Porous Thin-Wall Hollow Co3O4 Spheres for Supercapacitors with High Rate Capability
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Material Characterizations
2.3. Electrochemical Measurements
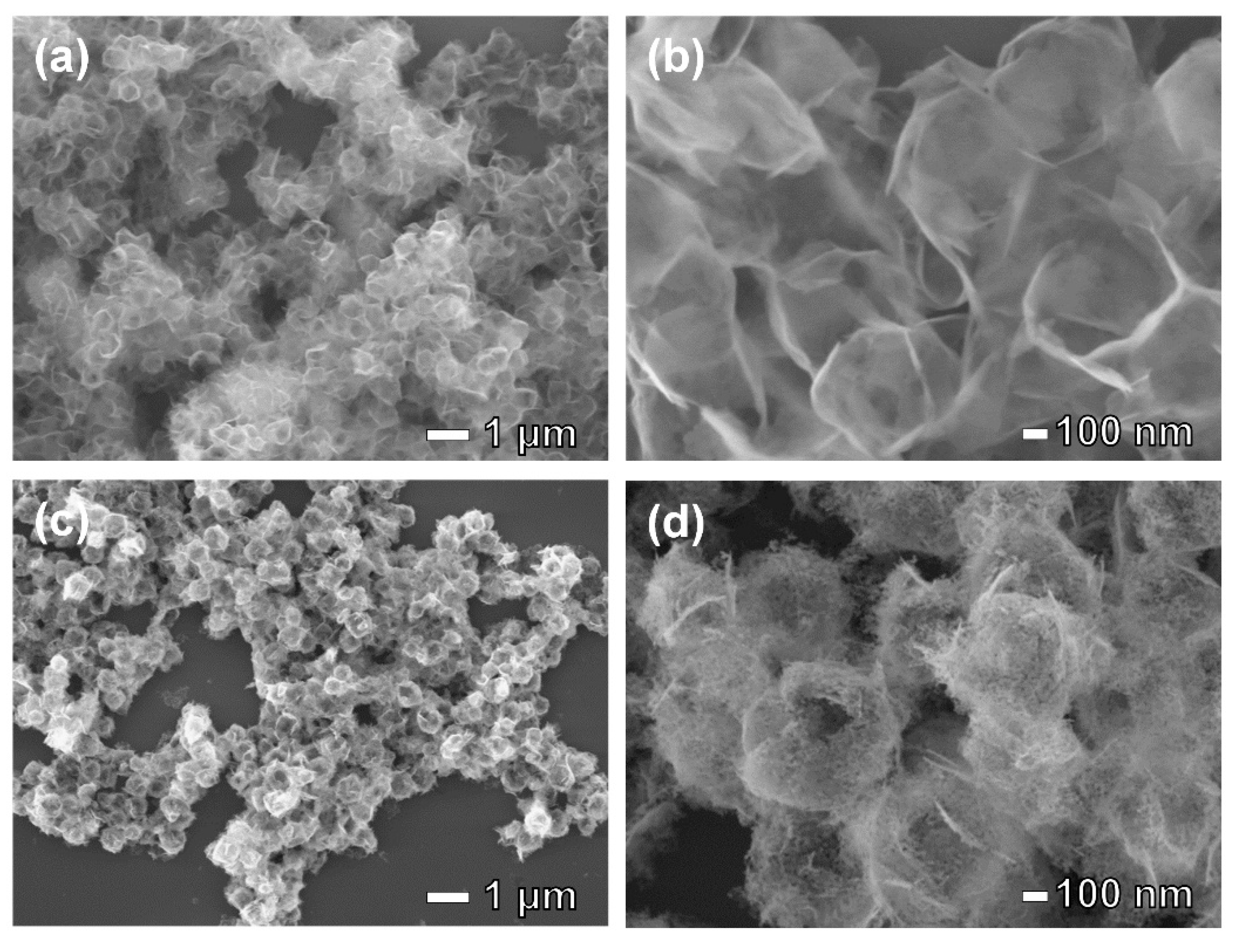
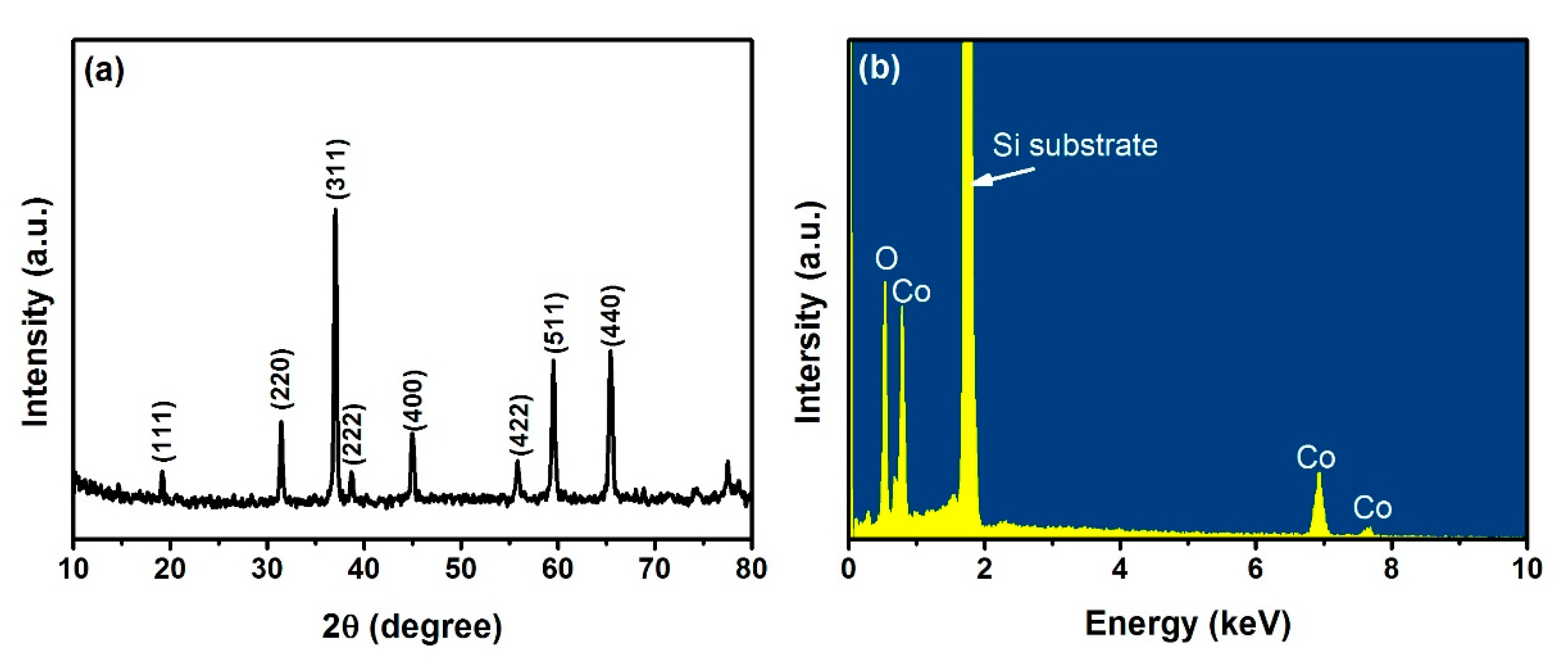
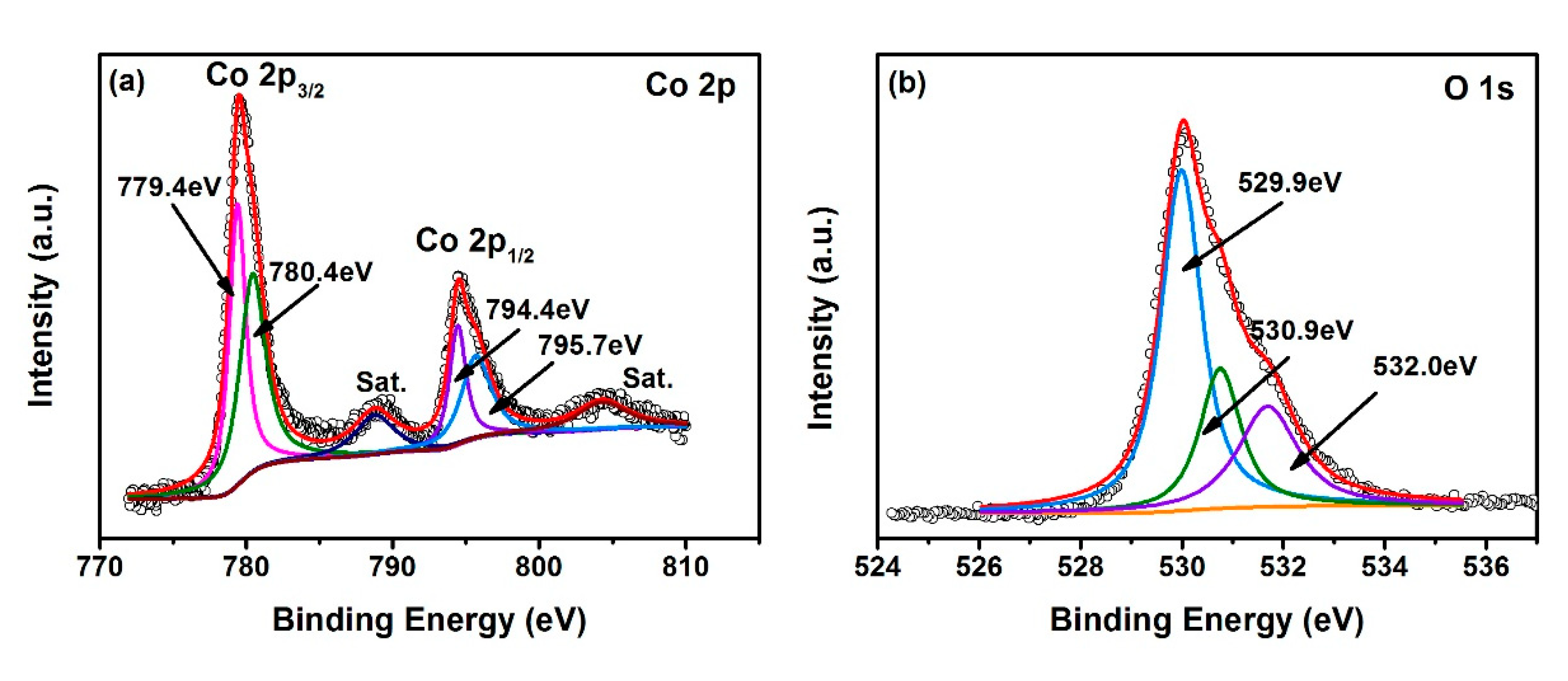
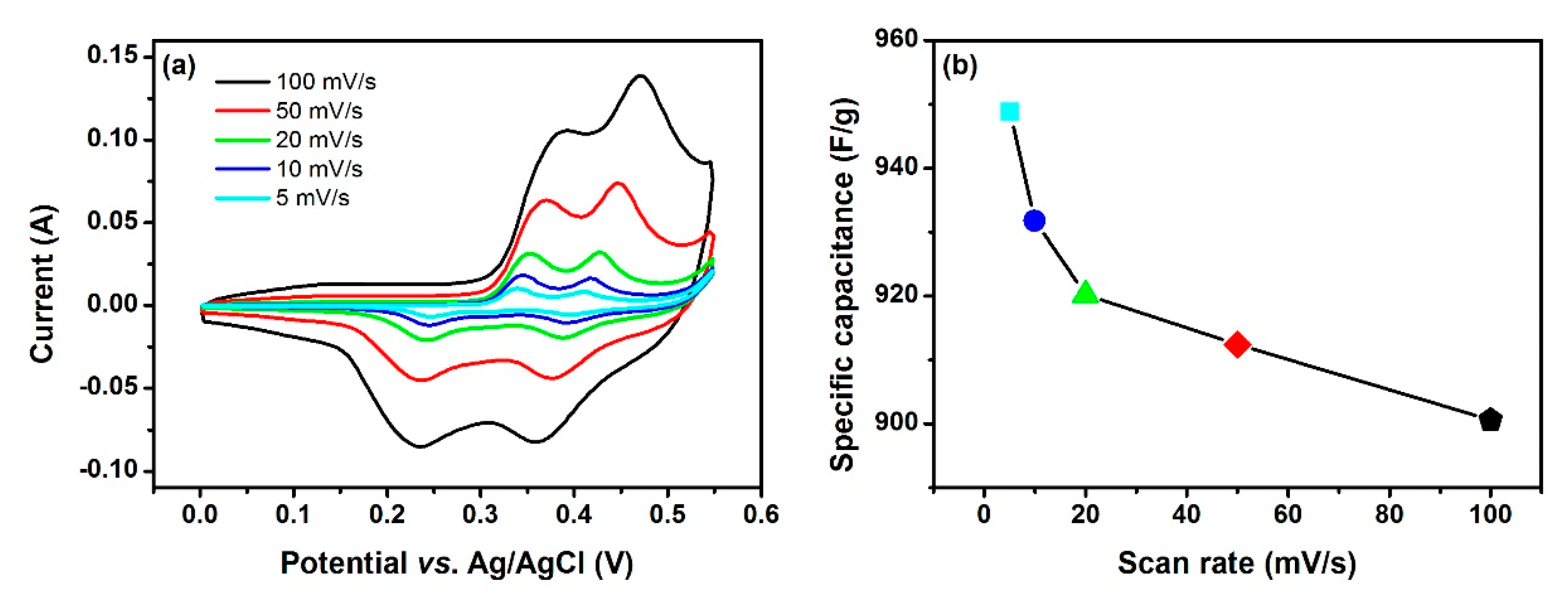
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hui, K.N.; San Hui, K.; Tang, Z.; Jadhav, V.V.; Xia, Q.X. Hierarchical chestnut-like MnCo2O4 nanoneedles grown on nickel foam as binder-free electrode for high energy density asymmetric supercapacitors. J. Power Sources 2016, 330, 195–203. [Google Scholar] [CrossRef]
- Cui, L.; Huang, L.; Ji, M.; Wang, Y.; Shi, H.; Zuo, Y.; Kang, S. High-performance MgCo2O4 nanocone arrays grown on three-dimensional nickel foams: Preparation and application as binder-free electrode for pseudo-supercapacitor. J. Power Sources 2016, 333, 118–124. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, C.H.; Gong, H. A high energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/Carbon nanotube electrodes. Adv. Funct. Mater. 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Thorat, G.M.; Seo, J.G. Electrochemical growth of Co(OH)2 nanoflakes on Ni foam for methanol electro-oxidation. New J. Chem. 2017, 41, 9546–9553. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, E.; He, W.; Deng, X.; Huang, J.; Ding, M.; Wei, X.; Liu, X.; Xu, X. NiCo2O4-based supercapacitor nanomaterials. Nanomaterials 2017, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of materials and fabrication methods. J. Energy Eng. 2012, 139, 72–79. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Lu, P.; Müller, L.; Hoffmann, M.; Chen, X. Taper silicon nano-scaffold regulated compact integration of 1D nanocarbons for improved on-chip supercapacitor. Nano Energy 2017, 41, 618–625. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Feng, Y. Carbon nanotubes for supercapacitor. Nanoscale Res. Lett. 2010, 5, 654. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.Y.; Ghosh, K.; Jena, R.K. Review on advances in porous nanostructured nickel oxides and their composite electrodes for high-performance supercapacitors. J. Power Sources 2016, 308, 121–140. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel–cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef]
- Lin, L.; Tang, S.; Zhao, S.; Peng, X.; Hu, N. Hierarchical three-dimensional FeCo2O4@MnO2 core-shell nanosheet arrays on nickel foam for high-performance supercapacitor. Electrochim. Acta 2017, 228, 175–182. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, P.; Shen, L.; Wang, K.; Song, S.; Yu, X.; Tan, S.; Zhao, C.; Mai, W. WO3 nanoflowers with excellent pseudo-capacitive performance and the capacitance contribution analysis. J. Mater. Chem. A 2016, 4, 7266–7273. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 nanowire@MnO2 ultrathin nanosheet core/shell arrays: A new class of high-performance pseudocapacitive materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Yang, L.; Hou, L.; Li, J.; Sun, Y.; Zhang, X.; Shen, L.; Lu, X.; Xiong, S.; Lou, X.W. Flexible hybrid paper made of monolayer Co3O4 microsphere arrays on rGO/CNTs and their application in electrochemical capacitors. Adv. Funct. Mater. 2012, 22, 2560–2566. [Google Scholar] [CrossRef]
