Assessing Spatial Distribution of Benthic Macroinvertebrate Communities Associated with Surrounding Land Cover and Water Quality
Abstract
1. Introduction
2. Materials and Methods
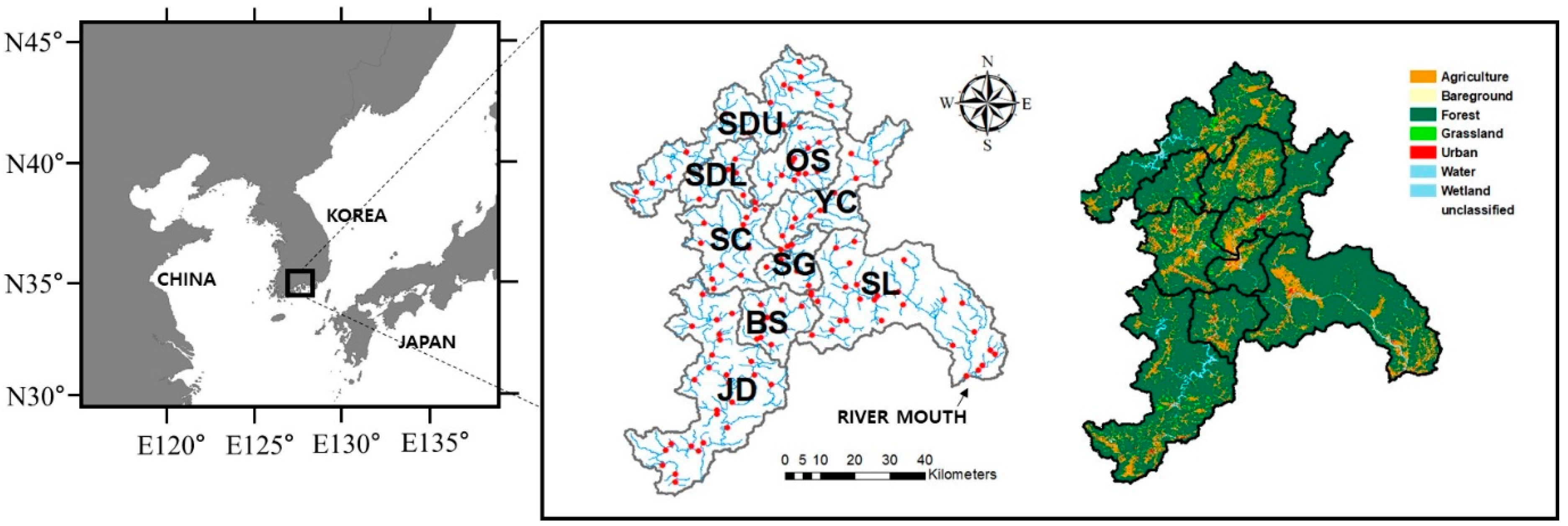
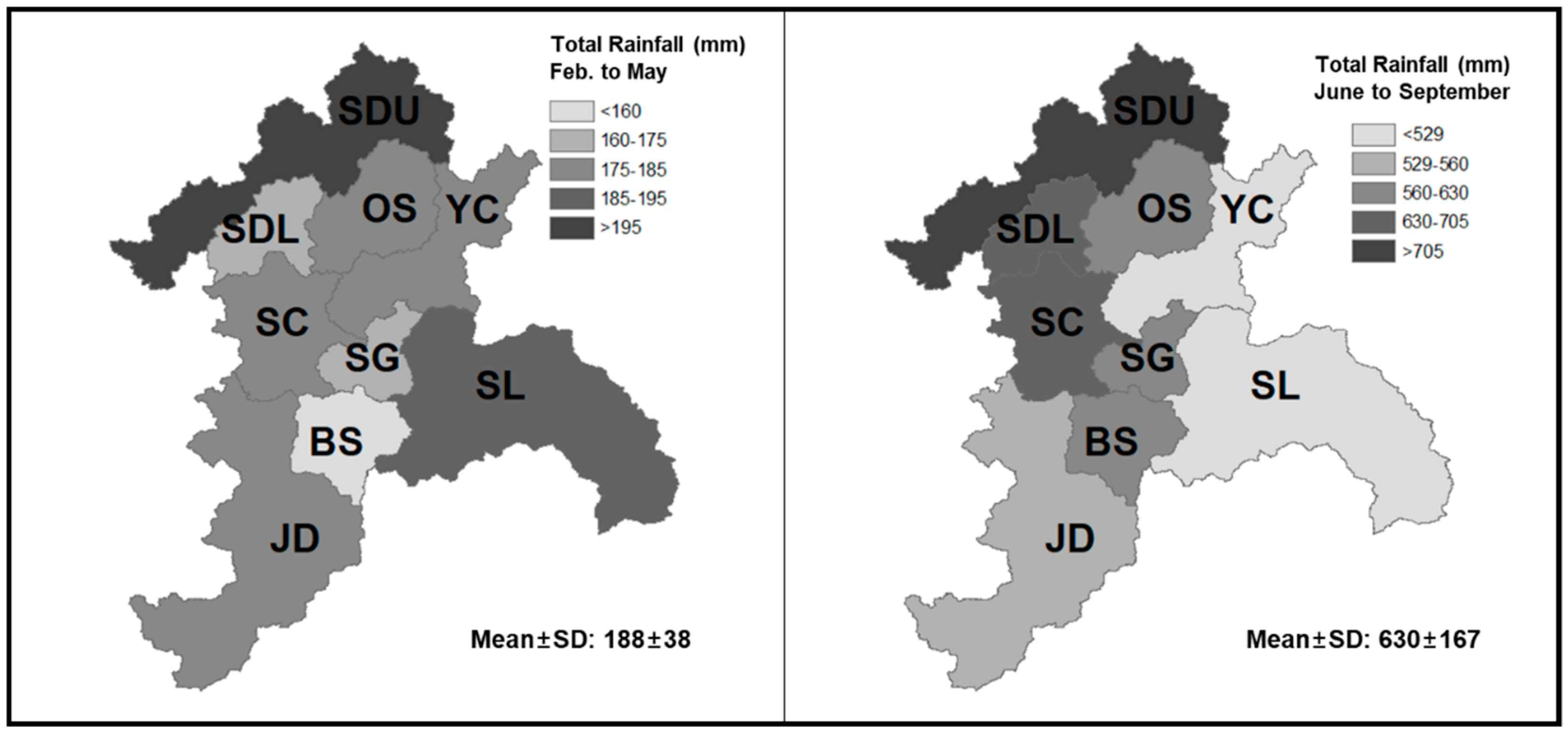
2.1. Site Description
2.2. Data Collection
2.3. Use of Biotic Indices
2.4. Multivariate Analysis for Data Ordination
3. Results and Discussion
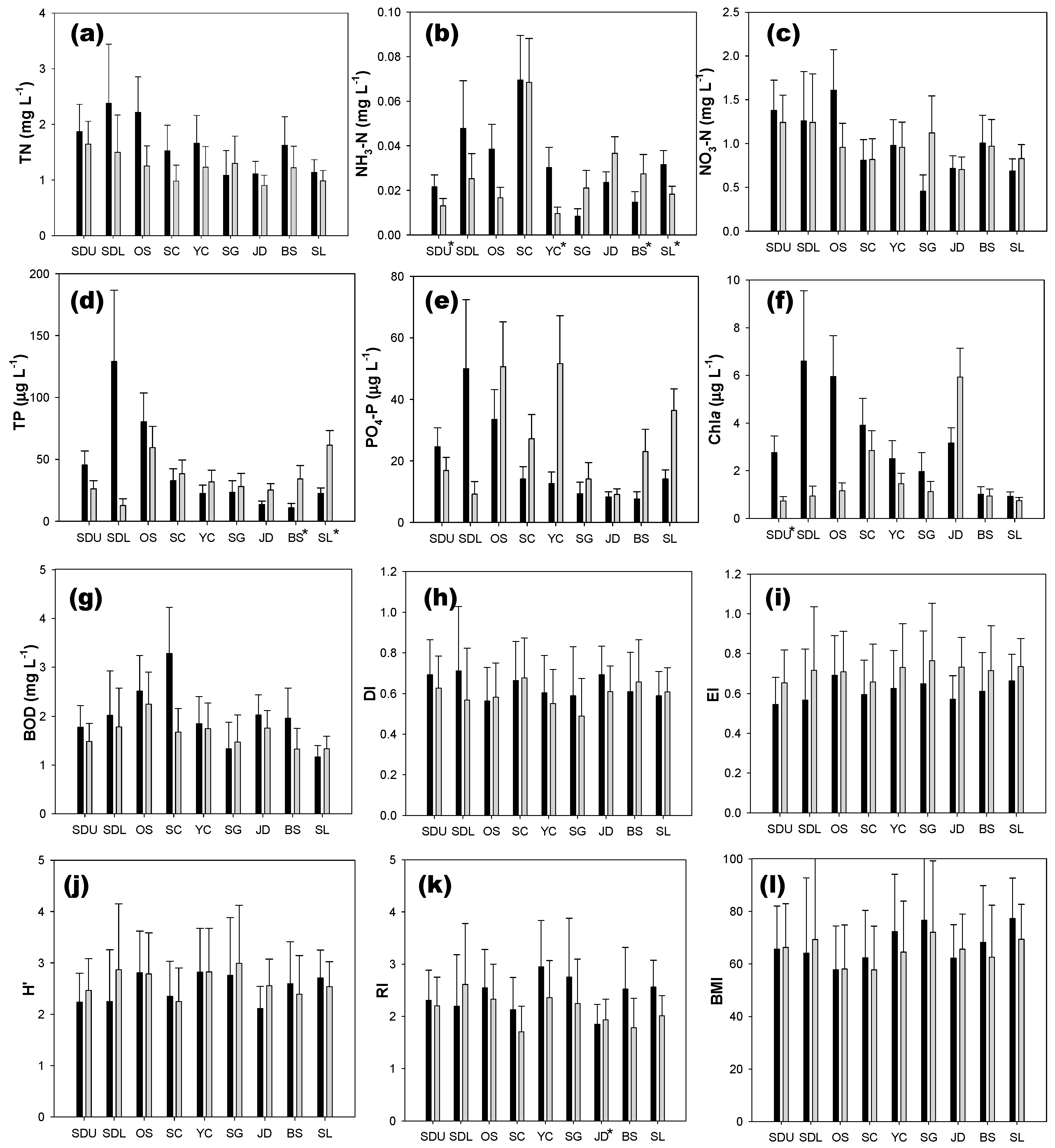
3.1. Comparison of Water Quality and Biotic Indices
3.2. Spatial Distribution of Benthic Macroinvertebrate Communities Before and After Summer Rainfall
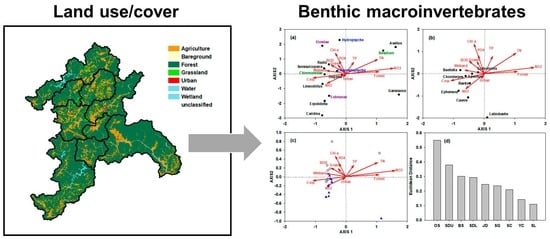
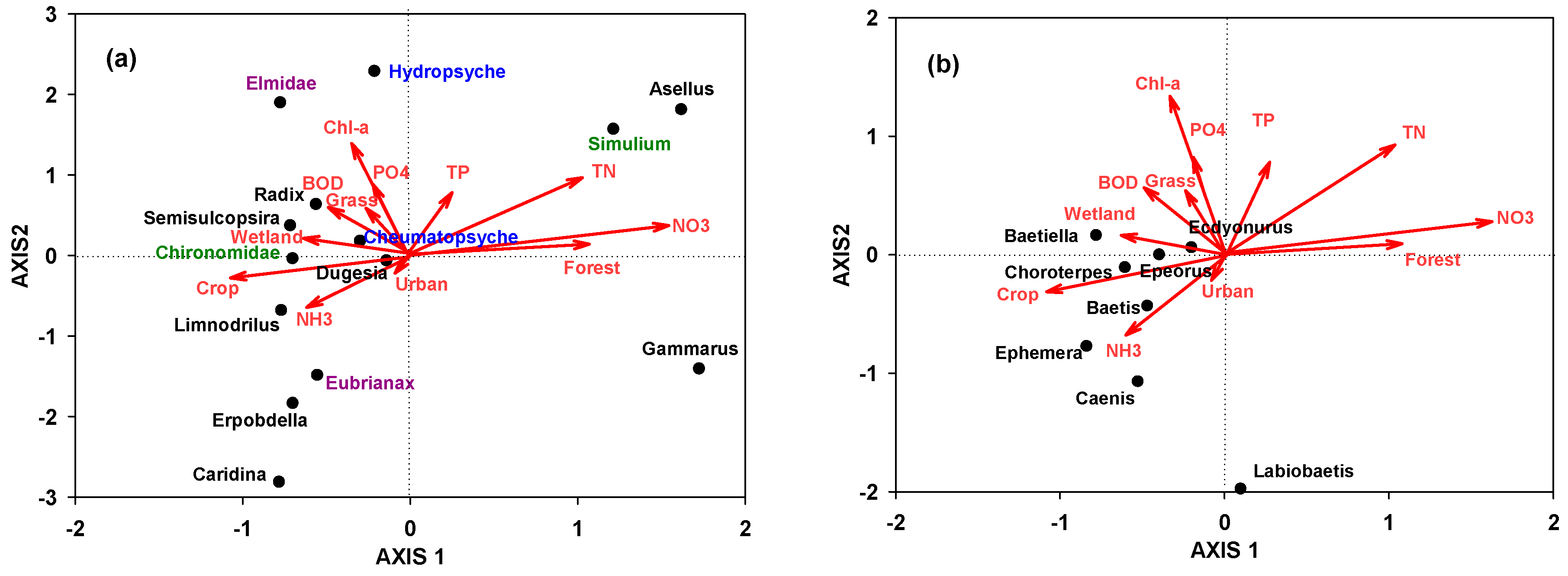
3.3. Association among Benthic Macroinvertebrates, Land-Use Coverage, and Ambient Water Quality
3.4. Identification of Spatiotemporal Characteristics in the Data from Seomjin River
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, D.M.; Resh, V.H. Freshwater Biomonitoring and Benthic Macroinvertebrates; Springer: Berlin/Heidelberg, Germany, 1993; p. 488. [Google Scholar]
- Baird, D.J.; Van den Brink, P.J. Using biological traits to predict species sensitivity to toxic substances. Ecotoxicol. Environ. Saf. 2007, 67, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Doledec, S.; Statzner, B. Invertebrate traits for the biomonitoring of large European rivers: An assessment of specific types of human impact. Freshwat. Biol. 2008, 53, 617–634. [Google Scholar] [CrossRef]
