α-Mangostin Hydrogel Film Based Chitosan–Alginate for Recurrent Aphthous Stomatitis
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
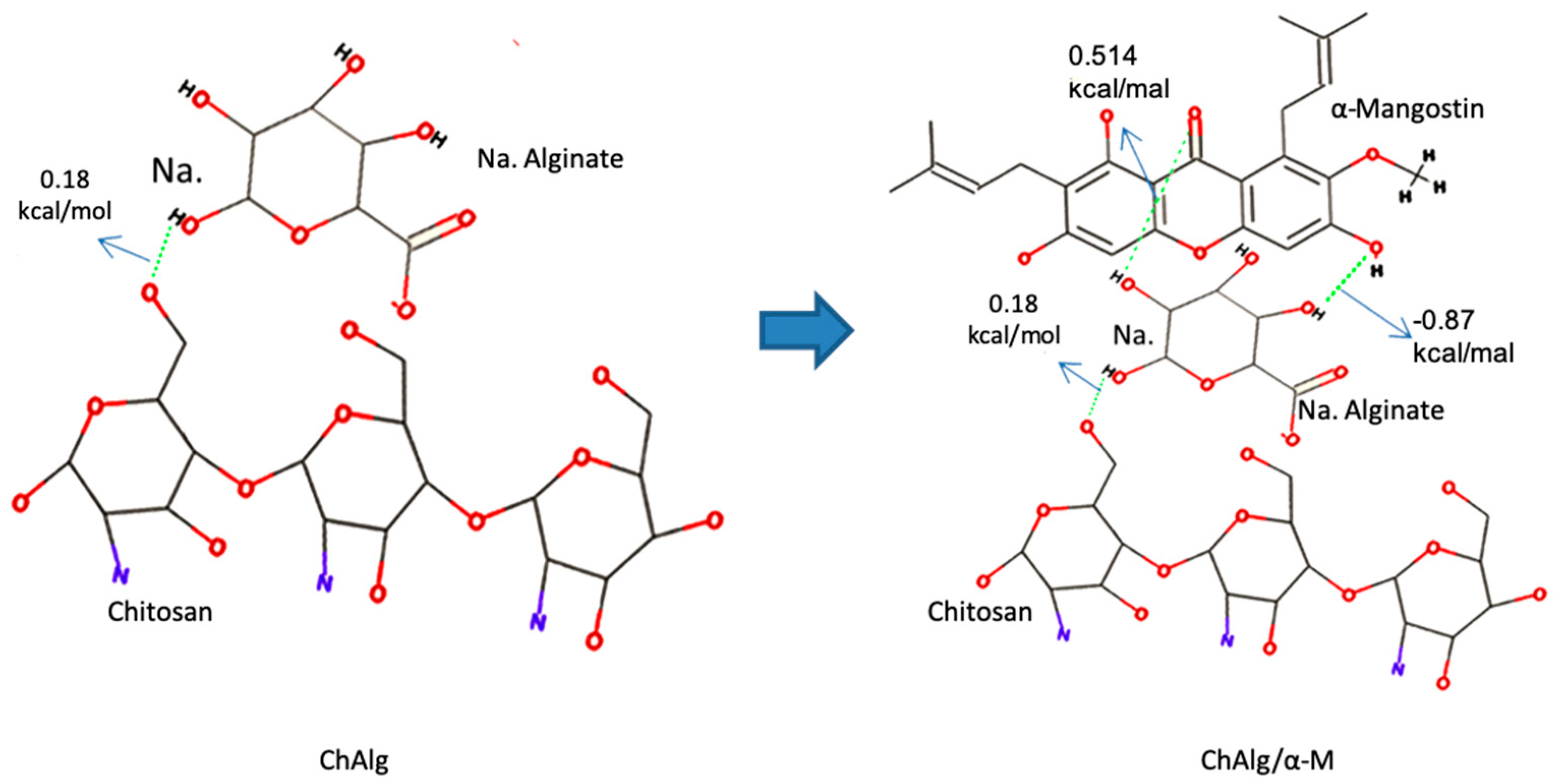
2.2.1. Molecular Docking
2.2.2. Preparation of ChAlg HF
2.2.3. Preparation of ChAlg/α-M HF
2.2.4. Scanning Electron Microscope (SEM)
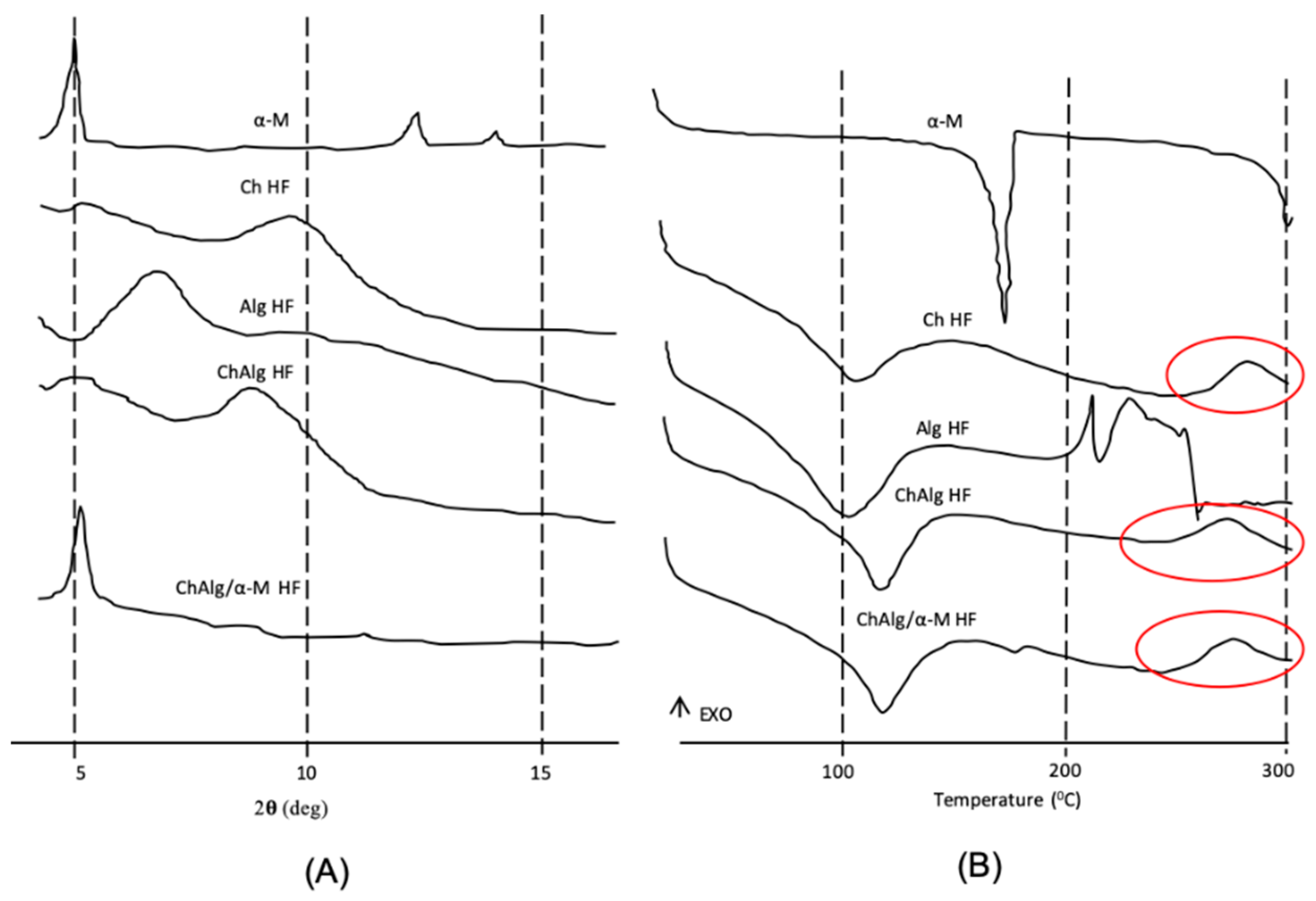
2.2.5. X-Ray Diffractometry (XRD)
2.2.6. Differential Scanning Calorimetry (DSC)
2.2.7. Mechanical Properties
2.2.8. Degradability Study
2.2.9. Swelling Ability
2.2.10. In Vitro Release
2.2.11. In Vitro Mucoadhesive Study
3. Statistical Analysis
4. Results and Discussion
4.1. Molecular Docking
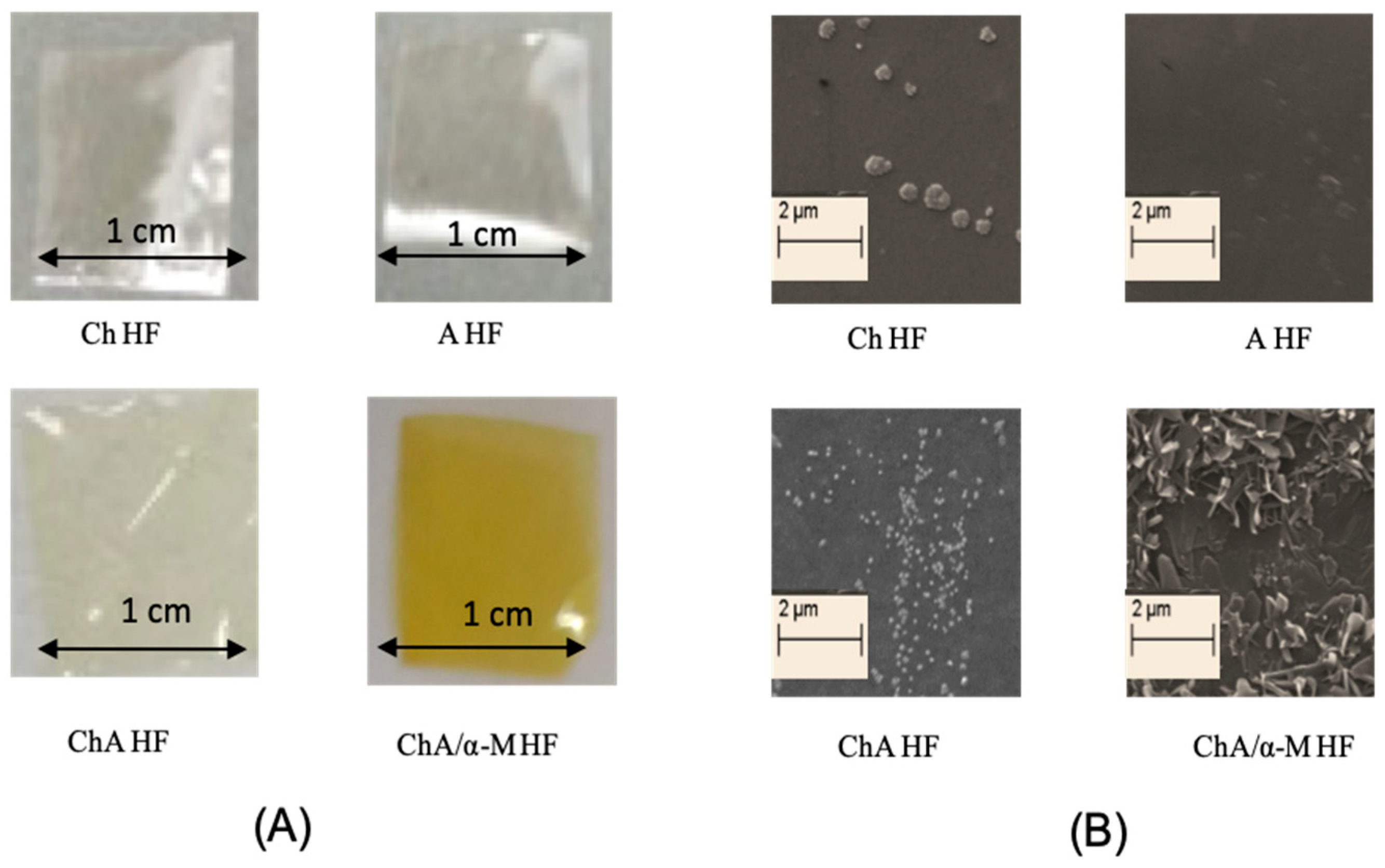
4.2. Preparation and Physicochemical Characterizations
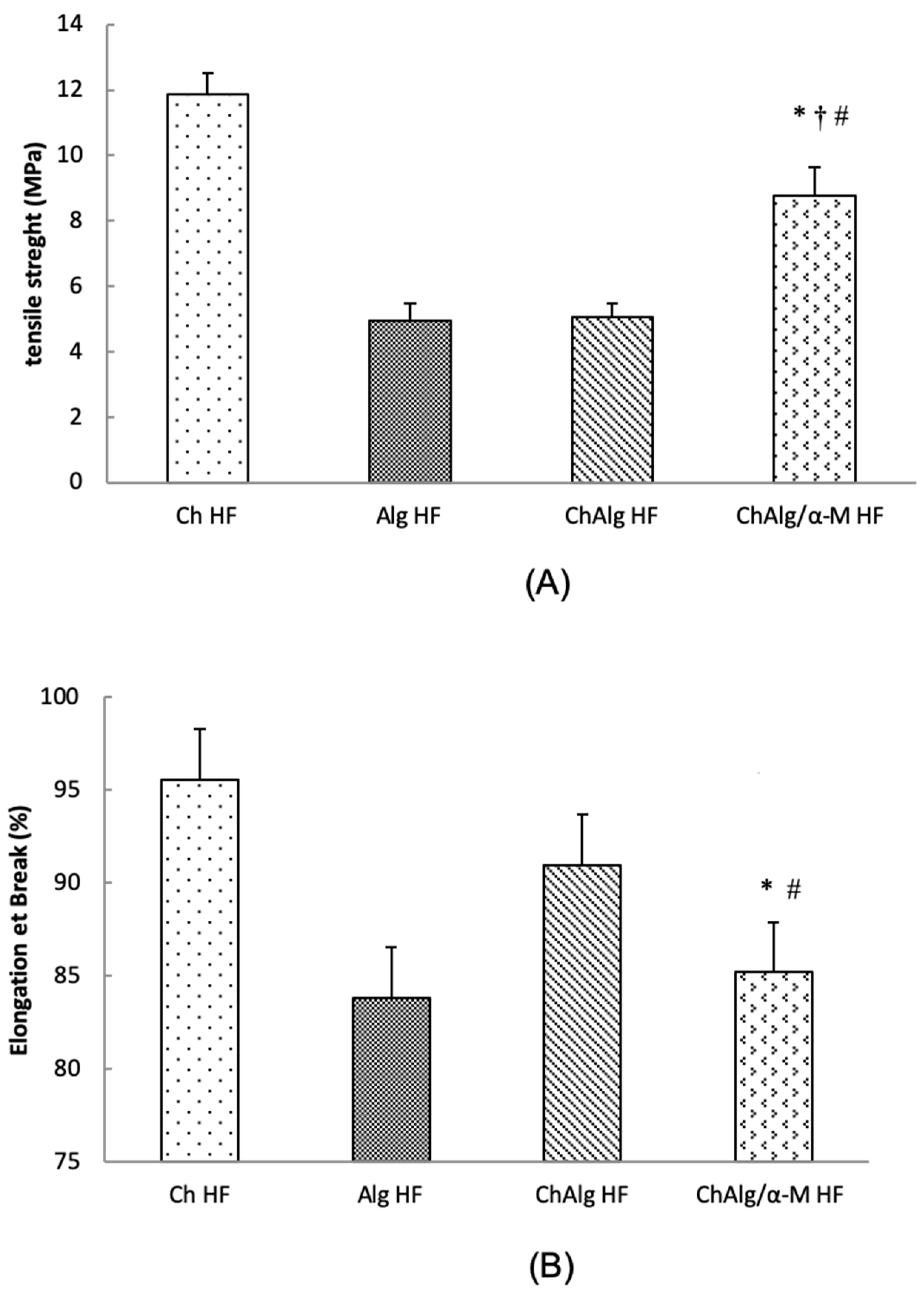
4.3. Mechanical Properties
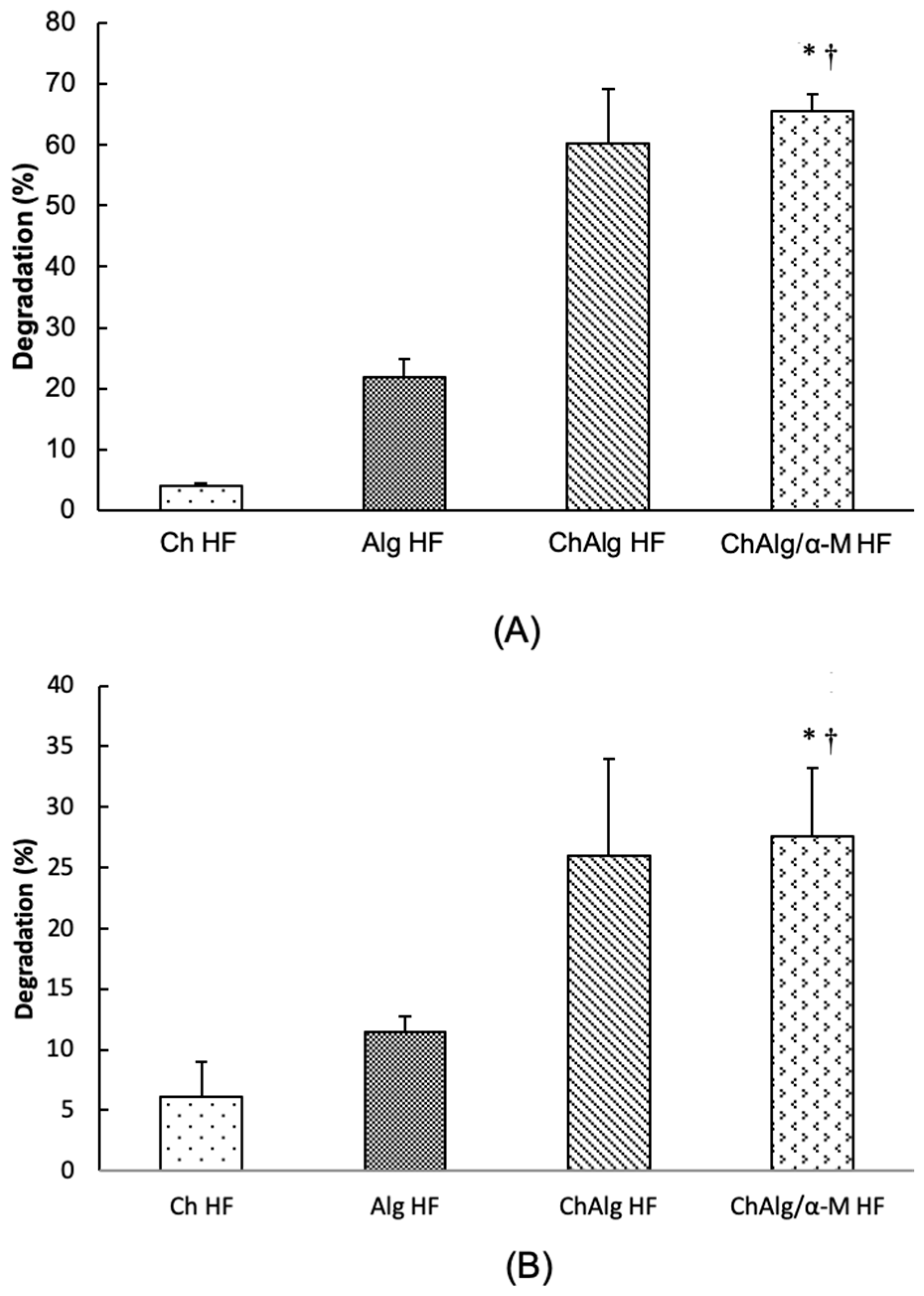
4.4. Degradability Study
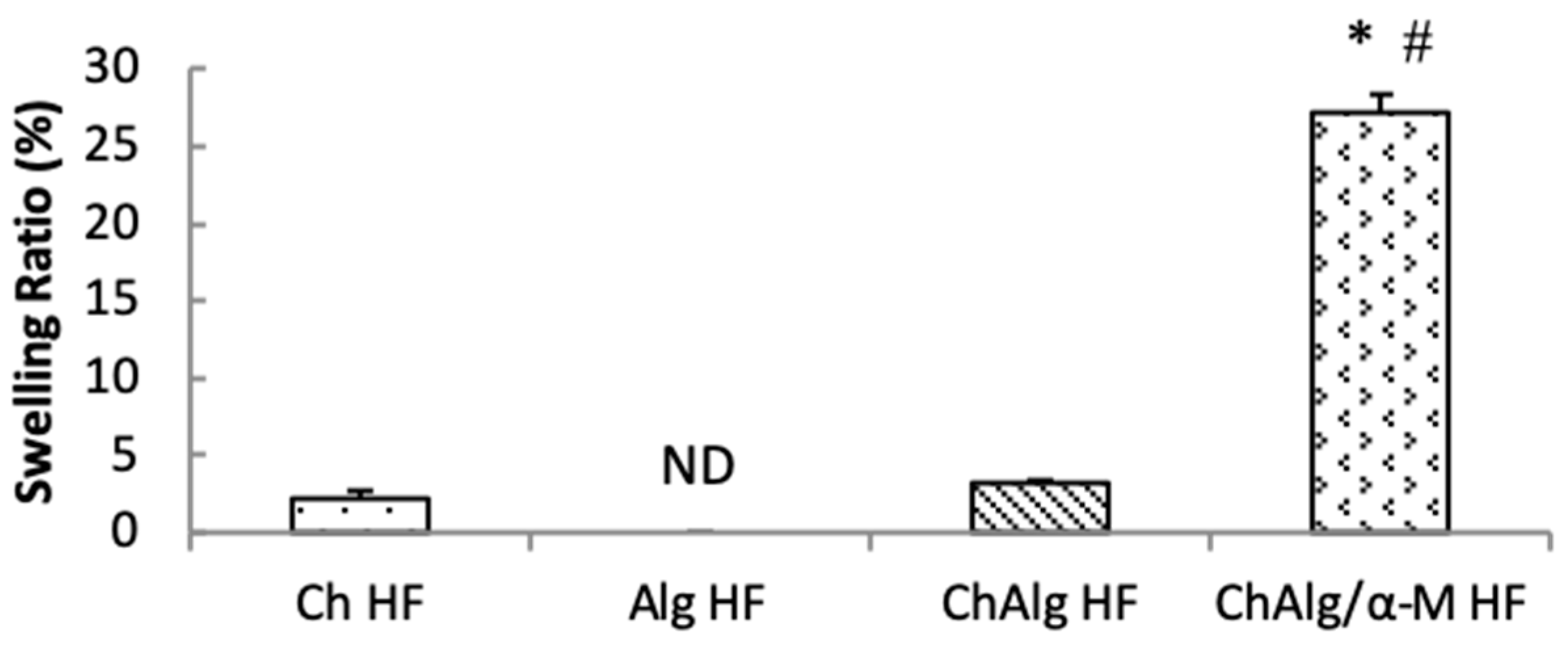
4.5. Swelling Ability
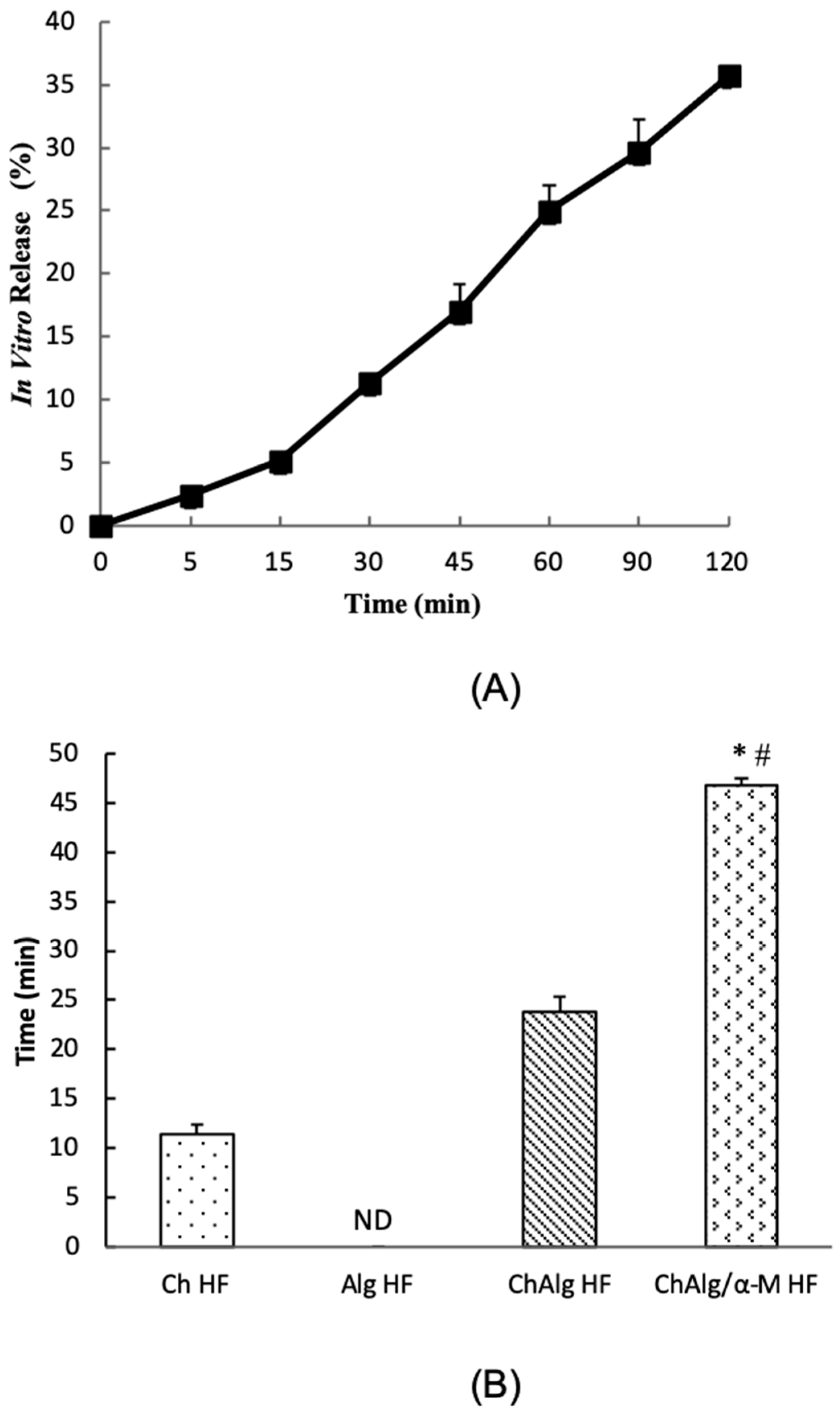
4.6. In Vitro Release Study
4.7. Mucoadhesive Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chavan, M.; Jain, H.; Diwan, N.; Khedkar, S.; Shete, A.; Durkar, S. Recurrent aphthous stomatitis: A review. J. Oral Pathol. Med. 2012, 41, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Sunarjo, L.; Hendari, R.; Rimbyastuti, H. Manfaat xanthone terhadap kesembuhan ulkus rongga mulut dilihat dari jumlah sel PMN dan fibroblast. Odonto Dent. J. 2015, 2, 14–21. [Google Scholar] [CrossRef]
