Stochastic Modeling and Optimal Time-Frequency Estimation of Task-Related HRV
Abstract
1. Introduction
2. Methods
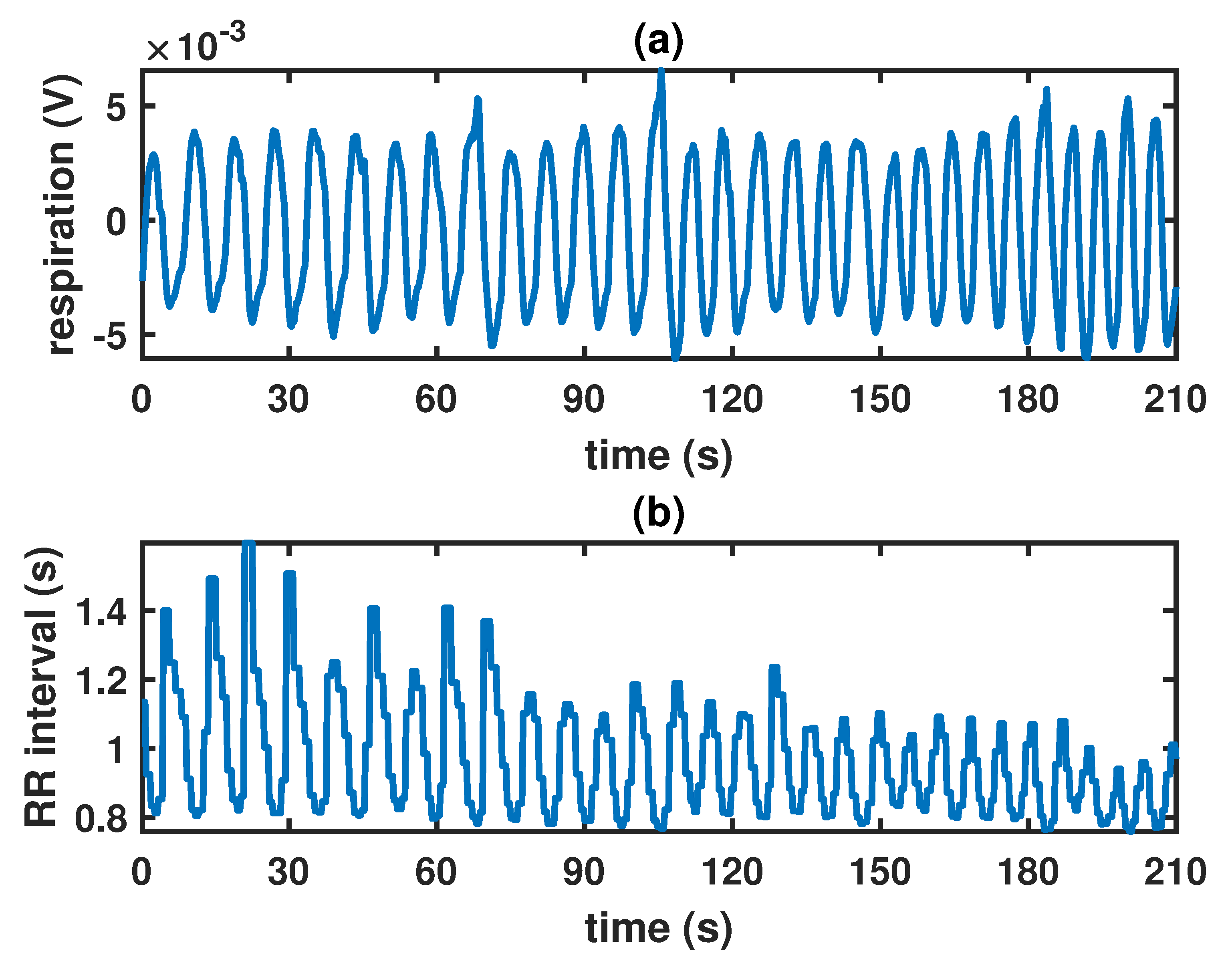
2.1. Data Acquisition and Preprocessing
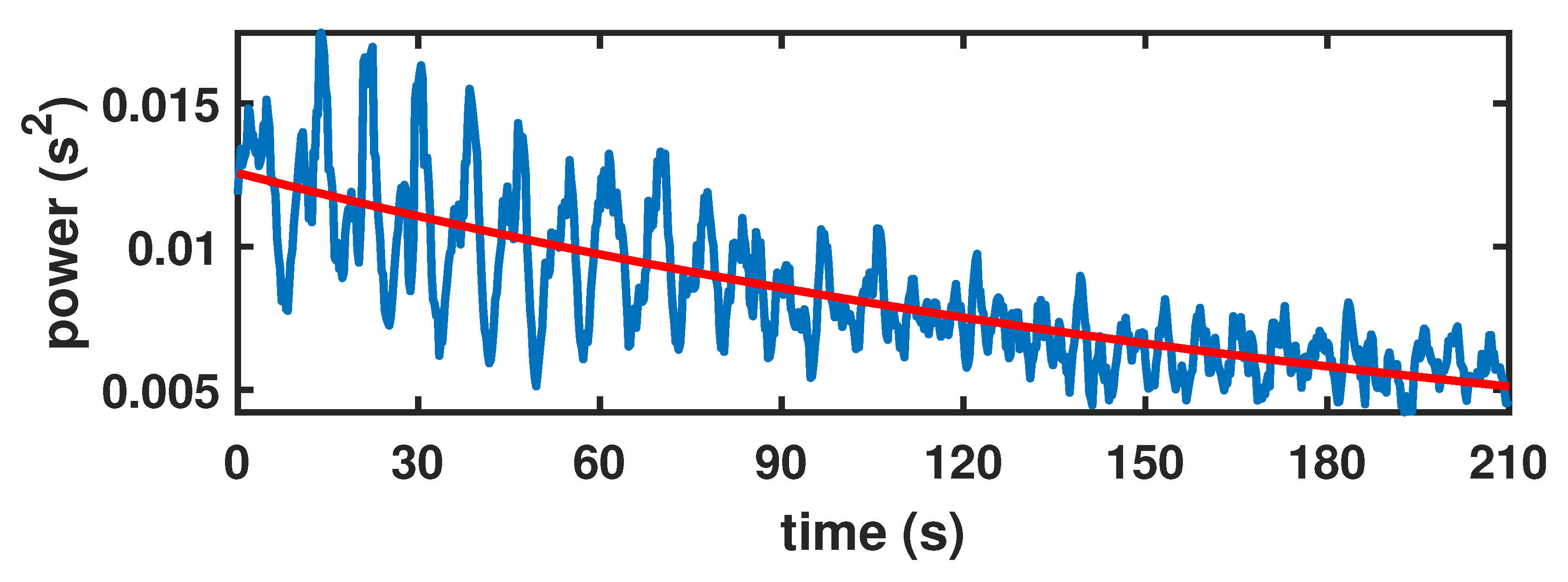
2.2. Locally Stationary Chirp Processes
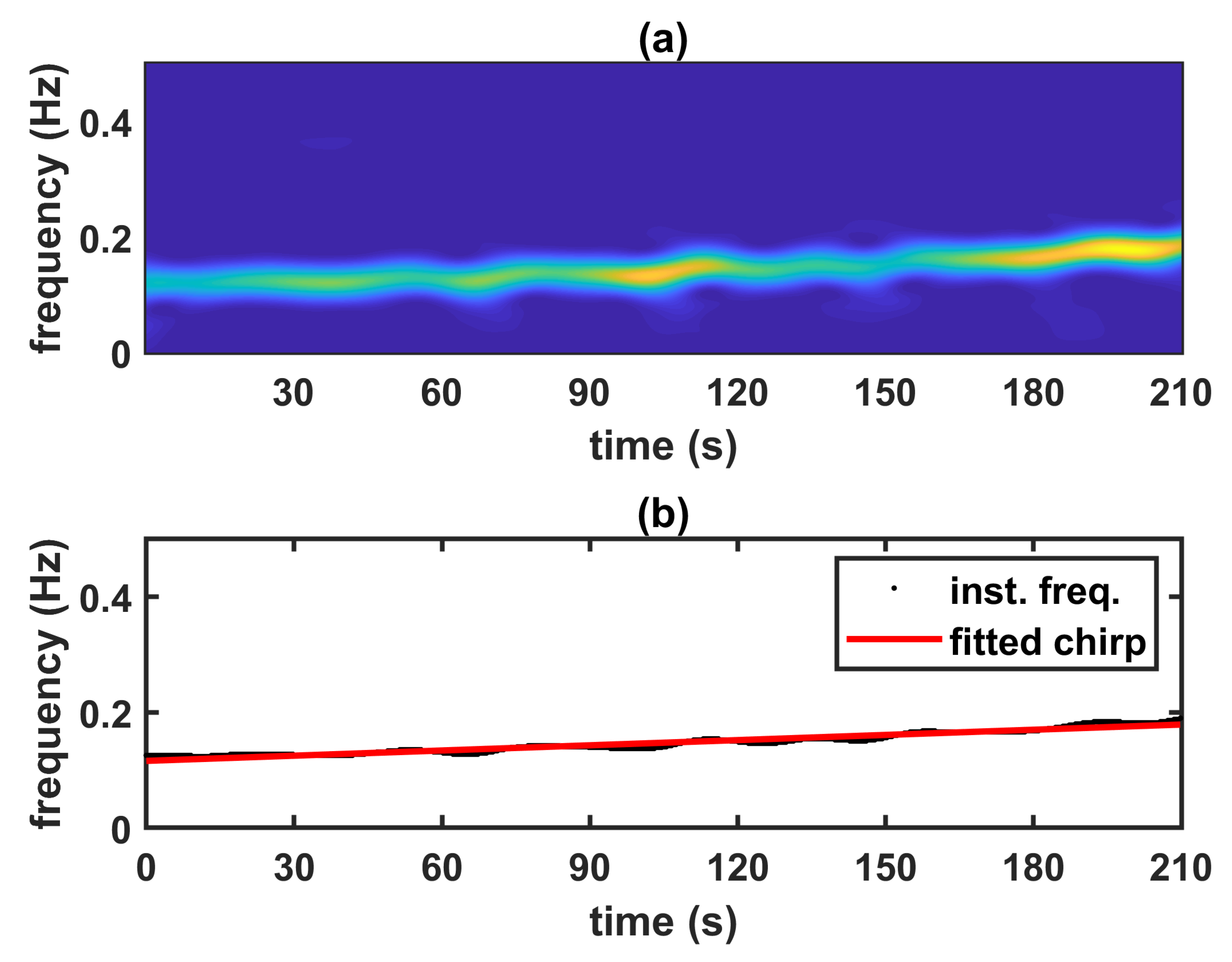
2.3. Inference Method
| Algorithm 1: Inference for LSCP parameters |
| Input: Zero mean time-series data, both respiratory signal and corresponding HRV. Output: Estimated chirp parameters and estimated LSP parameters . Chirp estimation 1 Estimate the instantaneous frequency of the chirp from the the respiratory signal, by taking the frequency of maximum power in the chirp range from a TF representation of the signal; 2 Find and the corresponding covariance matrix by fitting the linear chirp to the instantaneous frequency of the respiratory data; LSP estimation 3 Compute the instantaneous power from the HRV data; 4 Find by fitting the model function q to ; 5 Find by fitting to where R is the stationary covariance matrix of the model function r, is the estimated sample covariance matrix of the HRV data and is the corresponding Hankel matrix based on the model q with parameters ; Return: . |
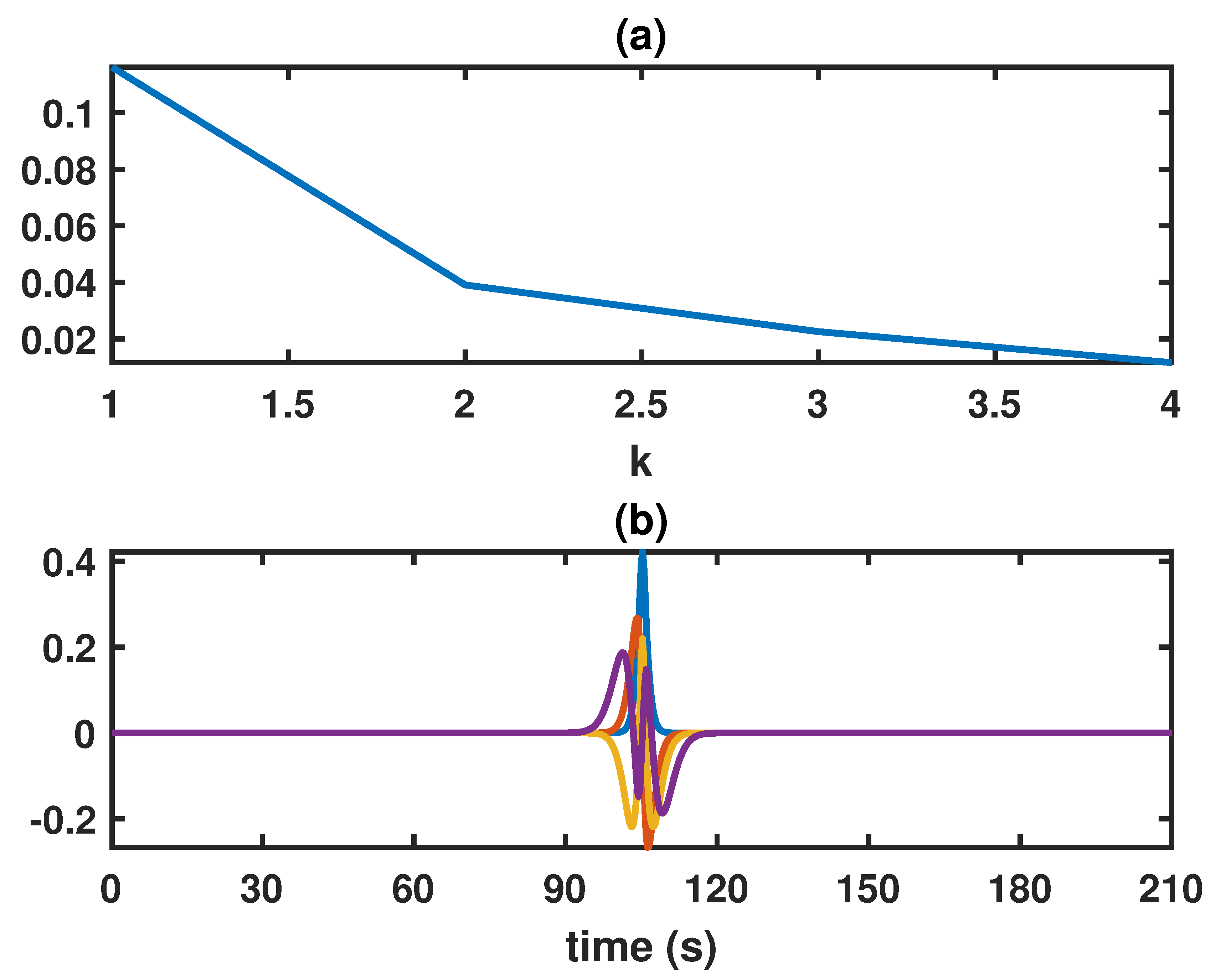
2.4. Mean Square Error Optimal Time-Frequency Kernel
2.5. Regression Analysis
3. Results and Discussion
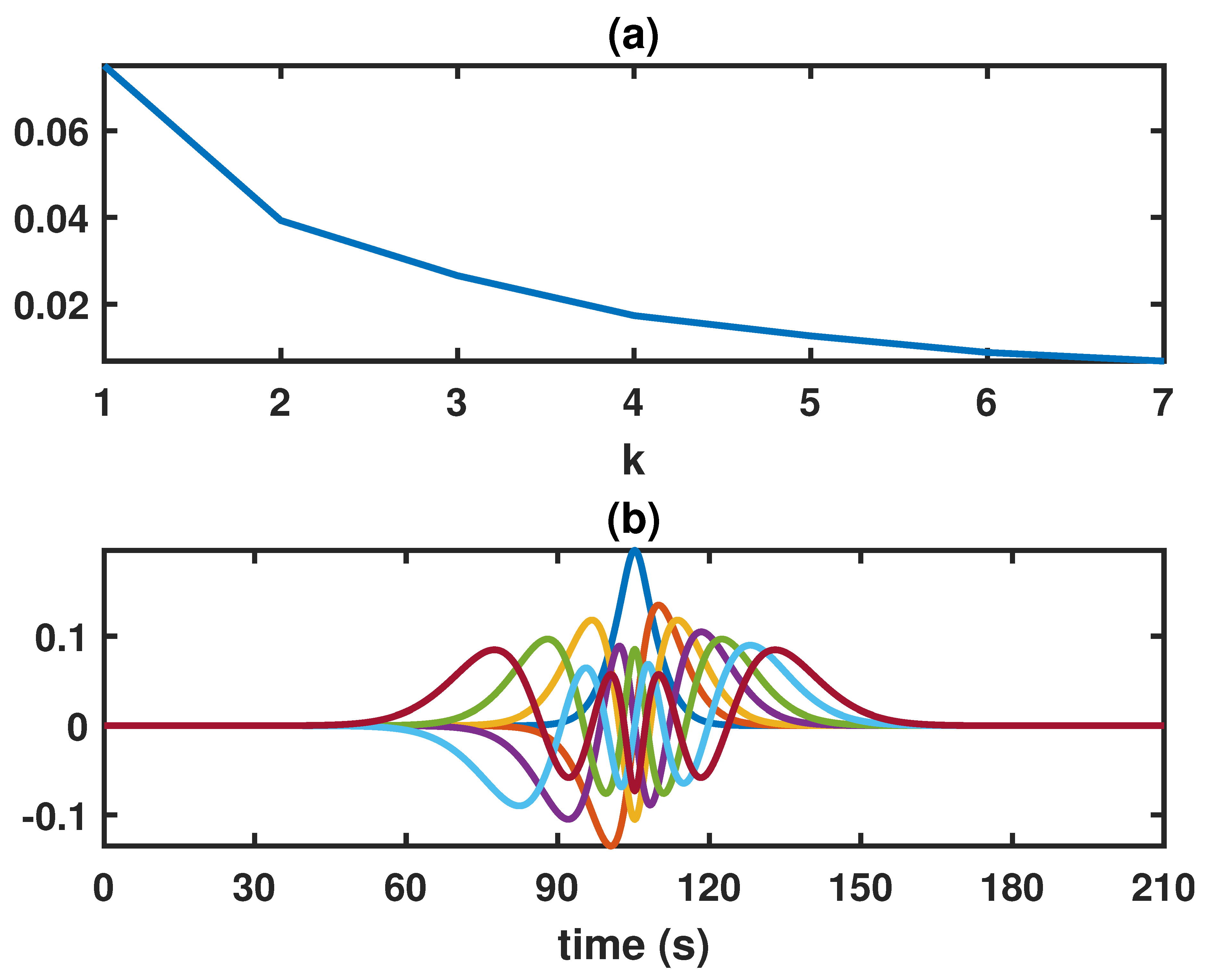
3.1. Inference on the Model Parameters
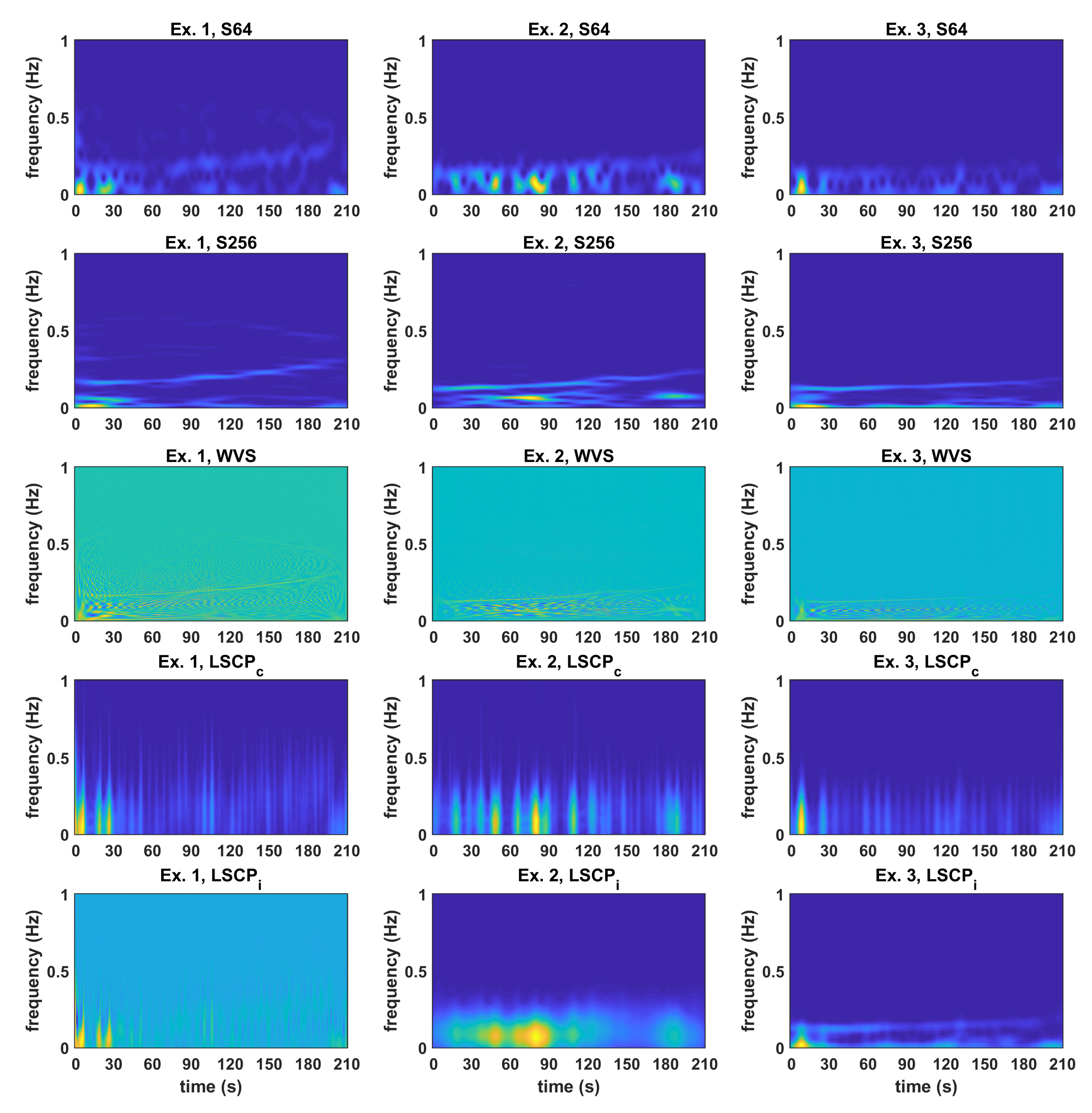
3.2. Comparison between Time-Frequency Estimates