- De Castro-Català, N.; Muñoz, I.; Armendáriz, L.; Campos, B.; Barceló, D.; López-Doval, J.; Pérez, S.; Petrovic, M.; Picó, Y.; Riera, J.L. Invertebrate community responses to emerging water pollutants in Iberian river basins. Sci. Total Environ. 2015, 503, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.B.; Webster, J.R. The Role of Macroinvertebrates in Stream Ecosystem Function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The Role of Benthic Invertebrate Species in Freshwater Ecosystems: Zoobenthic species influence energy flows and nutrient cycling. BioScience 1999, 49, 119–127. [Google Scholar] [CrossRef]
- McLenaghan, N.A.; Tyler, A.C.; Mahl, U.H.; Howarth, R.W.; Marino, R.M. Benthic macroinvertebrate functional diversity regulates nutrient and algal dynamics in a shallow estuary. Mar. Ecol. Prog. Ser. 2011, 426, 171–184. [Google Scholar] [CrossRef]
- Ogbeibu, A.E.; Oribhabor, B.J. Ecological impact of river impoundment using benthic macro-invertebrates as indicators. Water Res. 2002, 36, 2427–2436. [Google Scholar] [CrossRef]
- Arimoro, F.O.; Ikomi, R.B. Ecological integrity of upper Warri River, Niger Delta using aquatic insects as bioindicators. Ecol. Indic. 2009, 9, 455–461. [Google Scholar] [CrossRef]