- Kardono, L.B.S.; Basuki, T.; Padmawinata, K. Selected Indonesian Medical Plant Monograph and Description; Penerbit PT Grasindo Gramedia Widiasarana Indonesia: Jakarta, Indonesia, 2003. [Google Scholar]
- Zand, N.; Ataie-Fashtami, L.; Djavid, G.E.; Fateh, M.; Alinaghizadeh, M.R.; Fatemi, S.M.; Arbabi-Kalati, F. Relieving pain in minor aphthous stomatitis by a single session of non-thermal carbon dioxide laser irradiation. Lasers Med. Sci. 2009, 24, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garciana mangostana L.: A phytochemical and Pharmacological review. Phytoterapy Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.; Li, W.; Gao, P.; Xiang, D.; Ren, X.; Liu, D. Mucoadhesive buccal film containing ornidazole and dexamethasone for oral ulcers: in vitro and in vivo studies. Pharm. Dev. Technol. 2018, 24, 118–126. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Yaprak Karavana, S.; Güneri, P.; Ertan, G. Benzydamine hydrochloride buccal bioadhesive gels designed for oral ulcers: Preparation, rheological, textural, mucoadhesive and release properties. Pharm. Dev. Technol. 2009, 14, 623–631. [Google Scholar] [CrossRef]
