In Vivo Rat Brain Imaging through Full-Field Optical Coherence Microscopy Using an Ultrathin Short Multimode Fiber Probe
Abstract
1. Introduction
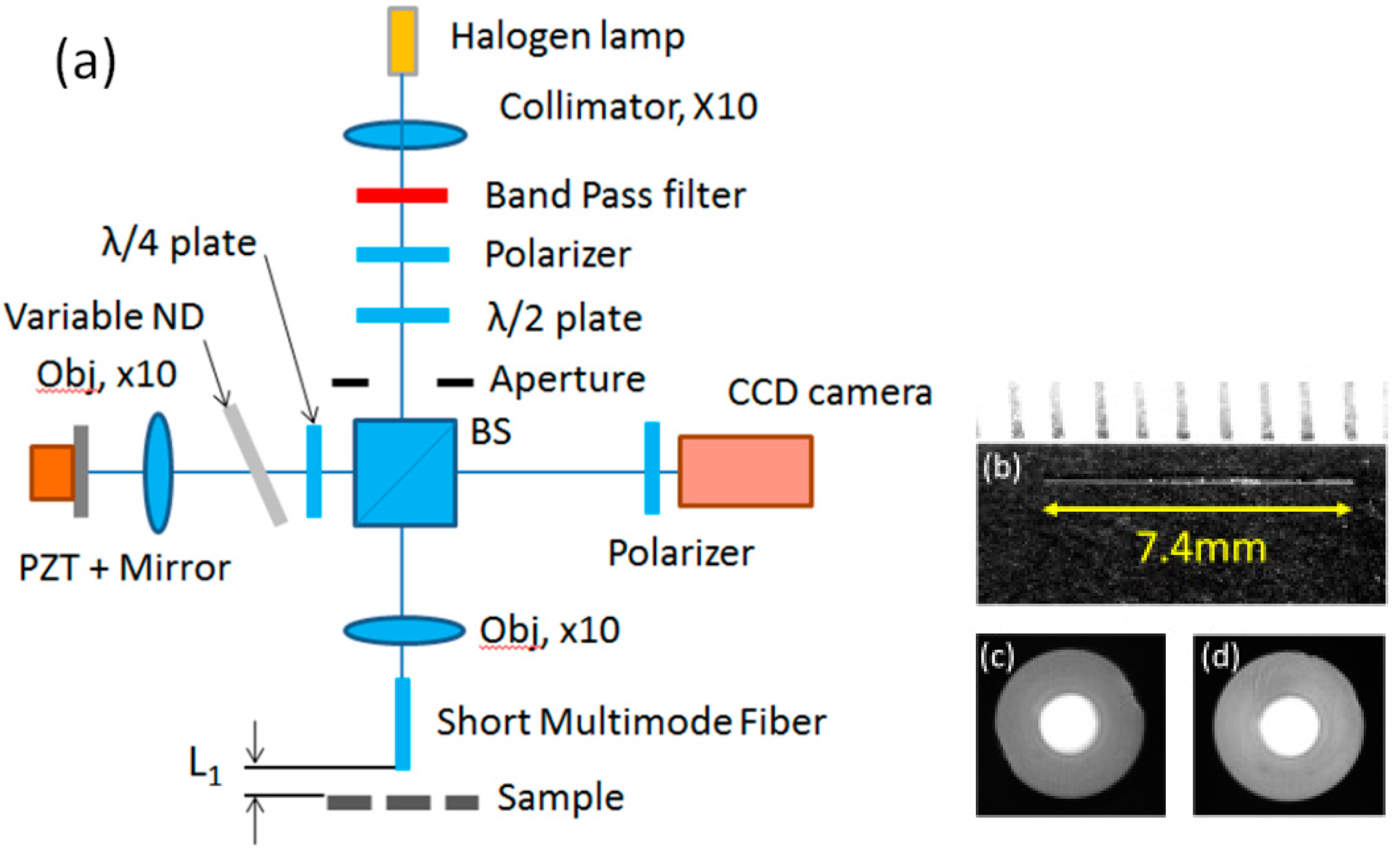
2. FF-OCM Using SMMF
3. Results
3.1. Basic Characteristics
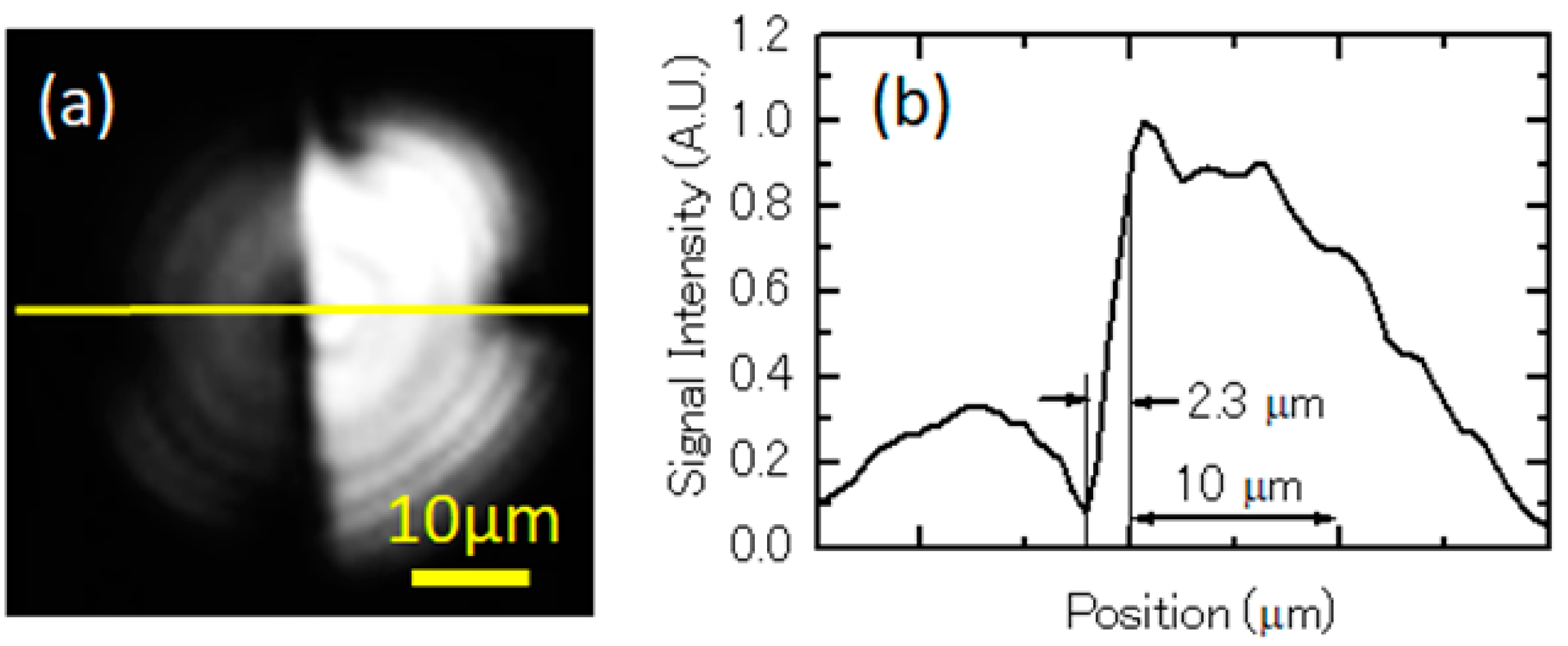
3.1.1. Spatial Resolution
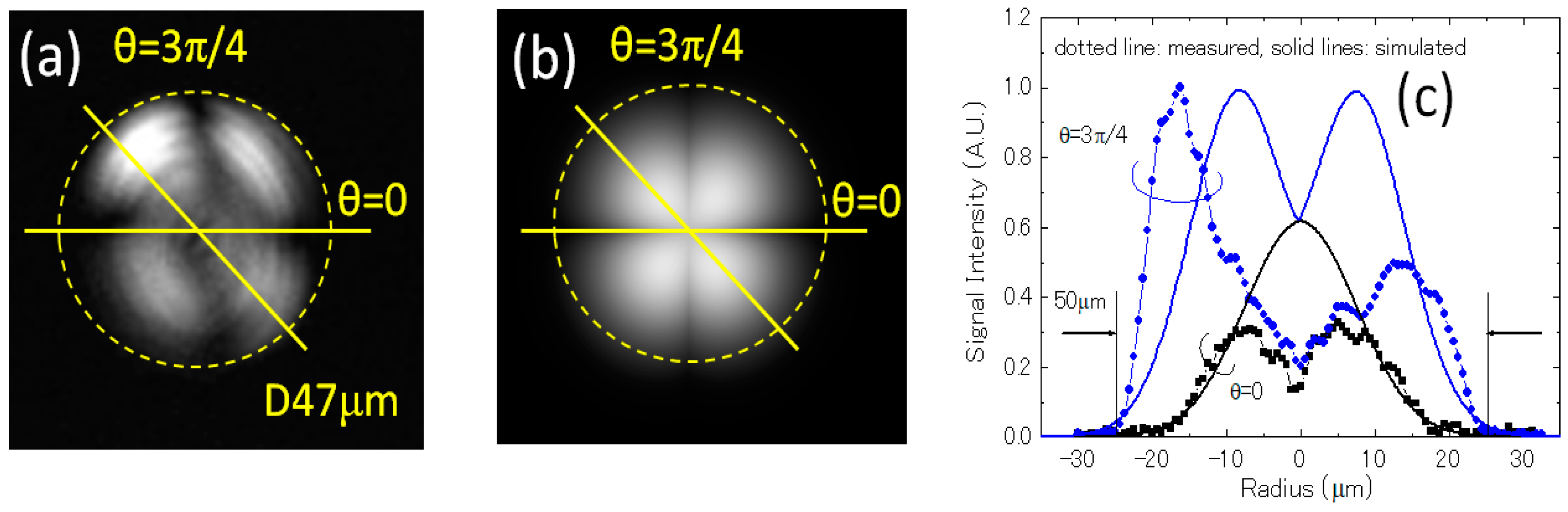
3.1.2. Mode Pattern
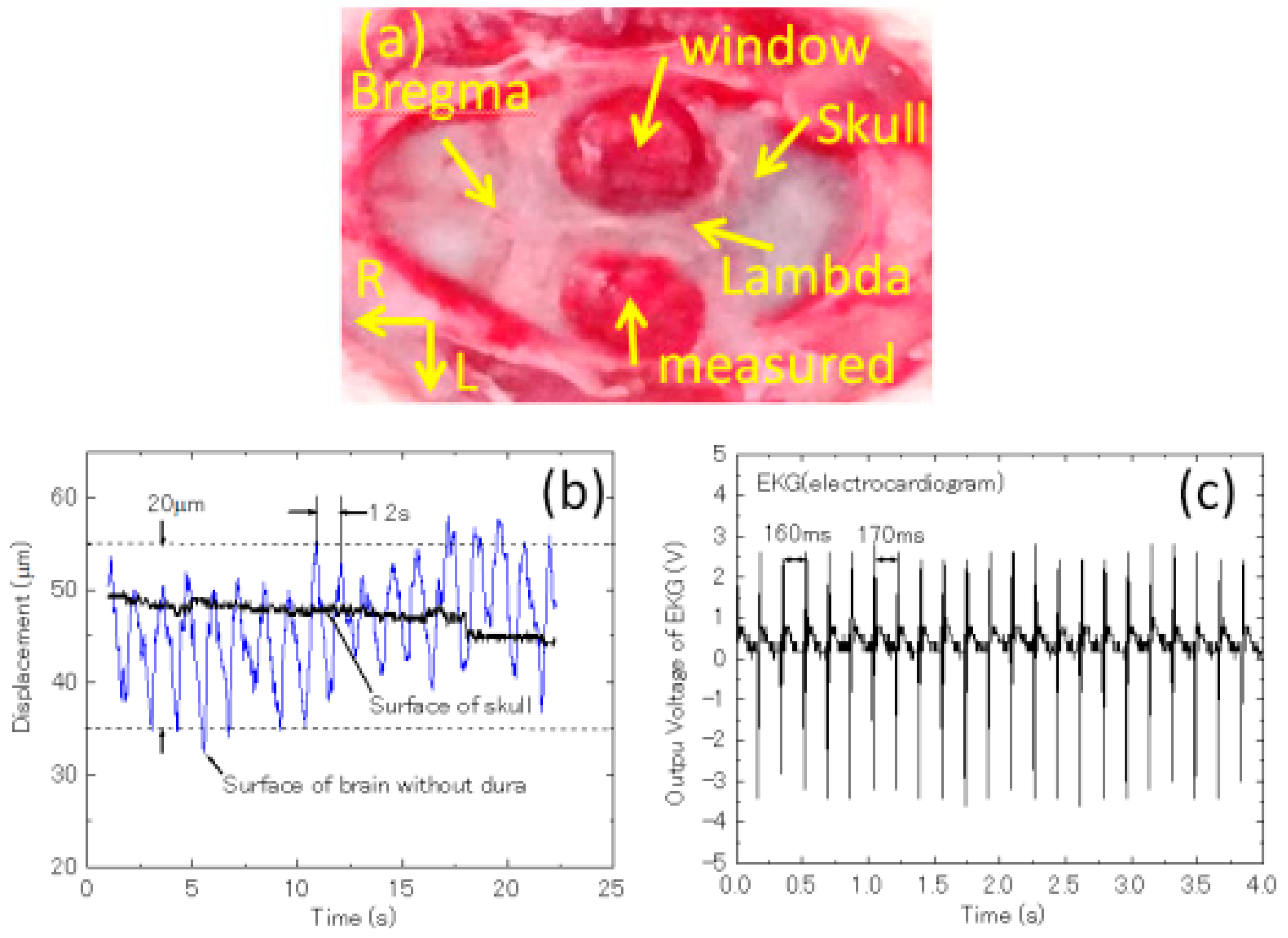
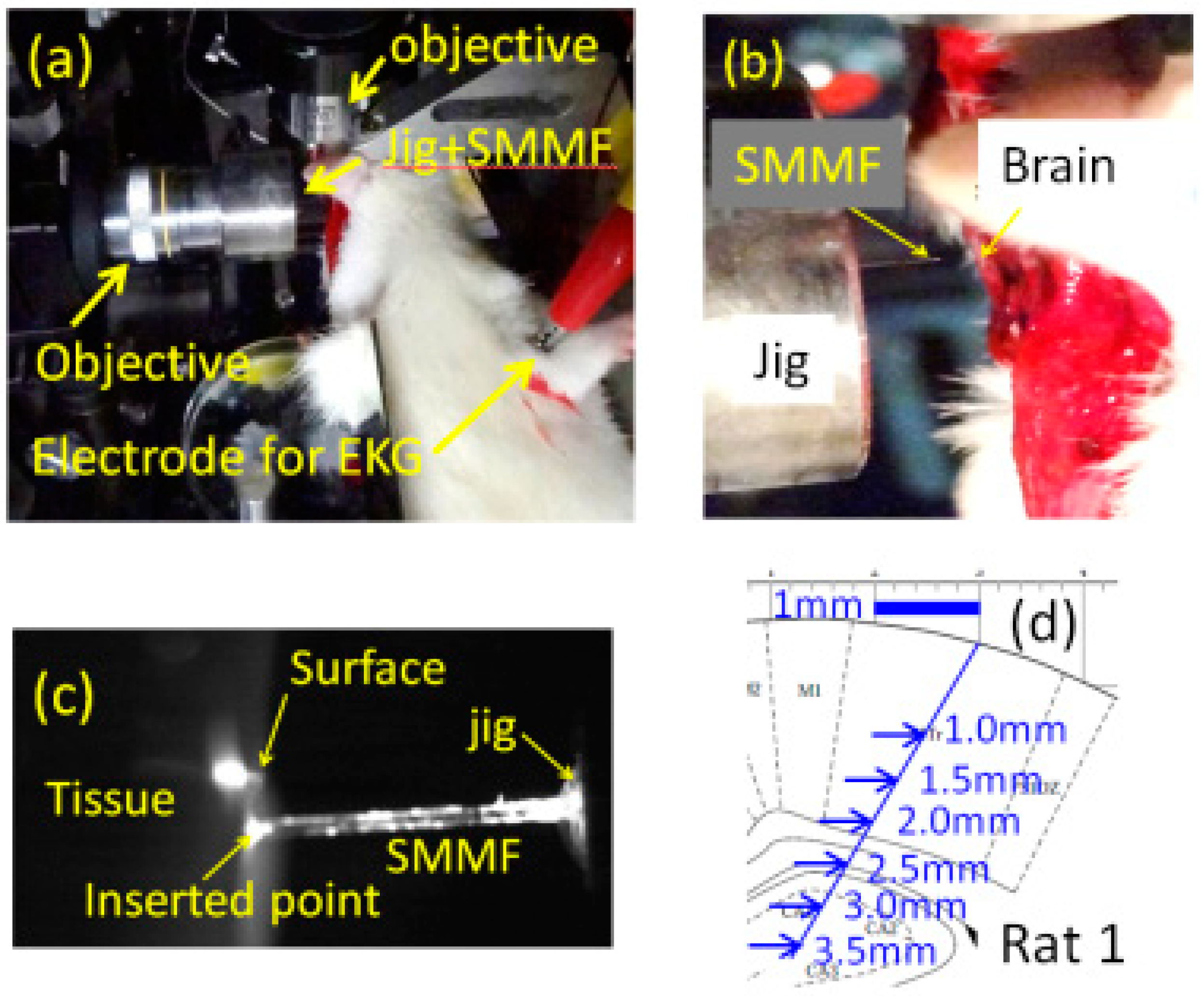
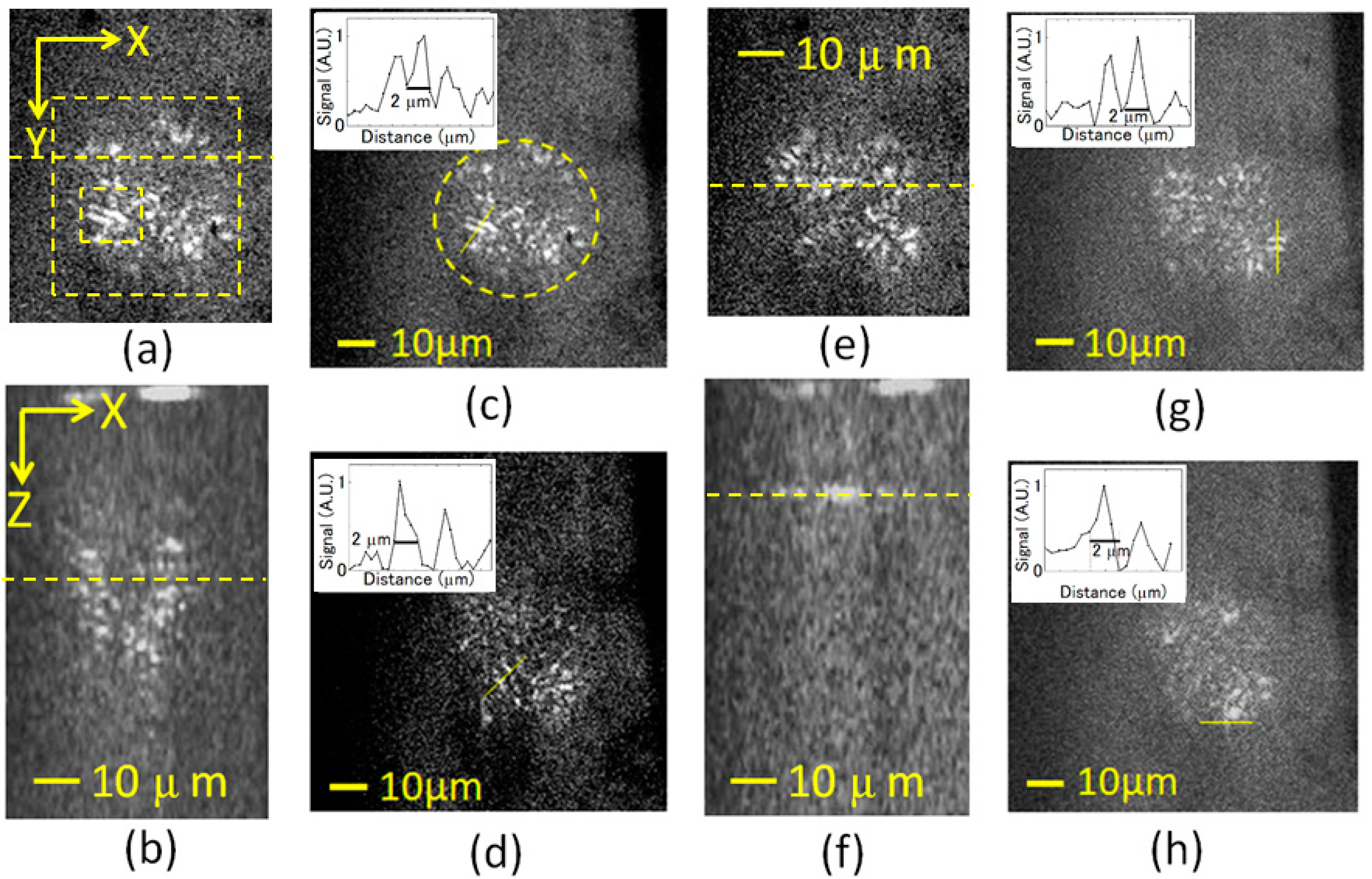
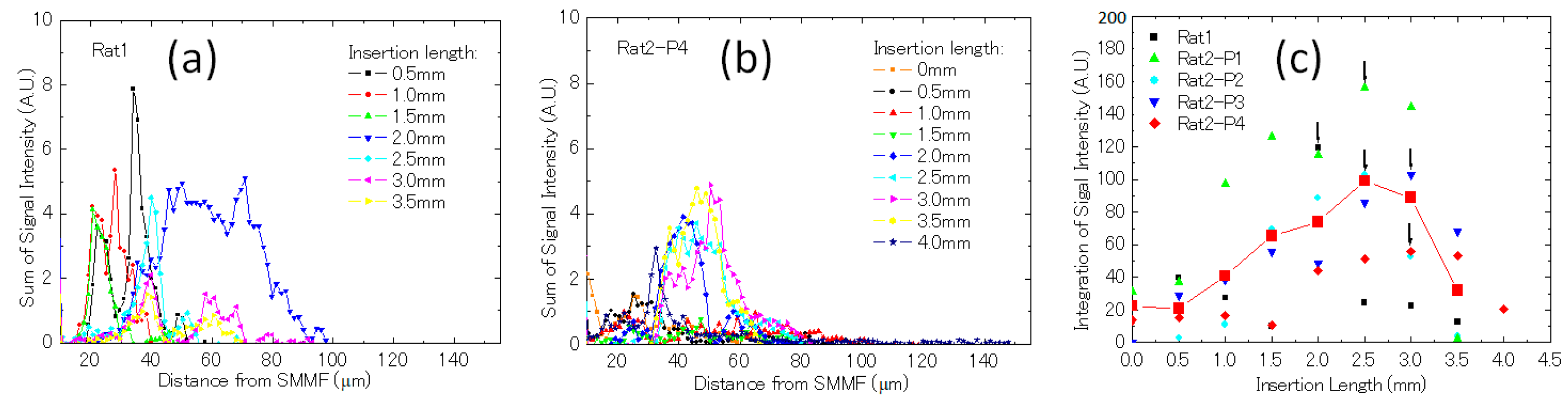
3.2. Imaging of Biological Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Bouma, B.; Tearney, J. (Eds.) Handbook of Optical Coherence Tomography; Marcel Dekker: New York, NY, USA, 2002; ISBN 0-8247-0558-0. [Google Scholar]
- Podoleanu, A.G. Optical coherence tomography. J. Microsc. 2012, 247, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, Z.; Wu, J.; McDowell, E.J.; Heng, X.; Yang, C. Methods and application areas of endoscopic optical coherence tomography. J. Biomed. Opt. 2006, 11, 063001-1–063001-19. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Min, E.J.; Kim, Y.H. Fiber-based optical coherence tomography for biomedical imaging sensing, and precision measurements. Opt. Fiber Technol. 2013, 19, 729–740. [Google Scholar] [CrossRef]
- Li, X.; Chudoba, C.; Ko, T.; Pitris, C.; Fujimoto, J.G. Imaging needle for optical coherence tomography. Opt. Lett. 2000, 25, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lorenser, D.; McLaughlin, R.A.; Kirk, R.W.; Edmond, M.; Simpson, M.C.; Grounds, M.D.; Sampson, D.D. Imaging