Comparison of Water Pretreatment Devices for the Measurement of Polar Odorous Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
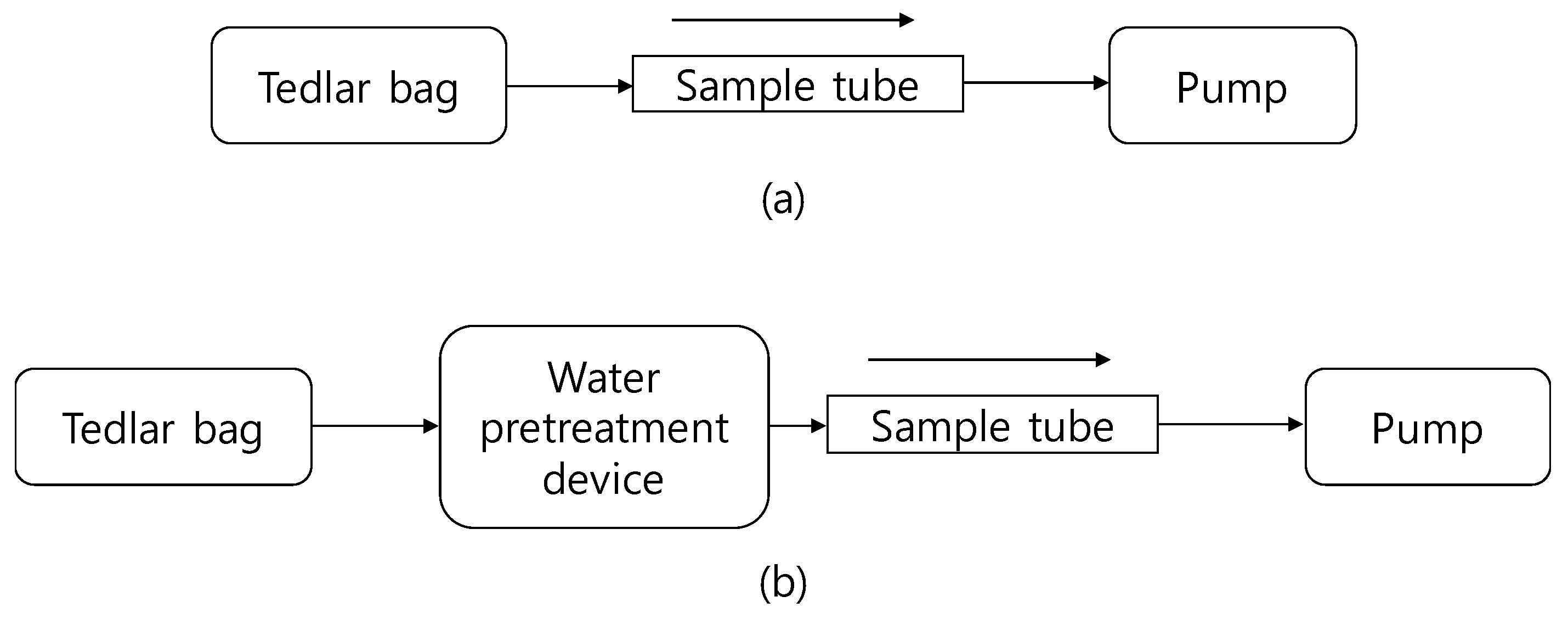
2.2. Water Pretreatement Device
2.3. Analytical System
2.4. Experiment Procedure
2.4.1. Analytical Methods
2.4.2. Water Vapor Removal
2.4.3. Odorous Compound Recovery by Water Pretreatment Device
3. Results and Discussion
3.1. Quality Assurance
3.2. Water Vapor Removal
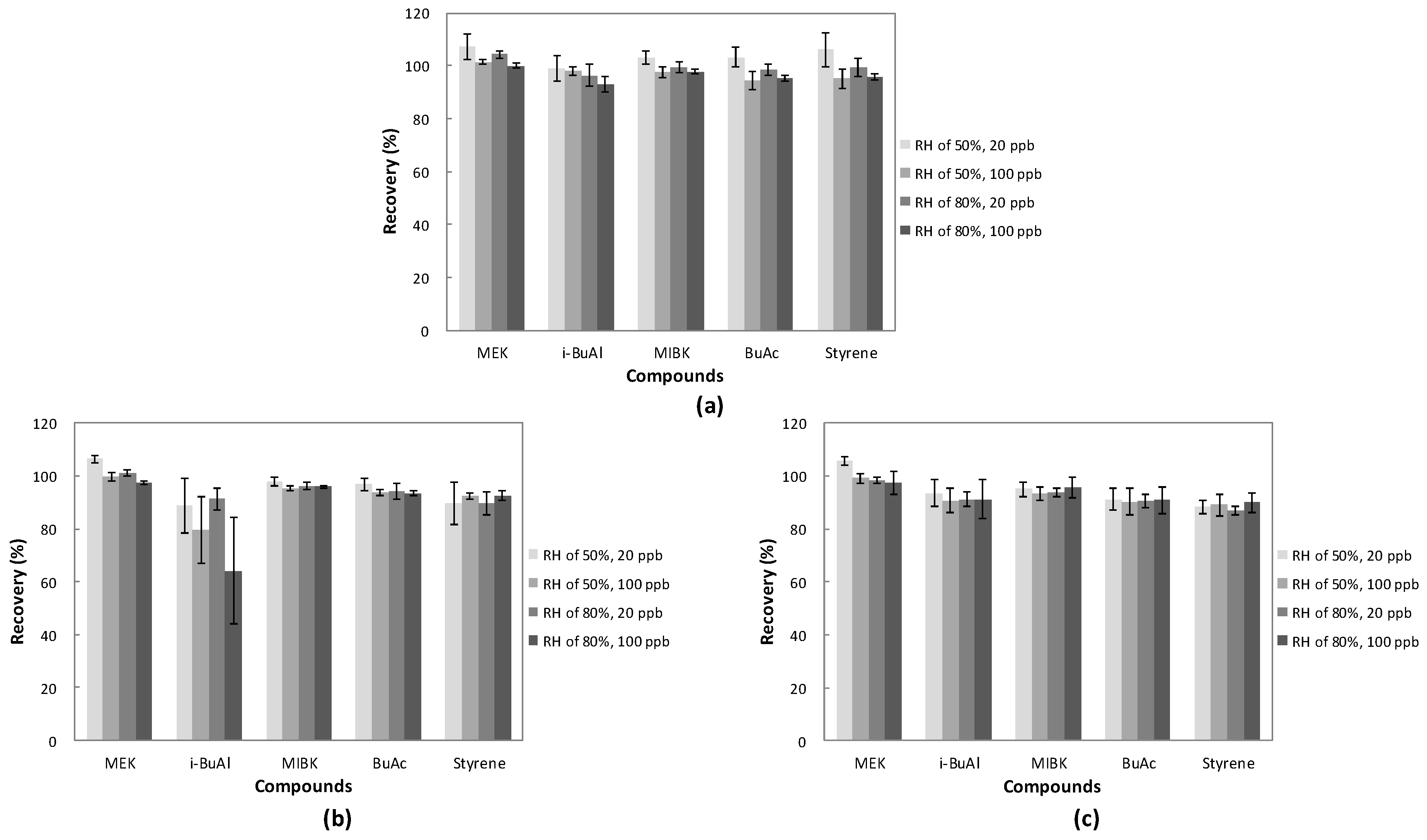
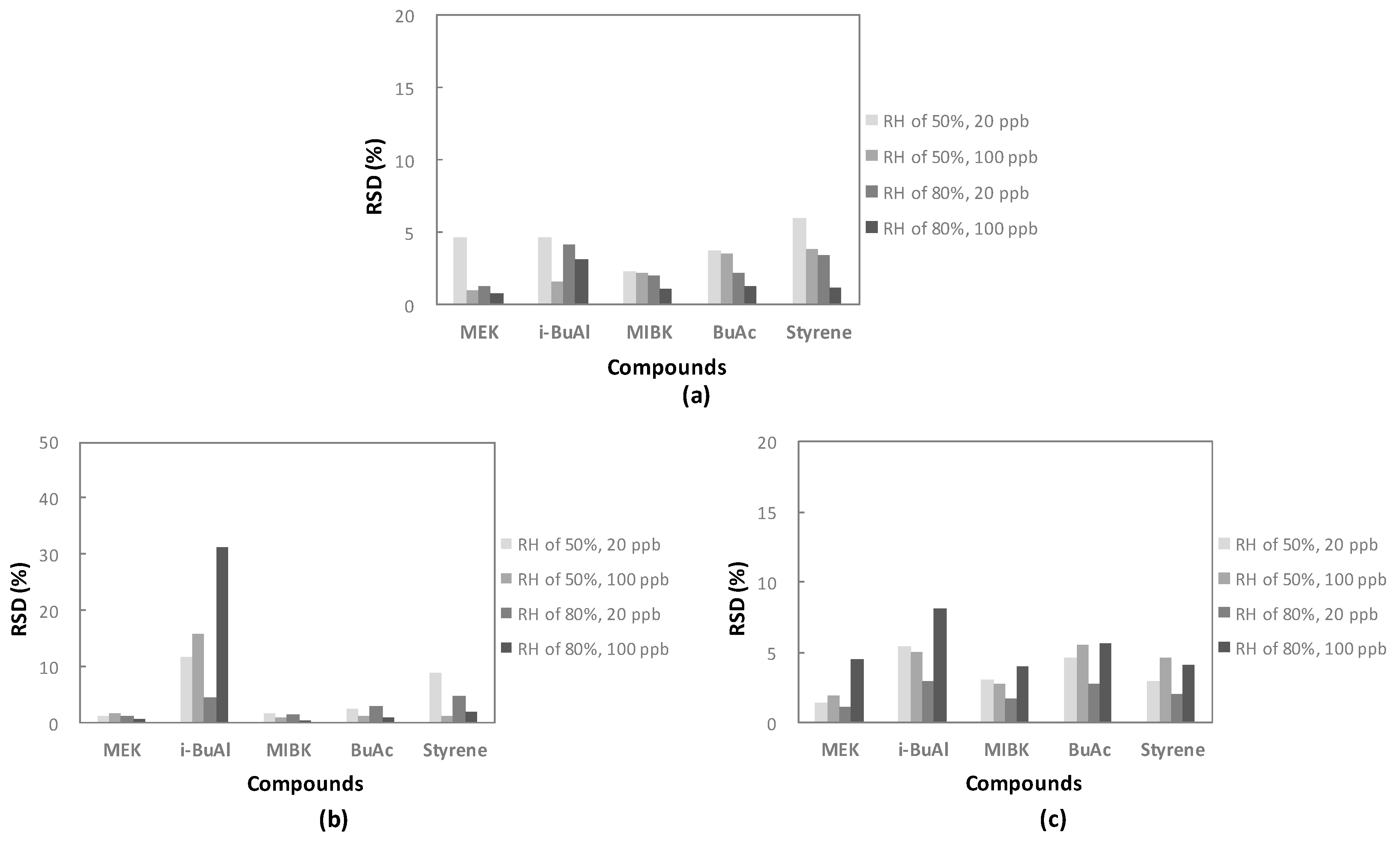
3.3. Odorous Compound Recovery of Water Pretreatment Device
3.3.1. Recovery of Odorous Compounds
3.3.2. Reproducibility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kistenev, Y.V.; Kuryak, A.N.; Makogon, M.M.; Ponomarev, Y.N. The system for dehumidification of samples in laser gas analysis. Atmos. Ocean. Opt. 2012, 25, 92–95. [Google Scholar] [CrossRef]
- Haberhauer-Troyer, C.; Rosenberg, E.; Grasserbauer, M. Investigation of membrane dryers and evaluation of a new ozone scrubbing material for the sampling of organosulphur compounds in air. J. Chromatogr. A 1999, 852, 589–595. [Google Scholar] [CrossRef]
- Sundin, N.G.; Tyson, J.F.; Hanna, C.P.; McIntosh, S.A. The use of nafion dryer tubes for moisture removal in flow injection chemical vapor generation atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1995, 50, 369–375. [Google Scholar] [CrossRef]
- U.S. EPA. Technical Assistance Document for Sampling and Analysis of Ozone Precursors; U.S. EPA: Research Triangle Park, NC, USA, 1998.
