Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Properties
2.2. Membrane Distillation and Crystallization Setup
2.3. Fouling/Scaling Analysis
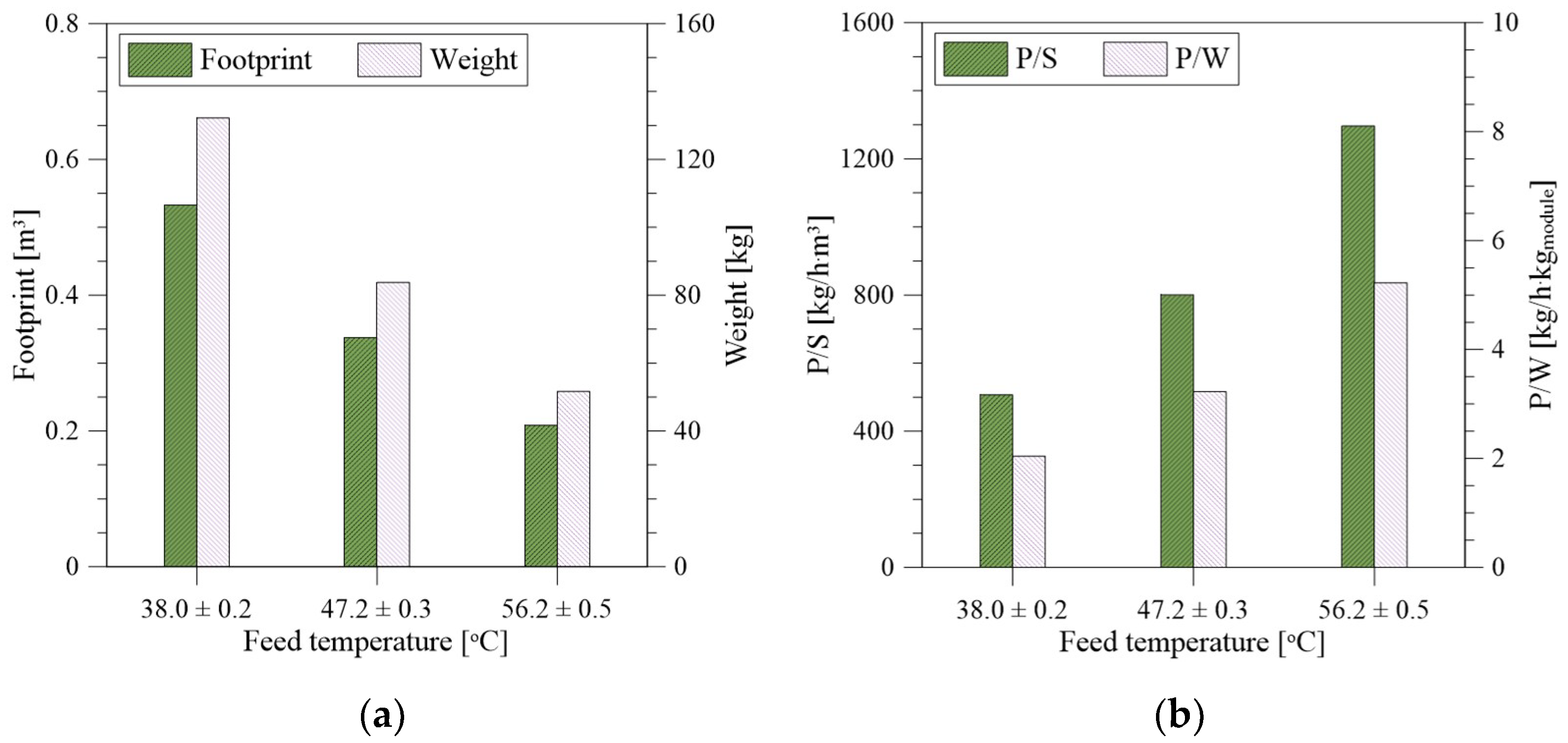
2.4. Footprint, Weight, and Waste Reduction Analysis
