Genipin Attachment of Conjugated Gold Nanoparticles to a Decellularized Tissue Scaffold
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Harvest and Decellularization
2.2. Genipin, Gold Nanoparticles and the Crosslinking Procedure
2.3. Experimental Groups
- Untreated: porcine diaphragm tendon that underwent decellularization protocol.
- 15 min, 1 h, 4 h, 8 h, 24 h Au Gen: decellularized tissue crosslinked with 0.25 mL of functionalized 20 nm gold nanoparticles at the stock and 1 mL of genipin at 3 mM. Crosslinking time ranged from 15 min to 24 h.
- 15 min, 1 h, 4 h, 8 h, 24 h Gen: decellularized tissue crosslinked with 0.25 mL of PBSand 1 mL of genipin at 3 mM. Crosslinking time ranged from 15 min to 24 h. This group was crosslinked with genipin but without the addition of AuNPs.
- 15 min, 1 h, 4 h, 8 h, 24 h Au: decellularized tissue crosslinked with 0.25 mL of functionalized 20 nm gold nanoparticles at the stock and 1 mL of PBS. Crosslinking time ranged from 15 min to 24 h. This group was conjugated with nanoparticles but without the addition of genipin.
- EDC/NHS crosslinked tissue: decellularized tissue that were crosslinked with the chemical crosslinkers 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) (Thermo Fisher Scientific, Waltham, MA, USA) and N-hydroxysuccinimide (NHS) (Thermo Fisher Scientific, Waltham, MA) according to a previously published protocol [26]. Briefly, 2mM EDC 2-(N-Morpholino)ethanesulfonic acid buffer and combined with 5mM NHS in dimethyl formamide. These are then added to a 50:50 (v/v) acetone:phosphate buffered saline mixture. The tissue is incubated