Abstract
In the process of coal mining, coal and gas outburst is one of the most severe disasters, which is accompanied by the outbursts of CO2, CH4, N2, and other gases. Since the intensity of the outburst is affected by the adsorption characteristics of different gases, the study of the adsorption characteristics of multi-gas in the coal body is of great significance for the prevention and control of coal and gas dynamical outbursts in coal mining. In this paper, the self-developed adsorption-instantaneous pressure relief test system for gas-containing coal was used to study the damage characteristics of multi-gas in briquette adsorption-instantaneous pressure relief and to characterize coal adsorption expansion deformation and instantaneous pressure relief deformation quantitatively. The results showed that under the same temperature and pressure conditions, the adsorption expansion deformation capacities of different gases were in the order of CO2 > CH4 > N2. With the increasing pressure of pressure relief, the damage of the coal body tended to be more significant. Although the briquette exhibited isotropic mechanical properties, the process of adsorption expansion deformation was not completely consistent with the isotropy of deformation, in which the axial strain was slightly larger than the radial strain. Moreover, it was found that the adsorption equilibrium time was positively correlated with gas pressure and gas adsorption characteristics.
1. Introduction
Coal and gas outburst is one of the common disasters in the mining process, which severely affects the production system and restricts the safe and efficient production of coal mines [1,2,3,4,5,6,7,8,9]. Since the first coal and gas outburst occurred in France in 1834 [10], many coal and gas outburst accidents have been reported around the world [11,12,13], of which about 50% occurred in China [14,15].
There are various factors affecting coal and gas outbursts, among which the pore structure of the coal body, the strength of the coal body, and the adsorption characteristics of the coal body significantly affect coal and gas outbursts [16,17,18,19,20]. As coal has a double pore structure, it is generally believed that the gas in coal has three states, namely free, adsorbed, and absorbed states. The adsorbed gas accounts for a large ratio, which is about 80% to 90% of the total. The form of physical adsorption is distributed on the surface of coal body fractures, micropores, and molecular particles. Because of the existence of adsorbed gas, the physical and mechanical properties of the coal body become very complicated, which not only changes the mechanical properties of the gas-containing coal but also causes changes in the ground stress field and the gas pressure field in the coal seam. Therefore, it is of particular significance to have an in-depth understanding of the adsorption characteristics of coal. A large number of laboratory studies have been carried out by scientists in China and overseas on the adsorption characteristics of coal [21,22,23,24,25,26]. The pore structure of coal [27,28], the adsorption characteristics of gas [29], the pore volume, and specific surface area of coal determine the adsorption performance of coal to some extent. At the same time, the water content, the microscopic composition of the coal, the temperature, and pressure have a great influence on the adsorption characteristics of the coal. Clarkson and Bustin [30] studied the effect of coal water content and composition on the adsorption characteristics of methane–carbon dioxide mixed gas and found that water could enhance the ability of the coal matrix to adsorb gas. The composition and the molecular structure of coal affect the adsorption capacity of coal to gas, whereas the microscopic composition of coal affects the diffusion rate of adsorbed gas [31]. Current studies have shown that gas adsorption capacity decreased with increasing temperature [32] and was positively correlated with pressure over a particular pressure range [33].
To further elucidate the adsorption characteristics of the coal body, the kinetics of gas adsorption in the coal body have been studied extensively, which theoretically described the adsorption characteristics of the coal body [34,35,36]. The French chemist Langmuir proposed a one-component gas adsorption model, which is also known as the Langmuir model [37]. Due to the simplicity and clear physical meaning of each parameter of this model, the corresponding equation of this model has been widely used to describe the adsorption kinetics of gas on the surface of coal [38]. Given that coal bed gas contains multiple gas ingredients, some researchers have proposed the multi-component adsorption equation. The widely used multi-component adsorption model includes the extended-Langmuir equation and the ideal adsorbed solution theory. Since the gas outburst from the coal seam of the mine is predominated with CO2 and CH4, the gases used in the laboratory simulation are mainly CO2 and N2. It is critical to understand the adsorption characteristics of different gases. Therefore, to understand the adsorption characteristics of CO2, CH4, and N2, the adsorption properties of coal to different gases have been intensively studied in China and overseas [39,40,41,42]. For example, experiments by Zhou Shining, Lin Baiquan et al. [43] demonstrated that the coal sample adsorbed gas and underwent expansion deformation, which is subjected to the Langmuir equation. Battistutta E. [44] quantitatively studied the adsorption capacity of different types of coal for CO2, CH4, and N2. Lehua Xu [17] analyzed the effects of different gas types and different gas pressures on the risk of coal and gas outbursts. Zhou Junping [45] studied the adsorption characteristics of coal on single gas, including CH4, CO2, and N2, and the effect of adsorption characteristics on coal rock permeability. Luo Mingkun [46] conducted experimental studies on the competitive adsorption relationship between CH4, N2, and CO2. In summary, considerably related studies on the expansion effect of coal gas adsorption and its influence on gas migration have been intensively conducted in China and overseas, which has yielded abundant research results. Nevertheless, there are still some related issues that need further research. For example, due to the relatively short occurrence time of gas outbursts, the studies on the coal body damage at the moment of coal body adsorption-instantaneous pressure relief have been seldom reported. At the same time, the quantitative research and test device for coal body adsorption-expansion deformation is also relatively rare.
With the self-developed gas-containing coal adsorption-instantaneous pressure relief test system, the gas parameters and the gas adsorption-instantaneous pressure relief test were carried out by using gas with different adsorption characteristics to observe the deformation and damage of the coal during the adsorption-instantaneous pressure relief process and to analyze the influencing factors of coal adsorption characteristics. Furthermore, the damage of coal under adsorption-decompression conditions was studied. Through monitoring the macroscopic deformation of coal samples with strain gauges and subsequent study of the damage of coal under different gas parameters by variation of the gas parameters, the damage evolution and gas migration law of coal were analyzed quantitatively. The results of this study are of great significance for clarifying the adsorption characteristics of different gases, as well as the prevention and treatment of coal and gas outburst disasters.