2.5. Crystal Characterization
3. Results and Discussion
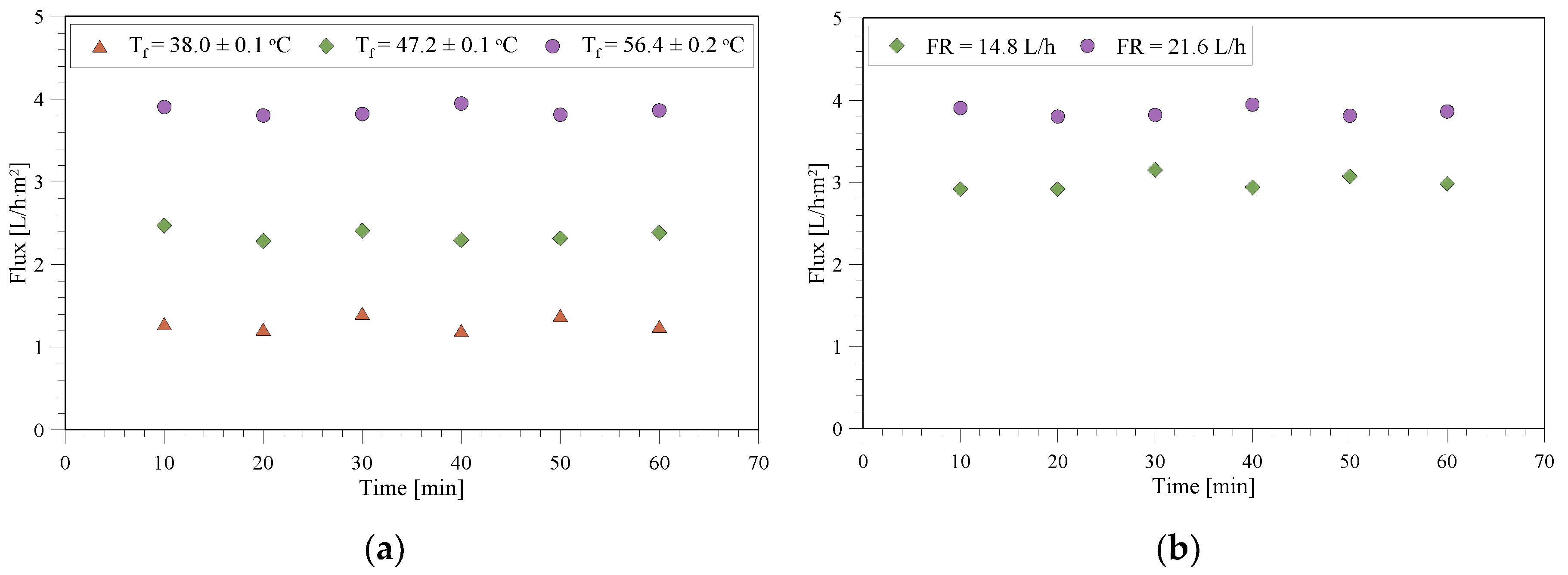
3.1. Membrane Characterization with Pure Water
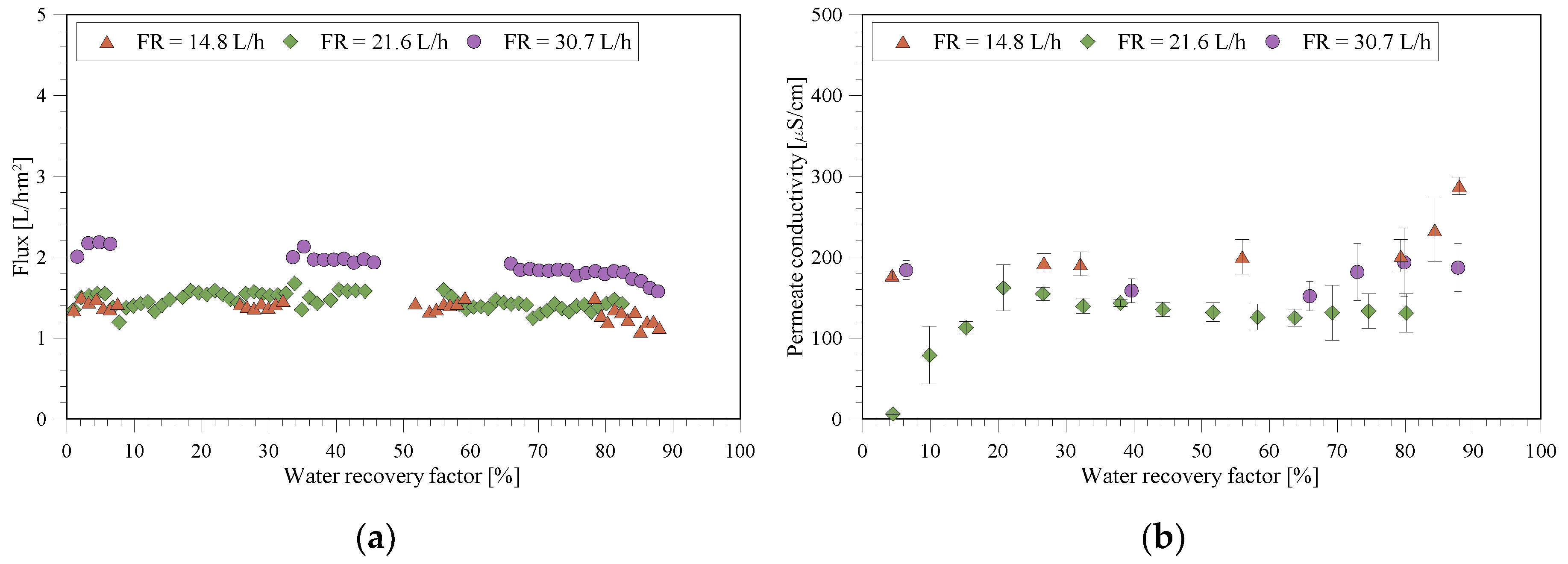
3.2. MD/MCr Tests with the Wastewater
3.3. Crystallization Study