- Li, Z.Y.; Bui, P.T.; Kwak, D.H.; Akhtar, M.S.; Yang, O.B. Enhanced electrochemical activity of low temperature solution process synthesized Co3O4 nanoparticles for pseudo-supercapacitors applications. Ceram. Int. 2016, 42, 1879–1885. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Chen, W.; Cha, D.; Alshareef, H.N. Substrate dependent self-organization of mesoporous cobalt oxide nanowires with remarkable pseudocapacitance. Nano Lett. 2012, 12, 2559–2567. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Xu, Y.; Gu, Y. Surfactant-assisted solvothermal synthesis of Co3O4 hollow spheres with oriented-aggregation nanostructures and tunable particle size. Langmuir 2004, 20, 8404–8408. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Wang, X.L.; Gu, C.D.; Zhao, X.B. Mesoporous Co3O4 monolayer hollow-sphere array as electrochemical pseudocapacitor material. Chem. Commun. 2011, 47, 5786–5788. [Google Scholar] [CrossRef]
- Yao, M.; Hu, Z.; Xu, Z.; Liu, Y. Template synthesis of 1D hierarchical hollow Co3O4 nanotubes as high performance supercapacitor materials. J. Alloys Compd. 2015, 644, 721–728. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, X.; Xia, Z.; Zhang, Z.; Li, J.; Ma, Y.; Qu, Y. Hollow fluffy Co3O4 cages as efficient electroactive materials for supercapacitors and oxygen evolution reaction. ACS Appl. Mater. Interfaces 2015, 7, 20322–20331. [Google Scholar] [CrossRef]
- Lu, P.; Ohlckers, P.; Müller, L.; Leopold, S.; Hoffmann, M.; Grigoras, K.; Ahopelto, J.; Prunnila, M.; Chen, X. Nano fabricated silicon nanorod array with titanium nitride coating for on-chip supercapacitors. Electrochem. Commun. 2016, 70, 51–55. [Google Scholar] [CrossRef]