3.3. Regression Analysis
3.3.1. Parameter m
3.3.2. Parameter d
3.3.3. Parameter a
3.3.4. Parameter b
3.3.5. Parameter c
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike information criterion |
| BMI | body-mass index |
| ECG | electrocardiography |
| HATS | Hankel-Toeplitz separation |
| HRV | heart rate variability |
| LSCP | locally stationary chirp process |
| LSCP with parameters estimated from a common process | |
| LSCP with individually estimated parameters | |
| LSP | locally stationary process |
| MSE | mean square error |
| adjusted coefficient of determination R square | |
| RSA | respiratory sinus arrhythmia |
| SMBQ | Shirom-Melamed Burnout Questionnaire |
| STAI | Spielberg State-Trait Anxiety Inventory |
| S64 | spectrogram with 64 samples Hanning window |
| S256 | spectrogram with 256 samples Hanning window |
| TF | time-frequency |
| TP | test participant |
| WVS | Wigner-Ville spectrum |
References
- Laborde, S.; Mosley, E.; Mertgen, A. A unifying conceptual framework of factors associated to cardiac vagal control. Heliyon 2018, 4, e01002. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Hernando, A.; Casajus, J.A.; Laguna, P.; Garatachea, N.; Bailón, R. Methodological framework for heart rate variability analysis during exercise: Application to running and cycling stress testing. Med. Biol. Eng. Comput. 2018, 56, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, P.; Österberg, K.; Wallergård, M.; Hansen, Å.M.; Garde, A.H.; Johansson, G.; Karlson, B. Exhaustion-related changes in cardiovascular and cortisol reactivity to acute psychosocial stress. Physiol. Behav. 