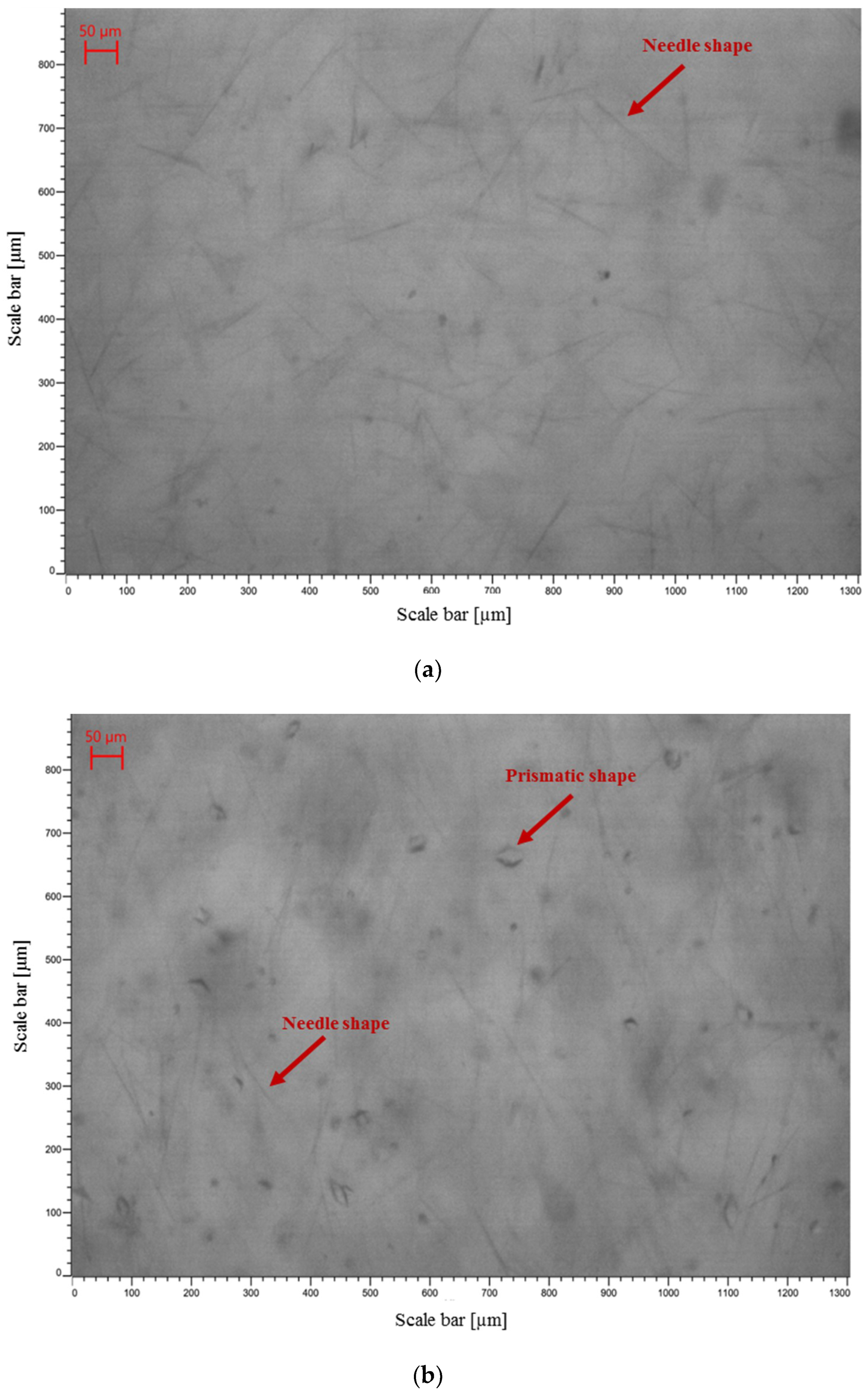
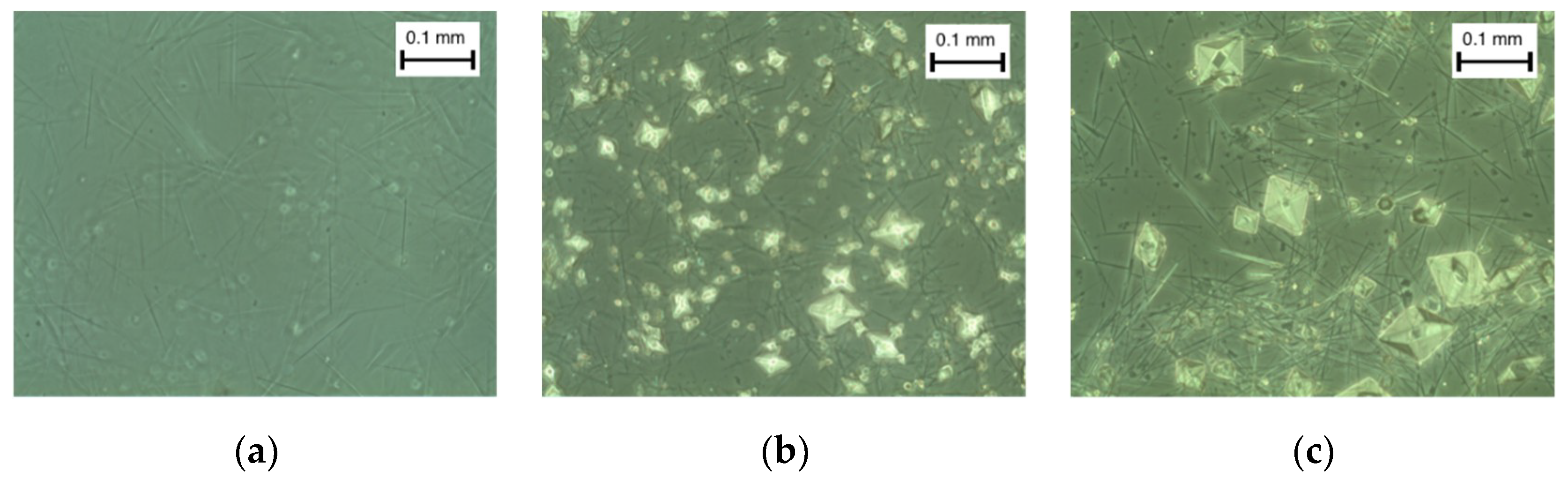
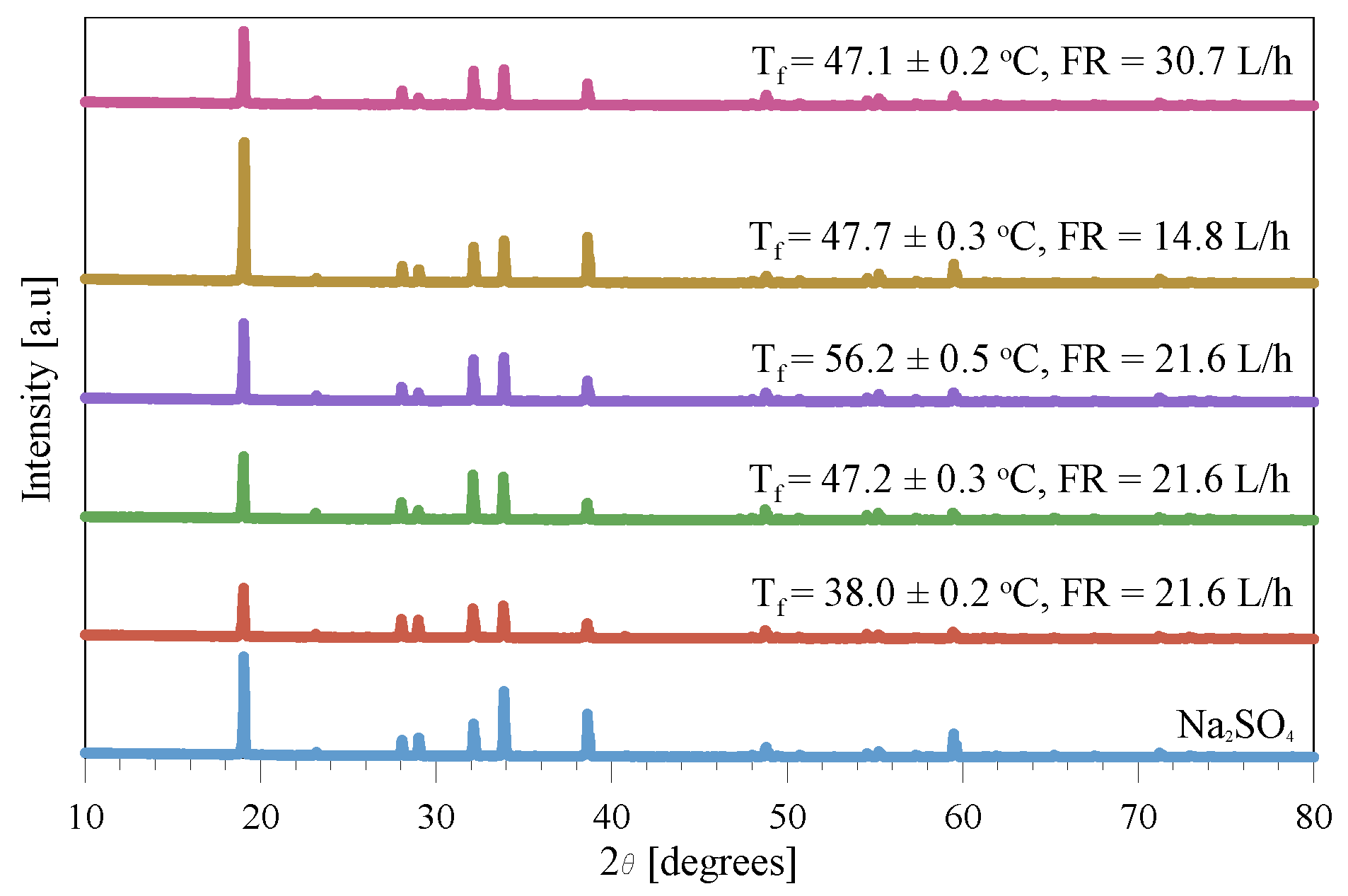
3.3.1. Crystal Morphology
3.3.2. Identification and Purity of the Crystals
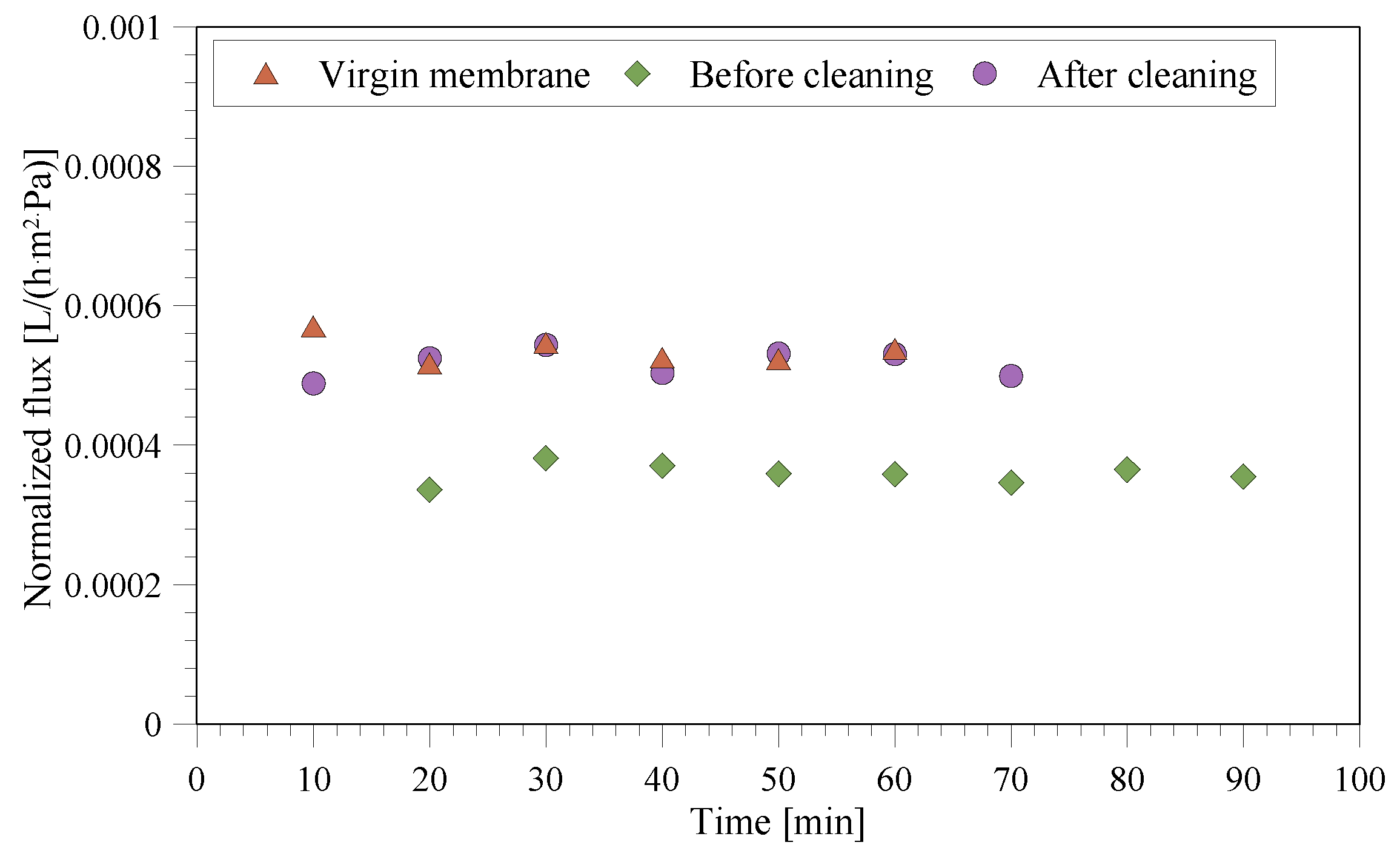
3.4. Fouling/Scaling Analysis
3.5. Weight, Size, and Waste Reduction Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevenson, M.F. Anodizing. In ASM Handbook; ASM Press: Washington, DC, USA, 1994; Volume 5, pp. 482–493. [Google Scholar]
- Patrick, G.; McCune, L. Aluminium Anodizer Implements Zero Liquid Discharge. Products Finishing. 2016. Available online: http://www.pfonline.com/articles/aluminum-anodizer-implements-zero-liquid-discharge (accessed on 14 October 2018).
- Amer, S. Treating Metal Finishing Wastewater; AQUACHEM INC.: Denver, CO, USA, 1998; pp. 1–7. [Google Scholar]
- CIE Waste Water Treatment & Zero Liquid Discharge Plants. Available online: http://www.cieeng.com/site/new/industries/aluminum/extrusion/ (accessed on 14 October 2018).