2. Sample and Methods
2.1. Preparation of Coal Samples and Selection of Gases
The briquette has similar isotropic mechanical properties, which avoids the issue that the raw coal has a large dispersion of research results due to the difference in pore fracture development. Therefore, briquettes are selected in the test. During the test, the sample was acquired from the 15# coal seam of Yuquan Coal Mine in Yangquan, Shanxi Province. The 15# coal seam has a high risk of outburst with a gas content of 4.71 m3/t. The basic parameters of coal samples from 15# coal seam are shown in Table 1. The coal sample preparation process is shown in Figure 1. Firstly, after crushing the coal, the coal powder with the particle size of 80–120 mesh (120–180 µm) was sieved. Subsequently, 260 g of coal powder and 20 g of water were mixed homogeneously. After that, the mixture was poured into a mold. Under a molding pressure of 8 MPa and a pressing time of 30 min, a cylindrical sample with a size of 50 mm × 100 mm was prepared with an error of ±1 mm. Finally, the treated sample was wrapped with plastic film and placed in a drying oven at 80 °C for 24 h. Before attaching the strain gauge, the surface of the coal sample was wiped with alcohol. The strain gauge was attached along the axial and radial directions of the coal sample with glue. During the pasting process, the flatness of the strain gauge was ensured.

Table 1.
The basic parameters of the coal sample.

Figure 1.
Preparation chart of the coal sample.
To study the deformation and damage of the coal body under different gas parameters, the instantaneous pressure relief experiments were carried out under different pressure conditions with CH4, CO2, and N2 gases separately. The purity of each gas was 99.99%.
2.2. Experimental Apparatus and Procedures
2.2.1. Experimental Apparatus
Figure 2A is a self-developed adsorption-instantaneous pressure relief test system for gas-containing coal. Figure 2B is a schematic diagram of the test equipment of the study, which consists of four parts, namely a pressure chamber, pressure relief device, gas control system, and data acquisition system. The volume of the pressure chamber is 5 L, with a maximum resisting pressure of ≤5 MPa. The pressure relief device has a solenoid valve that can quickly open the pressure chamber to trigger pressure relief. The gas control system includes a vacuum system and a gas pressurization system, wherein the gas pressurization system consists of a cylinder, a control valve, and a gas injection pipeline. The control valve has a main valve (range 0 MPa~16 MPa) and a pressure reducing valve. The main valve is the cylinder pressure, and the pressure reducing valve can control the injection pressure with a maximum output pressure of 2.5. MPa. The data acquisition system is used to collect strain and monitor pressure changes in the pressure chamber. All data are automatically collected and recorded by a computer-controlled data acquisition system.
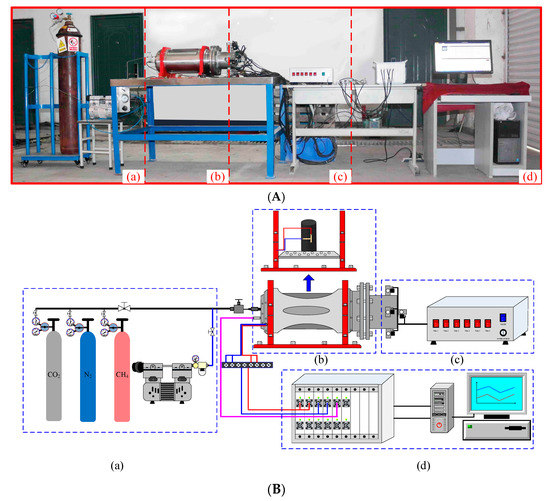
Figure 2.
(A) Pictorial diagram of testing equipment. (a) Gas control system; (b) pressure chamber; (c) pressure relief device; (d) data acquisition system; (B) Schematic diagram of the test equipment. (a) Gas control system; (b) pressure chamber; (c) pressure relief device; (d) data acquisition system.
2.2.2. Testing Process
The testing process is divided into four stages, namely vacuuming (stage I), inflation (stage II), adsorption equilibrium (stage III), and instantaneous pressure relief (stage IV). The specific operation of the test is carried out as follows:
- (a)
- Gas tightness detectionBefore the test, the airtightness of the chamber was tested with helium gas. The chamber was charged with helium gas to the pressure of 1.2 MPa. The pressure change in the chamber was monitored via a pressure sensor to record the pressure change in the chamber within 24 h. If the pressure inside the chamber remains constant, the airtightness is optimal.
- (b)
- In the prepared test piece, the strain gauge was attached to the strain data acquisition system with a soldering iron, followed by the resistance test. If the resistance value reached 120 ± 0.1 Ω, the next step could be performed.
- (c)
- The test piece was placed into the pressure chamber for fixing, sealing, and connecting with the pressure sensor;
- (d)
- Vacuum treatmentThe vacuum pump was turned on to evacuate the coal sample chamber for 1.5 h. The purpose of vacuuming is to remove air from the coal sample and reduce the test error. The initial vacuuming was fast and then gradually stabilized with the increase of time. During the vacuuming process, the macroscopic performance of the coal sample exhibited micro-shrinkage deformation. The pressure and strain were monitored to judge whether the air in the coal body was exhausted
- (e)
- The data acquisition system was turned on to monitor the coal strain and the pressure change in the pressure chamber.Simultaneously, the inlet valve was turned on to charge the gas. When reaching the set pressure, the inlet valve was closed to conduct the adsorption experiment at a constant temperature. At this stage, the pressure reducing valve was turned on to charge the pressure chamber with high-pressure gas, in which the gas underwent a seepage and adsorption process inside the coal body. In the meantime, the compression of the coal matrix caused compression deformation and reduced porosity. As a result, the characteristic of the coal sample continuously remained shrinkage deformation.