in 0.25 mL of crosslinking solution for 15 min. The tissue is then incubated overnight and then rinsed for 48 h in PBS at 225 rpm. EDC/NHS is a zero-length crosslinker commonly utilized to crosslinked tissue. It was utilized as a control in the DSC studies.
2.4. Neutron Activation Analysis
2.5. Modulated Differential Scanning Calorimetry
2.6. Cell Culture
2.7. Cell Viability
2.8. dsDNA Assay
2.9. Scanning Electron Microscopy
2.10. Statistical Analysis
3. Results
3.1. Neutron Activation Analysis
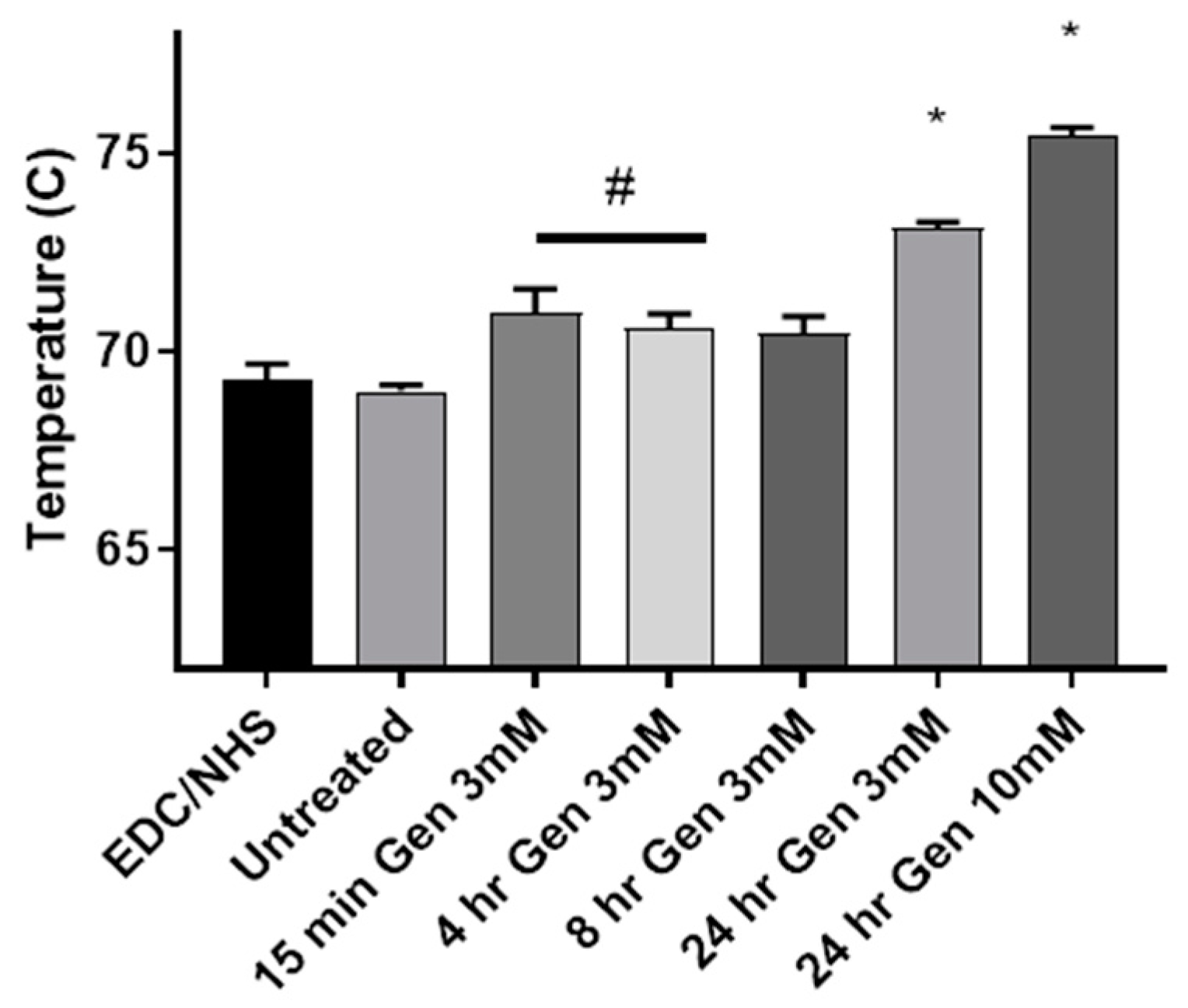
3.2. Modulated Differential Scanning Calorimetry
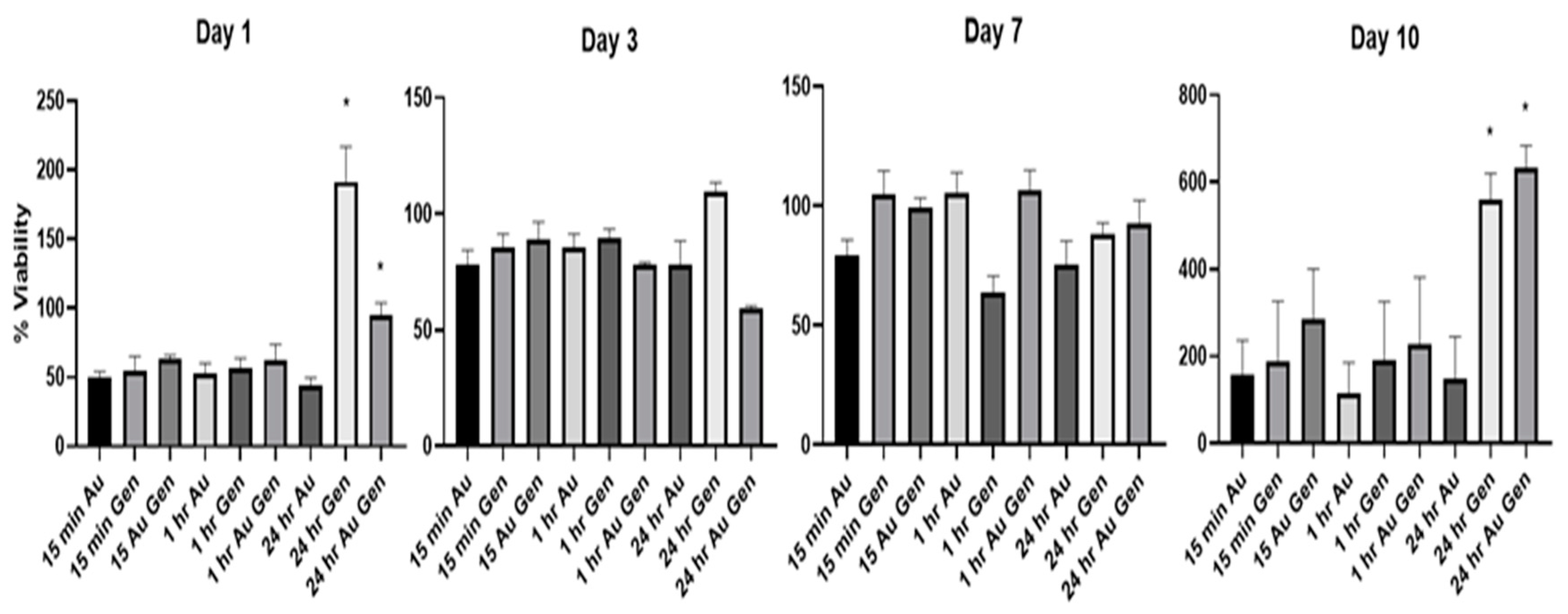
3.3. Cell Viability
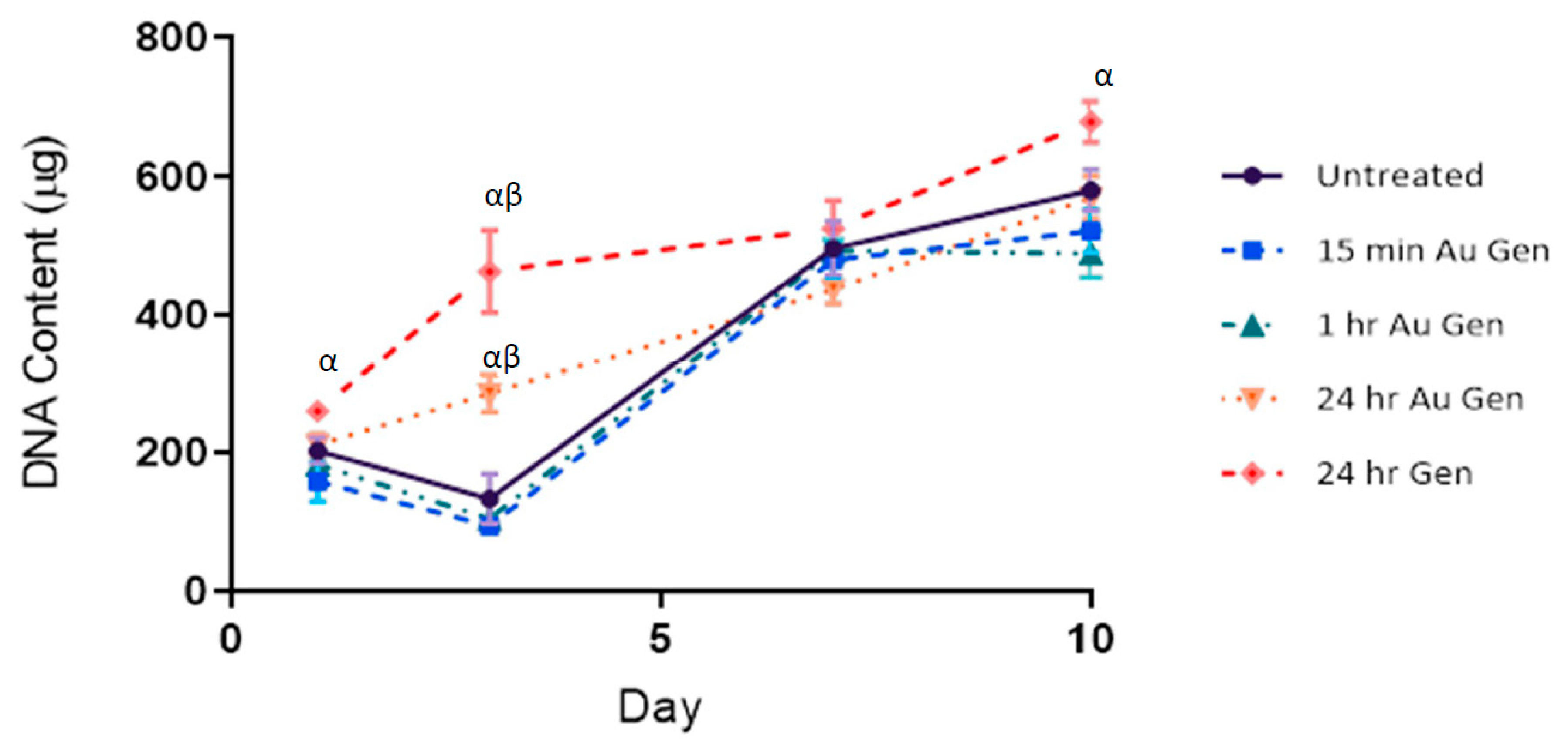
3.4. dsDNA Assay
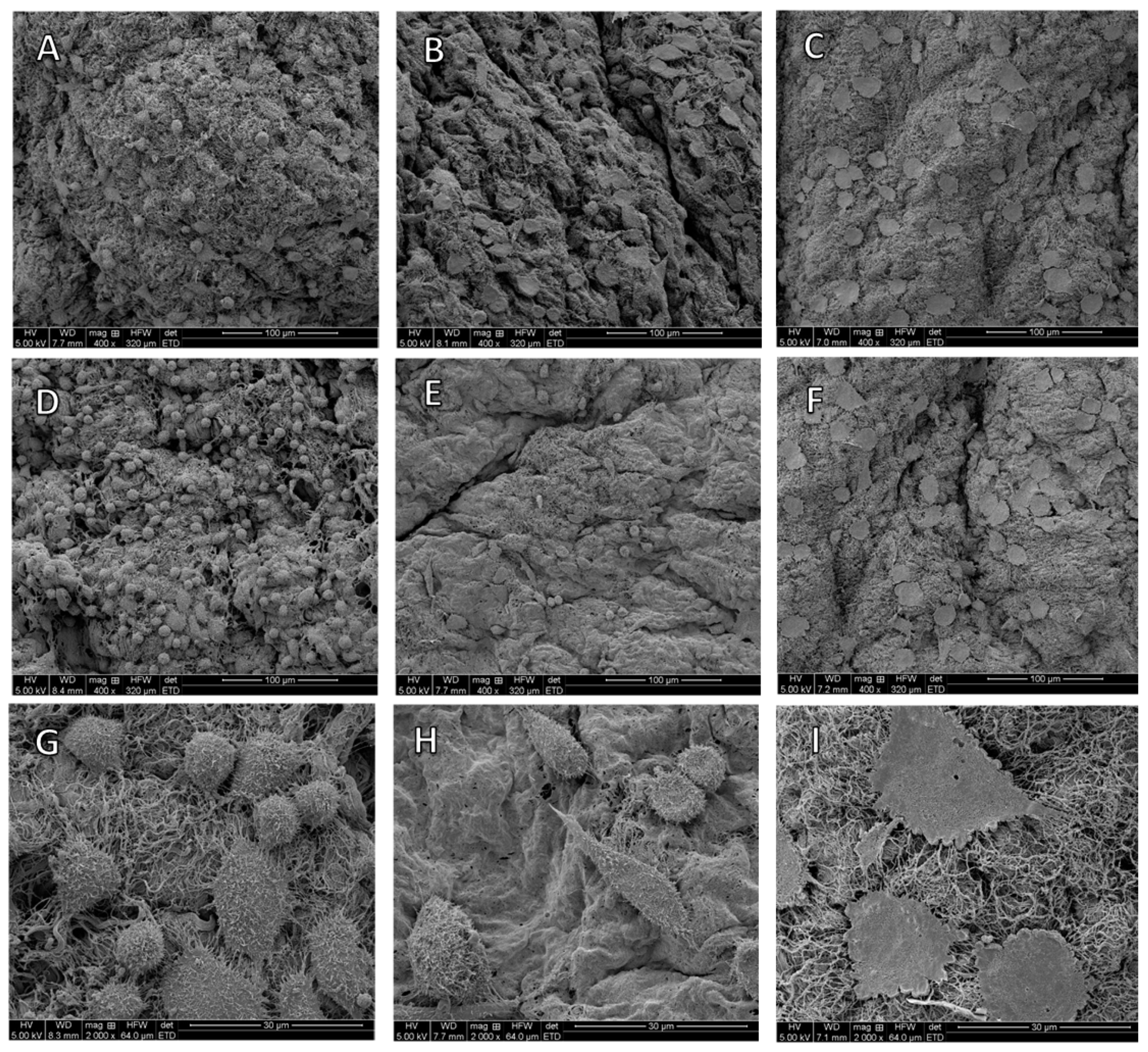
3.5. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.H.; Calle, E.A.; Zhao, L.; Lee, E.J.; Gui, L.; Raredon, M.B.; Gavrilov, K.; Yi, T.; Zhuang, Z.W.; Breuer, C. Tissue-engineered lungs for in vivo implantation. Science 2010, 329, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.-L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814. [Google Scholar] [CrossRef]
- Londono, R.; Badylak, S.F. Biologic Scaffolds for Regenerative Medicine: Mechanisms of In vivo Remodeling. Ann. Biomed. Eng. 2015, 43, 577–592. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Whitlock, P.W.; Smith, T.L.; Poehling, G.G.; Shilt, J.S.; Van Dyke, M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials 2007, 28, 4321–4329. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, J.; Wu, X.; Wu, Q.; Li, Y.; Zhou, Y.; Li, L.; Bu, H. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci. Rep. 2016, 6, 24779. [Google Scholar] [CrossRef]