2015, 151, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-Term Heart Rate Variability - Influence of Gender and Age in Healthy Subjects. PLoS ONE 2015, 10, 1–33. [Google Scholar] [CrossRef]
- Gurel, N.Z.; Carek, A.M.; Inan, O.T.; Levantsevych, O.; Abdelhadi, N.; Hammadah, M.; O’Neal, W.T.; Kelli, H.; Wilmot, K.; Ward, L.; et al. Comparison of autonomic stress reactivity in young healthy versus aging subjects with heart disease. PLoS ONE 2019, 14, e0216278. [Google Scholar] [CrossRef]
- Woo, J.M.; Kim, T.S. Gender Plays Significant Role in Short-Term Heart Rate Variability. Appl. Psychophysiol. Biofeedback 2015, 40, 297–303. [Google Scholar] [CrossRef]
- Lennartsson, A.; Jonsdottir, I.; Sjörs, A. Low heart rate variability in patients with clinical burnout. Int. J. Psychophysiol. 2016, 110, 171–178. [Google Scholar] [CrossRef]
- Lindgren, G.; Rootzén, H.; Sandsten, M. Stationary Stochastic Processes for Scientists and Engineers; Chapman and Hall/CRC: Boca Raton, FL, USA, 2014. [Google Scholar]
- Valderas, M.T.; Bolea, J.; Laguna, P.; Bailón, R.; Vallverdú, M. Mutual information between heart rate variability and respiration for emotion characterization. Physiol. Meas. 2019, 40, 084001. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Wang, X.; Xie, L.; Tong, J.; Zhao, F.; Di, X.; Yan, X.; Zhang, J. An improved method to evaluate heart rate variability based on time-variant cardiorespiratory relation. J. Appl. Physiol. (Bethesda Md. 1985) 2019, 127, 320–327. [Google Scholar] [CrossRef]
