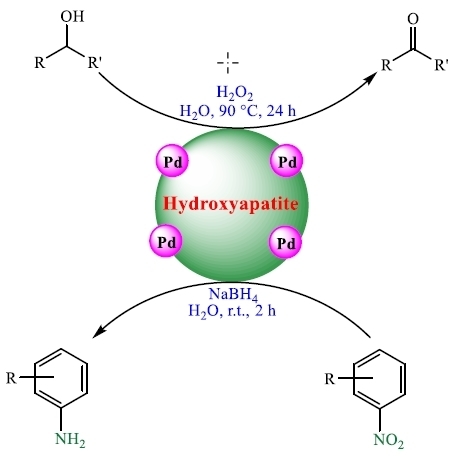
Palladium Nanocatalysts on Hydroxyapatite: Green Oxidation of Alcohols and Reduction of Nitroarenes in Water
Abstract
1. Introduction
2. Experimental Section
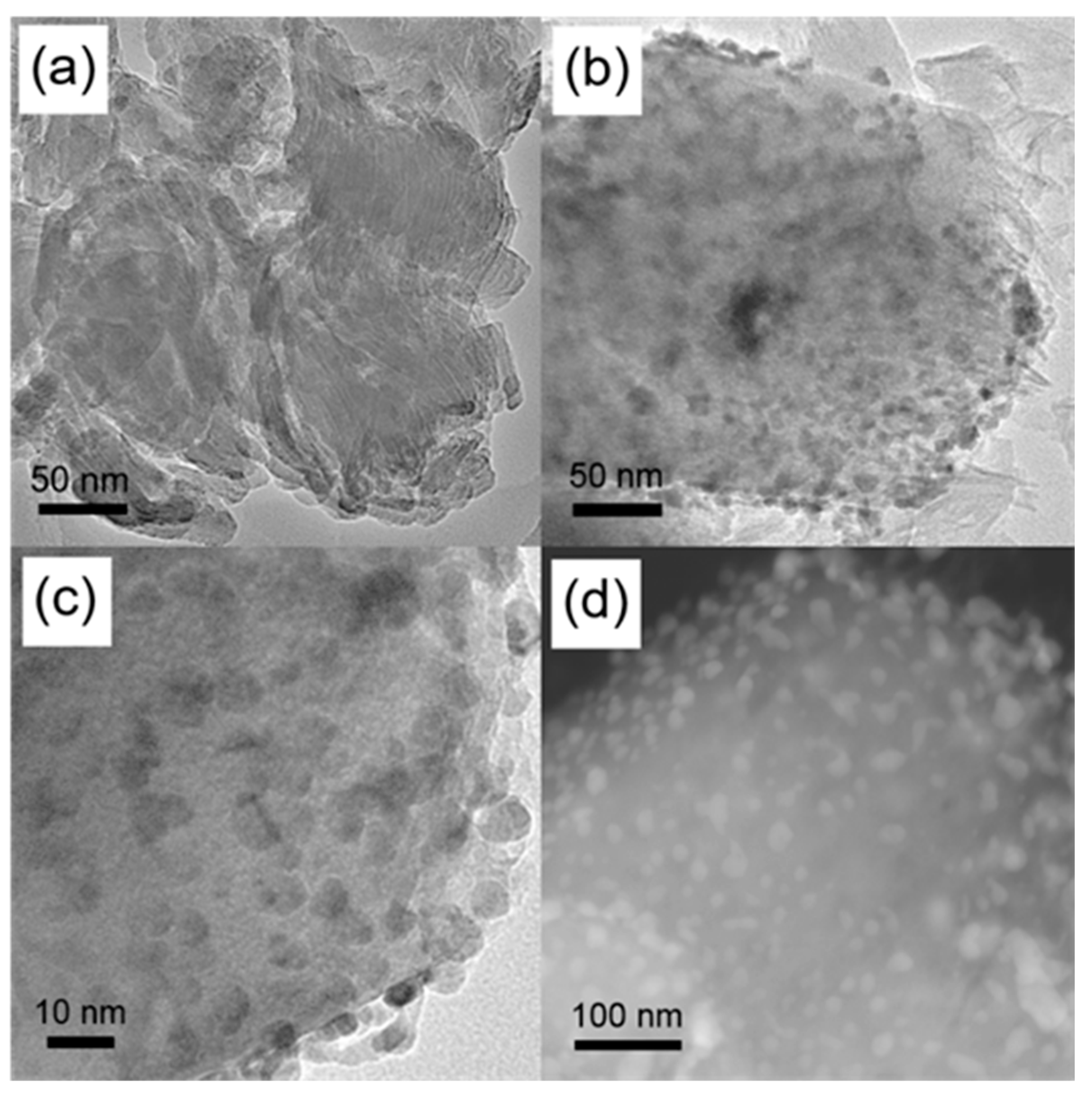
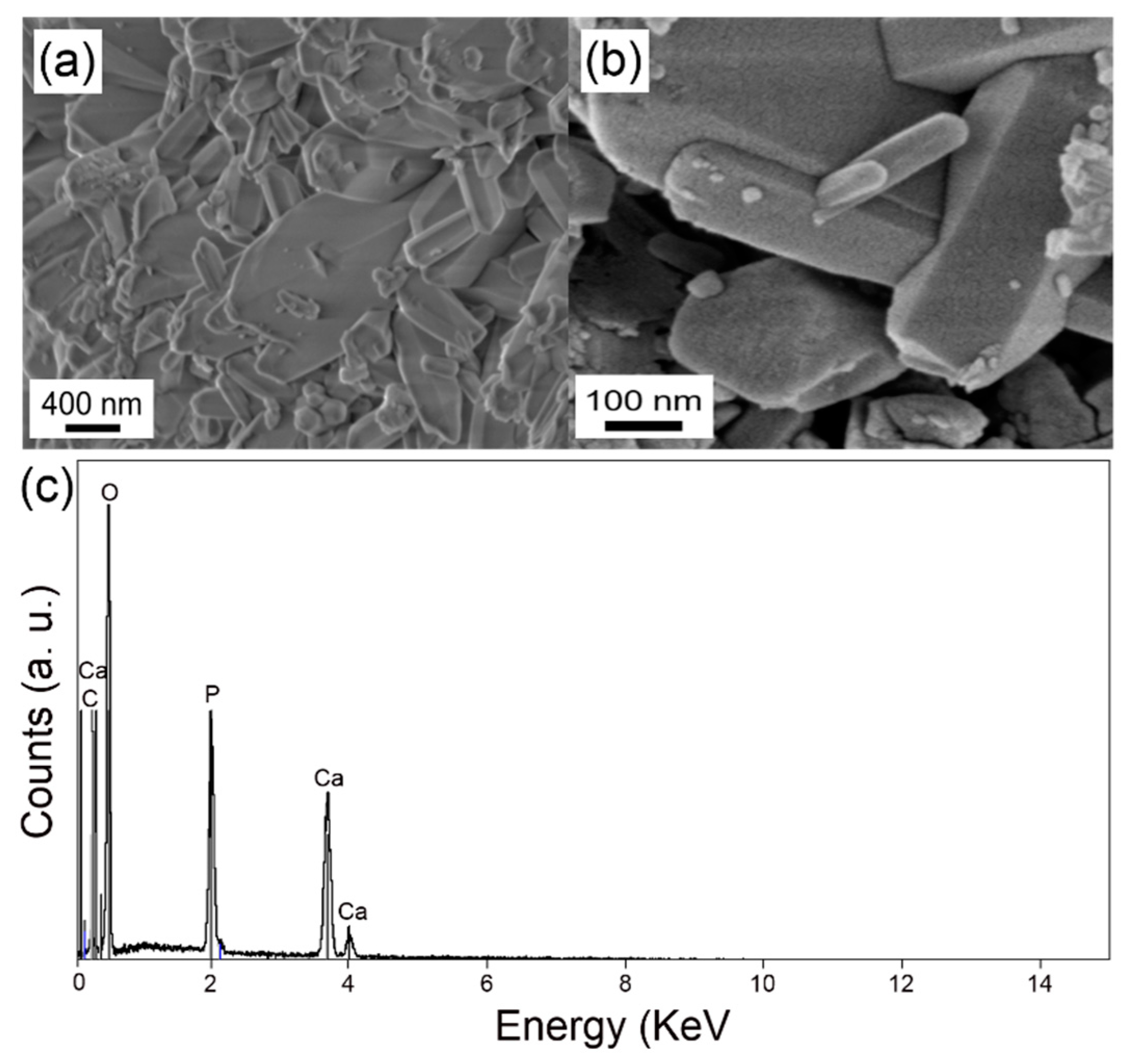
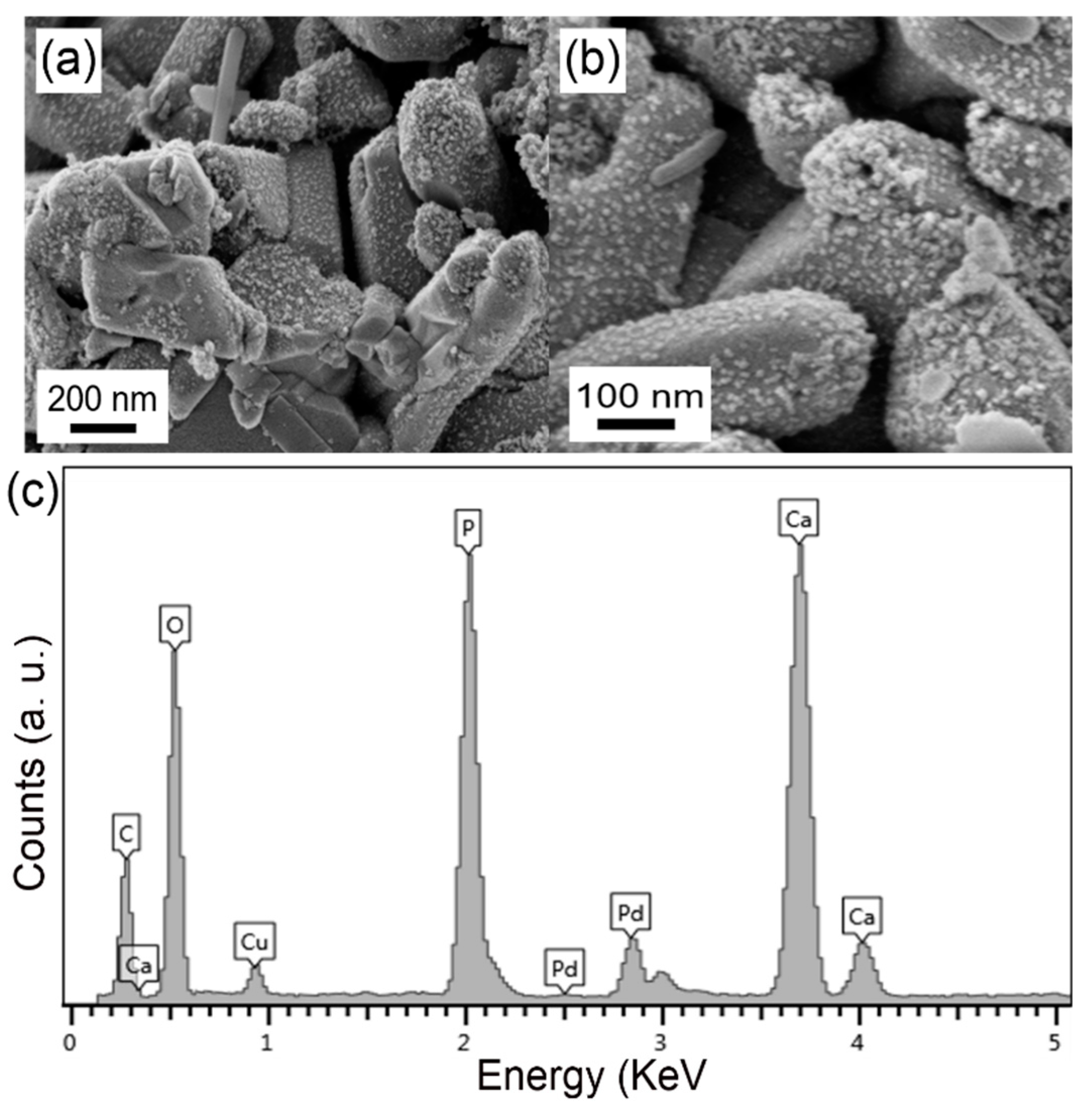
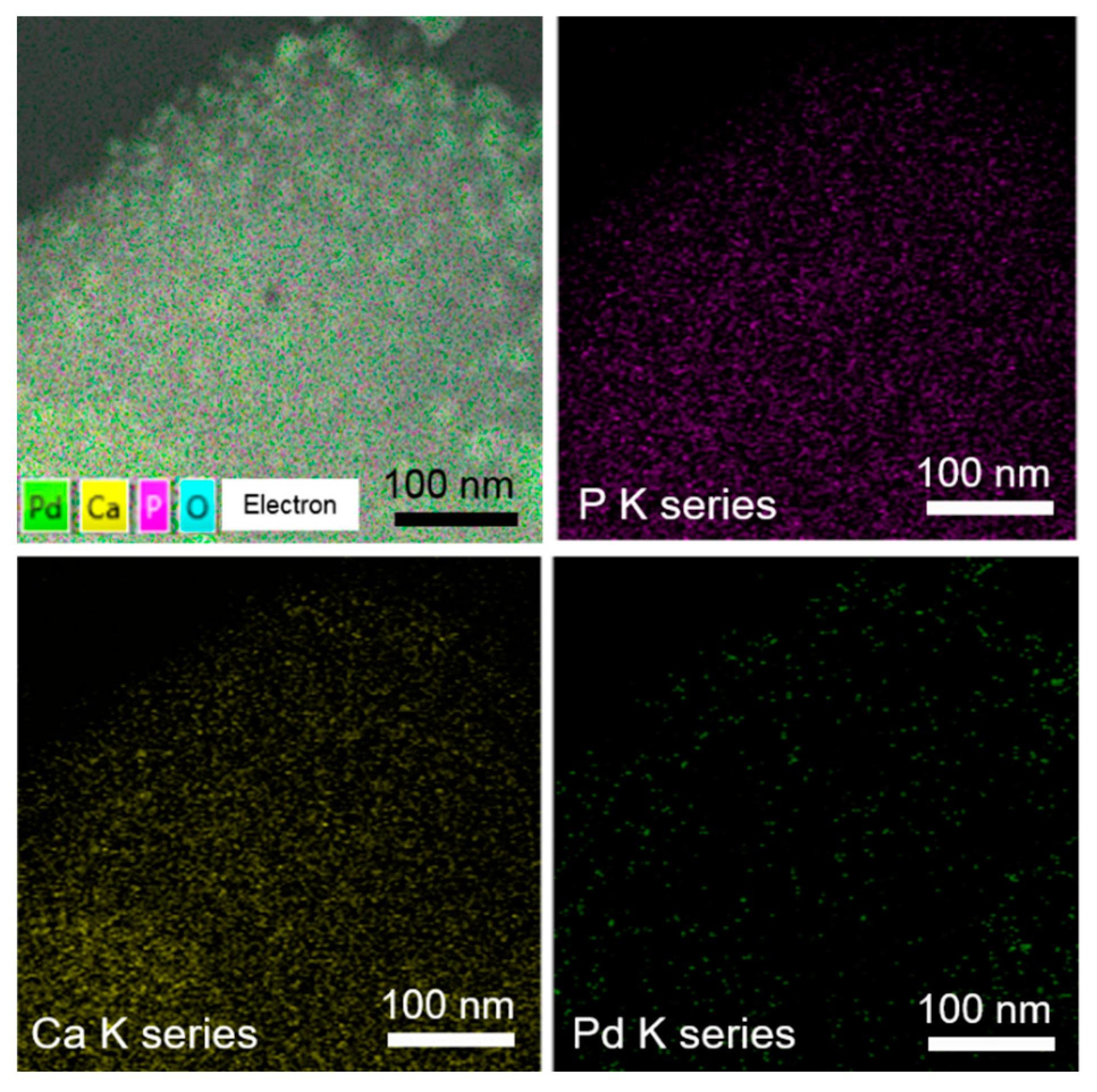
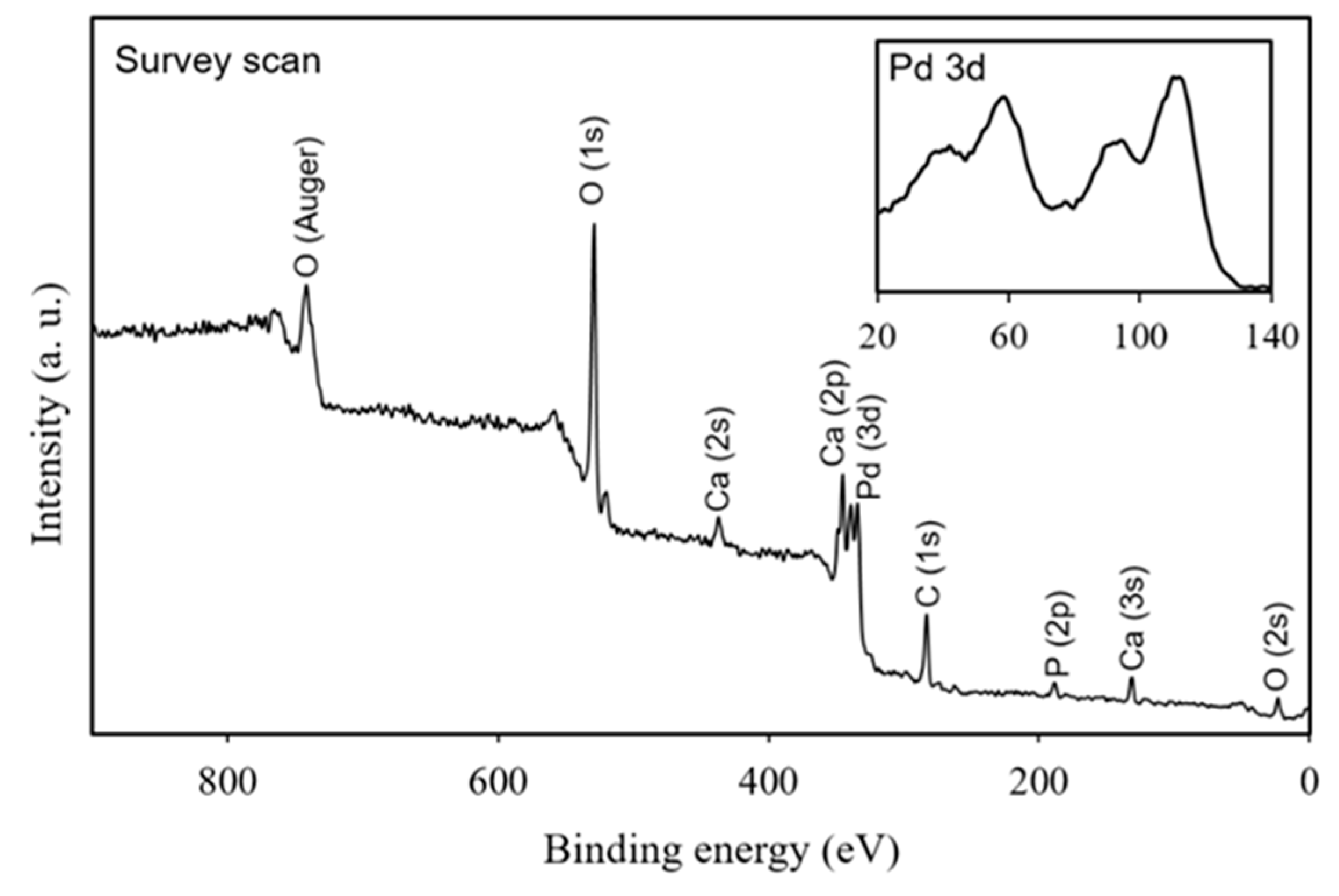
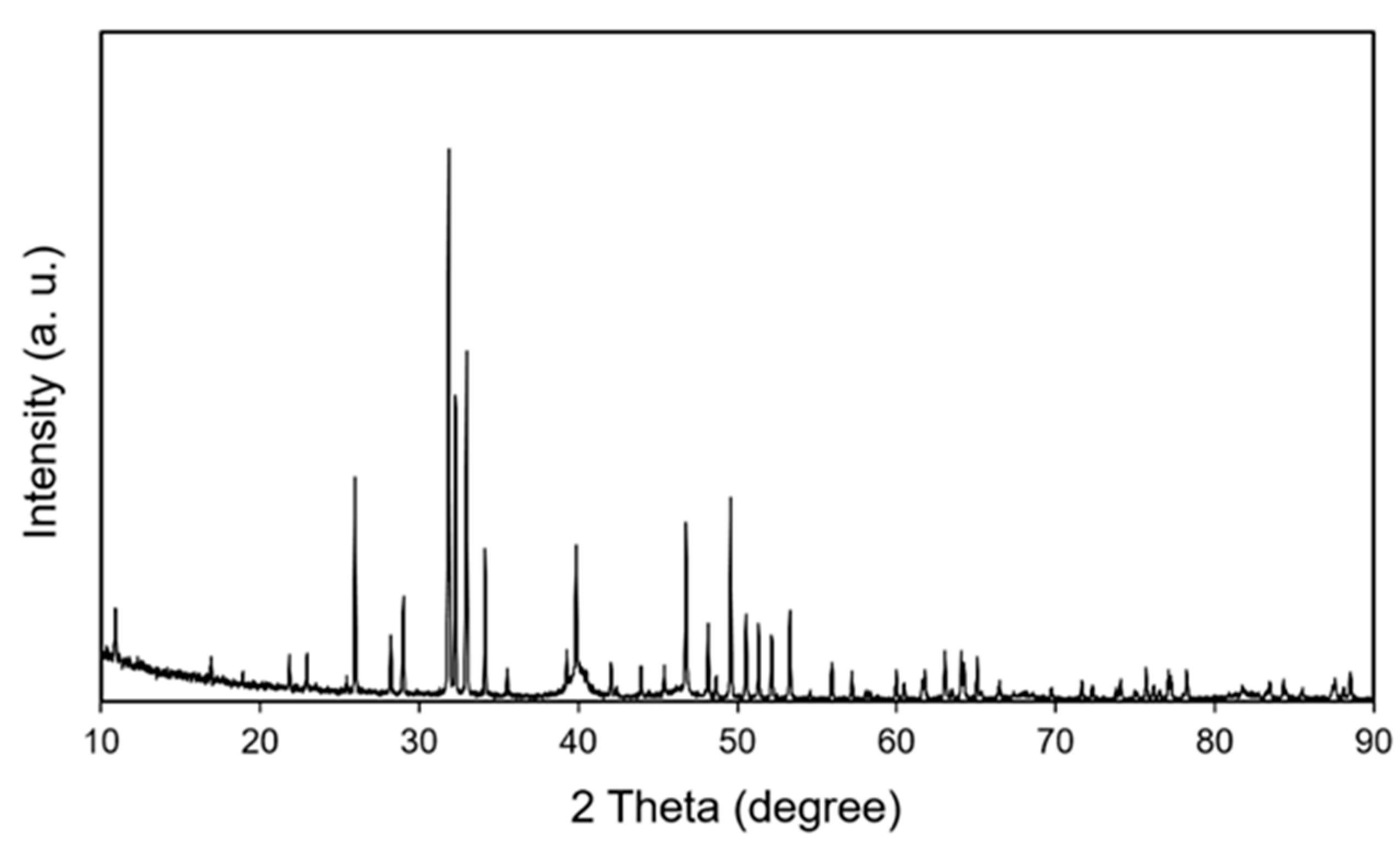
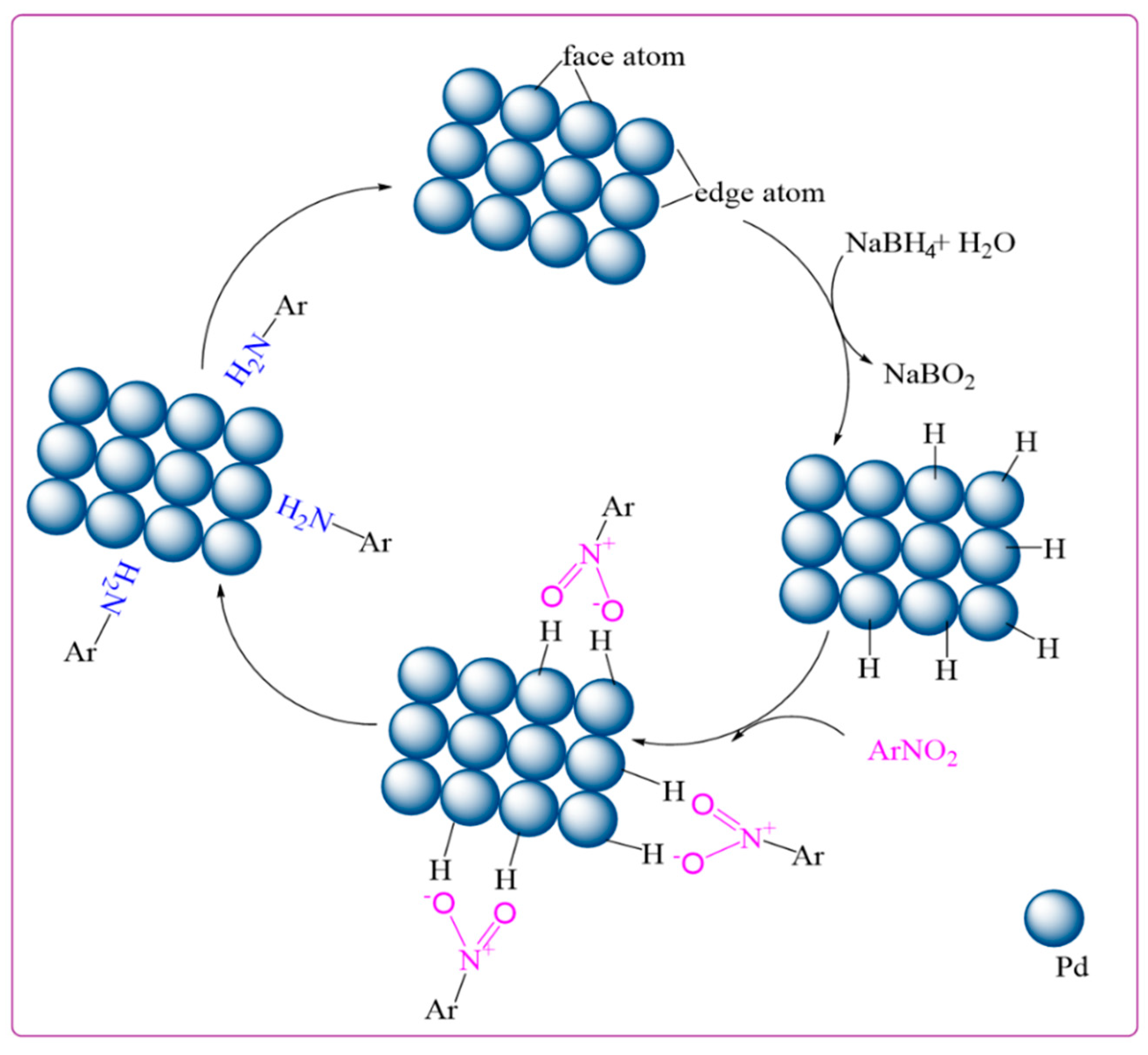
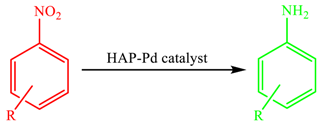
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Varma, R.S. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43–55. [Google Scholar] [CrossRef]
- Varma, R.S. Sustainability via nanocatalysis. Curr. Opin. Green Sustain. Chem. 2019, 15, A1. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Shylesh, S.; Schunemann, V.; Thiel, W.R. Magnetically separable nanocatalysts: Bridges between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Varma, R.S. Greener and sustainable trends in synthesis of organics and nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Tahsili, M.; Shokouhimehr, M.; Varma, R.S. Synthesis of 1-substituted 1H-1,2,3,4-tetrazoles using biosynthesized Ag/sodium borosilicate nanocomposite. ACS Omega 2019, 4, 8985–9000. [Google Scholar] [CrossRef]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, A.; Shokouhimehr, M. Palladium nanocatalysts confined in mesoporous silica for heterogeneous reduction of nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Laali, K.K.; Shokouhimehr, M. The Pschorr reaction, a fresh look at a classical transformation. Curr. Org. Synth. 2009, 6, 193–202. [Google Scholar] [CrossRef]
