Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean Calendula Spp. (Asteraceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Collection, Processing, and Preparation of Plant Extract
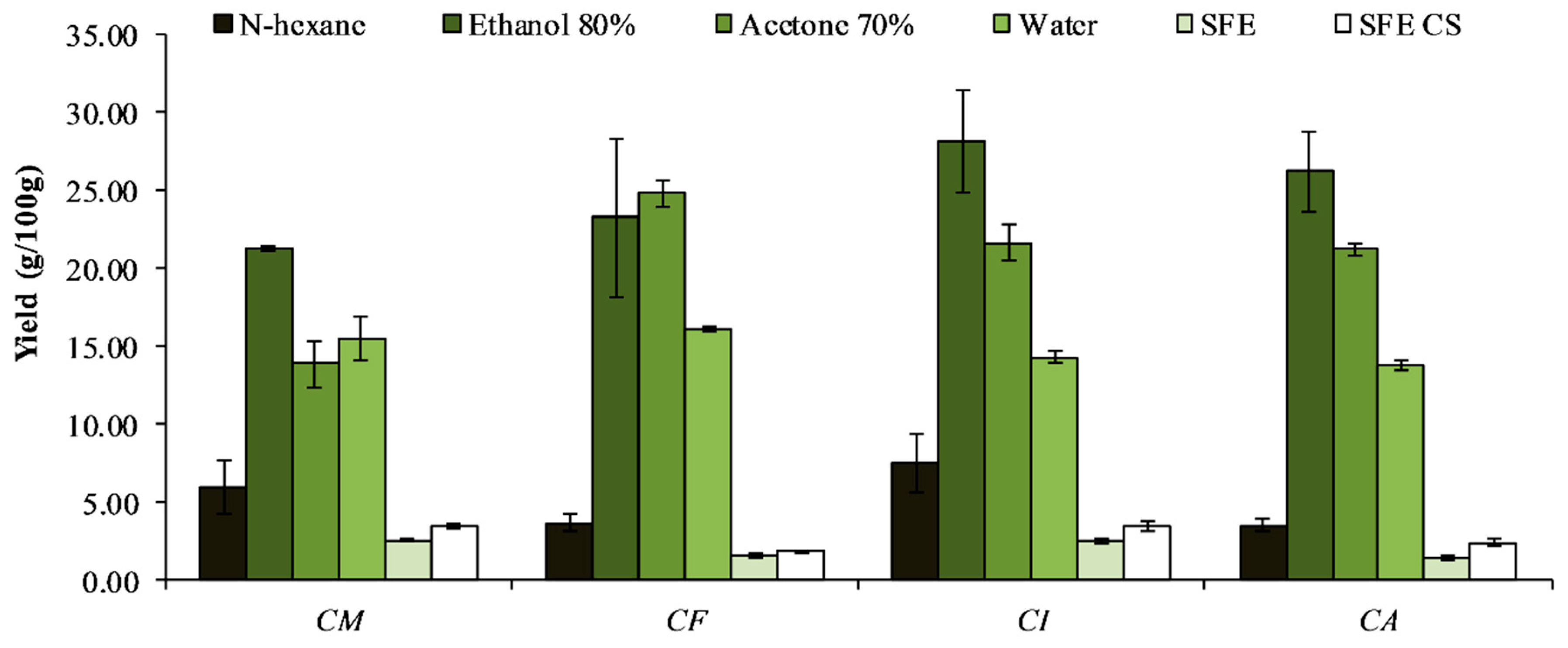
2.2.1. Extraction with Solvents
2.2.2. Extraction with Supercritical Fluid Extraction (SFE)
2.3. Characterization of the Antioxidant Power
2.3.1. Total Polyphenols Contents
2.3.2. DPPH Radical Scavenging Activity
2.3.3. Reducing Power
2.4. Evaluation of Bioactive Properties in Vitro
Protective Effect of Calendula Spp. Extracts in Fibroblast Cell Line HS-68 Exposed to Oxidative Stress
2.5. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Evaluation of Antioxidant Power
3.1.1. Total Phenolic Content
3.1.2. 1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
3.1.3. Reducing Power
3.2. Protective Effect of Calendula Spp. Extracts in Fibroblast Cell Line HS-68 Exposed to Oxidative Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonçalves, A.C.; Castro, S.; Paiva, J.; Santos, C.; Silveira, P. Taxonomic revision of the genus calendula (Asteraceae) in the Iberian Peninsula and the Balearic Islands. Phytotaxa 2018, 352, 1–91. [Google Scholar] [CrossRef]
- Khalid, K.A.; Silva, J.A.T. Biology of Calendula officinalis Linn.: Focus on Pharmacology, Biological Activities and Agronomic Practices. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 12–27. [Google Scholar]
- Chakraborthy, G.S. Antimicrobial activity of the leaf extracts of Calendula officinalis (Linn.). J. Herb. Med. Toxicol. 2008, 2, 65–66. [Google Scholar]
- Faustino, M.V.; Pinto, D.C.G.A.; Gonçalves, M.J.; Salgueiro, L.; Silveira, P.; Silva, A.M.S. Calendula L. species polyphenolic profile and in vitro antifungal activity. J. Funct. Foods 2018, 45, 254–267. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Masteikova, R.; Davalgiene, J.; Peciura, R.; Gauryliene, R.; Bernatoniene, R.; Majiene, D.; Lazauskas, R.; Civinskiene, G.; Velziene, S.; et al. Topical application of calendula officinalis (L.): Formulation and evaluation of hydrophilic cream with antioxidant activity. J. Med. Plants Res. 2011, 5, 868–877. [Google Scholar]
- Laughton, M.J.; Evans, P.J.; Moroney, M.A.; Hoult, J.R.S.; Halliwell, B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives: Relationship to antioxidant activity and to iron ion-reducing ability. Biochem. Pharmacol. 1991, 42, 1673–1681. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Mancuso, F.P.; Messina, C.M.; Santulli, A.; Laudicella, V.A.; Giommi, C.; Sarà, G.; Airoldi, L. Influence of ambient temperature on the photosynthetic activity and phenolic content of the intertidal Cystoseira compressa along the Italian coastline. J. Appl. Phycol. 2019, 1–8. [Google Scholar] [CrossRef]
- Messina, C.; Renda, G.; Laudicella, V.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A.; Messina, C.M.; Renda, G.; Laudicella, V.A.; et al. From Ecology to Biotechnology, Study of the Defense Strategies of Algae and Halophytes (from Trapani Saltworks, NW Sicily) with a Focus on Antioxidants and Antimicrobial Properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef]
- Arora, D.; Rani, A.; Sharma, A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn. Rev. 2013, 7, 179–187. [Google Scholar] [CrossRef]
- Troia, A.; Pasta, S. Calendula maritima. IUCN Red List Threat. Species 2006, 2006, T61618A12524417. [Google Scholar] [CrossRef]
- Troia, A. Strategie di gestione delle popolazioni di Calendula maritima Guss. (Asteraceae). Nat. sicil. 2011, 35, 51–63. [Google Scholar]