- Bonada, N.; Prat, N.; Resh, V.H.; Statzner, B. Developments in aquatic insect biomonitoring: A comparative analysis of recent approaches. Annu. Rev. Entomol. 2006, 51, 495–523. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Feld, C.K.; Hering, D. Community structure or function: Effects of environmental stress on benthic macroinvertebrates at different spatial scales. Freshwat. Biol. 2007, 52, 1380–1399. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Wang, X. Recognizing the Relationship between Spatial Patterns in Water Quality and Land-Use/Cover Types: A Case Study of the Jinghe Oasis in Xinjiang, China. Water 2018, 10, 646. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kaluskar, S.; Mugalingam, S.; Arhonditsis, G.B. Evaluating the relationships between watershed physiography, land use patterns, and phosphorus loading in the Bay of Quinte, Ontario, Canada. J. Great Lakes Res. 2016, 42, 972–984. [Google Scholar] [CrossRef]
- Sponseller, R.A.; Benfield, E.F.; Valett, H.M. Relationships between land use, spatial scale and stream macroinvertebrate communities. Freshwat. Biol. 2001, 46, 1409–1424. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kaluskar, S.; Mugalingam, S.; Blukacz-Richards, A.; Long, T.; Morley, A.; Arhonditsis, G.B. A Bayesian approach for estimating phosphorus export and delivery rates with the SPAtially Referenced Regression On Watershed attributes (SPARROW) model. Ecol. Inform. 2017, 37, 77–91. [Google Scholar] [CrossRef]