- Cohen, L. Time-Frequency Analysis; Signal Processing Series; Prentice-Hall: Upper Saddle River, NJ, USA, 1995. [Google Scholar]
- Gates, K.M.; Gatzke-Kopp, L.M.; Sandsten, M.; Blandon, A.Y. Estimating time-varying RSA to examine psychophysiological linkage of marital dyads. Psychophysiology 2015, 52, 1059–1065. [Google Scholar] [CrossRef]
- Anderson, R.; Jönsson, P.; Sandsten, M. Insights on Spectral Measures for HRV Based on a Novel Approach for Data Acquisition. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 510–513. [Google Scholar] [CrossRef]
- Billman, G.E. Heart Rate Variability—A Historical Perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Weippert, M.; Behrens, K.; Rieger, A.; Kumar, M.; Behrens, M. Effects of breathing patterns and light exercise on linear and nonlinear heart rate variability. Appl. Physiol. Nutr. Metab. 2015, 40, 762–768. [Google Scholar] [CrossRef]
- Hernando, A.; Lazaro, J.; Gil, E.; Arza, A.; Garzon, J.; Lopez-Anton, R.; De La Camara, C.; Laguna, P.; Aguilo, J.; Bailon, R. Inclusion of respiratory frequency information in heart rate variability analysis for stress assessment. IEEE J. Biomed. Health Inform. 2016, 20, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jönsson, P.; Sandsten, M. Performance Comparison of Time-Frequency Distributions for Estimation of Instantaneous Frequency of Heart Rate Variability Signals. Appl. Sci. 2017, 7, 221. [Google Scholar] [CrossRef]