- (f)
- When reaching the preset adsorption time (usually 24 h), the solenoid valve was turned on to relieve the pressure in the chamber, where the rapid desorption and migration of a large amount of gas caused damage to the coal body. After the test data and the coal sample images were saved, the next set of experiments was performed.
3. Results and Discussion
3.1. Adsorption-Instantaneous Pressure Relief Test Study for Gases with Different Adsorption Characteristics
It can be seen from the test results that the overall trend of the coal body in the adsorption process was consistent. Therefore, the strain variation law of the various directions in the adsorption process of the coal body can be characterized by the law of the volumetric strain variation. The volumetric strain is calculated by the following formula, wherein compression is positive while expansion is negative according to the conventional representation of rock mechanics.
wherein, , , and represent volumetric strain, axial strain, and radial strain, respectively.
Comparing the curves of different gas strains versus time, it can be seen that there were differences in the deformation and adsorption equilibrium time caused by the adsorption of different gases on coal samples. Table 2 lists the axial strains, radial strains, and volumetric strains of coal samples after adsorption equilibrium under different adsorption pressures of different gases. Table 3 illustrates the axial strain, radial strain, and volumetric strain of the damaged coal sample after the pressure relief expansion deformation. It can be seen from the test data that the coal briquettes exhibited similar isotropic mechanical properties. However, the axial strain and radial strain had different values in the adsorption equilibrium process, in which the axial strain was slightly larger than the radial strain. It can be seen from Figure 3, Figure 4 and Figure 5 that the adsorption equilibrium pressure significantly affected the adsorption and expansion deformation of the coal body. With increasing pressure, the adsorption expansion deformation of the coal sample increased. In addition, the increment varied with the pressure. Under N2 atmosphere, as can be seen from Figure 3, when the gas pressure increased from 0.4 MPa to 0.8 MPa, the axial strain, radial strain, and volumetric strain of coal increased by 26 με, 29 με, and 94 με, respectively. When the gas pressure increased from 0.8 MPa to 1.2 MPa, axial strain, radial strain, and volumetric strain increased by 132 με, 240 με, and 712 με, respectively. Under the CH4 atmosphere, as can be seen from Figure 4, as the gas pressure increased, the amount of adsorption expansion increased. When the gas pressure increased from 0.4 MPa to 0.8 MPa and 1.2 MPa, the axial strains increased by 53 με and 241 με, respectively. The radial strains increased by 45 με and 220 με, and the volumetric strain increased by 143 με and 681 με, respectively. Under CO2 gas atmosphere, as shown in Figure 5, the corresponding axial strain, radial strain, and volumetric strain were −458 με, −415 με, and −1288 με, respectively, when the gas pressure was 0.4 MPa. When the gas pressure increased to 0.8 MPa, the corresponding axial strain, radial strain, and volumetric strain were −551 με, −510 με, and −1571 με, which were increased by 93 με, 95 με, and 283 με, respectively. When the gas pressure was 1.2 MPa, the axial strain, radial strain, and volumetric strain were −747 με, −701 με, and −2149 με, which were increased by 196 με, 191 με, and 578 με, respectively.

Table 2.
Coal sample adsorption equilibrium strain.

Table 3.
The pressure relief strain of coal sample.
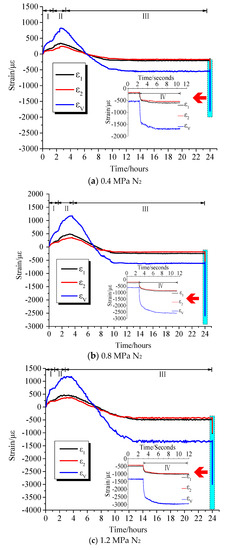
Figure 3.
Strain versus time curves under N2 atmosphere.
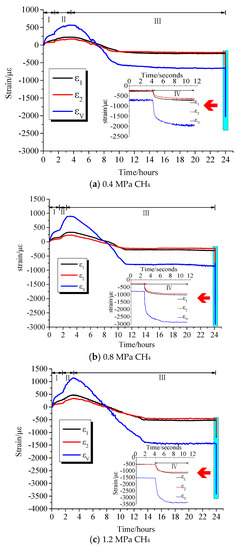
Figure 4.
Strain versus time curves under CH4 atmosphere.
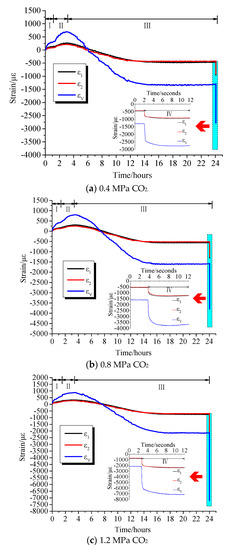
Figure 5.
Strain versus time curves under CO2 atmosphere.
When the gas containing coal was instantaneously relieved, the gas with different adsorption characteristics exhibited different deformation characteristics under different pressures. When the gas pressure was 0.4 MPa, the corresponding volumetric strains of the pressure relief in the N2, CH4, and CO2 gas environments were −1716 με, −1984 με, and −2785 με, respectively. When the adsorption equilibrium pressure at 0.8 MPa was suddenly relieved, their corresponding volumetric strains were −2612 με, −2633 με, and −3695 με, respectively. When the gas pressure was 1.2 MPa, their corresponding volumetric strains were −2999 με, −3438 με, and −7198 με, respectively. Therefore, it can be seen that with the increase of pressure relief pressure, the expansion deformation of the coal sample after pressure relief was more significant. Under the same relief pressure, the damage degree of the coal sample was in the order of CO2 > CH4 >N2.
