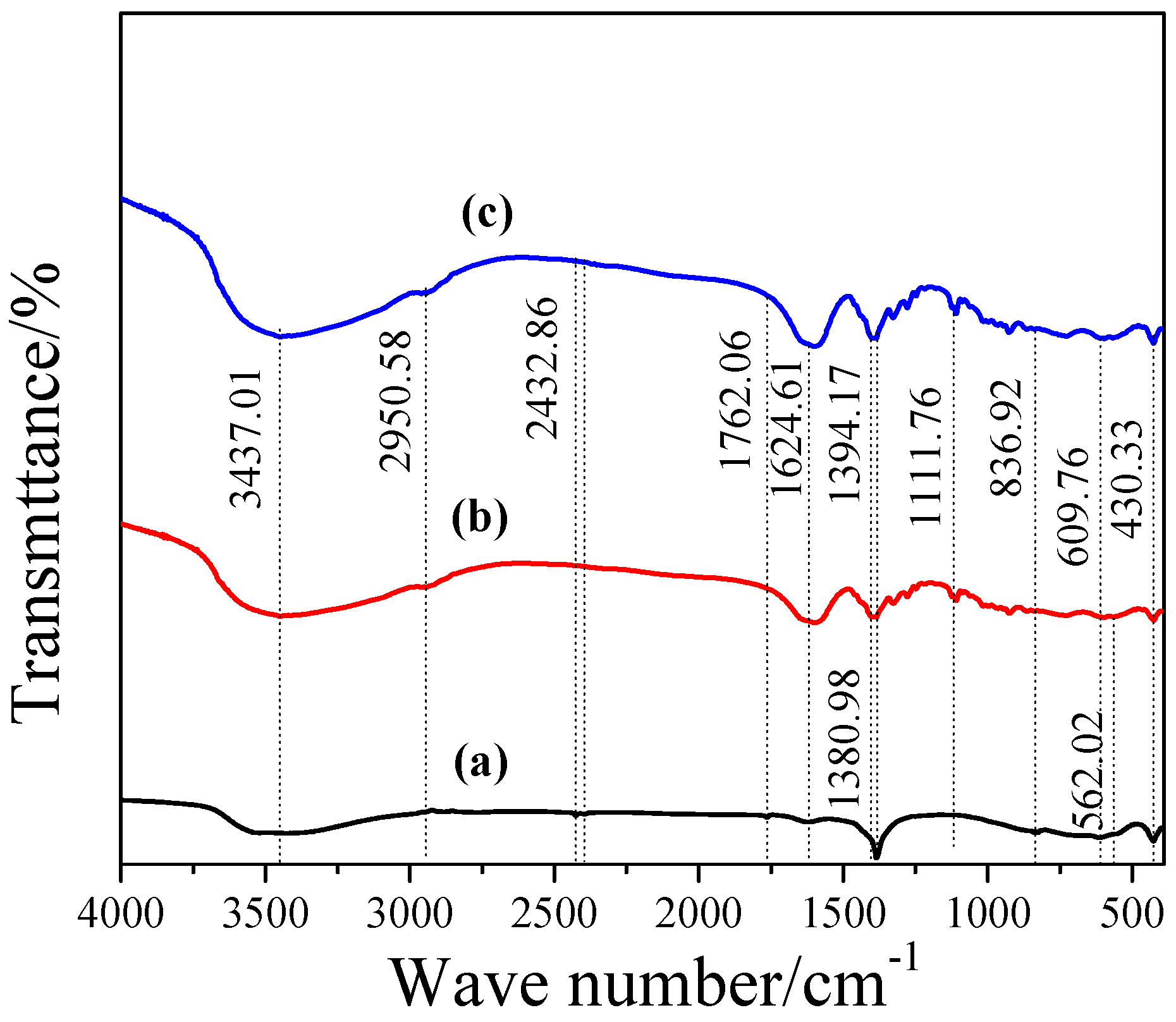
3.2. Characterization of L1 and L2
3.3. Regeneration Studies
3.4. Numerical Results of Adsorption and Desorption Rates
3.5. Quantum Chemistry Calculation
3.6. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Zou, J.; Zhao, X.R.; Jiang, X.Y.; Jiao, F.P.; Yu, J.G.; Liu, Q.; Teng, J. Facile assembly of three-dimensional cylindrical egg white embedded graphene oxide composite with good reusability for aqueous adsorption of rare earth elements. Colloids Surf. A 2019, 570, 127–140. [Google Scholar] [CrossRef]
- Toth, M.T.; Schubert, F.; Raucsik, B.; Fintor, K. Mineralogical and geochemical constraints of the REE accumulation in the almasfuzito red mud depository in northwest Hungary. Appl. Sci. 2019, 9, 3654. [Google Scholar] [CrossRef]
- Laurino, J.P.; Mustacato, J.; Huba, Z.J. Rare earth element recovery from acidic extracts of Florida Phosphate mining materials using Chelating polymer 1-Octadecene, polymer with 2,5-Furandione, Sodium salt. Minerals 2019, 9, 477. [Google Scholar] [CrossRef]
- Chour, Z.; Laubie, B.; Morel, J.L.; Tang, Y.; Qiu, R.L.; Simonnot, M.O.; Muhr, L. Recovery of rare earth elements from Dicranopteris dichotoma by an enhanced ion exchange leaching process. Chem. Eng. Process 2018, 130, 208–213. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Ramasamy, D.L.; Naseer, W.A.; Sillanpaa, M. A novel approach for synthesis of exfoliated biopolymeric-LDH hybrid nanocomposites via in-stiu coprecipitation with gum Arabic: Application towards REEs recovery. Chem. Eng. J. 2018, 347, 398–406. [Google Scholar] [CrossRef]
- Yang, M.J.; Liang, X.L.; Ma, L.Y.; Huang, J.; He, H.P.; Zhu, J.X. Adsorption of REEs on kaolinite and halloysite: A link to the REE distribution on clays in the weathering crust of granite. Chem. Geol. 2019, 525, 210–217. [Google Scholar] [CrossRef]
- Sakamoto, N.; Kano, N.; Wang, Y.; Gao, L.D.; Imaizumi, H. Biosorption of Lanthanides using three kinds of seaweed biomasses. Radioisotopes 2010, 59, 623–636. [Google Scholar] [CrossRef]
- Koto, Y.; Kano, N.; Wang, Y.; Sakamoto, N.; Imaizumi, H. Biosorption of lanthanides from aqueous solutions using pretreated Buccinum tenuissimum shell biomass. Bioinorg. Chem. Appl. 2010. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Gallo Stampino, P.; Cristiani, C.; Dotelli, G. Enhanced lanthanum adsorption by amine modified activated carbon. Chem. Eng. J. 2018, 341, 75–82. [Google Scholar] [CrossRef]