- Choi, J.; Gutierrez-Osuna, R. Removal of Respiratory Influences From Heart Rate Variability in Stress Monitoring. IEEE Sens. J. 2011, 11, 2649. [Google Scholar] [CrossRef]
- Silverman, R. Locally stationary random processes. IRE Trans. Inf. Theory 1957, 3, 182. [Google Scholar] [CrossRef]
- Wahlberg, P.; Hansson, M. Kernels and multiple windows for estimation of the Wigner-Ville spectrum of Gaussian locally stationary processes. IEEE Trans. Signal Process. 2007, 55, 73–84. [Google Scholar] [CrossRef]
- Hansson-Sandsten, M. Optimal Multitaper Wigner Spectrum Estimation of a Class of Locally Stationary Processes Using Hermite Functions. EURASIP J. Adv. Signal Process. 2011, 980805. [Google Scholar] [CrossRef]
- Anderson, R.; Jönsson, P.; Sandsten, M. Effects of Age, BMI, Anxiety and Stress on the Parameters of a Stochastic Model for Heart Rate Variability Including Respiratory Information. In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies—Volume 3: BIOSIGNALS; SciTePress: Funchal, Madeira, Portugal, 2018; pp. 17–25. [Google Scholar] [CrossRef]
- Anderson, R.; Sandsten, M. Inference for time-varying signals using locally stationary processes. J. Comput. Appl. Math. 2019, 347, 24–35. [Google Scholar] [CrossRef]