- The Korea Ministry of Environment; The Korea National Institute of Environmental Research. Air Pollution Monitoring Network Installation and Operation Guidelines; The Korea Ministry of Environment: Sejong, Korea, 2018; Available online: http://www.me.go.kr/home/web/public_info/read.do;jsessionid=ZoQ68eydUH63yakjSV1mZ90hrC6nKwmuIaIGNXrdIwh87RtigvMG7pOtmCAYSzFJ.meweb1vhost_servlet_engine1?pagerOffset=0&maxPageItems=10&maxIndexPages=10&searchKey=&searchValue=&menuId=10357&orgCd=&condition.publicInfoMasterId=2&condition.deleteYn=N&publicInfoId=426&menuId=10357 (accessed on 7 November 2018).
- The Korea Ministry of Environment. VOCs-Cold Trap-GC Method—on-Line Monitoring Method (ES 09906.a); The Korea National Institute of Environmental Research: Incheon, Korea, 2018.
- Perma Pure. Available online: http://www.permapure.com (accessed on 1 August 2019).
- The Korea National Institute of Environmental Research. Method for the Measurement of Odorants; The Korea National Institute of Environmental Research: Incheon, Korea, 2007.
- U.S. EPA. Compendium Method TO-17: Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling Onto Sorbent Tubes; U.S. EPA: Cincinnati, OH, USA, 1999.
- Yang, J.; Conver, T.S.; Koropchak, J.A.; Leighty, D.A. Use of a multi-tube Nafion® membrane dryer for desolvation with thermospray sample introduction to inductively coupled plasma-atomic emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1996, 51, 1491–1503. [Google Scholar] [CrossRef]
- Jahnke, J.A. Continuous Emission Monitoring, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Zielinska, B.; Sagebiel, J.C.; Harshfield, G.; Gertler, A.W.; Pierson, W.R. Volatile organic compounds up to C20 emitted from motor vehicles; measurement methods. Atmos. Environ. 1996, 30, 2269–2286. [Google Scholar] [CrossRef]
- McClenny, W.A.; Pleil, J.D.; Evans, G.F.; Oliver, K.D.; Holdren, M.W.; Winberry, W.T. Canister-based method for monitoring toxic vocs in ambient air. J. Air Waste Manag. Assoc. 1991, 41, 1308–1318. [Google Scholar] [CrossRef]
- Hsu, J.P.; Miller, G.; Moran, V. Analytical method for determination of trace organics in gas samples collected by Canister. J. Chromatogr. Sci. 1991, 29, 83–88. [Google Scholar] [CrossRef]
- Gong, Q.; Demerjian, K.L. Hydrocarbon losses on a regenerated nation® dryer. J. Air Waste Manag. Assoc. 1995, 45, 490–493. [Google Scholar] [CrossRef]
- Son, E.-S.; Seo, Y.-K.; Lee, D.-H.; Lee, M.-D.; Han, J.-S.; Baek, S.-O. A Study on the Performanc Optimization of A Continuous Monitoring Method for Hazardous VOCs in the Ambient Atmosphere. J. Korean Soc. Atmos. Environ. 2009, 25, 523–538. [Google Scholar] [CrossRef]
- U.S. EPA. Compendium Method TO-14A: Determination Of Volatile Organic Compounds (VOCs). In Ambient Air Using Specially Prepared Canisters With Subsequent Analysis By Gas Chromatography; U.S. EPA: Cincinnati, OH, USA, 1999. [Google Scholar]
- Seo, Y.-K.; Chung, S.-H.; Baek, S.-O. Current Status and Prospective of Hazardous VOC in Ambient Air. J. Korean Soc. Atmos. Environ. 2012, 27, 734–745. [Google Scholar] [CrossRef]
- Son, Y.S.; Lee, G.; Kim, J.C.; Han, J.S. Development of a pretreatment system for the analysis of atmospheric reduced sulfur compounds. Anal. Chem. 2013, 85, 10134–10141. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q. Mpemba Paradox: Hydrogen Bond Memory and Water-Skin Supersolidity. arXiv 2015, arXiv:1501.00765. [Google Scholar]
- Jeng, M. The Mpemba effect: When can hot water freeze faster than cold? Am. J. Phys. 2006, 74, 514–522. [Google Scholar] [CrossRef]
- The Korea Ministry of Environment. Annual Environmental Report; The Korea Ministry of Environment: Sejong, Korea, 2008.