- Sotiles, A.R.; Baika, L.M.; Grassi, M.T.; Wypych, F. Cation exchange reactions in layered double hydroxides intercalated with sulfate and alkaline cations (A(H2O)6)[M2+6Al3(OH)18(SO4)2]·6H2O (M2+ = Mn, Mg, Zn; A+ = Li, Na, K). J. Am. Chem. Soc. 2019, 141, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Sotiles, A.R.; Wypych, F. Converting Mn/Al layered double hydroxide anion exchangers into cation exchangers by topotactic reactions using alkali metal sulfate solutions. Chem. Commun. 2019, 55, 7824–7827. [Google Scholar] [CrossRef] [PubMed]
- Du, J.Z.; Jin, L.; Zeng, H.Y.; Shi, X.K.; Zhou, E.G.; Feng, B.; Sheng, X. Flame retardancy of organic-anion-intercalated layered double hydroxides in ethylene vinyl acetate copolymer. Appl. Clay Sci. 2019, 180, 105193. [Google Scholar] [CrossRef]
- Zhao, P.W.; Liu, X.H.; Tian, W.L.; Yan, D.P.; Sun, X.M.; Lei, X.D. Adsolubilization of 2,4,6-trichlorophenol from aqueous solution by surfactant intercalated ZnAl layered double hydroxides. Chem. Eng. J. 2015, 279, 597–640. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Ribeiro, C. [Mg-Al]-LDH and [Zn-Al]-LDH as matrices for removal of high loadings of phosphate. Mater. Res. 2018, 21, e20171001. [Google Scholar] [CrossRef]
- Oestreicher, V.; Jobbágy, M.; Regazzoni, A.E. Halide exchange on Mg(II)-Al(III) layered double hydroxides: Exploring affinities and electrostatic predictive models. Langmuir 2014, 30, 8408–8415. [Google Scholar] [CrossRef]
- Costantino, U.; Vivani, R.; Bastianini, M.; Costantino, F.; Nocchetti, M. Ion exchange and intercalation properties of layered double hydroxides towards halide anions. Dalton Trans. 2004, 30, 11587–11596. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Chen, H.; Lin, J.H.; Zhang, N.; Chen, L.Z.; Zhong, S.P.; Wang, Y.; Zhang, W.Q.; Ling, Q.D. Preparation of MgAl-EDTA-LDH based electrospun nanofiber membrane and its adsorption properties of copper(II) from wastewater. J. Hazard. Mater. 2018, 345, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zeng, H.Y.; Liu, X.J.; Xu, S.; Chen, C.R.; Du, J.Z. Modification of MgAl hydrotalcite by ammonium sulfate for enhancement of lead adsorption. J. Taiwan Inst. Chem. Eng. 2016, 60, 361–368. [Google Scholar] [CrossRef]