deep skeletal muscle structure using a high-sensitivity ultrathin side-viewing optical coherence tomography needle probe. Biomed. Opt. Express 2013, 5, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chang, S.; Sherif, S.; Flueraru, C. Graded-index fiber lens proposed for ultrasmall probes used in biomedical imaging. Appl. Opt. 2007, 46, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.A.; Yan, M.F.; Schnitzer, M.J. Gradient-index fiber-optic microprobes for minimally invasive in vivo low-coherence interferometry. Opt. Lett. 2002, 27, 1794–1796. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.; Petersen, C.L.; McNamara, E.; Lamport, R.B.; Kelly, D.L. Ultrasmall Optical Probes, Imaging Optics, and Methods for Using Same. U.S. Patent 6,445,939, 3 September 2002. [Google Scholar]
- Liu, X.; Cobb, M.J.; Chen, Y.; Kimmey, M.B.; Li, X. Rapid-scanning forward-imaging miniature endoscope for real-time optical coherence tomography. Opt. Lett. 2004, 29, 1763–1765. [Google Scholar] [CrossRef]
- Park, H.-C.; Song, C.; Kang, M.; Jeong, Y.; Jeong, K.-H. Forward imaging OCT endoscopic catheter based on MEMS lens scanning. Opt. Lett. 2012, 37, 2673–2675. [Google Scholar] [CrossRef]
- Wu, J.; Conry, M.; Gu, C.; Wang, F.; Yaqoob, Z.; Yang, C. Paired-angle-rotation scanning optical coherence tomography forward-imaging probe. Opt. Lett. 2006, 31, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Joos, K.M.; Shen, J.-H. Miniature real-time intraoperative forward-imaging optical coherence tomography probe. Biomed. Opt. Express 2013, 4, 1342–1350. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Z.; Wang, H.; Wang, Y.T.; Jenkins, M.W.; Rollins, A.M. Fiber-optic catheter-based polarization-sensitive OCT for radio-frequency ablation monitoring. Opt. Lett. 2014, 39, 5066–5069. [Google Scholar] [CrossRef]
- Fu, X.; Patel, D.; Zhu, H.; MacLennan, G.; Wang, Y.T.; Jenkins, M.W.; Rollins, A.M. Miniature forward-viewing common-path OCT probe for imaging the renal pelvis. Biomed. Opt. Express 2015, 6, 1164–1171. [Google Scholar] [CrossRef]
- Liang, C.-P.; Wierwille, J.; Moreira, T.; Schwartzbauer, G.; Jafri, M.S.; Tang, C.-M.; Chen, Y. A forward-imaging needle-type OCT probe for image guided stereotactic procedures. Opt. Express 2011, 19, 26283–26293. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Bouma, B.E.; Iftimia, N.; Yelin, R.; Tearney, G.J. Spectrally-modulated full-field optical coherence microscopy for ultrahigh-resolution endoscopic imaging. Opt. Express 2006, 14, 8675–8684. [Google Scholar] [CrossRef] [PubMed]
- Izatt, J.A.; Swanson, E.A.; Fujimoto, J.G.; Hee, M.R.; Owen, G.M. Optical coherence microscopy in scattering media. Opt. Lett. 1994, 19, 590–592. [Google Scholar] [CrossRef]
- Federici, A.; Gutierrez da Costa, H.S.; Ogien, J.; Ellerbee, A.K.; Dubois, A. Wide-field, full-field optical coherence microscopy for high-axial-resolution phase and amplitude imaging. Appl. Opt. 2015, 54, 8212–8220. [Google Scholar] [CrossRef] [PubMed]
- Federici, A.; Dubois, A. Full-field optical coherence microscopy with optimized ultrahigh spatial resolution. Opt. Lett. 2015, 40, 5347–5350. [Google Scholar] [CrossRef]