- Wellen, C.; Arhonditsis, G.B.; Labencki, T.; Boyd, D. Application of the SPARROW model in watersheds with limited information: A Bayesian assessment of the model uncertainty and the value of additional monitoring. Hydrol. Process. 2014, 28, 1260–1283. [Google Scholar] [CrossRef]
- Park, S.-B.; Lee, S.-K.; Chang, K.-H.; Jeong, K.-S.; Joo, G.-J. The impact of monsoon rainfall (Changma) on the changes of water quality in the lower Nakdong River (Mulgeum). Korean J. Limnol. 2002, 35, 161–170. [Google Scholar]
- Park, J.-S.; Kang, H.-S.; Lee, Y.S.; Kim, M.-K. Changes in the extreme daily rainfall in South Korea. Int. J. Climatol. 2011, 31, 2290–2299. [Google Scholar] [CrossRef]
- Wetzel, R.G.; Likens, G.E. Limnological Analysis; Springer: Berlin/Heidelberg, Germany, 1991; p. 429. [Google Scholar]
- Quigley, M. Invertebrates of Streams and Rivers; Edward A. Ltd.: Colchester, London, UK, 1977; p. 84. [Google Scholar]
- Pennak, R.W. Freshwater Invertebrates of the United States; John Wiley and Sons, Inc.: Hoboken, MY, USA, 1978; p. 803. [Google Scholar]
- Brighnam, A.R.; Brighnam, W.U.; Gnika, A. Aquatic Insects and Oligochaetea of North and South Carolina; Midwest Aquatic Enterprises: Seaford, UK, 1982. [Google Scholar]
- Yun, I.-B. Illustrated Encyclopedia of Fauna and Flora of Korea. Aquatic Insects; Ministry of Education: Seoul, Korea, 1988; Volume 30, p. 840.