- Abdullah, E.; Idris, A.; Saparon, A. Papr reduction using scs-slm technique in stfbc mimo-ofdm. Arpn J. Eng. Appl. Sci. 2017, 12, 3218–3221. [Google Scholar]
- Kumar, P.T.S.; Srinivasan, S.; Lakshmanan, V.K.; Tamura, H.; Nair, S.V.; Jayakumar, R. Synthesis, characterization and cytocompatibility studies of α-chitin hydrogel/nano hydroxyapatite composite scaffolds. Int. J. Biol. Macromol. 2011, 49, 20–31. [Google Scholar] [CrossRef]
- Qin, Y. Alginate fibers: An overwiew of the production processes and applications in wound management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Meng, X.; Tian, F.; Yang, J.; He, C.N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Salis, A.; Porcu, E.P.; Giunchedi, P.; Roldo, M.; Gavini, E. Composite chitosan/alginate hydrogel for controlled release of deferoxamine: A system to potentially treat iron dysregulation diseases. Carbohydr. Polym. 2016, 136, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Kole, S.G.; Gurav, P.B.; Vilegave, K.V. Natural polymer based mucoadhesive hydrogel beads of nizatidine: Preparation, characterization and evaluation. Indian J. Pharm. Educ. Res. 2016, 50, 159–169. [Google Scholar] [CrossRef]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 2018, 123, 441–451. [Google Scholar] [CrossRef]