3.2. Analysis of Macroscopic Damage Characteristics of Coal
The schematic diagram of the adsorption of coal samples under different gas atmospheres is shown in Figure 6. The damage of the coal body after the instantaneous pressure relief test is shown in Figure 7, Figure 8 and Figure 9. It can be seen from Figure 7, Figure 8 and Figure 9 that the damage of the coal body varied under different gas atmospheres and different pressures. When the experiment was carried out under N2 atmosphere, the coal sample was damaged to varying degrees when the air pressure increased from 0.4 MPa to 1.2 MPa. Under N2 atmosphere, when the instantaneous pressure relief pressure was 0.4 MPa, only small transverse cracks appeared in the axial direction. As shown in Figure 7c, no significant change in the radial direction was observed. When the gas pressure increased to 0.8 MPa, large transverse cracks appeared in the axial direction. Simultaneously, cracks began to appear in the radial direction, as shown in Figure 8c. When the air pressure increased to 1.2 MPa, as shown in Figure 9c, two cracks with different sizes appeared in the axial direction, and connected cracks began to appear in the radial direction. When the test was performed by charging CH4 gas and the gas pressure was suddenly relieved at 0.4 MPa, cracks with different sizes appeared on the surface of the coal sample, and fine cracks appeared in the radial direction. The deformation of the coal sample is shown in Figure 7b. When the gas pressure increased to 0.8 MPa, as shown Figure 8b), the surface of coal sample in radial direction varied significantly with the increase of pressure with the appearance of three small cracks. When the air pressure increased to 1.2 MPa, connected cracks in the axial direction penetrated the coal body, as shown in Figure 9b. When CO2 gas was charged for testing and the pressure was suddenly relieved under 0.4 MPa pressure, accompanied by the rapid desorption and seepage of the gas, the coal body was damaged with the appearance of cracks in both the axial and radial directions. The fracture distribution is shown in Figure 7a. When the gas pressure increased to 0.8 MPa, it can be seen from Figure 8a that the axial direction crack was obvious and the staggered cracks began to appear. The large cracks and densely small cracks appeared in radial direction. When the pressure increased to 1.2 MPa, as shown in Figure 9a, the coal sample exhibited a large area of damage with cracks in various sizes and intertwined with each other.
Figure 6.
Schematic diagram of coal sample adsorption under different gas atmospheres.
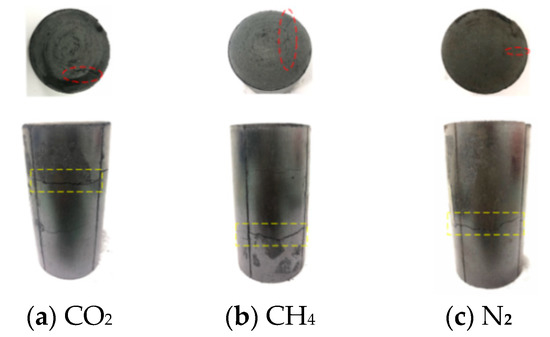
Figure 7.
Damage diagram of coal samples under 0.4 MPa gas pressure instantaneous pressure relief test.
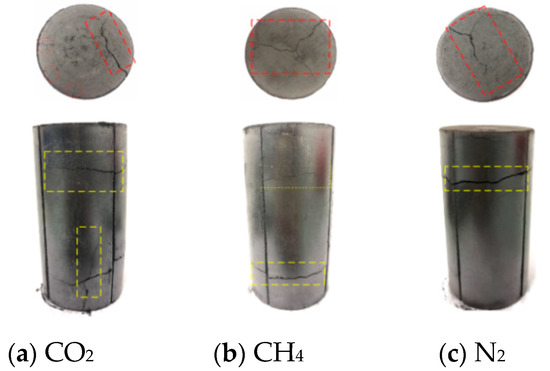
Figure 8.
Damage diagram of coal samples under 0.8 MPa gas pressure instantaneous pressure relief test.
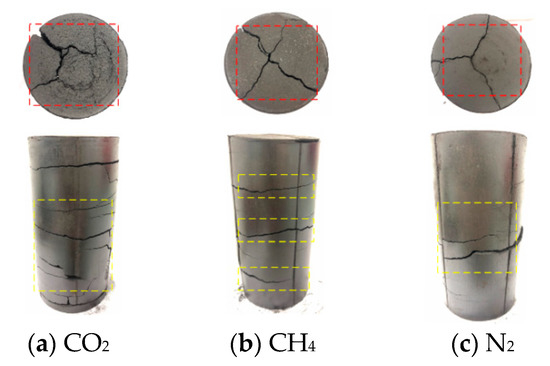
Figure 9.
Damage diagram of coal samples under 1.2 MPa gas pressure instantaneous pressure relief test.
It can be seen from the above test results that the degree of cracks increased with increasing gas pressure, and the crushing capacity of gas was in the order of CO2 > CH4 > N2.
3.3. Deformation Analysis of Coal Samples with Different Gas Pressures
3.3.1. Analysis of Compression Deformation of Coal Samples with Different Gas Pressures
The deformation characteristics of coal samples vary with different gas atmospheres. This difference is closely related to the adsorption characteristics of gases. Due to different adsorption characteristics, the deformation rate of coal samples and the maximum compression deformation variables are different during the charge compression process. During the process of compression and deformation of the coal sample during the charging process, accompanied by gas migration, a part of the gas molecules enter the pores of the coal body. As a result, the coal matrix adsorbs gas to expand and deform. Since CO2, CH4, and N2 have different adsorption characteristics, they exhibit different adsorption expansion deformations under the same gas pressure. It can be seen from Figure 10, Figure 11 and Figure 12 that as the gas pressure increased, the maximum compressive deformation increased. When the gas pressure increased from 0.4 MPa to 0.8 MPa, their corresponding maximum compressive strains in the CO2, CH4, and N2 atmospheres increased by 101 με, 243 με, and 335 με, respectively. When the gas pressure increased from 0.8 MPa to 1.2 MPa, their corresponding maximum compressive strains under the CO2, CH4, and N2 atmosphere increased by 84 με, 347 με, and 56 με, respectively. Under the pressure of 0.8 MPa and 1.2 MPa, N2 exhibited the most significant compression deformation, followed by CH4 and CO2, among which CO2 showed the minimum deformation. The compressive deformation of the coal sample under the atmospheres of CH4 and N2 varied considerably with the pressure change, whereas the influence of pressure change under the CO2 gas atmosphere was relatively insignificant. At the same time, the difference of the maximum compressive deformation was insignificant with the increase of CO2 pressure. By linearly fitting the gas adsorption equilibrium pressure with the maximum compressive deformation, the gas adsorption characteristics of the different gases were evident. According to the fitting results, linear fitness degree increases with increasing gas adsorption characteristics. As the gas pressure increased, the strain change rate of the coal sample in the CO2 gas environment was relatively insignificant.