- Tamborski, S.; Lyu, H.C.; Dolezyczek, H.; Malinowska, M.; Wilczynski, G.; Szlag, D.; Lasser, T.; Wojtkowski, M.; Szkulmowski, M. Extended-focus optical coherence microscopy for high-resolution imaging of the murine brain. Biomed. Opt. Express 2016, 7, 4400–4414. [Google Scholar] [CrossRef]
- Apelian, C.; Harms, F.; Thouvenin, O.; Boccara, A.C. Dynamic full field optical coherence tomography: Subcellular metabolic contrast revealed in tissues by interferometric signals temporal analysis. Biomed. Opt. Express 2016, 7, 1511–1522. [Google Scholar] [CrossRef]
- Sato, M.; Kanno, T.; Ishihara, S.; Suto, H.; Takahashi, T.; Nishidate, I. Reflectance Imaging by Graded-Index Short Multimode Fiber. Appl. Phys. Express 2013, 6, 052503-1–052503-3. [Google Scholar] [CrossRef]
- Sato, M.; Shouji, K.; Saito, D.; Nishidate, I. Imaging characteristics of a 8.8 mm long and 125μm thick graded-index short multimode fiber probe. Appl. Opt. 2016, 55, 3297–3305. [Google Scholar] [CrossRef]
- Sato, M.; Kanno, T.; Ishihara, S.; Suto, H.; Takahashi, T.; Kurotani, R.; Abe, H.; Nishidate, I. Imaging of rat brain using short graded-index multimode fiber. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 1–6 February 2014; Volume 8928, pp. 89281D-1–89281D-8. [Google Scholar]
- Sato, M.; Shouji, K.; Nishidate, I. Modulation transfer function of the imaging probe using an 8.8 mm-long and 125 μm-thick graded- index short multimode fiber. Opt. Commun. 2017, 385, 25–35. [Google Scholar] [CrossRef]