- Menchicchi, B.; Fuenzalida, J.P.; Bobbili, K.B.; Hensel, A.; Swamy, M.J.; Goycoolea, F.M. Structure of chitosan determines its interactions with mucin. Biomacromolecules 2014, 15, 3550–3558. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Yusuf, M. Molecular Docking, 1st ed.; Unpad Press: Umedang Regenc, Indonesia, 2018. [Google Scholar]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int. J. Biol. Macromol. 2016, 89, 465–470. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T. Enhancement of curcumin wound healing ability by complexation with 2-hydroxypropyl-Y-cyclodextrin in sacran hydrogel film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef]
- Wathoni, N.; Hasanah, A.N.; Mohammed, A.F.A.; Pratiwi, E.D.; Mahmudah, R. Accelerated wound healing ability of sacran hydrogel film by keratinocyte growth factor in alloxan-induced diabetic mice. Int. J. Appl. Pharm. 2018, 10, 57–61. [Google Scholar] [CrossRef][Green Version]
- Wathoni, N.; Insani, U.C. Characterization and optimization of natural maltodextrin-based niosome. J. Appl. Pharm. Sci. 2013, 3, 68–71. [Google Scholar]
- Wathoni, N.; Rusdiana, T.; Hasanah, A.N.; Muhtadi, A.; Pratiwi, E.D.; Mahmudah, R.; Mohammed, A.F.A.; Okajima, M.; Kaneko, T.; Arima, H. Sacran Hydrogel Film Containing Keratinocyte Growth Factor Accelerates Wound Healing by Stimulating Fibroblast Migration and Re-epithelization. Chem. Pharm. Bull. 2019, 67, 849–854. [Google Scholar] [CrossRef]