- Merritt, R.W.; Cummins, K.W. An Introduction to the Aquatic Insects of North America; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 1996; p. 862. [Google Scholar]
- McNaughton, S.J. Relationships among Functional Properties of Californian Grassland. Nature 1967, 216, 168–169. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Champaign, IL, USA, 1964; p. 125. [Google Scholar]
- Margalef, R. Temporal succession and spatial heterogeneity in phytoplankton. In Perspectives in Marine Biology; Buzzati-Traverso, A.A., Ed.; University of California Press: Berkeley, CA, USA, 1958; pp. 323–347. [Google Scholar]
- Pielou, E.C. Ecological Diversity; Wiley: New York, NY, USA, 1975; p. 165. [Google Scholar]
- Kong, D.; Son, S.-H.; Hwang, S.-J.; Won, D.H.; Kim, M.C.; Park, J.H.; Jeon, T.-S.; Lee, J.E.; Kim, J.H.; Kim, J.S.; et al. Development of benthic macroinvertebrates index (BMI) for biological assessment on stream environment. J. Korean Soc. Water Environ. 2018, 34, 183–201, (Written In Korean). [Google Scholar]
- Zelinka, M.; Marvan, P. Zur Präzisierung der biologischen Klassifikation der Reinheit fließender Gewässer. Arch. Hydrobiol. 1961, 57, 389–407. [Google Scholar]
- National Institute of Environmental Research (NIER). Biomonitoring Survey and Assessment Manual; NIER: Incheon, Korea, 2017.
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 269. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012; p. 496. [Google Scholar]
- Aschonitis, V.G.; Feld, C.K.; Castaldelli, G.; Turin, P.; Visonà, E.; Fano, E.A. Environmental stressor gradients hierarchically regulate macrozoobenthic community turnover in lotic systems of Northern Italy. Hydrobiologia 2016, 765, 131–147. [Google Scholar] [CrossRef]
- Osborne, J.W. Improving your data transformation: Applying the Box-Cox transformation. Pract. Assess. Res. Eval. 2010, 15, 1–9. [Google Scholar]
- Liu, R.; Wang, Q.; Xu, F.; Men, C.; Guo, L. Impacts of manure application on SWAT model outputs in the Xiangxi River watershed. J. Hydrol. 2017, 555, 479–488. [Google Scholar] [CrossRef]
- Tiessen, K.H.D.; Elliott, J.A.; Yarotski, J.; Lobb, D.A.; Flaten, D.N.; Glozier, N.E. Conventional and conservation tillage: Influence on seasonal runoff, sediment, and nutrient losses in the Canadian Prairies. J. Environ. Qual. 2010, 39, 964–980. [Google Scholar] [CrossRef]
- Ahn, K.S. The water pollution of Yocheon, uppermost stream of the Sumjin River. J. Korean Earth Sci. Soc. 2005, 26, 821–827. [Google Scholar]
- Liang, T.; Wang, S.; Cao, H.; Zhang, C.; Li, H.; Li, H.; Song, W.; Chong, Z. Estimation of ammonia nitrogen load from nonpoint sources in the Xitiao River catchment, China. J. Environ. Sci. 2008, 20, 1195–1201. [Google Scholar] [CrossRef]
- Chen, A.; Lei, B.; Hu, W.; Lu, Y.; Mao, Y.; Duan, Z.; Shi, Z. Characteristics of ammonia volatilization on rice grown under different nitrogen application rates and its quantitative predictions in Erhai Lake Watershed, China. Nutr. Cycl. Agroecosyst. 2015, 101, 139–152. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Heenan, D.P.; Marschner, P.; McNeill, A.M. Long-term effects of crop rotation, stubble management and tillage on soil phosphorus dynamics. Aust. J. Soil Res. 2006, 44, 611–618. [Google Scholar] [CrossRef]