- Partington, L.; Mordan, N.; Mason, C.; Knowles, J.; Kim, H.; Lowdell, M.; Birchall, M.; Wall, I. Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater. 2013, 9, 5251–5261. [Google Scholar] [CrossRef]
- Jackson, D.W.; Corsetti, J.; Simon, T.M. Biologic incorporation of allograft anterior cruciate ligament replacements. Clin. Orthop. Relat. Res. 1996, 324, 126–133. [Google Scholar] [CrossRef]
- Norton, S. A brief history of potable gold. Mol. Interv. 2008, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Ionita, P.; Spafiu, F.; Ghica, C. Dual behavior of gold nanoparticles, as generators and scavengers for free radicals. J. Mater. Sci. 2008, 43, 6571–6574. [Google Scholar] [CrossRef]
- BarathManiKanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.K.; Youn, H.-s.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-h.; Tang, C.-M.; Tseng, H.-J. Gold nanoparticles induce surface morphological transformation in polyurethane and affect the cellular response. Biomacromolecules 2007, 9, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.A.; Spradling, C.S.; Grant, D.N.; Fox, D.B.; Jimenez, L.; Grant, D.A.; Rone, R.J. Assessment of the biocompatibility and stability of a gold nanoparticle collagen bioscaffold. J. Biomed. Mater. Res. Part A 2014, 102, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.M.; Anseth, K.S.; van den Beucken, J.J.; Chan, C.K.; Ercan, B.; Jansen, J.A.; Laurencin, C.T.; Li, W.J.; Murugan, R.; Nair, L.S. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Nishi, C.; Nakajima, N.; Ikada, Y. In vitro evaluation of cytotoxicity of diepoxy compounds used for biomaterial modification. J. Biomed. Mater. Res. Part A 1995, 29, 829–834. [Google Scholar] [CrossRef]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Deeken, C.; Esebua, M.; Bachman, S.; Ramshaw, B.; Grant, S. Assessment of the biocompatibility of two novel, bionanocomposite scaffolds in a rodent model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Kim, Y.J.; Kim, S.H.; Choi, S.H. Study on genipin: A new alternative natural crosslinking agent for fixing heterograft tissue. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 197. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.W.; Huang, R.N.; Huang, L.L.; Tsai, C.C.; Chiu, C.T. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J. Biomed. Mater. Res. Part A 1998, 42, 560–567. [Google Scholar] [CrossRef]
- Koo, H.-J.; Lim, K.-H.; Jung, H.-J.; Park, E.-H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef]
- Nam, K.N.; Choi, Y.-S.; Jung, H.-J.; Park, G.H.; Park, J.-M.; Moon, S.-K.; Cho, K.-H.; Kang, C.; Kang, I.; Oh, M.S. Genipin inhibits the inflammatory response of rat brain microglial cells. Int. Immunopharmacol. 2010, 10, 493–499. [Google Scholar] [CrossRef]
- Deeken, C.; White, A.; Bachman, S.; Ramshaw, B.; Cleveland, D.; Loy, T.; Grant, S. Method of preparing a decellularized porcine tendon using tributyl phosphate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 199–206. [Google Scholar] [CrossRef]
- Leonavičienė, L.; Kirdaitė, G.; Bradūnaitė, R.; Vaitkienė, D.; Vasiliauskas, A.; Zabulytė, D.; Ramanavičienė, A.; Ramanavičius, A.; Ašmenavičius, T.; Mackiewicz, Z. Effect of gold nanoparticles in the treatment of established collagen arthritis in rats. Medicina 2012, 48, 16. [Google Scholar] [CrossRef]