- Pramanik, B.K.; Thangavadivel, K.; Shu, L.; Jegatheesan, V. A critical review of membrane crystallization for the purification of water and recovery of minerals. Rev. Environ. Sci. Biotechnol. 2016, 15, 411–439. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Drioli, E.; Macedonio, F. Evaluation of integrated microfiltration and membrane distillation/crystallization processes for produced water treatment. Desalination 2018, 434, 161–168. [Google Scholar] [CrossRef]
- Criscuoli, A.; Drioli, E. New Metrics for Evaluating the Performance of Membrane Operations in the Logic of Process Intensification. Ind. Eng. Chem. Res. 2007, 46, 2268–2271. [Google Scholar] [CrossRef]
- Ali, A.; Quist-jensen, C.A.; Macedonio, F.; Drioli, E. Application of Membrane Crystallization for Minerals’ Recovery from Produced Water. Membranes (Basel) 2015, 5, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Quist-Jensen, C.A.; Macedonio, F.; Conidi, C.; Cassano, A.; Aljlil, S.; Alharbi, O.A.; Drioli, E. Direct contact membrane distillation for the concentration of clarified orange juice. J. Food Eng. 2016, 187, 37–43. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Kargari, A. A Review on Applications of Membrane Distillation (MD) Process for Wastewater Treatment. J. Membr. Sci. Res. 2015, 1, 101–112. [Google Scholar]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Sørensen, J.M.; Svenstrup, A.; Scarpa, L.; Carlsen, T.S.; Jensen, H.C.; Wybrandt, L.; Christensen, M.L. Membrane crystallization for phosphorus recovery and ammonia stripping from reject water from sludge dewatering process. Desalination 2018, 440, 156–160. [Google Scholar] [CrossRef]
- Lu, D.; Li, P.; Xiao, W.; He, G.; Jiang, X. Simultaneous Recovery and Crystallization Control of Saline Organic Wastewater by Membrane Distillation Crystallization. AIChE J. 2017, 63, 2187–2197. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Integrated membrane desalination systems with membrane crystallization units for resource recovery: A new approach for mining from the sea. Crystals 2016, 6, 36. [Google Scholar] [CrossRef]
- Ji, X.; Curcio, E.; Al Obaidani, S.; Di Profio, G.; Fontananova, E.; Drioli, E. Membrane distillation-crystallization of seawater reverse osmosis brines. Sep. Purif. Technol. 2010, 71, 76–82. [Google Scholar] [CrossRef]
- Li, W.; Van der Bruggen, B.; Luis, P. Integration of reverse osmosis and membrane crystallization for sodium sulphate recovery. Chem. Eng. Process. Process Intensif. 2014, 85, 57–68. [Google Scholar] [CrossRef]
- Curcio, E.; Ji, X.; Matin, A.; Barghi, S.; Di, G.; Fontananova, E.; Macleod, T.; Drioli, E. Hybrid nanofiltration-membrane crystallization system for the treatment of sulfate wastes. J. Membr. Sci. 2010, 360, 493–498. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Horbez, D.; Drioli, E. Reclamation of sodium sulfate from industrial wastewater by using membrane distillation and membrane crystallization. Desalination 2017, 401, 112–119. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Optimization of module length for continuous direct contact membrane distillation process. Chem. Eng. Process. Process Intensif. 2016, 110, 188–200. [Google Scholar] [CrossRef]
- Gill, W.N.; Wiley, D.E.; Fell, C.J.D.; Fane, A.G. Effect of viscosity on concentration polarization in ultrafiltration. AIChE J. 1988, 34, 1563–1567. [Google Scholar] [CrossRef]