- Easton, Z.M.; Gérard-Marchant, P.; Walter, M.T.; Petrovic, A.M.; Steenhuis, T.S. Identifying dissolved phosphorus source areas and predicting transport from an urban watershed using distributed hydrologic modeling. Water Resour. Res. 2007, 43. [Google Scholar] [CrossRef]
- Ahn, S.R.; Jeong, J.H.; Kim, S.J. Assessing drought threats to agricultural water supplies under climate change by combining the SWAT and MODSIM models for the Geum River basin, South Korea. Hydrol. Sci. J. 2016, 61, 2740–2753. [Google Scholar] [CrossRef]
- Jeong, K.-S.; Hong, D.-G.; Byeon, M.-S.; Jeong, J.-C.; Kim, H.-G.; Kim, D.-K.; Joo, G.-J. Stream modification patterns in a river basin: Field survey and self-organizing map (SOM) application. Ecol. Inform. 2010, 5, 293–303. [Google Scholar] [CrossRef]
- Jäch, M.A.; Balke, M. Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia 2008, 595, 419–442. [Google Scholar] [CrossRef]
- Jung, S.W.; Jäch, M.A.; Bae, Y.J. Review of the Korean Elmidae (Coleoptera: Dryopoidea) with descriptions of three new species. Aquat. Insects 2014, 36, 93–124. [Google Scholar] [CrossRef]
- Criado, F.G.; Alaez, M.F. Aquatic Coleoptera (Hydraenidae and Elmidae) as indicators of the chemical characteristics of water in the Orbigo River basin (N-W Spain). Ann. Limnol. Int. J. Lim. 1995, 31, 185–199. [Google Scholar] [CrossRef]
- Wallace, J.B. Food Partitioning in Net-spinning Trichoptera Larvae: Hydropsyche venularis, Cheumatopsyche etrona, and Macronema zebratum (Hydropsychidae). Ann. Entomol. Soc. Am. 1975, 68, 463–472. [Google Scholar] [CrossRef]
- Fuller, R.L.; Mackay, R.J. Effects of food quality on the growth of three Hydropsyche species (Trichoptera: Hydropsychidae). Can. J. Zool. 1981, 59, 1133–1140. [Google Scholar] [CrossRef]
- Osborne, L.L.; Herricks, E.E. Microhabitat Characteristics of Hydropsyche (Trichoptera:Hydropsychidae) and the Importance of Body Size. J. N. Am. Benthol. Soc. 1987, 6, 115–124. [Google Scholar] [CrossRef]
- Beketov, M. Different sensitivity of mayflies (Insecta, Ephemeroptera) to ammonia, nitrite and nitrate: Linkage between experimental and observational data. Hydrobiologia 2004, 528, 209–216. [Google Scholar] [CrossRef]
- Lammers-Campbell, R. Ordination of Chironomid (Diptera: Chironomidae) Communities Characterizing Habitats in a Minnesota Peatland. J. Kans. Entomol. Soc. 1998, 71, 414–425. [Google Scholar]
- Principe, R.E.; Boccolini, M.F.; Corigliano, M.C. Structure and Spatial-Temporal Dynamics of Chironomidae Fauna (Diptera) in Upland and Lowland Fluvial Habitats of the Chocancharava River Basin (Argentina). Int. Rev. Hydrobiol. 2008, 93, 342–357. [Google Scholar] [CrossRef]
- Campbell, B.D.; Haro, R.J.; Richardson, W.B. Effects of agricultural land use on chironomid communities: Comparisons among natural wetlands and farm ponds. Wetlands 2009, 29, 1070–1080. [Google Scholar] [CrossRef]
