β-Nitroacrylates: New Key Precursors of Indole-2-Carboxylates via Fischer Indole Synthesis
Abstract
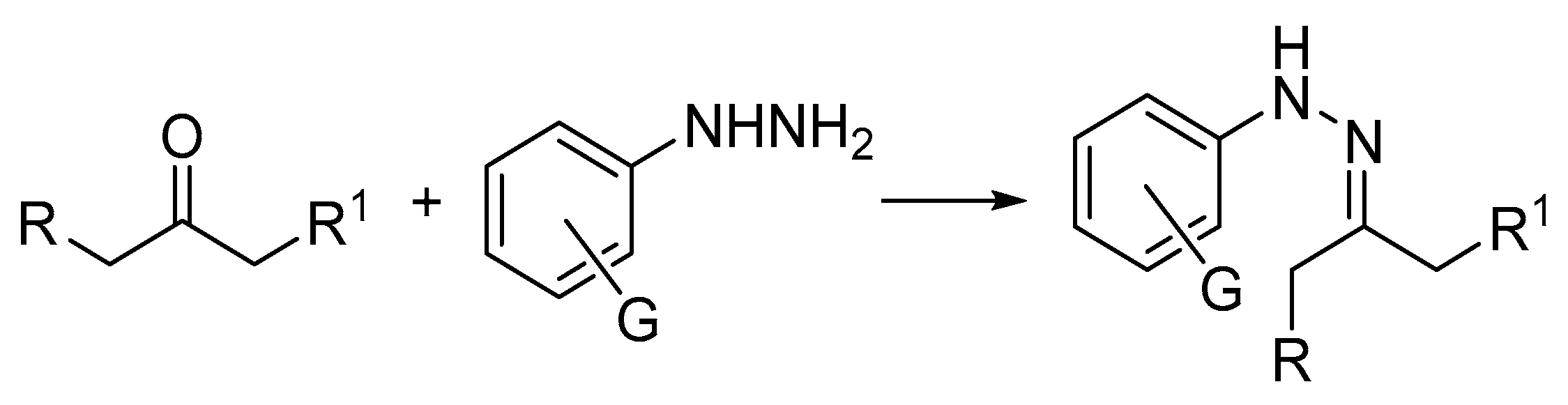
1. Introduction
2. Materials and Methods
2.1. General Section
2.2. Preparation of Starting Materials
2.3. General Procedure for the Preparation of Compounds 5
3. Results and Discussion
3.1. Optimization of the 1st Step
3.2. Optimization of the 2nd Step
3.3. Substrate Scope Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TMG | 1,1,3,3-Tetramethylguanidine |
| DBU | 1,5-Diazabiciclo[5.4.0]undec-5-ene |
| PS-TBD | 1,5,7-Triazabicyclo[4.4.0]dec-5-ene bound to polystyrene (Sigma-Aldrich code: 01961) |
| PS-Carbonate | Carbonate on polymer support (Sigma-Aldrich code: 21850) |
| NNRTI | Non-nucleoside reverse transcriptase inhibitor |
| PPA | Poly Phosphoric Acid |
References
- De Sá Alves, F.R.; Barreiro, E.J.; Fraga, C.A. From nature to drug discovery: The indole scaffold as a ‘privileged structure’. Mini Rev. Med. Chem. 2009, 9, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Lancianesi, S.; Palmieri, A.; Petrini, M. Synthetic approaches to 3-(2-nitroalkyl) indoles and their use to access tryptamines and related bioactive compounds. Chem. Rev. 2014, 114, 7108–7149. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. In Indole Ring Synthesis: From Natural Products to Drug Discovery; Wiley-VCH: Chichester, UK, 2016. [Google Scholar]
- Palmieri, A.; Gabrielli, S.; Lanari, D.; Vaccaro, L.; Ballini, R. A New one-pot synthesis of polysubstituted indoles from pyrroles and β-nitroacrylates. Adv. Synth. Catal. 2011, 353, 1425–1428. [Google Scholar] [CrossRef]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Fischer, E.; Jourdan, F. The hydrazone of pyruvic acid. Ber. Dtsch. Chem. Ges. 1883, 16, 2241–2245. [Google Scholar] [CrossRef]
- Fischer, E.; Hess, O. The synthesis of indole derivatives. Ber. Dtsch. Chem. Ges. 1884, 17, 559–568. [Google Scholar] [CrossRef]
- Heravi, M.M.; Rohani, S.; Zadsirjan, V.; Zahedi, N. Fischer indole synthesis applied to the total synthesis of natural products. RSC Adv. 2017, 7, 52852–52887. [Google Scholar] [CrossRef]