- Sato, M.; Eto, K.; Masuta, J.; Nishidate, I. Depolarization characteristics of spatial modes in imaging probe using short multimode fiber. Appl. Opt. 2018, 57, 10083–10091. [Google Scholar] [CrossRef]
- Sato, M.; Saito, D.; Shouji, K.; Kurotani, R.; Abe, H.; Nishidate, I. Ultrathin forward-imaging short multimode fiber probe for full-field optical coherence microscopy. Opt. Commun. 2016, 381, 296–308. [Google Scholar] [CrossRef]
- Sato, M.; Saito, D.; Kurotani, R.; Abe, H.; Kawauchi, S.; Sato, S.; Nishidate, I. Rat brain imaging using full field optical coherence microscopy with short multimode fiber probe. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 17 February 2017; Volume 10053, pp. 1005338-1–1005338-10. [Google Scholar]
- Mem, J.; Huang, Y.; Solanki, J.; Zeng, X.; Alex, A.; Jerwick, J.; Zhang, Z.; Tanzi, R.E.; Li, A.; Zhou, C. Optical coherence tomography for brain imaging and developmental biology. IEEE J. Quantum. Electron. 2016, QE-22, 6803213. [Google Scholar]
- Srinivasan, V.J.; Sakadzic, S.; Gorczynska, I.; Ruvinskaya, S.; Wu, W.; Fujimoto, J.G.; Boas, D.A. Quantitative cerebral blood flow with optical coherence tomography. Opt. Express 2010, 18, 2477–2494. [Google Scholar] [CrossRef]
- Srinivasan, V.J.; Radhakrishnan, H. Optical coherence tomography angiography reveals laminar microvascular hemodynamics in the rat somatosensory cortex during activation. Neuroimage 2014, 102, 393–406. [Google Scholar] [CrossRef]
- Binding, J.; Arous, J.B.; Leger, J.F.; Gigan, S.; Boccara, C.; Brourdieu, L. Brain refractive index measured in vivo with high-NA defocus-corrected full-field OCT and consequences for two-photon microscopy. Opt. Express 2011, 19, 4833–4847. [Google Scholar] [CrossRef]