- Ali, A.; Tsai, J.-H.; Tung, K.-L.; Drioli, E. On designing and optimization of continuous direct contact membrane distillation process. Desalination 2018, 426, 97–107. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. On designing of membrane thickness and thermal conductivity for large scale membrane distillation modules. J. Membr. Sci. Res. 2016, 2, 179–185. [Google Scholar]
- Rodriguez-Navarro, C.; Doehne, E.; Sebastian, E. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cem. Concr. Res. 2000, 30, 1527–1534. [Google Scholar] [CrossRef]
- Naruse, H.; Tanaka, K.; Morikawa, H.; Marumo, F.; Mehrotra, B.N. Structure of Na2SO4 (I) at 693 K. Acta Crystallogr. Sect. B Struct. Sci. 1987, 43, 143–146. [Google Scholar] [CrossRef]
- Eyser, W.; Components, T. Grystal Ghemistry of the System NarSOn-KrSOn-KrCrOn-Na,CrOn and of the Glaserite Phase. Am. Mineral. 1973, 58, 736–747. [Google Scholar]
- Rasmussen, S.E.; Jørgensen, J.E.; Lundtoft, B. Structures and Phase Transitions of Na2SO4. J. Appl. Crystallogr. 1996, 29, 42–47. [Google Scholar] [CrossRef]
- Bouchrit, R.; Boubakri, A.; Mosbahi, T.; Hafiane, A.; Bouguecha, S.A.T. Membrane crystallization for mineral recovery from saline solution: Study case Na2SO4 crystals. Desalination 2017, 412, 1–12. [Google Scholar] [CrossRef]





| pH | 7.36 |
| Redox potential [mV] | 102 |
| Conductivity [mS/cm] | 37.61 |
| Dry matter [g/L] | 64.4 ± 0.4 |
| Inorganic matter [%] | 88.7 |
| Organic matter [%] | 11.3 |
| Supernatant [mg/L] | |
|---|---|
| Dry matter [g/L] | 43.3 |
| Inorganic matter [%] | 97.9 |
| Organic matter [%] | 2.1 |
| Conductivity [ms/cm] | 39 |
| Mn2+ | 3.648 |
| Fe2+ | 6 |
| Mg2+ | 7.813 |
| P3− | 90 |
| Na+ | 2302 |
| SO42− | 18990 |
| Material | Polypropylene |
| Type | Hollow fiber |
| No. of fibers | 19 |
| Length of fibers (cm) | 42 |
| Inner fiber diameter (mm) | 1.8 |
| Outer fiber diameter (mm) | 2.7 |
| Membrane thickness (mm) | 0.45 |
| Average pore size (μm) | 0.2 |
| Porosity (%) | 73 |
| Surface area (cm2) | 45.1 |
| Concentration [mg/L] | |
|---|---|
| Mn2+ | 0.0043 |
| Mg2+ | 0.076 |
| P3− | 0.0367 |
| Na+ | 16.6 |
| Experiment | Al | Fe | Mg | Mn | Na | P |
|---|---|---|---|---|---|---|
| 47 °C-21.6 L/h | nd | nd | 0.00042 | 0.0021 | 28.47 | nd |
| 56 °C-21.6 L/h | 0.065 | nd | 0.0233 | 0.0032 | 32.84 | 0.0053 |
| 38 °C-21.6 L/h | 0.012 | 0.00005 | 0.016 | 0.0023 | 26.21 | 0.0048 |
| 47 °C-30.7 L/h | 0.0032 | 0.0008 | 0.015 | 0.0026 | 30.53 | 0.0028 |
| 47 °C-14.8 L/h | 0.146 | 0.0027 | 0.045 | 0.0059 | 23.32 | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Hvid Jacobsen, J.; Casper Jensen, H.; Lykkegaard Christensen, M.; Quist-Jensen, C.A. Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization. Appl. Sci. 2019, 9, 287. https://doi.org/10.3390/app9020287
Ali A, Hvid Jacobsen J, Casper Jensen H, Lykkegaard Christensen M, Quist-Jensen CA. Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization. Applied Sciences. 2019; 9(2):287. https://doi.org/10.3390/app9020287
Chicago/Turabian StyleAli, Aamer, Josephine Hvid Jacobsen, Henriette Casper Jensen, Morten Lykkegaard Christensen, and Cejna Anna Quist-Jensen. 2019. "Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization" Applied Sciences 9, no. 2: 287. https://doi.org/10.3390/app9020287
APA StyleAli, A., Hvid Jacobsen, J., Casper Jensen, H., Lykkegaard Christensen, M., & Quist-Jensen, C. A. (2019). Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization. Applied Sciences, 9(2), 287. https://doi.org/10.3390/app9020287