- Kiasat, A.R.; Kazemi, F.; Nourbakhsh, K. H2SO4/SiO2 as an efficient catalyst for the preparation of phenylhydrazones and 2,4-dinitrophenylhydrazones under solvent-free conditions. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 569–573. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Polystyrene sulfonic acid–catalyzed greener synthesis of hydrazones in aqueous medium using microwaves. Tetrahedron Lett. 2007, 48, 5649–5652. [Google Scholar] [CrossRef]
- Zhang, M.; Shang, Z.-R.; Li, X.-T.; Zhang, J.-N.; Wang, Y.; Li, K.; Li, Y.-Y.; Zhang, Z.-H. Simple and efficient approach for synthesis of hydrazones from carbonyl compounds and hydrazides catalyzed by meglumine. Synth. Commun. 2017, 47, 178–187. [Google Scholar] [CrossRef]
- Sudhakara, A.; Jayadevappa, H.; Mahadevan, K.M.; Hulikal, V. Efficient synthesis of 2-ethoxycarbonyl indoles. Synth. Commun. 2009, 39, 2506–2515. [Google Scholar] [CrossRef]
- Brands, M.; Ergüden, J.-K.; Hashimoto, K.; Heimbach, D.; Schröder, C.; Siegel, S.; Stasch, J.-P.; Weigand, S. Novel, selective indole-based ECE inhibitors: Lead optimization via solid-phase and classical synthesis. Bioorg. Med. Chem. Lett. 2005, 15, 4201–4205. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Kato, M.; Inuki, S.; Ohno, H.; Evans, B.; Wang, Z.-X.; Peiper, S.C.; Izumi, K.; Kodama, E.; Matsuoka, M. Identification of novel non-peptide CXCR4 antagonists by ligand-based design approach. Bioorg. Med. Chem. Lett. 2008, 18, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.L.; Morge, R.A.; Genin, M.J.; Biles, C.; Busso, M.; Resnick, L.; Althaus, I.W.; Reusser, F.; Thomas, R.C.; Tarpleyt, W.G. Bis(heteroary1)piperazine (BHAP) reverse transcriptase inhibitors: Structure-activity relationships of novel substituted indole analogues and the identification of 1-[(5-methanesulfonamido-1H-indol-2-yl)-carbonyl]-4-[3-[(l-methylethyl)amino]-pyridinyl]piperazine monomethanesulfonate (U-90152S), a second-generation clinical candidate. J. Med. Chem. 1993, 36, 1505–1508. [Google Scholar] [PubMed]