- Srinivasan, V.J.; Radhakrishnan, H.; Jiang, J.Y.; Barry, S.; Cable, A.E. Optical coherence microscopy for deep tissue imaging of the cerebral cortex with intrinsic contrast. Opt. Express 2012, 20, 2220–2239. [Google Scholar] [CrossRef] [PubMed]
- Arous, J.B.; Binding, J.; Leger, J.F.; Casado, M.; Topilko, P.; Gigan, S.; Boccara, A.C.; Brourdieu, L. Single myelin fiber imaging in living rodents without labeling by deep optical coherence microscopy. J. Biomed. Opt. 2011, 16, 11116012-1–11116012-9. [Google Scholar] [CrossRef]
- Leahy, C.; Radhakrishnan, H.; Srinivasan, V.J. Volumetric imaging and quantification of cytoarchitecture and myeloarchitecture with intrinsic scattering contrast. Biomed. Opt. Express 2013, 4, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.C.; de Leeuw, F.E.; Oudkerk, M.; van Gijn, J.; Hofman, A.; Jolles, J.; Breteler, M.M. Cerebral white matter lesions and cognitive function: The Rotterdam scan study. Ann. Neurol. 2000, 47, 145–151. [Google Scholar] [CrossRef]
- Kramer, J.H.; Mungas, D.; Reed, B.R.; Wetzel, M.E.; Burnett, M.M.; Miller, B.L.; Weiner, M.W.; Chui, H.C. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology 2007, 21, 412–418. [Google Scholar] [CrossRef]
- Shu, Y.; Wei, L.; De-Shan, Z.; Tang, Y. Enriched environment and white matter in aging brain. Anat. Rec. 2012, 295, 1406–1414. [Google Scholar]
