In-Situ Continuous Monitoring of the Viscosity of Surfactant-Stabilized and Nanoparticles-Stabilized Pickering Emulsions
Abstract
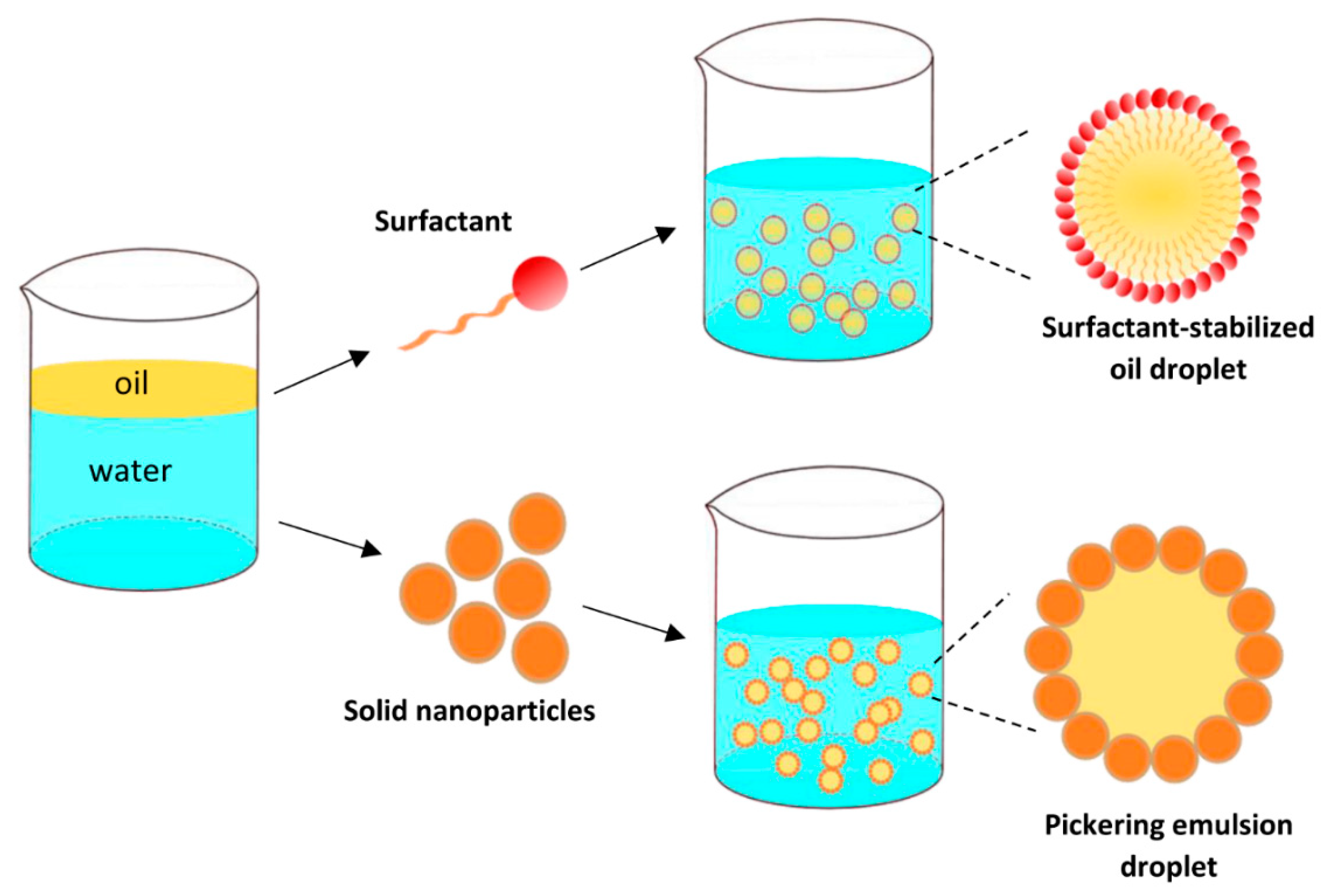
:1. Introduction
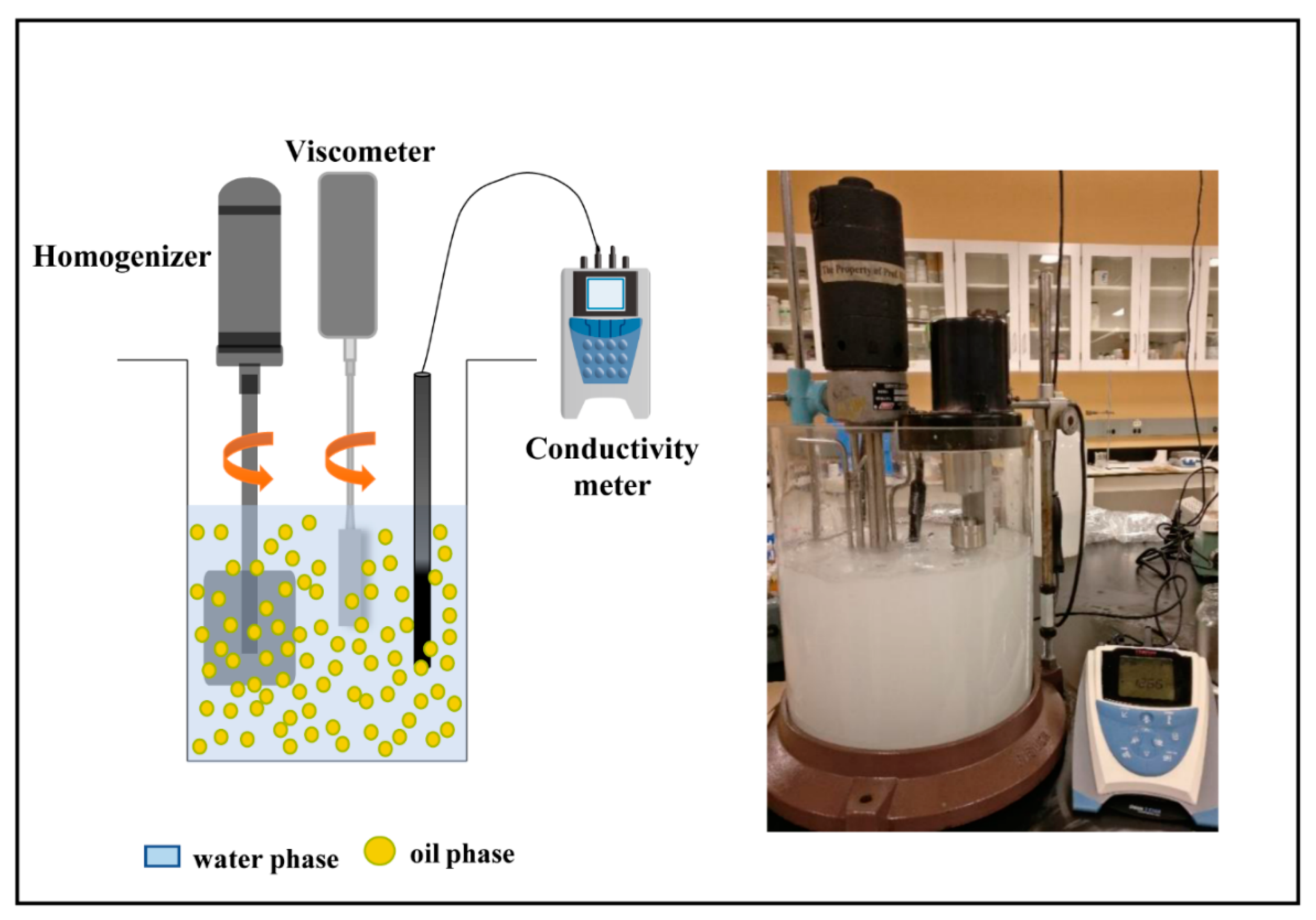
2. Experimental Setup