- Don, M.-J.; Lewis, D.F.V.; Wang, S.-Y.; Tsai, M.-W.; Ueng, Y.-F. Effect of structural modification on the inhibitory selectivity of rutaecarpine derivatives on human CYP1A1, CYP1A2, and CYP1B1. Bioorg. Med. Chem. Lett. 2003, 13, 2535–2538. [Google Scholar] [CrossRef]
- Urwyler, S.; Floersheim, P.; Roy, B.L.; Koller, M. Drug design, in vitro pharmacology, and structure−activity relationships of 3-acylamino-2-aminopropionic acid derivatives, a novel class of partial agonists at the glycine site on the N-methyl-d-aspartate (NMDA) receptor complex. J. Med. Chem. 2009, 52, 5093–5107. [Google Scholar] [CrossRef] [PubMed]
- Ballini, R.; Gabrielli, S.; Palmieri, A. β-Nitroacrylates as an emerging, versatile class of functionalized nitroalkenes for the synthesis of a variety of chemicals. Curr. Org. Chem. 2010, 14, 65–83. [Google Scholar] [CrossRef]
- Gabrielli, S.; Chiurchiù, E.; Palmieri, A. β-Nitroacrylates: A versatile and growing class of functionalized nitroalkenes. Adv. Synth. Catal. 2019, 361, 630–653. [Google Scholar] [CrossRef]
- Gabrielli, S.; Giardinieri, A.; Sampaolesi, S.; Ballini, R.; Palmieri, A. A new one-pot synthesis of quinoline-2-carboxylates under heterogeneous conditions. Molecules 2016, 21, 776. [Google Scholar] [CrossRef]
- Chiurchiù, E.; Patehebieke, Y.; Gabrielli, S.; Ballini, R.; Palmieri, A. β-Nitroacrylates as starting materials of thiophene-2-carboxylates under continuous flow conditions. Adv. Synth. Catal. 2019, 361, 2042–2047. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Maggi, R.; Ballini, R. β-Nitroacrylates as useful building blocks for the synthesis of alkyl indole-2-carboxylates. Synlett 2014, 25, 128–132. [Google Scholar] [CrossRef]
- Ballini, R.; Fiorini, D.; Palmieri, A. Nitroalkanes and ethyl glyoxalate as common precursors for the preparation of both β-keto esters and α,β-unsaturated esters. Tetrahedron Lett. 2004, 45, 7027–7029. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Ballini, R. Low impact synthesis of β-nitroacrylates under fully heterogeneous conditions. Green Chem. 2013, 15, 2344–2348. [Google Scholar] [CrossRef]