- Fujikawa, S.; Nakamura, S.; Koga, K. Genipin, a new type of protein crosslinking reagent from gardenia fruits. Agric. Biol. Chem. 1988, 52, 869–870. [Google Scholar]
- Lima, E.G.; Tan, A.R.; Tai, T.; Marra, K.G.; DeFail, A.; Ateshian, G.A.; Hung, C.T. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 91, 692–700. [Google Scholar] [CrossRef]
- Haag, J.; Baiguera, S.; Jungebluth, P.; Barale, D.; Del Gaudio, C.; Castiglione, F.; Bianco, A.; Comin, C.E.; Ribatti, D.; Macchiarini, P. Biomechanical and angiogenic properties of tissue-engineered rat trachea using genipin cross-linked decellularized tissue. Biomaterials 2012, 33, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.W.; Chang, Y.; Chiu, C.T.; Chen, C.N.; Liang, H.C. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J. Biomed. Mater. Res. 1999, 47, 116–126. [Google Scholar] [CrossRef]
- Gattazzo, F.; De Maria, C.; Rimessi, A.; Donà, S.; Braghetta, P.; Pinton, P.; Vozzi, G.; Bonaldo, P. Gelatin–genipin-based biomaterials for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2763–2777. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Mu, J.; Huang, Z.; Han, Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.W.; Huang, D.M.; Chang, W.H.; Huang, R.N.; Hsu, J.C. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: In vitro study. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 1999, 46, 520–530. [Google Scholar]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y. Effects of genipin cross-linking of chitosan hydrogels on cellular adhesion and viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405. [Google Scholar] [CrossRef]