- Pasta, S.; Garfi, G.; Carimi, F.; Marcenò, C. Human disturbance, habitat degradation and niche shift: The case of the endemic Calendula maritima Guss. (W Sicily, Italy). Rend. Lincei 2017, 28. [Google Scholar] [CrossRef]
- Plume, O.; Raimondo, F.M.; Troia, A. Hybridization and competition between the endangered sea marigold (Calendula maritima Asteraceae) and a more common congener. Plant Biosyst. 2015, 149, 68–77. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Preethia, K.C.; Kuttanb, G.; Kuttan, R. Anti-inflammatory activity of flower extract of Calendula officinalis Linn. and its possible mechanism of action. Indian J. Exp. Biol. 2009, 47, 113–120. [Google Scholar]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Muley, B.; Khadabadi, S.; Banarase, N. Phytochemical Constituents and Pharmacological Activities of Calendula officinalis Linn (Asteraceae): A Review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Kashani, H.H. Pot marigold (Calendula officinalis) medicinal usage and cultivation. Sci. Res. Essays 2012, 7, 1468–1472. [Google Scholar]
- Parente, L.M.L.; Lino Júnior, R.D.S.; Tresvenzol, L.M.F.; Vinaud, M.C.; De Paula, J.R.; Paulo, N.M. Wound healing and anti-inflammatory effect in animal models of calendula officinalis L. growing in Brazil. Evidence-based Complement. Altern. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.Á.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables - Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Li, H.B.; Cheng, K.W.; Chen, F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Choi, Y.; Jeong, H.S.; Lee, J. Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem. 2007, 103, 130–138. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Oueslati, S.; Guyot, S.; Magné, C.; Abdelly, C. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem. Toxicol. 2009, 47, 2308–2313. [Google Scholar]
- Odabasoglu, F.; Aslan, A.; Cakir, A.; Suleyman, H.; Karagoz, Y.; Bayir, Y.; Halici, M. Antioxidant activity, reducing power and total phenolic content of some lichen species. Fitoterapia 2005, 76, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. South African J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef]
- Prinsloo, G.; Nogemane, N. The effects of season and water availability on chemical composition, secondary metabolites and biological activity in plants. Phytochem. Rev. 2018, 17, 889–902. [Google Scholar] [CrossRef]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Nomizo, A.; Gerlach, R.F.; Fonseca, M.J.V. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Sineiro, J.; Rubilar, M.; Sánchez, M.; Jerez, M.; Pinelo, M.; Costoya, N.; Núñez, M.J. Polyphenols from plant materials: Extraction and antioxidant power. Electron. J. Environ. Agric. Food Chem. 2008, 7, 3210–3216. [Google Scholar]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Ferreira, S.R.S.; Parada-Alfonso, F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2010, 51, 319–324. [Google Scholar] [CrossRef]
- Trabelsi, N.; Megdiche, W.; Ksouri, R.; Falleh, H.; Oueslati, S.; Soumaya, B.; Hajlaoui, H.; Abdelly, C. Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT - Food Sci. Technol. 2010, 43, 632–639. [Google Scholar] [CrossRef]