- Lu, P.; Halvorsen, E.; Ohlckers, P.; Müller, L.; Leopold, S.; Hoffmann, M.; Grigoras, K.; Ahopelto, J.; Prunnila, M.; Chen, X. Ternary composite Si/TiN/MnO2 taper nanorod array for on-chip supercapacitor. Electrochim. Acta 2017, 248, 397–408. [Google Scholar] [CrossRef]
- Li, W.Y.; Xu, L.N.; Chen, J. Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Li, G.; Chen, M.; Ouyang, Y.; Yao, D.; Lu, L.; Wang, L.; Xia, X.; Lei, W.; Chen, S.M.; Hao, Q. Manganese doped Co3O4 mesoporous nanoneedle array for long cycle-stable supercapacitors. Appl. Surf. Sci. 2019, 469, 941–950. [Google Scholar] [CrossRef]
- Wu, H.B.; Pang, H.; Lou, X.W.D. Facile synthesis of mesoporous Ni0.3Co2.7O4 hierarchical structures for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 3619–3626. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Wang, T. Morphology-controllable synthesis of cobalt oxalates and their conversion to mesoporous Co3O4 nanostructures for application in supercapacitors. Inorg. Chem. 2011, 50, 6482–6492. [Google Scholar] [CrossRef]
- Du, H.; Jiao, L.; Wang, Q.; Yang, J.; Guo, L.; Si, Y.; Wang, Y.; Yuan, H. Facile carbonaceous microsphere templated synthesis of Co3O4 hollow spheres and their electrochemical performance in supercapacitors. Nano Res. 2013, 6, 87–98. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, B.; Fu, W.; Chen, J.; Zhou, J.; Xie, E. Fabrication of porous nanosheet-based Co3O4 hollow nanocubes for electrochemical capacitors with high rate capability. Electrochim. Acta 2015, 178, 555–563. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-nanonet hollow structured Co3O4 for high capacity supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef]