- Dey, R.; Kumar, P.; Banerjee, P. Lewis acid catalyzed annulation of cyclopropane carbaldehydes and aryl hydrazines: Construction of tetrahydropyridazines and application toward a one-pot synthesis of hexahydropyrrolo[1,2-b]pyridazines. J. Org. Chem. 2018, 83, 5438–5449. [Google Scholar] [CrossRef]
- Bie, J.; Liu, S.; Li, Z.; Mu, Y.; Xu, B.; Shen, Z. Discovery of novel indole derivatives as allosteric inhibitors of fructose-1,6-bisphosphate. Eur. J. Med. Chem. 2015, 90, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, R.; Jacob-Roetne, R.; Peters, J.-U.; Wichmann, J. Indol-Carboxamide Derivatives. Patent WO2015036412 (A1), 19 March 2015. [Google Scholar]
- Yasui, E.; Wada, M.; Takamura, N. Novel approach to arylhydrazones, the precursor for Fischer indole synthesis, via diazo esters derived from α-amino acid esters. Tetrahderon Lett. 2006, 47, 743–746. [Google Scholar] [CrossRef]
- Kostenis, E.; Spinrath, A.; Hennen, S.; Peters, L.; Müller, C.E.; Akkari, R.; Baqi, Y.; Ritter, K. GPR 17 Agonists and Screening Assay. Patent EP2567698 (A1), 7 January 2013. [Google Scholar]
- Pal, R.; Sarkar, T.; Khasnobis, S. Amberlyst-15 in organic synthesis. ARKIVOC 2012, 2012, 570–609. [Google Scholar] [CrossRef]


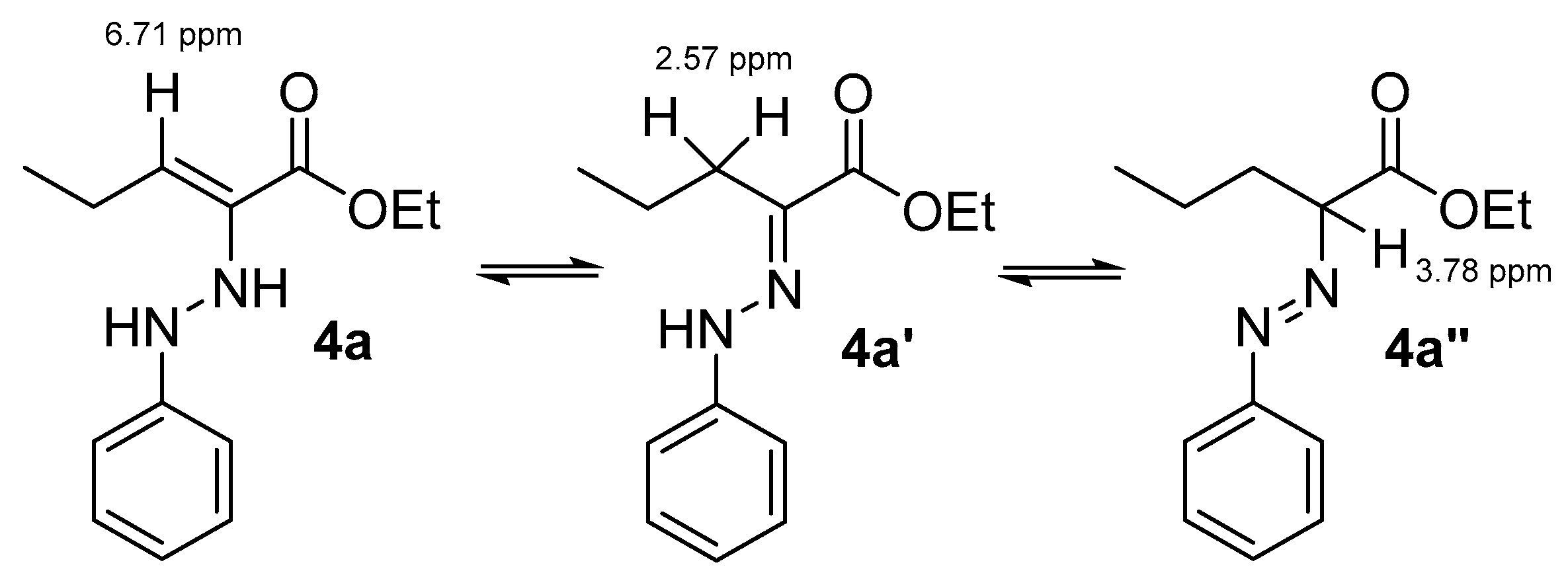

| Entry | Base | Solvent | Temp. | Time (h) | Yield (%) of 4a-a″ |
|---|---|---|---|---|---|
| A | DBU (1 eq.) | MeCN | rt | 2 | 23 |
| B | TMG (1 eq.) | MeCN | rt | 4 | 55 |
| C | KF/Al2O3 (1 eq.) | MeCN | rt | 24 | --- 1 |
| D | PS-Carbonate (1 eq.) | MeCN | rt | 24 | --- 1 |
| E | PS-TBD (1 eq.) | MeCN | rt | 4 | 35 |
| F | TMG (2 eq.) | MeCN | rt | 4 | 75 |
| G | TMG (2 eq.) | MeCN | 50 °C | 1 | 60 |
| H | TMG (3 eq.) | MeCN | rt | 4 | 71 |
| I | TMG (2 eq.) | THF | rt | 4 | 35 |
| J | TMG (2 eq.) | EtOAc | rt | 4 | 49 |
| K | TMG (2 eq.) | DCM | rt | 4 | 21 |
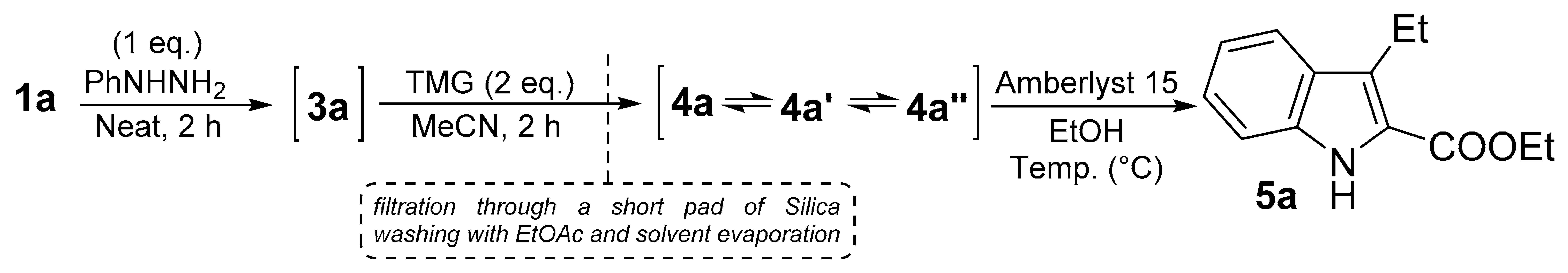