- Ren, J.Q.; Aika, Y.; Heizmann, C.W.; Kosaka, T. Quantitative analysis of neurons and glial cells in the rat somatosensory cortex, with special reference to GABAergic neurons and parvalbumin-containing neurons. Exp. Brain Res. 1992, 92, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Steven, W.S. The Scientist and Engineer’s Guide to Digital Signal Processing; California Technical Publishing: Poway, CA, USA, 1997; pp. 423–430. ISBN 0-9660176-3-3. [Google Scholar]
- Saleh, B.E.A.; Teich, M.C. Fundamental of Photonics; John Wiley & Sons: Hoboken, NJ, USA, 1991; ISBN 0-471-83965-5. [Google Scholar]
- Mahalati, R.N.; Gu, R.Y.; Kahn, J.M. Resolution limits for imaging through multi-mode fiber. Opt. Express 2013, 21, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Synder, A.W.; Love, J.D. Optical Waveguide Theory; Chapman and Hall: London, UK, 1983; ISBN 0-412-24250-8. [Google Scholar]
- Okamoto, K. Fundamentals of Optical Waveguides; Academic Press: Cambridge, MA, USA, 2006; ISBN 0-12-525096-7. [Google Scholar]
- Marcuse, D.; Presby, H.M. Effects of profile deformations on fiber bandwidth. Appl. Opt. 1979, 18, 3758–3763. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain; Academic Press: Cambridge, MA, USA, 2007; ISBN 0-12-547612-4. [Google Scholar]
- Paxinos, G. (Ed.) The Rat Nervous System, 3rd ed.; Academic Press: Cambridge, MA, USA, 2004; ISBN 0-12-547638-8. [Google Scholar]
- Chomiak, T.; Hu, B. What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? PLoS ONE 2009, 4, e7754. [Google Scholar] [CrossRef]