- Kumar, R.D.; Andou, Y.; Karuppuchamy, S. Microwave-assisted synthesis of Zn–WO3 and ZnWO4 for pseudocapacitor applications. J. Phys. Chem. Solids 2016, 92, 94–99. [Google Scholar] [CrossRef]
- Ede, S.R.; Ramadoss, A.; Nithiyanantham, U.; Anantharaj, S.; Kundu, S. Bio-molecule assisted aggregation of ZnWO4 nanoparticles (NPs) into chain-like assemblies: Material for high performance supercapacitor and as catalyst for benzyl alcohol oxidation. Inorg. Chem. 2015, 54, 3851–3863. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Supercapacitor electrode of nano-Co3O4 decorated with gold nanoparticles via in-situ reduction method. J. Power Sources 2017, 363, 1–8. [Google Scholar] [CrossRef]
- Pang, M.; Long, G.; Jiang, S.; Ji, Y.; Han, W.; Wang, B.; Liu, X.; Xi, Y.; Wang, D.; Xu, F. Ethanol-assisted solvothermal synthesis of porous nanostructured cobalt oxides (CoO/Co3O4) for high-performance supercapacitors. Chem. Eng. J. 2015, 280, 377–384. [Google Scholar] [CrossRef]
- Yang, G.W.; Xu, C.L.; Li, H.L. Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem. Commun. 2008, 6537–6539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, A.; Zhu, Q.; Nie, Z.; Zhang, Y.; Tang, Y.; Liang, S.; Cao, G. Facile synthesis of nanorod-assembled multi-shelled Co3O4 hollow microspheres for high-performance supercapacitors. J. Power Sources 2014, 272, 107–112. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, C.; Li, T.T.; Hu, Y.; Qian, J.; Huang, S. MOF-templated syntheses of porous Co3O4 hollow spheres and micro-flowers for enhanced performance in supercapacitors. CrystEngComm 2018, 20, 3812–3816. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Chen, X.; Liu, J.; Lu, F.; Chen, L.; Shao, G. Facile precursor conversion synthesis of hollow coral-shaped Co3O4 nanostructures for high-performance supercapacitors. Colloids Surf. A 2019, 570, 63–72. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Mai, Y.J.; Wang, X.L.; Gu, C.D.; Zhao, X.B. Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance. J. Mater. Chem. 2011, 21, 9319–9325. [Google Scholar] [CrossRef]
- Du, W.; Liu, R.; Jiang, Y.; Lu, Q.; Fan, Y.; Gao, F. Facile synthesis of hollow Co3O4 boxes for high capacity supercapacitor. J. Power Sources 2013, 227, 101–105. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Y.; Xie, Y.L.; Cheng, T.; Lai, W.Y.; Pang, H.; Huang, W. Porous hollow Co3O4 with rhombic dodecahedral structures for high-performance supercapacitors. Nanoscale 2014, 6, 14354–14359. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Wang, S.; Du, W.; Zhang, H.; Ding, C.; Du, Y.; Zhu, L. Facile synthesis of homogeneous core-shell Co3O4 mesoporous nanospheres as high performance electrode materials for supercapacitor. J. Alloys Compd. 2019, 774, 137–144. [Google Scholar] [CrossRef]
- Guo, D.; Song, X.; Li, F.; Tan, L.; Ma, H.; Zhang, L.; Zhao, Y. Oriented synthesis of Co3O4 core-shell microspheres for high-performance asymmetric supercapacitor. Colloids Surf. A 2018, 546, 1–8. [Google Scholar] [CrossRef]