- Rawlings, J.O.; Pantula, S.G.; Dickey, D.A. Applied Regression Analysis—A Research Tool, 2nd ed.; Springer: New York, NY, USA; London, UK, 1998. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L. Manual for the State-Trait Anxiety Inventory, STAI (Form Y); Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Shirom, A. Burnout in work organization. In International Review of Industrial and Organizational Psychology; Cooper, C.L., Robertson, I., Eds.; Wiley: New York, NY, USA, 1989; pp. 25–48. [Google Scholar]
- Melamed, S.; Kushnir, T.; Shirom, A. Burnout and risk factors for cardiovascular diseases. Behav. Med. 1992, 18, 53–60. [Google Scholar] [CrossRef]
- Melamed, S.; Shirom, A.; Toker, S.; Berliner, S.; Shapira, I. Burnout and Risk of Cardiovascular Disease: Evidence, Possible Causal Paths, and Promising Research Directions. Psychol. Bull. 2006, 132, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Hansen, Å.M.; Hogh, A.; Persson, R.; Karlson, B.; Garde, A.H.; Ørbæk, P. Original article: Bullying at work, health outcomes, and physiological stress response. J. Psychosom. Res. 2006, 60, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Persson, R.; Ørbæk, P. The influence of personality traits on neuropsychological test performance and self-reported health and social context in women. Personal. Individ. Differ. 2003, 34, 295–313. [Google Scholar] [CrossRef]
- Persson, R.; Österberg, K.; Karlson, B.; Ørbæk, P. The Meta-Contrast Technique: Relationships with personality traits and cognitive abilities in healthy women. Scand. J. Psychol. 2005, 46, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Grossi, G.; Perski, A.; Evengård, B.; Blomkvist, V.; Orth-Gomér, K. Physiological correlates of burnout among women. J. Psychosom. Res. 2003, 55, 309–316. [Google Scholar] [CrossRef]