- Choi, K.H.; Shokouhimehr, M.; Sung, Y.E. Heterogeneous Suzuki cross-coupling reaction catalyzed by magnetically recyclable nanocatalysts. Bull. Korean Chem. Soc. 2013, 34, 1477–1480. [Google Scholar] [CrossRef]
- Rafiaei, S.M.; Kim, A.; Shokouhimehr, M. Gadolinium triflate immobilized on magnetic nanocomposites as recyclable Lewis acid catalyst for acetylation of phenols. Nanosci. Nanotechnol. Lett. 2014, 6, 309–313. [Google Scholar] [CrossRef]
- Choi, K.H.; Shokouhimehr, M.; Kang, Y.S.; Chung, D.Y.; Chung, Y.H.; Ahn, M.; Sung, Y.E. Preparation and characterization of palladium nanoparticles supported on nickel hexacyanoferrate for fuel cell application. Bull. Korean Chem. Soc. 2013, 34, 1195–1198. [Google Scholar] [CrossRef][Green Version]
- Shokouhimehr, M.; Shahedi Asl, M.; Mazinani, B. Modulated large-pore mesoporous silica as an efficient base catalyst for the Henry reaction. Res. Chem. Intermed. 2018, 44, 1617–1626. [Google Scholar] [CrossRef]
- Maleki, M.; Beitollahi, A.; Shokouhimehr, M. Three-dimensionally interconnected porous boron nitride foam derived from polymeric foams. RSC Adv. 2016, 6, 51426–51434. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Choi, J.W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent advances in the nanocatalysts-assisted NaBH4 reduction of nitroaromatics in water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hong, K.; Suh, J.M.; Lee, T.H.; Kwon, O.; Shokouhimehr, M.; Jang, H.W. Facile synthesis of monodispersed Pd nanocatalysts decorated on graphene oxide for reduction of nitroaromatics in aqueous solution. Res. Chem. Intermed. 2019, 45, 599–611. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Zhang, S.; Ahadi, A.; Rostamnia, S.; Banaei, R.; Li, Z.; Liu, X.; Shokouhimehr, M. Synthesis of Bimetallic 4-PySI-Pd@Cu(BDC) via open metal site Cu-MOF: Effect of metal and support of Pd@Cu-MOFs in H2 generation from formic Acid. Mol. Catal. 2019, 467, 30–37. [Google Scholar] [CrossRef]
- Fihri, S.A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Amer, W.; Abdelouahdi, K.; Ramananarivo, H.R.; Zahouily, M.; Fihri, A.; Djessas, K.; Zahouily, K.; Varma, R.S.; Solhy, A. Microwave-assisted synthesis of mesoporous nano-hydroxyapatite using surfactant templates. CrystEngComm 2014, 16, 543–549. [Google Scholar] [CrossRef]
- Amer, W.; Abdelouahdi, K.; Ramananarivo, H.; Zahouily, M.; Fihri, A.; Coppel, Y.; Varma, R.S.; Solhy, A. Synthesis of mesoporous nano-hydroxyapatite by using zwitterions surfactant. Mater. Lett. 2013, 107, 189–193. [Google Scholar] [CrossRef]
- Gawande, M.B.; Bonifacio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.W.; Park, J.; Hong, S.P.; Sohn, W.; Andoshe, D.M.; Shokouhimehr, M.; Jang, H.W. Decoration of metal oxide surface with {111} form Au nanoparticles using PEGylation. RSC Adv. 2018, 8, 18442–18450. [Google Scholar] [CrossRef]