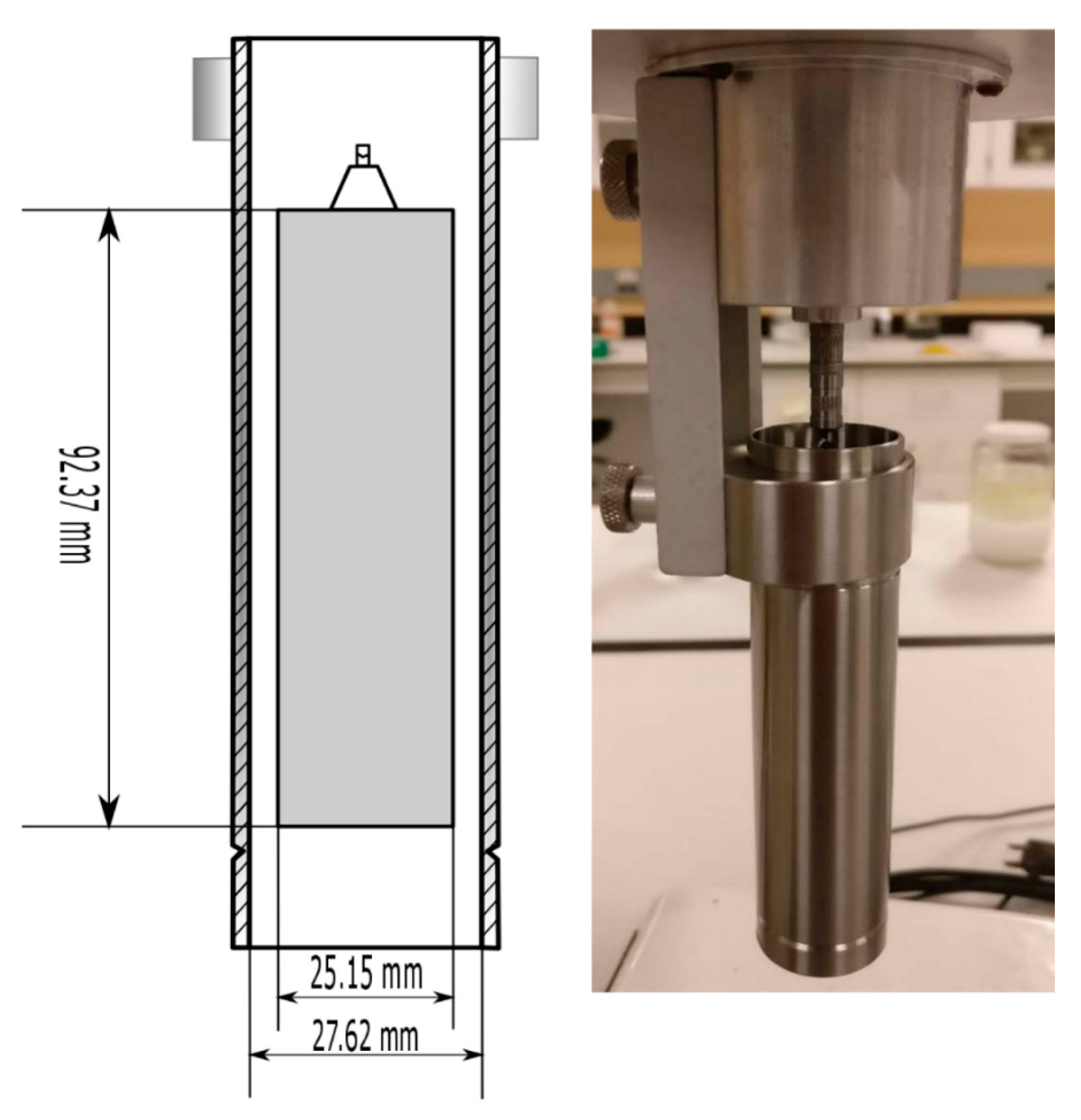
2.1. In-Situ Measurement of the Viscosity of Emulsions
2.2. Viscosity versus