| Entry | Amberlyst 15 (g/mmol) | Temp. (°C) | Time (h) | Yield (%) 1 of 5a |
|---|---|---|---|---|
| a | 1 | reflux | 8 | 30 |
| b | 1.5 | reflux | 8 | 34 |
| c | 2 | reflux | 8 | 38 |
| d | 2.5 | reflux | 8 | 39 |
| e | 2 | 60 | 8 | 15 |
| f | 2 | 100 2 | 8 | 40 |
| g | 2 | reflux | 16 | 45 |
| h | 2 | reflux | 24 | 53 |
| i | 2 | reflux | 32 | 51 |
| j | 2 | 80 3 | 4 | 42 |
| k | 2 | 90 3 | 4 | 54 |
| l | 2 | 100 3 | 4 | 49 |
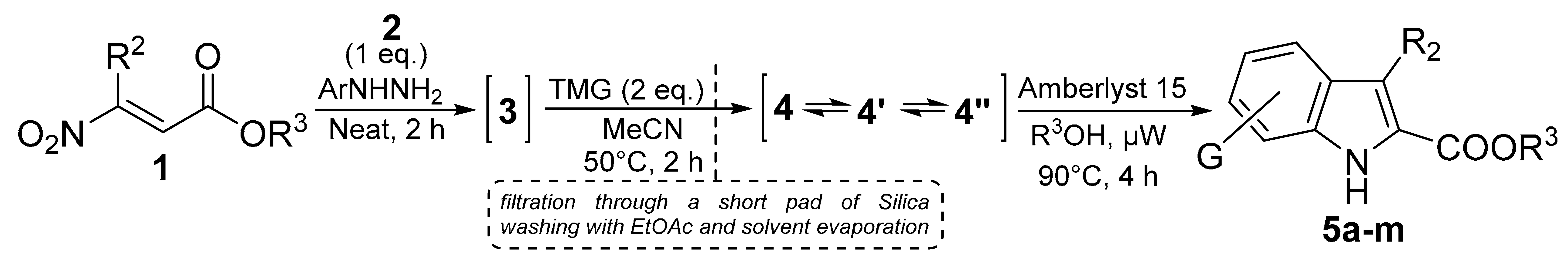
| Entry | R2 | R3 | G | Solvent | Yield (%) 1 of 5 | |
|---|---|---|---|---|---|---|
| a | Et | Et | H | EtOH | 5a | 54 |
| b | CH3(CH2)4 | Et | H | EtOH | 5b | 52 |
| c | CH3(CH2)8 | Et | H | EtOH | 5c | 50 |
| d | Et | Et | 7-Me | EtOH | 5d | 55 |
| e | Ph(CH2)2 | Et | H | EtOH | 5e | 43 |
| f | CH2=CH(CH2)2 | Et | 7-Me | EtOH | 5f | 43 |
| g | CH≡C(CH2)3 | Et | H | EtOH | 5g | 41 |
| h | Et | Me | H | MeOH | 5h | 42 |
| i | Et | Me | 5-Br | MeOH | 5i | 41 |
| j | MeOOC(CH2)4 | Me | H | MeOH | 5j | 36 |
| k | Et | Pr | H | PrOH | 5k | 46 |
| l | Et | Pr | 7-Me | PrOH | 5l | 45 |
| m | CH3(CH2)6 | Pr | H | PrOH | 5m | 38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrielli, S.; Panmand, D.; Ballini, R.; Palmieri, A. β-Nitroacrylates: New Key Precursors of Indole-2-Carboxylates via Fischer Indole Synthesis. Appl. Sci. 2019, 9, 5168. https://doi.org/10.3390/app9235168
Gabrielli S, Panmand D, Ballini R, Palmieri A. β-Nitroacrylates: New Key Precursors of Indole-2-Carboxylates via Fischer Indole Synthesis. Applied Sciences. 2019; 9(23):5168. https://doi.org/10.3390/app9235168
Chicago/Turabian StyleGabrielli, Serena, Deepak Panmand, Roberto Ballini, and Alessandro Palmieri. 2019. "β-Nitroacrylates: New Key Precursors of Indole-2-Carboxylates via Fischer Indole Synthesis" Applied Sciences 9, no. 23: 5168. https://doi.org/10.3390/app9235168
APA StyleGabrielli, S., Panmand, D., Ballini, R., & Palmieri, A. (2019). β-Nitroacrylates: New Key Precursors of Indole-2-Carboxylates via Fischer Indole Synthesis. Applied Sciences, 9(23), 5168. https://doi.org/10.3390/app9235168