- Ahadi, A.; Rostamnia, S.; Panahi, P.; Wilson, L.D.; Kong, Q.; An, Z.; Shokouhimehr, M. Palladium comprising dicationic bipyridinium supported periodic mesoporous organosilica (PMO): Pd@Bipy-PMO as an efficient hybrid catalyst for Suzuki-Miyaura cross-coupling reaction in water. Catalysts 2019, 9, 140. [Google Scholar] [CrossRef]
- Kwon, K.C.; Suh, J.M.; Varma, R.S.; Shokouhimehr, M.; Jang, H.W. Electrocatalytic water splitting and CO2 reduction: Sustainable solutions via single-atom catalysts supported on 2D materials. Small Methods 2019, 3. [Google Scholar] [CrossRef]
- Haghighatzadeha, A.; Mazinani, B.; Shokouhimehr, M.; Samiee, L. Preparation of mesoporous TiO2-SiO2 by ultrasonic impregnation method and effect of its calcination temperature on photocatalytic activity. Desalin. Water Treat. 2017, 92, 145–151. [Google Scholar] [CrossRef]
- Tafesh, A.M.; Weiguny, J. A review of the selective catalytic reduction of aromatic nitro compounds into aromatic amines, isocyanates, carbamates, and ureas using CO. Chem. Rev. 1996, 96, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M.; Piao, Y.; Kim, J.; Jang, Y.; Hyeon, T. A magnetically recyclable nanocomposite catalyst for olefin epoxidation. Angew. Chem. Int. Ed. 2007, 46, 7039–7043. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Kim, T.; Jun, S.W.; Shin, K.; Jang, Y.; Kim, B.H.; Kim, J.; Hyeon, T. Magnetically separable carbon nanocomposite catalysts for efficient nitroarene reduction and Suzuki reactions. Appl. Catal. A Gen. 2014, 476, 133–139. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Shokouhimehr, M.; Varma, R.S. Recent developments in palladium (nano)catalysts supported on polymers for selective and sustainable oxidation processes. Coord. Chem. Rev. 2019, 397, 54–75. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Lee, J.E.; Han, S.I.; Hyeon, T. Magnetically recyclable hollow nanocomposite catalysts for heterogeneous reduction of nitroarenes and Suzuki reactions. Chem. Commun. 2013, 49, 4779–4781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Suh, J.M.; Lee, T.H.; Cha, J.H.; Choi, J.W.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Copper oxide-graphene oxide nanocomposite: Efficient catalyst for hydrogenation of nitroaromatics in water. Nano Converg. 2019, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Joo, J.B.; Shokouhimehr, M. Graphene derivatives supported nanocatalysts for oxygen reduction reaction. Chin. J. Catal. 2015, 36, 1799–1810. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Kim, J.H.; Lee, Y.S. Heterogeneous Heck reaction catalyzed by recyclable polymer-supported N-heterocyclic carbene-palladium complex. Synlett 2006, 4, 618–620. [Google Scholar] [CrossRef]
- Uozumi, Y.; Nakao, R. Catalytic oxidation of alcohols in water under atmospheric oxygen by use of an amphiphilic resin-dispersion of a nanopalladium catalyst. Angew. Chem. Int. Ed. 2003, 42, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Hara, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Hydroxyapatite-supported palladium nanoclusters: A highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. J. Am. Chem. Soc. 2004, 126, 10657–10666. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Kamieniak, J.; Bernalte, E.; Foster, C.W.; Doyle, A.M.; Kelly, P.J.; Banks, C.E. High yield synthesis of hydroxyapatite (HAP) and palladium doped HAP via a wet chemical synthetic route. Catalysts 2016, 6, 119. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium borohydride stabilizes very active gold. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef]
- Osako, T.; Torii, K.; Uozumi, Y. Aerobic flow oxidation of alcohols in water catalyzed by platinum nanoparticles dispersed in an amphiphilic polymer. RSC Adv. 2015, 5, 2647–2654. [Google Scholar] [CrossRef]
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Selective oxidation of alcohols using photoactive VO@g-C3N4. ACS Sustain. Chem. Eng. 2016, 4, 1094–1098. [Google Scholar] [CrossRef]
- Jachuck, R.J.J.; Selvaraj, D.K.; Varma, R.S. Process intensification: Oxidation of benzyl alcohol using a continuous isothermal reactor under microwave irradiation. Green Chem. 2006, 8, 29–33. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Shin, K.Y.; Lee, J.S.; Hackett, M.J.; Jun, S.W.; Oh, M.H.; Jang, J.; Hyeon, T. Magnetically recyclable core-shell nanocatalysts for efficient heterogeneous oxidation of alcohols. J. Mater. Chem. A 2014, 2, 7593–7599. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, M.; Monga, Y.; Gaur, R.; Adholeya, A.; Zboril, R.; Varma, R.S.; Gawande, M.B. Silica-based magnetic manganese nanocatalyst-Applications in the oxidation of organic halides and alcohols. ACS Sustain. Chem. Eng. 2016, 4, 1123–1130. [Google Scholar] [CrossRef]
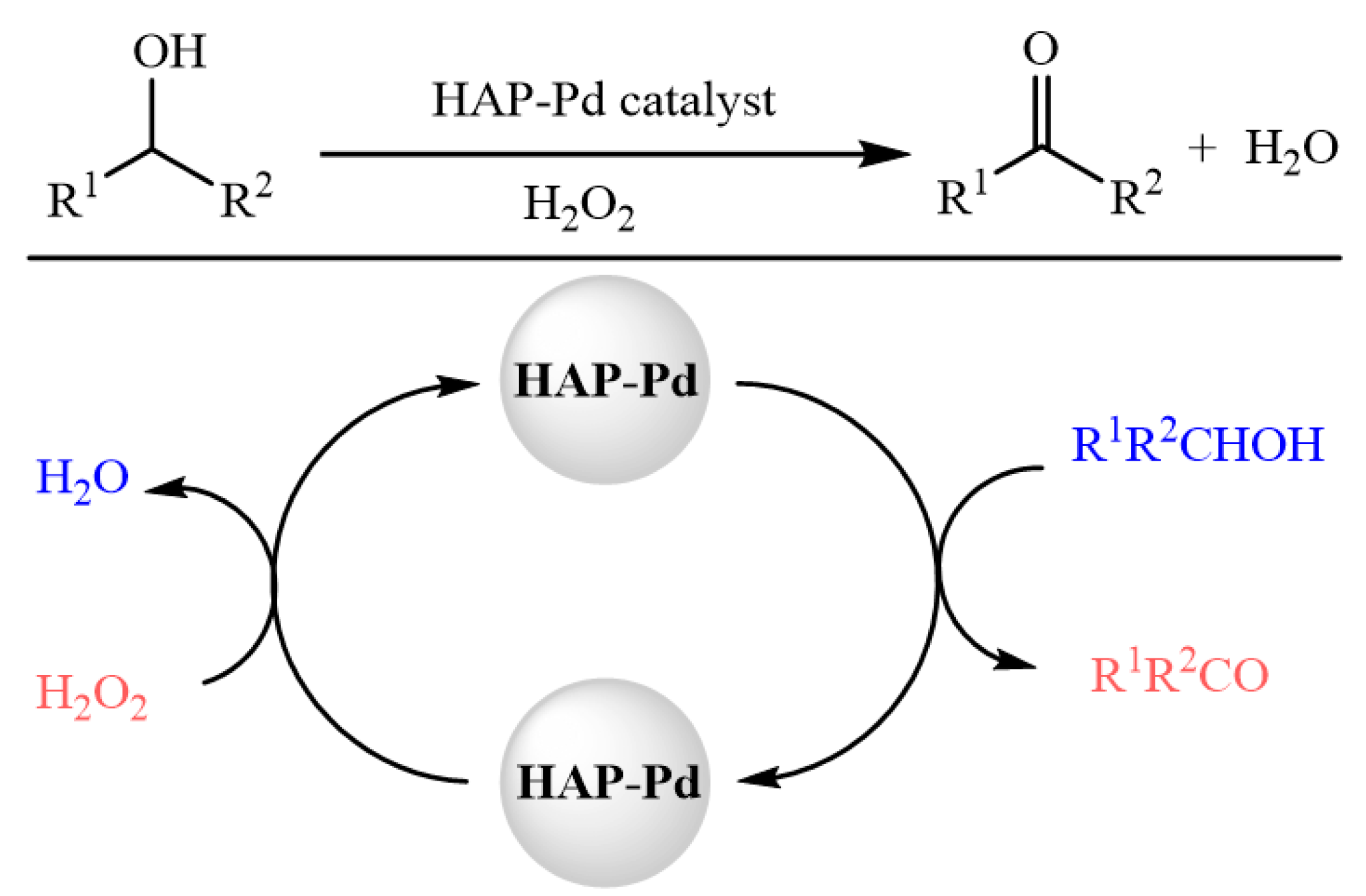

 | |||
|---|---|---|---|
| Entry | Substrate | Product | Yield (%) b |
| 1 | 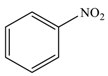 | 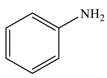 | 98 |
| 2 |  | 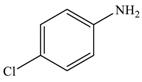 | 95 |
| 3 | 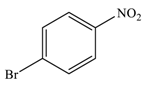 | 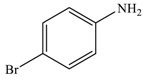 | 94 |
| 4 | 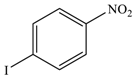 | 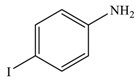 | 94 |
| 5 | 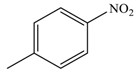 | 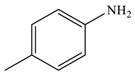 | 91 |
| 6 | 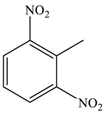 | 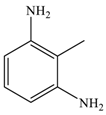 | 90 c |
| 7 | 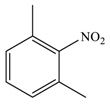 |  | 87 |
| Entry | Substrate | Product | Yield (%) b |
|---|---|---|---|
| 1 | 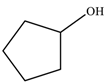 | 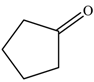 | 87 |
| 2 | 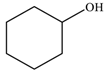 | 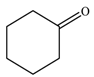 | 82 |
| 3 | 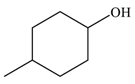 | 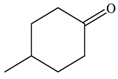 | 80 |