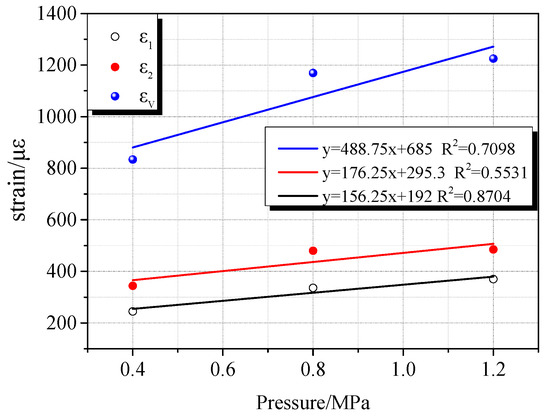
Figure 10.
Relationship between gas adsorption equilibrium pressure and maximum compressive deformation under N2 atmosphere.
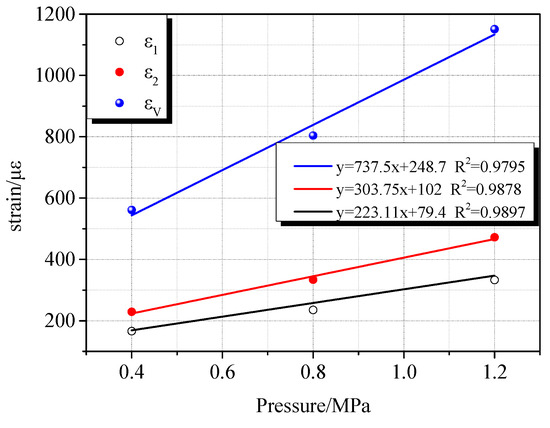
Figure 11.
Relationship between gas adsorption equilibrium pressure and maximum compressive deformation under the CH4 atmosphere.
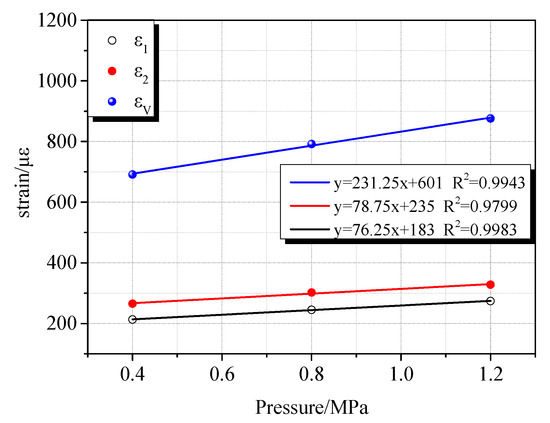
Figure 12.
Relationship between gas adsorption equilibrium pressure and maximum compressive deformation under CO2 atmosphere.
3.3.2. Effect of Different Gas Pressures on Coal Sample Strain and Adsorption Equilibrium Time
Although the adsorption equilibrium times of different gases with different adsorption characteristics are different, different gases exhibit a similar variation law under the different gas atmospheres. Under the same pressure, the overall trend of the strain in the adsorption process is consistent. In other words, the strain rate is high in the initial stage. As the adsorption progressed, the strain rate tended to be gradual. The equilibrium strains of the coal sample in axial and radial directions increased with the increase of the pressure. The effect of different gas pressures on coal sample strain and adsorption equilibrium time is shown in Figure 13. When the adsorption pressure was 0.4 MPa, the corresponding adsorption equilibrium times under N2, CH4, and CO2 gas atmospheres were 9.5 h, 10 h, and 13 h, respectively. When the gas pressure increased to 0.8 MPa, their corresponding adsorption equilibrium times were 10.6 h, 11 h, and 15 h, which were increased by 1.1 h, 1 h, and 2 h, respectively. When the gas pressure was 1.2 MPa, the adsorption equilibrium times were 12.3 h, 13.5 h, and 17 h, which were increased by 1.7 h, 2.5 h, and 2 h, respectively. It can be seen from Figure 13 that under the same pressure conditions, the equilibrium times with different gas environments were in the order of CO2 > CH4 > N2. The equilibrium time difference between N2 and CH4 gas environments was insignificant, whereas the equilibrium time of CO2 was considerably different. In other words, the stronger the gas adsorption characteristic of gas, the longer it took to reach the adsorption equilibrium state. The adsorption equilibrium time increased gradually with increasing gas adsorption pressure.
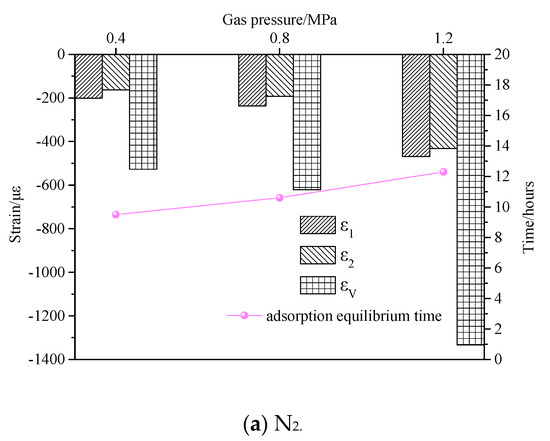
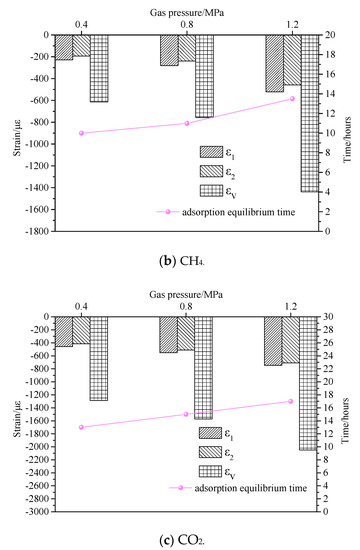
Figure 13.