- Zhang, S.; Kano, N.; Imaizumi, H. Adsorption of Cu (II), Pb (II) by Mg-Al Layered Double Hydroxides (LDHs): Intercalated with the chelating agents EDTA and EDDS. J. Chem. Eng. Jpn. 2014, 47, 324–328. [Google Scholar] [CrossRef]
- Mishima, K.; Zhang, S.; Minagawa, S.; Kano, N. Combined experimental and quantum chemical study on the adsorption mechanism of phosphorous anions on the hydrotalcite surfaces. Funct. Mater. Lett. 2016, 9, 1650061. [Google Scholar] [CrossRef]
- Smith, Y.R.; Bhattacharyya, D.; Willhard, T.; Misra, M. Adsorption of aqueous rare earth elements using carbon black derived from recycled tires. Chem. Eng. J. 2016, 296, 102–111. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Meenakshi, S. Preparation and characterization of La(III) encapsulated silica gel/chitosan composite and its metal uptake studies. J. Hazard. Mater. 2012, 203–204, 29–37. [Google Scholar] [CrossRef]
- Ren, X.M.; Chen, C.L.; Nagatsu, M.; Wang, X.K. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Yao, T.; Wu, X.W.; Chen, X.; Xiao, Y.P.; Zhang, Y.G.; Zhao, Y.L.; Li, F.B. Biosorption of Eu(III) and U(VI) on Bacillus subtilis: Macroscopic and modeling investigation. J. Mol. Liq. 2016, 219, 32–38. [Google Scholar] [CrossRef]
- Pu, M.; Zhang, B.F. Theoretical study on the microstructures of hydrotalcite lamellae with Mg/Al ratio of two. Mater. Lett. 2005, 59, 3343–3347. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Farouq, R.; Yousef, N.S. Equilibrium and kinetics studies of adsorption of copper (II) ions on natural biosorbent. Int. J. Chem. Eng. Appl. 2015, 6, 319–324. [Google Scholar] [CrossRef]
- Narita, E.; Yamagishi, T.; Tazawa, K.; Ichijo, O.; Umetsu, Y. Uptake behavior of chelating agents by magnesium-aluminum oxide precursor with reconstruction of hydrotalcite-like layer structure. Clay Sci. 1995, 9, 187–197. [Google Scholar]
- Machida, M.; Aikawa, M.; Tatsumoto, H. Prediction of simultaneous adsorption of Cu(II) and Pb(II) onto activated carbon by conventional Langmuir type equations. J. Hazard. Mater. 2005, 120, 271–275. [Google Scholar] [CrossRef] [PubMed]
- FORTRAN GA Version 1.7.1. Available online: http://www.cuaerospace.com/Technology/GeneticAlgorithm/PurchaseGeneticAlgorithm.aspx (accessed on 24 February 2019).