- Garman, R.H. Histology of the Central Nervous System. Toxicol. Pathol. 2011, 39, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, V.L.; Nikonenko, I.R.; Skibo, G.G.; Mckanna, J.A. Distribution of microglia and astrocytes in different regions of the normal adult rat brain. Neurophysiology 1997, 29, 343–351. [Google Scholar] [CrossRef]
- Li, Y.; Jay, V.; Wong, C.S. Oligodendrocytes in the adult rat spinal cord undergo radiation-induced–apoptosis. Cancer Res. 1996, 56, 5417–5422. [Google Scholar] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, M.; Eto, K.; Masuta, J.; Inoue, K.; Kurotani, R.; Abe, H.; Nishidate, I. In Vivo Rat Brain Imaging through Full-Field Optical Coherence Microscopy Using an Ultrathin Short Multimode Fiber Probe. Appl. Sci. 2019, 9, 216. https://doi.org/10.3390/app9020216
Sato M, Eto K, Masuta J, Inoue K, Kurotani R, Abe H, Nishidate I. In Vivo Rat Brain Imaging through Full-Field Optical Coherence Microscopy Using an Ultrathin Short Multimode Fiber Probe. Applied Sciences. 2019; 9(2):216. https://doi.org/10.3390/app9020216
Chicago/Turabian StyleSato, Manabu, Kai Eto, Junpei Masuta, Kenji Inoue, Reiko Kurotani, Hiroyuki Abe, and Izumi Nishidate. 2019. "In Vivo Rat Brain Imaging through Full-Field Optical Coherence Microscopy Using an Ultrathin Short Multimode Fiber Probe" Applied Sciences 9, no. 2: 216. https://doi.org/10.3390/app9020216
APA StyleSato, M., Eto, K., Masuta, J., Inoue, K., Kurotani, R., Abe, H., & Nishidate, I. (2019). In Vivo Rat Brain Imaging through Full-Field Optical Coherence Microscopy Using an Ultrathin Short Multimode Fiber Probe. Applied Sciences, 9(2), 216. https://doi.org/10.3390/app9020216