- U.S. EPA ISO 16000-6. Indoor Air—Part 6: Determination of Volatile Organic Compounds in Indoor and Test Chamber Air by Active Sampling on Tenax TA® Sorbent, Thermal Desorption and Gas Chromatography Using MS/FID; ISO Copyright Office: Geneva, Switzerland, 2004.
- U.S. EPA ISO 16017-2. Indoor, Ambient and Workplace Air—Sampling and Analysis of Volatile Organic Compounds by Sorbent Tube/Thermal Desorption/Capillary Gas Chromatography—Part 2: Diffusive Sampling; ISO Copyright Office: Geneva, Switzerland, 2003.
- U.S. EPA ISO 16017-1. Indoor, Ambient and Workplace Air—Sampling and Analysis of Volatile Organic Compounds by Sorbent Tube/Thermal Desorption/Capillary Gas Chromatography—Part 1: Pumped Sampling; ISO Copyright Office: Geneva, Switzerland, 2000.
- The Korea Ministry of Environment. Toluene, Xylene, Methyl ethyl Ketone, Methyl Isobutyl Ketone, Butyl Actate, Stylene and i-Butyl Alcohol-Cold Trap/Thermal Desorption—GC Method (ES09307.a); The Korea National Institute of Environmental Research: Incheon, Korea, 2018.
- The Korea Ministry of Environment. Methods for Determination of Hazardous and Volatile Organic Compounds in Ambient Air-Adsorbent Trap Method (ES 01804.2); The Korea National Institute of Environmental Research: Incheon, Korea, 2016.
- Deming, B.; Pagonis, D.; Liu, X.; Day, D.; Talukdar, R.; Krechmer, J.; de Gouw, J.A.; Jimenez, J.L.; Ziemann, P.J. Measurements of Delays of Gas-Phase Compounds in a Wide Variety of Tubing Materials due to Gas-Wall Interactions. Atmos. Meas. Tech. Discuss. 2019, 12, 3453–3461. [Google Scholar] [CrossRef]
- Dunder, T.A.; Leighty, D.A. Comparison of Thermoelectric and Permeation Dryers for Sulfur Dioxide Removal during Sample Conditioning of Wet Gas Streams; No. CONF-970677-.; Air and Waste Management Association: Pittsburgh, PA, USA, 1997. [Google Scholar]
- Kim, D.-J. The Effect of Water Pretreatment Device on Environmental AIR pollutants (O3, SO2, CO) Measurements and Analysis; Konkuk University: Seoul, Korea, 2018. [Google Scholar]
- Im, M.-S.; Ju, D.-W.; Kim, H.-S.; Song, K.-P.; Park, K.-H. A Study of Analytical Method for 4 Legally-designated Compounds [MEK, MIBK, n-Butyl acetate, i-Butyl alcohol] in Ambient Air Using On-line Thermal Desorber with GC/FID. J. Korean Soc. Dor Res. Eng. 2007, 6, 145–153. [Google Scholar]

| Compound | CAS No. | Molecular Weight | Solubility * | Vapor Pressure | |
|---|---|---|---|---|---|
| Full Name | Short Name | (g/mol) | (g/L) | (mmHg, 20 °C) | |