Effect of gas pressure on coal sample strain and adsorption equilibrium time.
3.4. Study on the Difference of Coal Body Damage under Different Pressure Reliefs
Figure 14, Figure 15 and Figure 16 show the variation of the volumetric strain of coal pressure relief with time. The pressure in the pressure chamber experiences four stages, including vacuum reduction, pressure rise, stable adsorption equilibrium, and instantaneous pressure relief. Figure 14, Figure 15 and Figure 16 show the expansion deformation caused by the instantaneous pressure relief of the coal body under different gas pressures of 0.4 MPa, 0.8 MPa, and 1.2 MPa, as well as the pressure change in the pressure chamber at the moment of pressure relief. It can be seen from Figure 14, Figure 15 and Figure 16 that the simultaneous opening of six pressure valves could reduce the pressure inside the pressure chamber to atmospheric pressure within 0.05 s, which is a very fast pressure relief rate. After the gas adsorption reaches equilibrium, the gas pressure inside the coal body has a pressure difference from the external pressure. When the pressure is instantaneously released, the gas molecules in the coal body are rapidly desorbed, accompanied by the diffusion of the seepage. As a result, the pores in the coal body expand to form new cracks, causing damage to the test piece. Comparing the test results, it can be concluded that due to the different adsorption characteristics of coal to different gases, the deformation and damage caused by instantaneous pressure relief of coal samples were different under the same gas pressure. The large adsorption equilibrium strain corresponds to a large pressure relief deformation strain. Under different gas pressures, the pressure of pressure relief should be positively correlated with the deformation of coal samples.
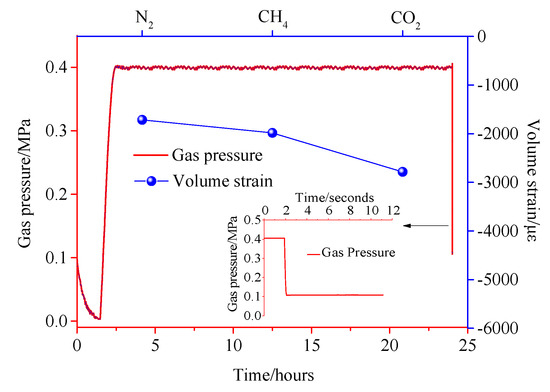
Figure 14.
Pressure−strain time curve under 0.4 MPa.
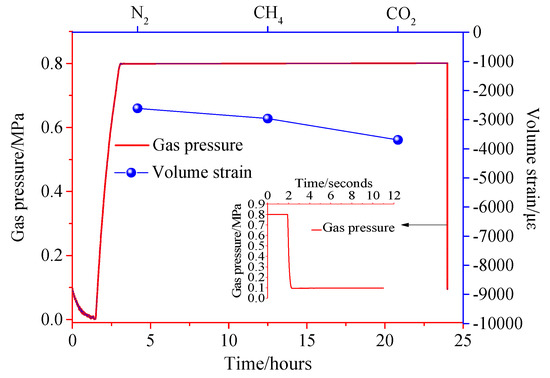
Figure 15.
Pressure−strain time curve under 0.8 MPa.
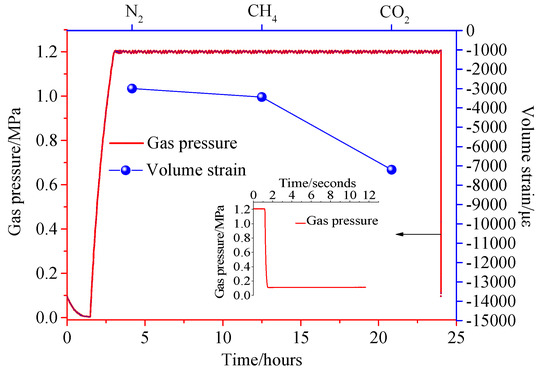
Figure 16.
Pressure−strain time curve under 1.2 MPa.
4. Conclusions
In this paper, the self-developed gas-containing coal adsorption-instantaneous pressure relief test system was used to conduct the gas-containing coal adsorption-instantaneous pressure relief experiment by changing the gas parameters and employing various gases with different adsorption characteristics. The conclusions of this study are as follows:
- (1)
- The gas adsorption-instantaneous pressure relief process of coal samples under different pressures of N2, CH4, and CO2 was studied to investigate the deformation and damage of coal samples. The strain characteristics of gas-containing coal in the adsorption-instantaneous pressure relief conditions were characterized for quantitative analysis of coal sample adsorption expansion deformation and pressure relief deformation.
- (2)
- The damage of coal samples varied under different gas atmospheres and different pressures. The degree of crushing increased with an increase in gas pressure. The crushing capacities of different gases were in the order of CO2 > CH4 > N2;
- (3)
- Coal exhibited different adsorption characteristics for different gases. The overall trend of coal strain was consistent in the adsorption process. The adsorption equilibrium time of the coal sample increased with increasing adsorption equilibrium pressure. Under the same pressure, the adsorption equilibrium time of CO2 was the longest, followed by CH4 and N2, among which N2 had the shortest equilibrium time;
- (4)
- Although briquette exhibited the isotropic mechanical properties, it did not completely conform to the isotropic deformation during the process of adsorption expansion deformation. The axial strain was slightly larger than the radial strain. With increasing gas pressure, the difference variation between axial strain and radial strain tended to be stable.