- Wang, C.; Lau, T.T.; Loh, W.L.; Su, K.; Wang, D.A. Cytocompatibility study of a natural biomaterial crosslinker—Genipin with therapeutic model cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 58–65. [Google Scholar] [CrossRef]
| Conjugation Time | Au | Au and Genipin |
|---|---|---|
| 15 min | 51 | 100 * |
| 1 h | 86 | 92 |
| 4 h | 153 | 190 |
| 8 h | 224 | 228 |
| 24 h | 373 | 389 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellrichard, M.; Snider, C.; Dittmar, C.; Brockman, J.; Grant, D.; A. Grant, S. Genipin Attachment of Conjugated Gold Nanoparticles to a Decellularized Tissue Scaffold. Appl. Sci. 2019, 9, 5231. https://doi.org/10.3390/app9235231
Bellrichard M, Snider C, Dittmar C, Brockman J, Grant D, A. Grant S. Genipin Attachment of Conjugated Gold Nanoparticles to a Decellularized Tissue Scaffold. Applied Sciences. 2019; 9(23):5231. https://doi.org/10.3390/app9235231
Chicago/Turabian StyleBellrichard, Mitch, Colten Snider, Cornelia Dittmar, John Brockman, Dave Grant, and Sheila A. Grant. 2019. "Genipin Attachment of Conjugated Gold Nanoparticles to a Decellularized Tissue Scaffold" Applied Sciences 9, no. 23: 5231. https://doi.org/10.3390/app9235231
APA StyleBellrichard, M., Snider, C., Dittmar, C., Brockman, J., Grant, D., & A. Grant, S. (2019). Genipin Attachment of Conjugated Gold Nanoparticles to a Decellularized Tissue Scaffold. Applied Sciences, 9(23), 5231. https://doi.org/10.3390/app9235231