- Corkum, L.D. Responses of chlorophyll-a, organic matter, and macroinvertebrates to nutrient additions in rivers flowing through agricultural and forested land. Arch. Hydrobiol. 1996, 136, 391–411. [Google Scholar]
- Hemphill, N. Competition between two stream dwelling filter-feeders, Hydropsyche oslari and Simulium virgatum. Oecologia 1988, 77, 73–80. [Google Scholar] [CrossRef]
- Azrina, M.Z.; Yap, C.K.; Rahim Ismail, A.; Ismail, A.; Tan, S.G. Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Langat River, Peninsular Malaysia. Ecotoxicol. Environ. Saf. 2006, 64, 337–347. [Google Scholar] [CrossRef]
- Scrimgeour, G.J.; Winterbourn, M.J. Effects of floods on epilithon and benthic macroinvertebrate populations in an unstable New Zealand river. Hydrobiologia 1989, 171, 33–44. [Google Scholar] [CrossRef]
- Robinson, C.T.; Uehlinger, U.; Monaghan, M.T. Effects of a multi-year experimental flood regime on macroinvertebrates downstream of a reservoir. Aquat. Sci. 2003, 65, 210–222. [Google Scholar] [CrossRef]


| Order | Family or Genus | Month | SDU | SDL | OS | SC | YC | SG | JD | BS | SL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphipoda | Gammarus spp. | May | 26.6% | 0.0% | 0.0% | 0.0% | 0.1% | 0.0% | 0.0% | 0.0% | 0.6% |
| Sep | 69.6% | 0.0% | 0.0% | 0.0% | 0.1% | 0.0% | 0.0% | 0.0% | 0.0% | ||
| Arhynchobdellida | Erpobdella spp. | May | 0.1% | 0.0% | 0.3% | 0.2% | 0.3% | 0.2% | 0.1% | 0.1% | 0.3% |
| Sep | 0.0% | 0.2% | 12.0% | 2.1% | 2.7% | 2.0% | 1.0% | 0.2% | 0.8% | ||
| Coleoptera | Elmidae spp. | May | 0.2% | 1.9% | 8.3% | 0.7% | 0.6% | 10.6% | 0.5% | 0.5% | 0.3% |
| Sep | 0.0% | 9.0% | 2.4% | 0.3% | 2.9% | 0.1% | 1.9% | 0.7% | 1.7% | ||
| Eubrianax spp. | May | 0.0% | 0.0% | 0.0% | 0.1% | 0.1% | 0.1% | 0.0% | 0.1% | 0.5% | |
| Sep | 0.9% | 0.6% | 1.2% | 6.6% | 0.5% | 4.2% | 9.6% | 11.8% | 1.1% | ||
| Decapoda | Caridina spp. | May | 0.0% | 0.5% | 0.1% | 0.3% | 0.0% | 0.1% | 0.0% | 0.1% | 0.0% |
| Sep | 0.0% | 4.5% | 10.9% | 1.1% | 0.3% | 0.2% | 0.0% | 0.7% | 0.3% | ||
| Diptera | Chironomidae | May | 9.6% | 21.9% | 23.5% | 61.7% | 51.6% | 44.3% | 66.3% | 36.1% | 37.8% |
| Sep | 5.9% | 24.1% | 19.5% | 30.3% | 30.3% | 19.1% | 28.1% | 19.5% | 24.4% | ||
| Simulium spp. | May | 7.4% | 0.5% | 0.4% | 0.7% | 0.5% | 0.3% | 0.1% | 0.7% | 1.9% | |
| Sep | 0.7% | 0.0% | 0.3% | 0.0% | 0.0% | 0.1% | 0.2% | 0.0% | 1.1% | ||
| Ephemeroptera | Baetiella spp. | May | 0.1% | 0.0% | 0.2% | 0.0% | 3.8% | 0.7% | 0.0% | 3.3% | 3.4% |
| Sep | 0.3% | 1.5% | 0.1% | 0.0% | 2.0% | 0.4% | 0.0% | 0.0% | 5.1% | ||
| Baetis spp. | May | 1.5% | 13.7% | 4.6% | 8.8% | 10.9% | 1.8% | 4.7% | 18.4% | 9.5% | |
| Sep | 6.5% | 4.0% | 8.2% | 16.1% | 7.6% | 12.8% | 9.7% | 2.4% | 20.2% | ||
| Caenidae | May | 0.1% | 3.2% | 0.0% | 1.1% | 0.0% | 0.1% | 0.4% | 0.4% | 0.7% | |
| Sep | 0.4% | 0.6% | 0.0% | 2.2% | 0.0% | 1.5% | 1.8% | 15.9% | 0.9% | ||
| Choroterpes spp. | May | 1.4% | 2.6% | 0.7% | 2.7% | 2.1% | 0.8% | 6.0% | 7.2% | 4.0% | |