Author Contributions
Conceptualization, C.D.; methodology, C.D.; resources, G.W.; data curation, Y.G.; writing—original draft preparation, L.S.; writing—review and editing, C.D.; supervision, W.C.; project administration, L.S.; funding acquisition, L.S.
Funding
This research was funded by National Natural Science Foundation of China, grant number 51704187; Shandong Province key research and development plan, China, grant number GG201809190180; Shandong Province Natural Science Foundation, China, grant number ZR2017BEE054; Post−Doctoral Innovation Project of Shandong Province, China, grant number 201703024; and Post−Doctoral Application Research Project of Qingdao, China grant number 2017−267. Thanks for all of the support for this basic research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beamish, B.; Crosdale, P. Instantaneous outbursts in underground coal mines: An overview and association with coal type. Int. J. Coal Geol. 1998, 35, 27–55. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Li, S.; Lin, H.; Jia, Y.; Yan, M.; Lin, J. Experimental Investigation of the Adsorption Characteristics of Mixed Coal and Variations of Specific Surface Areas before and after CH4 Adsorption. Appl. Sci. 2019, 9, 524. [Google Scholar] [CrossRef]
- Mastalerz, M.; Hampton, L.; Drobniak, A. Significance of analytical particle size in low-pressure N2 and CO2 adsorption of coal and shale. Int. J. Coal Geol. 2017, 178, 122–131. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Li, Z.; Li, J.; Zhang, X.; Liu, L.; Zhou, Y. Influence of soluble organic matter on mechanical properties of coal and occurrence of coal and gas outburst. Powder Technol. 2018, 332, 8–17. [Google Scholar] [CrossRef]
- Wang, G.; Shen, J.; Liu, S.; Jiang, C.; Qin, X. Three-dimensional modeling and analysis of macro-pore structure of coal using combined X-ray CT imaging and fractal theory. Int. J. Rock Mech. Min. Sci. 2019, 123, 104082. [Google Scholar] [CrossRef]
- Zuo, S.; Ge, Z.; Zhou, Z.; Wang, L.; Zhao, H. A Novel Hydraulic Mode to Promote Gas Extraction: Pressure Relief Technologies for Tectonic Regions and Fracturing Technologies for Nontectonic Regions. Appl. Sci. 2019, 9, 1404. [Google Scholar] [CrossRef]
- Lama, R.; Bodziony, J. Management of outburst in underground coal mines. Int. J. Coal Geol. 1998, 35, 83–115. [Google Scholar] [CrossRef]
- Tu, Q.; Cheng, Y.; Guo, P.; Jiang, J.; Wang, L.; Zhang, R. Experimental study of coal and gas outbursts related to gas-enriched areas. Rock Mech. Rock Eng. 2016, 49, 3769–3781. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, C.; Shen, J.; Han, D.; Qin, X. Deformation and water transport behaviors study of heterogenous coal using CT-based 3D simulation. Int. J. Coal Geol. 2019, 211. [Google Scholar] [CrossRef]
- Flores, R. Coalbed methane: From hazard to resource. Int. J. Coal Geol. 1998, 35, 3–26. [Google Scholar] [CrossRef]
- Fan, C.; Li, S.; Luo, M.; Du, W.; Yang, Z. Coal and gas outburst dynamic system. Int. J. Min. Sci. Technol. 2017, 27, 49–55. [Google Scholar] [CrossRef]
- Jin, K.; Cheng, Y.; Ren, T.; Zhao, W.; Tu, Q.; Dong, J.; Hu, B. Experimental investigation on the formation and transport mechanism of outburst coal-gas flow: Implications for the role of gas desorption in the development stage of outburst. Int. J. Coal Geol. 2018, 194, 45–58. [Google Scholar] [CrossRef]
- Black, D. Review of coal and gas outburst in Australian underground coal mines. Int. J. Min. Sci. Technol. 2019, 29, 815–824. [Google Scholar] [CrossRef]
- Yuan, L. Control of coal and gas outbursts in Huainan mines in China: A review. J. Rock Mech. Geotech. Eng. 2016, 8, 559–567. [Google Scholar] [CrossRef]
- Jin, K.; Cheng, Y.; Wang, W.; Liu, H.; Liu, Z.; Zhang, H. Evaluation of the remote lower protective seam mining for coal mine gas control: A typical case study from the Zhuxianzhuang coal mine, Huaibei coalfield, China. J. Nat. Gas Sci. Eng. 2016, 33, 44–55. [Google Scholar] [CrossRef]
- Sobczyk, J. The influence of sorption processes on gas stresses leading to the coal and gas outburst in the laboratory conditions. Fuel 2011, 190, 1018–1023. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, C. Initial desorption characterization of methane and carbon dioxide in coal and its influence on coal and gas outburst risk. Fuel 2017, 203, 700–706. [Google Scholar] [CrossRef]
- Zhao, W.; Cheng, Y.; Guo, P.; Jin, K.; Tu, Q.; Wang, H. An analysis of the gas-solid plug flow formation: New insights into the coal failure process during coal and gas outbursts. Powder Technol. 2017, 305, 39–47. [Google Scholar] [CrossRef]
- Wang, G.; Qin, X.; Shen, J.; Zhang, Z.; Han, D.; Jiang, C. Quantitative analysis of microscopic structure and gas seepage characteristics of low-rank coal based on CT three-dimensional reconstruction of CT images and fractal theory. Fuel 2019, 256, 115900. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Du, C.A.; Sun, L.; Liu, Z.; Wang, Y.; Cao, J. Experimental Study on Damage and Gas Migration Characteristics of Gas-Bearing Coal with Different Pore Structures under Sorption-Sudden Unloading of Methane. Geofluids 2019, 2019, 7287438. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, M.; Xian, X.; Jiang, Y.; Liu, Q.; Wang, X. Measurements and modeling of CH4 and CO2 adsorption behaviors on shales: Implication for CO2 enhanced shale gas recovery. Fuel 2019, 251, 293–306. [Google Scholar] [CrossRef]