- Wathoni, N.; Yuan Shan, C.; Yi Shan, W.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.I.; Haider, M.; Ali, M.A.M. Buccal Mucoadhesive Films Containing Antihypertensive Drug. J. Chem. Pharm. Res. 2011, 3, 665–686. [Google Scholar]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; Arima, H. Potential Use of a Megamolecular Polysaccharide Sacran as a Hydrogel-Based Sustained Release System. Chem. Pharm. Bull. 2014, 62, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Lala, R.; Thorat, A.A.; Gargote, C.S.; Awari, N.G. Preparation of buccoadhesive polymeric film of ketoprofen and its evaluation. Asian J. Pharm. Sci. 2011, 6, 267–274. [Google Scholar]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan-sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005. [Google Scholar] [CrossRef]
- Sæther, H.V.; Holme, H.K.; Maurstad, G.; Smidsrød, O.; Stokke, B.T. Polyelectrolyte complex formation using alginate and chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar] [CrossRef]
- Yu, C.Y.; Yin, B.C.; Zhang, W.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloids Surf. B Biointerfaces 2009, 68, 245–249. [Google Scholar] [CrossRef]
- Mulia, K.; Halimah, N.; Krisanti, E. Effect of alginate composition on profile release and characteristics of chitosanalginate microparticles loaded with mangosteen extract. In Proceedings of the AIP Conference, Vladivostok, Russia, 18–22 September 2016; American Institute of Physics: College Park, MD, USA, 2017; p. 020010. [Google Scholar]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate-chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Silk fibroin protein and chitosan polyelectrolyte complex porous scaffolds for tissue engineering applications. Carbohydr. Polym. 2011, 85, 325–333. [Google Scholar] [CrossRef]
- Neto, C.G.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Jacobsen, J.; Meng-Lund, E.; Muff-Westergaard, C.; Sander, C.; Madelung, P. A mechanistic based approach for enhancing buccal mucoadhesion of chitosan. Int. J. Pharm. 2014, 461, 280–285. [Google Scholar]
- Sarheed, O.; Rasool, B.K.A.; Abu-gharbieh, E.; Aziz, U.S. An Investigation and Characterization on Alginate Hydogel Dressing Loaded with Metronidazole Prepared by Combined Inotropic Gelation and Freeze-Thawing Cycles for Controlled Release. Aaps Pharmscitech. 2015, 16, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Özyazıcı, M.; Fırlak, M.; Tanrıverdı, S.T.; Rençber, S.; Karavana, Y.; Kahraman, M.V. Bioadhesive Gel and Hydrogel Systems for Buccal Delivery of Ketoprofen: Preparation and In vitro Evaluation Studies. Am. J. Drug Deliv. 2015, 2, 078–091. [Google Scholar]