- Lundgren-Nilsson, Å.; Jonsdottir, I.H.; Pallant, J.; Ahlborg, G. Internal construct validity of the Shirom-Melamed Burnout Questionnaire (SMBQ). BMC Public Health 2012, 12. [Google Scholar] [CrossRef]
- Shirom, A. Job-related burnout: A review. In Handbook of Occupational Health Psychology; American Psychological Association: Washington, DC, USA, 2003; pp. 245–264. [Google Scholar]
| Women | Men | |||||
|---|---|---|---|---|---|---|
| (n = 43) | (n = 49) | |||||
| Median | Mean | sd | Median | Mean | sd | |
| Age | 25 | 28.05 | 7.86 | 27 | 30.84 | 10.42 |
| Weight | 62 | 63.56 | 9.70 | 79 | 79.14 | 10.39 |
| BMI | 21.77 | 21.97 | 2.70 | 23.45 | 24.23 | 3.05 |
| State Anxiety | 33 | 34.30 | 7.02 | 30 | 31.73 | 7.76 |
| Trait Anxiety | 39 | 40.25 | 10.33 | 33 | 35.79 | 9.55 |
| SMBQ | 3.05 | 3.40 | 1.20 | 2.59 | 2.95 | 1.16 |
| Parameter | Median | Mean | sd | ||
|---|---|---|---|---|---|
| m | 0.0003 | 0.0003 | 0.0001 | 0.3139 | 0.0003 |
| d | −396.36 | −379.16 | 68.71 | −0.1812 | −402.62 |
| a | 0.0111 | 0.0155 | 0.0162 | 1.0423 | 0.0126 |
| b | 0.0052 | 0.0146 | 0.0462 | 3.1709 | 0.0043 |
| c | 4.2023 | 3.9032 | 1.8951 | 0.4855 | 5.6208 |
| Coeff. Est. | S.E. | p-Value | |
|---|---|---|---|
| Gender (M) | 0.0353 | ||
| Age | < | ||
| SMBQ | 0.0443 |
| Coeff. Est. | S.E. | p-Value | |
|---|---|---|---|
| Age | 5.6738 | 0.4896 | < |
| Coeff. Est. | S.E. | p-Value | |
|---|---|---|---|