- Wang, K.; Wang, G.; Ren, T.; Cheng, Y. Methane and CO2 sorption hysteresis on coal: A critical review. Int. J. Coal Geol. 2014, 132, 60–80. [Google Scholar] [CrossRef]
- Billemont, P.; Coasne, B.; De Weireld, G. An experimental and molecular simulationstudy of the adsorption of carbon dioxide and methane in nanoporous carbons in the presence of water. Langmuir 2010, 27, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, S. Experimental and theoretical characterization of methane and CO2 sorption hysteresis in coals based on Langmuir desorption. Int. J. Coal Geol. 2017, 171, 49–60. [Google Scholar] [CrossRef]
- Czerw, K. Methane and carbon dioxide sorption/desorption on bituminous coal—Experiments on cubicoid sample cut from the primal coal lump. Int. J. Coal Geol. 2011, 85, 72–77. [Google Scholar] [CrossRef]
- Majewska, Z.; Ceglarska-Stefańska, G.; Majewski, S.; Ziętek, J. Binary gas sorption/desorption experiments on a bituminous coal: Simultaneous measurements on sorption kinetics, volumetric strain and acoustic emission. Int. J. Coal Geol. 2009, 77, 90–102. [Google Scholar] [CrossRef]
- Tao, S.; Chen, S.; Tang, D.; Zhao, X.; Xu, H.; Li, S. Material composition, pore structure and adsorption capacity of low-rank coals around the first coalification jump: A case of eastern Junggar Basin, China. Fuel 2018, 211, 804–815. [Google Scholar] [CrossRef]
- Debelak, K.; Schrodt, J. Comparison of pore structure in Kentucky coals by mercury penetration and carbon dioxide adsorption. Fuel 1979, 58, 732–736. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Tang, D.; Mathews, J.P.; Li, S.; Tao, S. A comparative evaluation of coal specific surface area by CO2 and N2 adsorption and its influence on CH4 adsorption capacity at different pore sizes. Fuel 2016, 183, 420–431. [Google Scholar] [CrossRef]
- Clarkson, C.; Bustin, R. Binary gas adsorption/desorption isotherms: Effect of moisture and coal composition upon carbon dioxide selectivity over methane. Int. J. Coal Geol. 2000, 42, 241–271. [Google Scholar] [CrossRef]
- Keshavarz, A.; Sakurovs, R.; Grigore, M.; Sayyafzadeh, M. Effect of maceral composition and coal rank on gas diffusion in Australian coals. Int. J. Coal Geol. 2017, 173, 65–75. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.; Shao, Q.; Lu, L.; Lu, X.; Jiang, S.; Shen, W. Simulations of binary mixture adsorption of carbon dioxide and methane in carbon nanotubes: Temperature, pressure, and pore size effects. J. Phys. Chem. C 2007, 111, 11912–11920. [Google Scholar] [CrossRef]
- Wang, T.; Tian, S.; Li, G.; Sheng, M. Selective adsorption of supercritical carbon dioxide and methane binary mixture in shale kerogen nanopores. J. Nat. Gas Sci. Eng. 2018, 50, 181–188. [Google Scholar] [CrossRef]
- Goodman, A.; Favors, R.; Larsen, J. Argonne coals rearrangement caused by sorption of CO2. Energy Fuels 2006, 20, 2537–2543. [Google Scholar] [CrossRef]
- Siemons, N.; Busch, A.; Bruining, H.; Krooss, B.; Gensterblum, Y. Assessing the kinetics capacity of gas adsorption in coals by combined adsorption/diffusion method. In Proceedings of the SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers, New Orleans, LA, USA, 30 September–2 October 2003. [Google Scholar]
- Li, Z.; Liu, D.; Cai, Y.; Shi, Y. Investigation of methane diffusion in low-rank coals by a multiporous diffusion model. J. Nat. Gas Sci. Eng. 2016, 33, 97–107. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Li, Y. Comparative Research on Deformation Law of Sorption-Desorption of Briquettes and Coal Samples. Master’s Thesis, Chongqing University, Chongqing, China, 2012. [Google Scholar]
- Chen, Y.; Wei, L.; Mastalerz, M.; Schimmelmann, A. The effect of analytical particle size on gas adsorption porosimetry of shale. Int. J. Coal Geol. 2015, 138, 103–112. [Google Scholar] [CrossRef]
- Lutynski, M.; González, M. Characteristics of carbon dioxide sorption in coal and gas shale—The effect of particle size. J. Nat. Gas Sci. Eng. 2016, 28, 558–565. [Google Scholar] [CrossRef]
- Merkel, A.; Gensterblum, Y.; Krooss, B.; Amann, A. Competitive sorption of CH4, CO2 and H2O on natural coals of different rank. Int. J. Coal Geol. 2015, 150, 181–192. [Google Scholar] [CrossRef]
- Siemons, N.; Busch, A. Measurement and interpretation of supercritical CO2 sorption on various coals. Int. J. Coal Geol. 2007, 69, 229–242. [Google Scholar] [CrossRef]
- Zhou, S.; Lin, B. Theory of Coal Seam Gas Occurrence and Flow; Coal Industry Press: Beijing, China, 1999. [Google Scholar]
- Battistutta, E.; Van Hemert, P.; Lutynski, M.; Bruining, H.; Wolf, K. Swelling and sorption experiments on methane, nitrogen and carbon dioxide on dry Selar Cornish coal. Int. J. Coal Geol. 2010, 84, 39–48. [Google Scholar] [CrossRef]
- Zhou, J.; Xian, X.; Li, X.; Xu, J.; Gu, D. Influence of adsorption of different gases on the permeability of coal and rock. Chin. J. Rock Mech. Eng. 2010, 29, 2256–2262. [Google Scholar]
- Luo, M.; Li, S.; Rong, H.; Fan, C.; Yang, Z. NMR experimental study on the competitive adsorption relationship between CH4 and N2, CO2. J. China Coal Soc. 2018, 43, 490–497. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).