- Castel-Molieres, M.; Conzatti, G.; Torrisani, J.; Rouilly, A.; Cavalie, S.; Carrere, N.; Tourrette, A. Influence of Homogenization Technique and Blend Ratio on Chitosan/Alginate Polyelectrolyte Complex Properties. J. Med. Biol. Eng. 2017, 38, 10–21. [Google Scholar] [CrossRef]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 15, 142–151. [Google Scholar] [CrossRef]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Goh, M.C.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Lazim, A.M.; Eastoe, J.; Bradley, M.; Trickett, K.; Rogers, S.E. Recovery of gold nanoparticles using pH-sensitive microgels. Soft Matter 2010, 6, 2050–2055. [Google Scholar] [CrossRef]
- Ahmad, M.; Yamin, B.M.; Lazim, A.M. A study on dispersion and characterisation of α -mangostin loaded pH sensitive microgel systems. Chem. Cent. J. 2013, 7, 1. [Google Scholar] [CrossRef]
- Rajendran, A.; Basu, S.K. Alginate-Chitosan Particulate System for Sustained Release of Nimodipine. Trop. J. Pharm. Res. 2009, 8, 433–440. [Google Scholar] [CrossRef][Green Version]
- Das Neves, J.; Sarmento, B. Mucosal delivery of biopharmaceuticals: Biology, challenges and strategies. Mucosal Deliv. Biopharm. Biol. Chall. Strat. 