- Cheng, G.; Kou, T.; Zhang, J.; Si, C.; Gao, H.; Zhang, Z. O22−/O− functionalized oxygen-deficient Co3O4 nanorods as high performance supercapacitor electrodes and electrocatalysts towards water splitting. Nano Energy 2017, 38, 155–166. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.B.; Yang, Z.; Zheng, G. Reduced mesoporous Co3O4 nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Xiao, A.; Zhou, S.; Zuo, C.; Zhuan, Y.; Ding, X. Controllable synthesis of mesoporous Co3O4 nanoflake array and its application for supercapacitor. Mater. Res. Bull. 2014, 60, 674–678. [Google Scholar] [CrossRef]
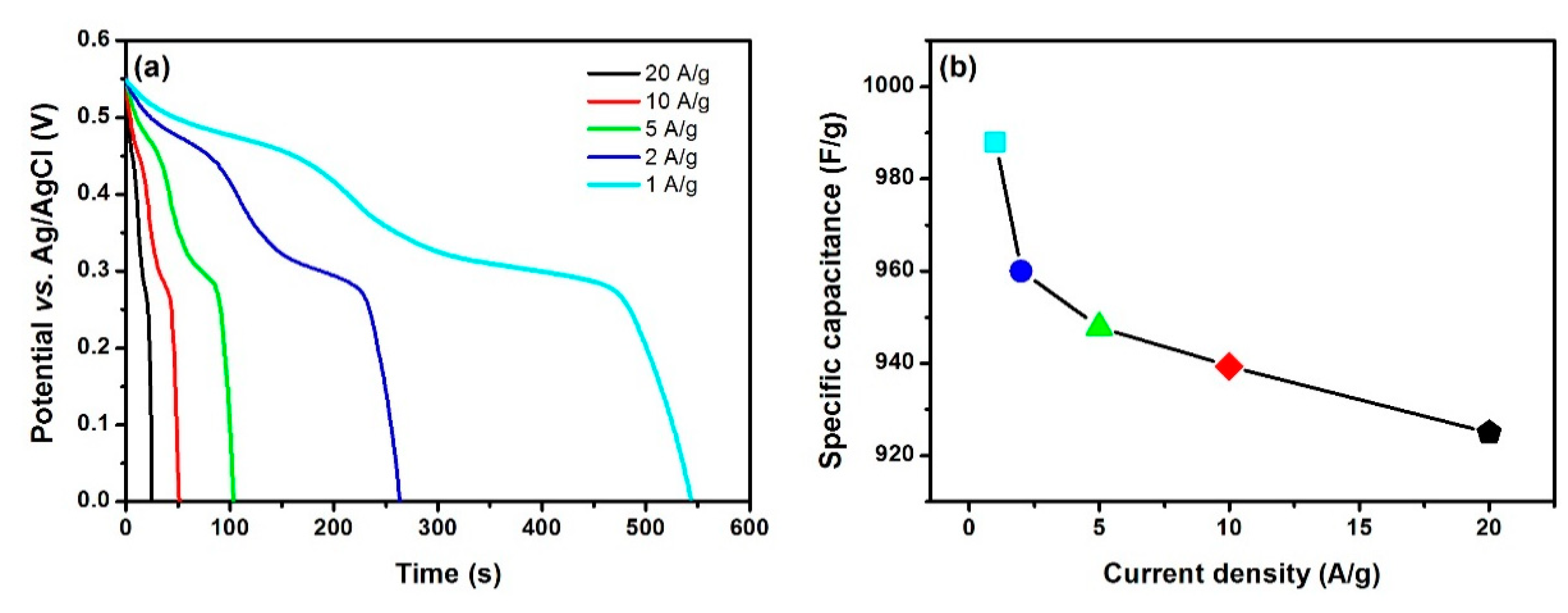
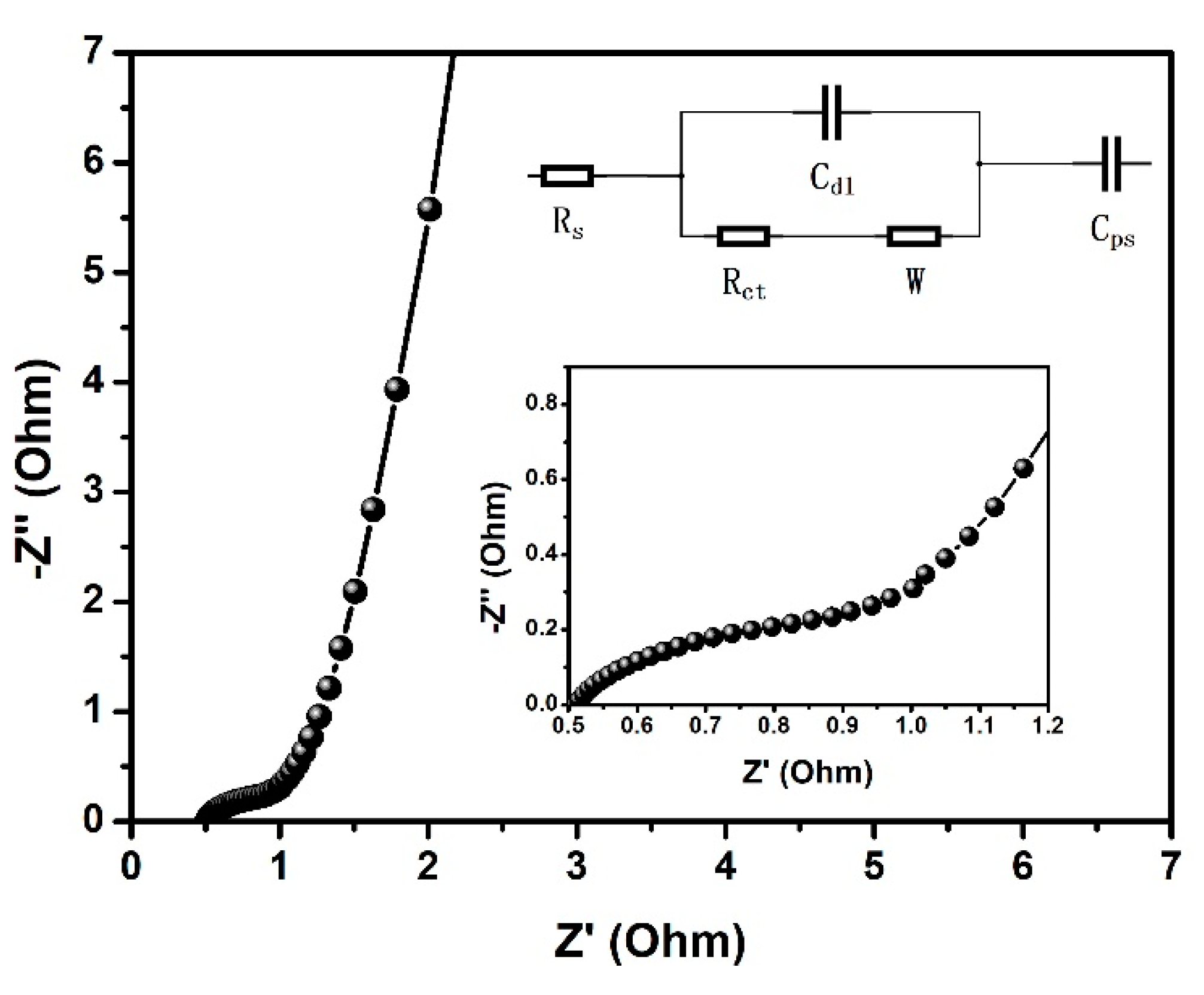

| Material | Specific Capacitance | Rate Capability | Stability-Cycles | Ref. |
|---|---|---|---|---|
| hollow Co3O4 spheres | 474.8 F/g at 1 A/g | 79% at 10 A/g | 99%-1000 | [37] |
| hollow Co3O4 spheres | 394.4 F/g at 2 A/g | 81% at 20 A/g | 92%-500 | [45] |
| hollow Co3O4 spheres | 342.1 F/g at 0.5 A/g | 69% at 10 A/g | 90%-2000 | [46] |
| hollow Co3O4 nanotubes | 1006 F/g at 1 A/g | 51% at 10 A/g | 91%-1000 | [29] |
| hollow Co3O4 cages | 948.9 F/g at 1 A/g | 56.6% at 40 A/g | – | [30] |
| hollow Co3O4 nanotubes | 404.9 F/g at 0.5 A/g | 87.8% at 20 A/g | 95%-2000 | [38] |
| hollow Co3O4 3D-nanonet | 739 F/g at 1 A/g | 72% at 15 A/g | 90.2%-1000 | [39] |
| hollow Co3O4 flowers | 210 F/g at 0.5 A/g | 86% at 10 A/g | – | [46] |
| hollow Co3O4 corals | 527 F/g at 1 A/g | 78% at 10 A/g | 99%-5000 | [47] |
| hollow Co3O4 nanowires | 599 F/g at 2 A/g | 73% at 40 A/g | 91%-7500 | [48] |
| hollow Co3O4 boxes | 278 F/g at 0.5 A/g | 63% at 5 A/g | – | [49] |