| Methyl ethyl ketone | MEK | 78-93-3 | 72.11 | 275 | 78 |
| Isobutyl alcohol | i-BuAl | 78-83-1 | 74.12 | 95 | 9 |
| Methyl isobutyl ketone | MIBK | 108-10-1 | 100.16 | 19.1 | 16 |
| Butyl acetate | BuAc | 123-86-4 | 116.16 | 6.8 | 9 |
| Styrene | S | 100-42-5 | 104.15 | 3 | 5 |
| TD (Unity 2, Markes International, UK) | ||||
| Predesorption | Prepurge time | 1 | (min) | |
| Split | 0 | (mL/min) | ||
| Sample tube Desorption | Tube hold | 10 | (min) | |
| Oven temperature | 300 | (°C) | ||
| Split | 0 | (mL/min) | ||
| Cold trap Desorption | Per-trap fire purge/min | 1 | (°C) | |
| Trap low | −10 | (°C) | ||
| Trap high | 300 | (°C) | ||
| Trap hold | 5 | (min) | ||
| Split | 0 | (mL/min) | ||
| GC (6890, Agilent Technologies, USA) | ||||
| MS (5975, Agilent Technologies, USA) | ||||
| Rate | Temp | Hold | Run Time | |
| (°C/min) | (°C) | (min) | (min) | |
| Initial | - | 40 | 5 | 5 |
| Ramp 1 | 10 | 240 | 5 | 30 |
| Carrier gas: 1.5 mL/min for He gas. | ||||
| Concentration | Volume | Working Gas Concentration (ppb) | ||
|---|---|---|---|---|
| Primary Standard Gas (ppm) | RH (%) | Humid Air (mL) | Primary Standard Gas (mL) | |
| 10 * | 50 | 9980 | 20 | 20 range (20.6–20.8) |
| 9900 | 100 | 100 range (103–104) | ||
| 10 * | 80 | 9980 | 20 | 20 range (20.6–20.8) |
| 9900 | 100 | 100 range (103–104) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-Y.; Dinh, T.-V.; Kim, D.-J.; Choi, I.-Y.; Ahn, J.-W.; Park, S.-Y.; Jung, Y.-J.; Kim, J.-C. Comparison of Water Pretreatment Devices for the Measurement of Polar Odorous Compounds. Appl. Sci. 2019, 9, 4045. https://doi.org/10.3390/app9194045
Lee J-Y, Dinh T-V, Kim D-J, Choi I-Y, Ahn J-W, Park S-Y, Jung Y-J, Kim J-C. Comparison of Water Pretreatment Devices for the Measurement of Polar Odorous Compounds. Applied Sciences. 2019; 9(19):4045. https://doi.org/10.3390/app9194045
Chicago/Turabian StyleLee, Joo-Yeon, Trieu-Vuong Dinh, Dong-June Kim, In-Young Choi, Ji-Won Ahn, Shin-Young Park, Yoo-Jin Jung, and Jo-Chun Kim. 2019. "Comparison of Water Pretreatment Devices for the Measurement of Polar Odorous Compounds" Applied Sciences 9, no. 19: 4045. https://doi.org/10.3390/app9194045
APA StyleLee, J.-Y., Dinh, T.-V., Kim, D.-J., Choi, I.-Y., Ahn, J.-W., Park, S.-Y., Jung, Y.-J., & Kim, J.-C. (2019). Comparison of Water Pretreatment Devices for the Measurement of Polar Odorous Compounds. Applied Sciences, 9(19), 4045. https://doi.org/10.3390/app9194045