| Age 30–40 | −0.5222 | 0.2294 | 0.0253 |
| Age | −1.8662 | 0.3121 | |
| Stress (In-Between) | −0.3341 | 0.2297 | 0.1494 |
| Stress (Pre-ED) | −0.4953 | 0.2284 | 0.0328 |
| Coeff. Est. | S.E. | p-Value | |
|---|---|---|---|
| SMBQ | −0.007082 | 0.003994 | 0.07959 |
| Coeff. Est. | S.E. | p-Value | |
|---|---|---|---|
| Gender (M) | 0.0417 | ||
| Age | 0.0215 | ||
| Stress (Pre-ED) | 0.0310 |
| Coeff. Est. | S.E. | p-Value | |
|---|---|---|---|
| Gender (M) | −0.85140 | 0.36593 | 0.02233 |
| Age | −0.06354 | 0.01897 | 0.00121 |
| Fit (Underweight) | 1.59192 | 0.97033 | 0.10453 |
| Fit (Overweight) | −0.12594 | 0.43446 | 0.77262 |
| Fit (Obese) | −2.43821 | 0.98378 | 0.01515 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, R.; Jönsson, P.; Sandsten, M. Stochastic Modeling and Optimal Time-Frequency Estimation of Task-Related HRV. Appl. Sci. 2019, 9, 5154. https://doi.org/10.3390/app9235154
Anderson R, Jönsson P, Sandsten M. Stochastic Modeling and Optimal Time-Frequency Estimation of Task-Related HRV. Applied Sciences. 2019; 9(23):5154. https://doi.org/10.3390/app9235154
Chicago/Turabian StyleAnderson, Rachele, Peter Jönsson, and Maria Sandsten. 2019. "Stochastic Modeling and Optimal Time-Frequency Estimation of Task-Related HRV" Applied Sciences 9, no. 23: 5154. https://doi.org/10.3390/app9235154
APA StyleAnderson, R., Jönsson, P., & Sandsten, M. (2019). Stochastic Modeling and Optimal Time-Frequency Estimation of Task-Related HRV. Applied Sciences, 9(23), 5154. https://doi.org/10.3390/app9235154