2014, 9781461495, 1–601. [Google Scholar]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Sizílio, R.H.; Galvão, J.G.; Trindade, G.G.G.; Pina, L.T.S.; Andrade, L.N.; Gonsalves, J.K.M.C.; Lira, A.A.M.; Chaud, M.V.; Alves, T.F.R.; Arguelho, M.L.P.M.; et al. Chitosan/pvp-based mucoadhesive membranes as a promising delivery system of betamethasone-17-valerate for aphthous stomatitis. Carbohydr. Polym. 2018, 190, 339–345. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wathoni, N.; Yuniarsih, N.; Cahyanto, A.; Muhctaridi, M. α-Mangostin Hydrogel Film Based Chitosan–Alginate for Recurrent Aphthous Stomatitis. Appl. Sci. 2019, 9, 5235. https://doi.org/10.3390/app9235235
Wathoni N, Yuniarsih N, Cahyanto A, Muhctaridi M. α-Mangostin Hydrogel Film Based Chitosan–Alginate for Recurrent Aphthous Stomatitis. Applied Sciences. 2019; 9(23):5235. https://doi.org/10.3390/app9235235
Chicago/Turabian StyleWathoni, Nasrul, Nia Yuniarsih, Arief Cahyanto, and Muhctaridi Muhctaridi. 2019. "α-Mangostin Hydrogel Film Based Chitosan–Alginate for Recurrent Aphthous Stomatitis" Applied Sciences 9, no. 23: 5235. https://doi.org/10.3390/app9235235
APA StyleWathoni, N., Yuniarsih, N., Cahyanto, A., & Muhctaridi, M. (2019). α-Mangostin Hydrogel Film Based Chitosan–Alginate for Recurrent Aphthous Stomatitis. Applied Sciences, 9(23), 5235. https://doi.org/10.3390/app9235235