- Kameda, T.; Saito, S.; Umetsu, Y. Mg-Al layered double hydroxide intercalated with Ethylene diaminetetraacetate anion: Synthesis and application to the uptake of heavy metal ions from an aqueous solution. Sep. Purif. Technol. 2005, 47, 20–26. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Meng, Y.; Wang, X.; Yin, D.; Sillanpaa, M. An EDTA-β-cyclodextrin material for the adsorption of rare earth elements and its application in preconcentration of rare earth elements in seawater. J. Colloid Interface Sci. 2016, 465, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kim, K.H. Nanomaterials for the adsorptive treatment of Hg(II) ions from water. Chem. Eng. J. 2019, 358, 264–282. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Connolly, J.P. The effect of concentration of adsorbing solids on the partition coefficient. Water Res. 1980, 14, 1517–1523. [Google Scholar] [CrossRef]
- Na, C.J.; Yoo, M.J.; Tsang, D.C.W.; Kim, H.W.; Kim, K.H. High-performance materials for effective sorptive removal of formaldehyde in air. J. Hazard. Mater. 2019, 366, 452–465. [Google Scholar] [CrossRef]
- Barnabas, M.J.; Parambadath, S.; Mathew, A. Highly efficient and selective adsorption of ln3+ on pristine Zn/Al layered double hydroxide (Zn/Al-LDH) from aqueous solutions. J. Solid State Chem. 2016, 233, 133–142. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Cheng, B.; You, W.; Yu, J.G.; Ho, W.K. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr(VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. FT-Raman and FT-IR spectroscopic study of synthetic Mg/Zn/Al-hydrotalcites. J. Raman Spectrosc. 2004, 35, 967–974. [Google Scholar] [CrossRef]
- Dolatyari, L.; Yaftian, M.R.; Rostamnia, S. Adsorption characteristics of Eu(III) and Th(IV) ions onto modified mesoporous silica SBA-15 materials. J. Taiwan Inst. Chem. Eng. 2016, 60, 174–184. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, X.; Zhang, X.; Li, S.; Liu, D.; Fan, L. Removal of Cu2+ from the aqueous solution by tartrate-intercalated layered double hydroxide. Desalin. Water Treat. 2016, 57, 2064–2072. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Sillanpää, M. Synthesis and application of LDH intercalated cellulose nanocomposite for separation of rare earth elements (REEs). Chem. Eng. J. 2017, 309, 130–139. [Google Scholar] [CrossRef]
- Kano, N.; Pang, M.L.; Deng, Y.L.; Imaizumi, H. Adsorption of Rare Earth Elements (REEs) onto activated carbon modified with Potassium Permanganate (KMnO4). J. Appl. Solut. Chem. Model. 2017, 6, 51–61. [Google Scholar] [CrossRef]
- Saito, G.P.; Romero, J.H.S.; Cebim, M.A.; Davolos, M.R. Eu (III) doped LDH intercalated with cinnamate anion as multifunctional sunscreens. J. Lumin. 2018, 203, 160–164. [Google Scholar] [CrossRef]
- Shao, D.D.; Fan, Q.H.; Li, J.X.; Niu, Z.W.; Wu, W.S.; Chen, Y.X.; Wang, X.K. Removal of Eu(III) from aqueous solution using ZSM-5 zeolite. Micropor. Mesopor. Mat. 2009, 123, 1–9. [Google Scholar] [CrossRef]
- Chevinli, A.S.; Najafi, M.; Sillanpaa, M. Removal of La (III) ions from aqueous solution by Lanthanide MOF; characterization, synthesizing and process conditions study. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100216. [Google Scholar]

| Radio Frequency Power | 1400 W |
|---|---|
| Plasma gas flow | 15 L·min−1 |
| Carrier gas flow | 1.2 L·min−1 |
| Sampling depth | 6.5 mm |
| Sample uptake rate | 0.5 mL·min−1 |
| Measurement point | 3 points/peak |
| Integration time | 1.0 sec/point |
| Measured Isotope | 139La, 153Eu |