- Gharbi, S.; Renda, G.; La Barbera, L.; Amri, M.; Messina, C.M.; Santulli, A. Tunisian tomato by-products, as a potential source of natural bioactive compounds. Nat. Prod. Res. 2017, 31, 626–631. [Google Scholar] [CrossRef]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Manuguerra, S.; Caccamo, L.; Mancuso, M.; Arena, R.; Rappazzo, A.C.; Genovese, L.; Santulli, A.; Messina, C.M.; Maricchiolo, G. The antioxidant power of horseradish, Armoracia rusticana, underlies antimicrobial and antiradical effects, exerted in vitro. Nat. Prod. Res. 2018, 1–4. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Furuta, s.; Nishiba, y.; Terahara, N.; Suda, I. Involvement of Anthocyanins and other Phenolic Compounds in Radical-Scavenging Activity of Purple-Fleshed Sweet Potato Cultivars. J. Food Sci. 2002, 67, 1752–1756. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.; Guyot, S.; Sotin, H.; Ayadi, M. Comparative Study of Antioxidant Power, Polyphenols, Flavonoids and Betacyanins of the Peel and Pulp of Three Tunisian Opuntia Forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ksouri, R.; Medini, F.; Guyot, S.; Abdelly, C.; Magné, C. Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind. Crops Prod. 2011, 34, 1066–1071. [Google Scholar] [CrossRef]
- Messina, C.M.; Pizzo, F.; Santulli, A.; Bušelić, I.; Boban, M.; Orhanović, S.; Mladineo, I. Anisakis pegreffii (Nematoda: Anisakidae) products modulate oxidative stress and apoptosis-related biomarkers in human cell lines. Parasites and Vectors 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Antioxidant properties of marigold extracts. Food Res. Int. 2004, 37, 643–650. [Google Scholar] [CrossRef]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef]
- Ercetin, T.; Senol, F.S.; Erdogan Orhan, I.; Toker, G. Comparative assessment of antioxidant and cholinesterase inhibitory properties of the marigold extracts from Calendula arvensis L. and Calendula officinalis L. Ind. Crops Prod. 2012, 36, 203–208. [Google Scholar] [CrossRef]
- Messina, C.; Renda, G.; Randazzo, M.; Laudicella, A.; Gharbi, S.; Pizzo, F.; Morghese, M.; Santulli, A. Extraction of bioactive compounds from shrimp waste. Bull. Inst. Natl. Sci. Technol. Mer Salammbô 2015 2015, 42, 27–29. [Google Scholar]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 243–259. ISBN 9780128137680. [Google Scholar]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Matysik, G.; Wójciak-Kosior, M.; Paduch, R. The influence of Calendulae officinalis flos extracts on cell cultures, and the chromatographic analysis of extracts. J. Pharm. Biomed. Anal. 2005, 38, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Schlieve, C.R.; Lieven, C.J.; Levin, L.A. Biochemical activity of reactive oxygen species scavengers do not predict retinal ganglion cell survival. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3878–3886. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, C.M.; Troia, A.; Arena, R.; Manuguerra, S.; Ioannou, T.; Curcuraci, E.; Renda, G.; Hellio, C.; Santulli, A. Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean Calendula Spp. (Asteraceae). Appl. Sci. 2019, 9, 4627. https://doi.org/10.3390/app9214627
Messina CM, Troia A, Arena R, Manuguerra S, Ioannou T, Curcuraci E, Renda G, Hellio C, Santulli A. Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean Calendula Spp. (Asteraceae). Applied Sciences. 2019; 9(21):4627. https://doi.org/10.3390/app9214627
Chicago/Turabian StyleMessina, Concetta Maria, Angelo Troia, Rosaria Arena, Simona Manuguerra, Theodora Ioannou, Eleonora Curcuraci, Giuseppe Renda, Claire Hellio, and Andrea Santulli. 2019. "Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean Calendula Spp. (Asteraceae)" Applied Sciences 9, no. 21: 4627. https://doi.org/10.3390/app9214627
APA StyleMessina, C. M., Troia, A., Arena, R., Manuguerra, S., Ioannou, T., Curcuraci, E., Renda, G., Hellio, C., & Santulli, A. (2019). Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean Calendula Spp. (Asteraceae). Applied Sciences, 9(21), 4627. https://doi.org/10.3390/app9214627