| hollow Co3O4 dodecahedron | 1100 F/g at 1.25 A/g | 40% at 12.5 A/g | 95.1%-6000 | [50] |
| Co3O4 spheres | 837.7 F/g at 1 A/g | 93.6% at 10 A/g | 87%-2000 | [51] |
| Co3O4 spheres | 261.1 F/g at 0.5 A/g | 42% at 5 A/g | 90.2%-2000 | [52] |
| Co3O4 nanorods | 739 F/g at 5 mV/s | 52.5% at 100 mV/s | 103%-50000 | [53] |
| Co3O4 nanowires | 977 F/g at 2 A/g | 49.5% at 10 A/g | 90%-2000 | [54] |
| Co3O4 nanoflakes | 450 F/g at 1 A/g | 81% at 20 A/g | 92%-5000 | [55] |
| Mn doped Co3O4 | 668.4 F/g at 1 A/g | 62% at 10 A/g | 104%-10000 | [34] |
| Au decorated Co3O4 | 681 F/g at 0.5 A/g | 58% at 10 A/g | 83.1%-13000 | [42] |
| CoO/Co3O4 | 451 F/g at 1 A/g | 68.3% at 20 A/g | 108%-5000 | [43] |
| hollow Co3O4 spheres | 988 F/g at 1 A/g | 93.6% at 20 A/g | 96.6%-6000 | Ours |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Sun, Y.; Ohlckers, P.; Chen, X. Porous Thin-Wall Hollow Co3O4 Spheres for Supercapacitors with High Rate Capability. Appl. Sci. 2019, 9, 4672. https://doi.org/10.3390/app9214672
Fan X, Sun Y, Ohlckers P, Chen X. Porous Thin-Wall Hollow Co3O4 Spheres for Supercapacitors with High Rate Capability. Applied Sciences. 2019; 9(21):4672. https://doi.org/10.3390/app9214672
Chicago/Turabian StyleFan, Xiao, Yongjiao Sun, Per Ohlckers, and Xuyuan Chen. 2019. "Porous Thin-Wall Hollow Co3O4 Spheres for Supercapacitors with High Rate Capability" Applied Sciences 9, no. 21: 4672. https://doi.org/10.3390/app9214672
APA StyleFan, X., Sun, Y., Ohlckers, P., & Chen, X. (2019). Porous Thin-Wall Hollow Co3O4 Spheres for Supercapacitors with High Rate Capability. Applied Sciences, 9(21), 4672. https://doi.org/10.3390/app9214672