| Sep | 0.0% | 0.8% | 0.9% | 0.7% | 1.6% | 1.3% | 2.4% | 4.0% | 1.1% | ||
| Ecdyonurus spp. | May | 4.0% | 0.0% | 0.7% | 2.6% | 2.9% | 8.7% | 2.3% | 3.2% | 5.0% | |
| Sep | 2.2% | 23.1% | 3.9% | 1.4% | 9.3% | 11.2% | 13.5% | 14.0% | 12.4% | ||
| Epeorus spp. | May | 0.4% | 0.1% | 0.0% | 0.0% | 1.7% | 0.1% | 0.3% | 0.1% | 4.4% | |
| Sep | 1.1% | 0.0% | 0.1% | 0.1% | 2.4% | 1.5% | 0.1% | 0.0% | 3.2% | ||
| Ephemera spp. | May | 0.1% | 0.2% | 0.4% | 1.1% | 1.4% | 1.1% | 4.5% | 1.2% | 0.4% | |
| Sep | 0.3% | 0.3% | 0.3% | 4.5% | 2.7% | 0.1% | 4.9% | 3.8% | 0.6% | ||
| Labiobaetis spp. | May | 0.0% | 0.3% | 0.0% | 0.1% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Sep | 0.5% | 8.7% | 1.9% | 0.0% | 0.1% | 0.0% | 0.2% | 0.0% | 0.1% | ||
| Gastropoda | Semisulcospira spp. | May | 1.5% | 40.4% | 16.6% | 8.1% | 7.0% | 22.2% | 3.0% | 15.3% | 16.3% |
| Sep | 0.6% | 11.6% | 19.4% | 7.1% | 18.1% | 19.6% | 16.2% | 11.8% | 15.6% | ||
| Radix spp. | May | 0.4% | 0.1% | 2.1% | 0.7% | 1.3% | 1.7% | 0.3% | 0.3% | 2.0% | |
| Sep | 0.1% | 0.0% | 0.3% | 0.0% | 0.2% | 1.0% | 0.8% | 6.6% | 1.4% | ||
| Haplotaxida | Limnodrilus spp. | May | 0.5% | 1.7% | 2.1% | 7.7% | 6.2% | 3.1% | 9.3% | 5.4% | 1.5% |
| Sep | 0.5% | 2.9% | 1.7% | 25.6% | 2.2% | 15.2% | 6.9% | 6.0% | 2.6% | ||
| Isopoda | Asellus sp. | May | 41.0% | 0.0% | 2.1% | 0.2% | 0.5% | 1.1% | 1.2% | 0.0% | 0.0% |
| Sep | 3.3% | 0.2% | 0.6% | 0.0% | 0.1% | 4.8% | 0.0% | 0.0% | 0.1% | ||
| Trichoptera | Cheumatopsyche spp. | May | 0.2% | 8.4% | 10.4% | 0.1% | 2.6% | 0.0% | 0.0% | 0.1% | 0.5% |
| Sep | 2.2% | 5.0% | 13.6% | 0.0% | 0.2% | 0.0% | 0.2% | 0.0% | 0.4% | ||
| Hydropsyche spp. | May | 2.6% | 3.2% | 24.5% | 0.2% | 0.6% | 0.1% | 0.0% | 0.7% | 7.4% | |
| Sep | 1.2% | 2.9% | 1.7% | 0.0% | 2.4% | 0.0% | 0.0% | 0.0% | 0.1% | ||
| Tricladida | Dugesia spp. | May | 2.3% | 1.3% | 2.9% | 3.0% | 5.9% | 2.9% | 0.9% | 6.8% | 3.5% |
| Sep | 3.7% | 0.0% | 0.9% | 1.8% | 14.3% | 5.1% | 2.5% | 2.8% | 6.6% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-K.; Jo, H.; Park, K.; Kwak, I.-S. Assessing Spatial Distribution of Benthic Macroinvertebrate Communities Associated with Surrounding Land Cover and Water Quality. Appl. Sci. 2019, 9, 5162. https://doi.org/10.3390/app9235162
Kim D-K, Jo H, Park K, Kwak I-S. Assessing Spatial Distribution of Benthic Macroinvertebrate Communities Associated with Surrounding Land Cover and Water Quality. Applied Sciences. 2019; 9(23):5162. https://doi.org/10.3390/app9235162
Chicago/Turabian StyleKim, Dong-Kyun, Hyunbin Jo, Kiyun Park, and Ihn-Sil Kwak. 2019. "Assessing Spatial Distribution of Benthic Macroinvertebrate Communities Associated with Surrounding Land Cover and Water Quality" Applied Sciences 9, no. 23: 5162. https://doi.org/10.3390/app9235162
APA StyleKim, D.-K., Jo, H., Park, K., & Kwak, I.-S. (2019). Assessing Spatial Distribution of Benthic Macroinvertebrate Communities Associated with Surrounding Land Cover and Water Quality. Applied Sciences, 9(23), 5162. https://doi.org/10.3390/app9235162