| Target Ions | Adsorbent | Effect Factor | Final Concentration (μg·L−1) | Adsorption Capacity (μg·g−1) | Partition Coefficient (μg·g−1·μM−1) | |
|---|---|---|---|---|---|---|
| Eu | L1 | Contact time (min) | 10 | 96.2 | 5.67 | 8.95 |
| 30 | 93.5 | 9.83 | 16.0 | |||
| 60 | 91.3 | 13.1 | 21.9 | |||
| 120 | 83.4 | 24.9 | 45.5 | |||
| L2 | Contact time (min) | 10 | 61.2 | 93 | 230 | |
| 30 | 38.2 | 127 | 506 | |||
| 60 | 31.0 | 138 | 676 | |||
| 120 | 26.4 | 145 | 834 | |||
| Initial concentration (μg·L−1) | 20 | 7.8 | 24.0 | 468 | ||
| 50 | 18.2 | 45.0 | 376 | |||
| 200 | 54.6 | 165 | 275 | |||
| 300 | 153 | 222 | 220 | |||
| La | L1 | Contact time (min) | 10 | 98.8 | 1.8 | 2.53 |
| 30 | 88.7 | 17 | 26.6 | |||
| 60 | 86.9 | 19.7 | 31.5 | |||
| 120 | 86.1 | 20.8 | 33.6 | |||
| L2 | Contact time (min) | 10 | 23.9 | 114 | 341 | |
| 30 | 46.3 | 115 | 346 | |||
| 60 | 46.3 | 119 | 345 | |||
| 120 | 40.8 | 124 | 405 | |||
| Initial concentration (μg·L−1) | 20 | 40.7 | 17.4 | 422 | ||
| 50 | 20.9 | 39.6 | 263 | |||
| 200 | 101 | 148 | 203 | |||
| 300 | 163 | 205 | 174 | |||
| Sample | L1 | L2 |
|---|---|---|
| Zeta potentials | 35.3 mV | 20.2 mV |
| Sample | %wt | Atomic Ratios | Ln Absorbed (μg·g−1) | Proposed Formula | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | N | H | Zn/Al | C/N | H/N | La | Eu | ||
| L1 | 0.06 | 4.26 | 2.38 | 2.10 | 0.00 | 7.82 | 15.9 | 18.4 | [Zn2Al (OH)6]NO3 |
| L2 | 13.7 | 3.35 | 3.60 | 1.67 | 0.31 | 15.1 | 87.6 | 103 | [Zn2Al(OH)6]2[C10H14N2O8] |
| Target Ion | ||
|---|---|---|
| Eu(III) | 0.001535 | 0.5686 |
| La(III) | 0.001001 | 0.2176 |
| Target Ions | Materials | Final Concentration (μg·L−1) | Adsorption Capacity (μg·g−1) | Partition Coefficient (μg·g−1·μM−1) | Reference |
|---|---|---|---|---|---|
| Eu | Activated carbon | 61.2 | 92.7 | 230 | [45] |
| Buccinum tenuissimum | 34.7 | 98 | 429 | [8] | |
| Sargassum hemiphyllum | 20.7 | 119 | 746 | [7] | |
| Fe3O4@MnOx | 28.1 × 103 | 138 × 103 | 745 | [46] | |
| ZSM-5 zeolite | 30.4 | 24.2 | 121 | [47] | |
| L1 (ZnAl-NO3) | 83.4 | 24.9 | 45.5 | This study | |
| L2 (ZnAl-EDTA) | 26.4 | 145 | 834 | This study | |
| La | Activated carbon | 52.7 | 71.0 | 205 | [45] |
| Buccinum tenuissimum | 34.0 | 99.0 | 401 | [8] | |
| Sargassum hemiphyllum | 35.1 | 97.3 | 421 | [7] | |
| Schizymenia dubyi | 39.7 | 90.5 | 392 | [7] | |
| Lanthanide MOF | 62.0 × 103 | 73.0 × 103 | 179 | [48] | |
| L1 (ZnAl-NO3) | 86.1 | 20.8 | 33.6 | This study | |
| L2 (ZnAl-EDTA) | 17.7 | 124 | 422 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Kano, N.; Mishima, K.; Okawa, H. Adsorption and Desorption Mechanisms of Rare Earth Elements (REEs) by Layered Double Hydroxide (LDH) Modified with Chelating Agents. Appl. Sci. 2019, 9, 4805. https://doi.org/10.3390/app9224805
Zhang S, Kano N, Mishima K, Okawa H. Adsorption and Desorption Mechanisms of Rare Earth Elements (REEs) by Layered Double Hydroxide (LDH) Modified with Chelating Agents. Applied Sciences. 2019; 9(22):4805. https://doi.org/10.3390/app9224805
Chicago/Turabian StyleZhang, Shuang, Naoki Kano, Kenji Mishima, and Hirokazu Okawa. 2019. "Adsorption and Desorption Mechanisms of Rare Earth Elements (REEs) by Layered Double Hydroxide (LDH) Modified with Chelating Agents" Applied Sciences 9, no. 22: 4805. https://doi.org/10.3390/app9224805
APA StyleZhang, S., Kano, N., Mishima, K., & Okawa, H. (2019). Adsorption and Desorption Mechanisms of Rare Earth Elements (REEs) by Layered Double Hydroxide (LDH) Modified with Chelating Agents. Applied Sciences, 9(22), 4805. https://doi.org/10.3390/app9224805