| 4 | 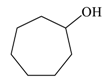 | 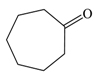 | 95 |
| 5 | 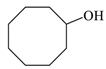 | 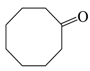 | 93 |
| 6 | 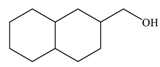 | 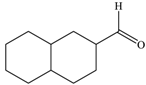 | 90 |
| 7 | 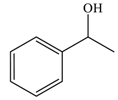 | 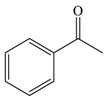 | 91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokouhimehr, M.; Yek, S.M.-G.; Nasrollahzadeh, M.; Kim, A.; Varma, R.S. Palladium Nanocatalysts on Hydroxyapatite: Green Oxidation of Alcohols and Reduction of Nitroarenes in Water. Appl. Sci. 2019, 9, 4183. https://doi.org/10.3390/app9194183
Shokouhimehr M, Yek SM-G, Nasrollahzadeh M, Kim A, Varma RS. Palladium Nanocatalysts on Hydroxyapatite: Green Oxidation of Alcohols and Reduction of Nitroarenes in Water. Applied Sciences. 2019; 9(19):4183. https://doi.org/10.3390/app9194183
Chicago/Turabian StyleShokouhimehr, Mohammadreza, Samaneh Mahmoudi-Gom Yek, Mahmoud Nasrollahzadeh, Aejung Kim, and Rajender S. Varma. 2019. "Palladium Nanocatalysts on Hydroxyapatite: Green Oxidation of Alcohols and Reduction of Nitroarenes in Water" Applied Sciences 9, no. 19: 4183. https://doi.org/10.3390/app9194183
APA StyleShokouhimehr, M., Yek, S. M.-G., Nasrollahzadeh, M., Kim, A., & Varma, R. S. (2019). Palladium Nanocatalysts on Hydroxyapatite: Green Oxidation of Alcohols and Reduction of Nitroarenes in Water. Applied Sciences, 9(19), 4183. https://doi.org/10.3390/app9194183