Shear Rate Behavior of Emulsions
3. Experimental Work
3.1. Materials
3.2. Preparation of Starch Dispersions
3.3. Preparation of Emulsions
4. Results and Discussion
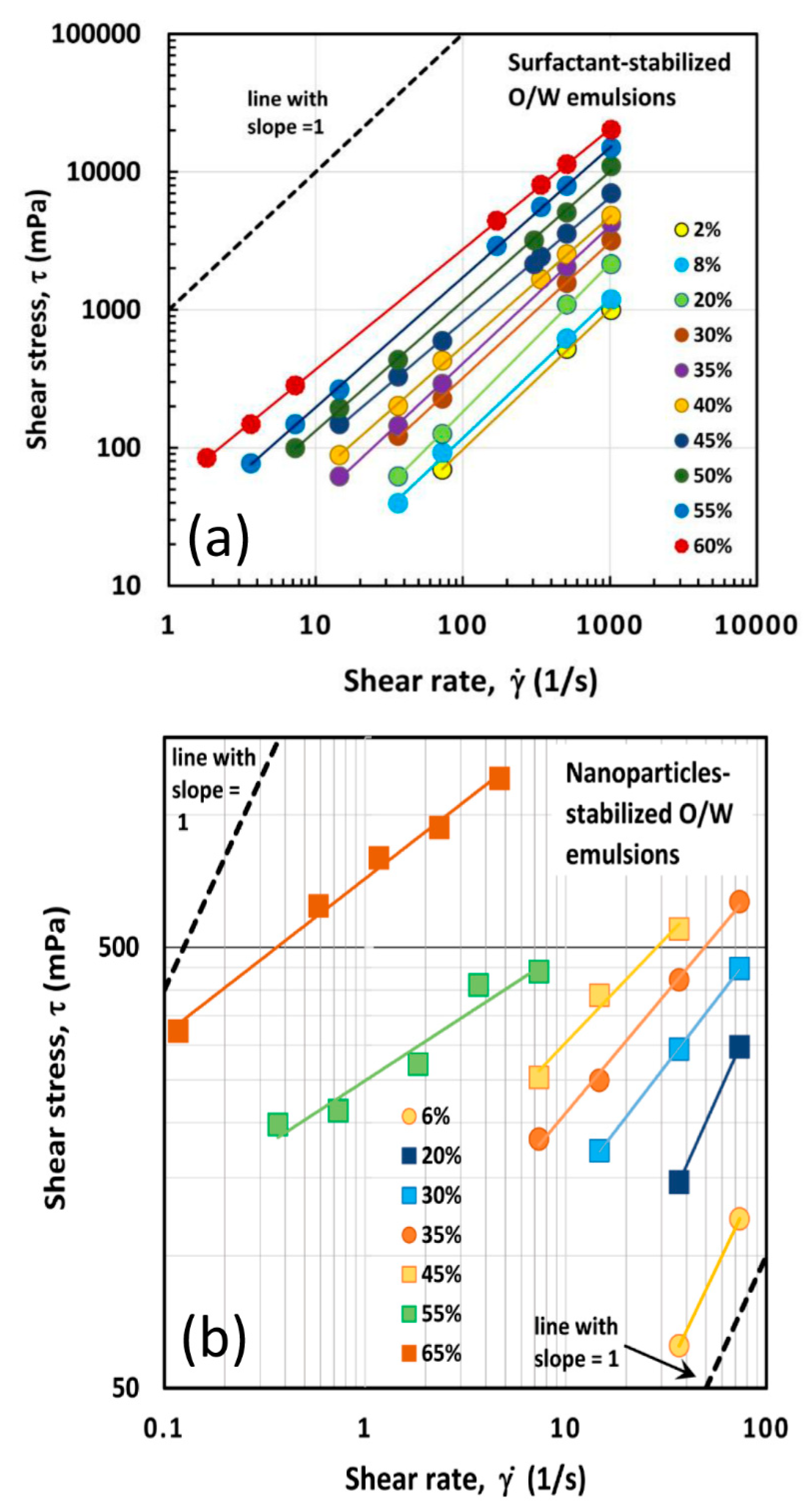
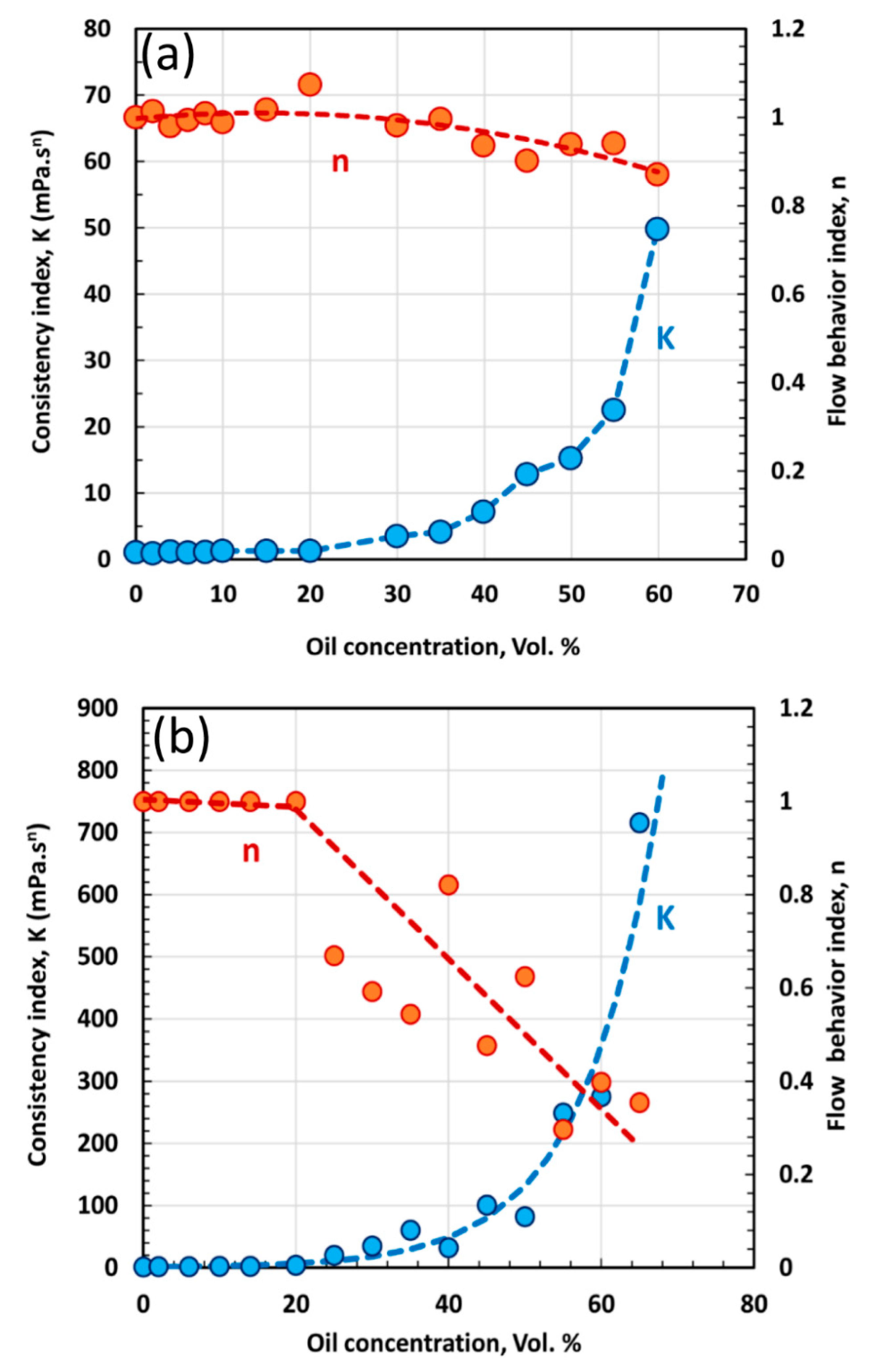
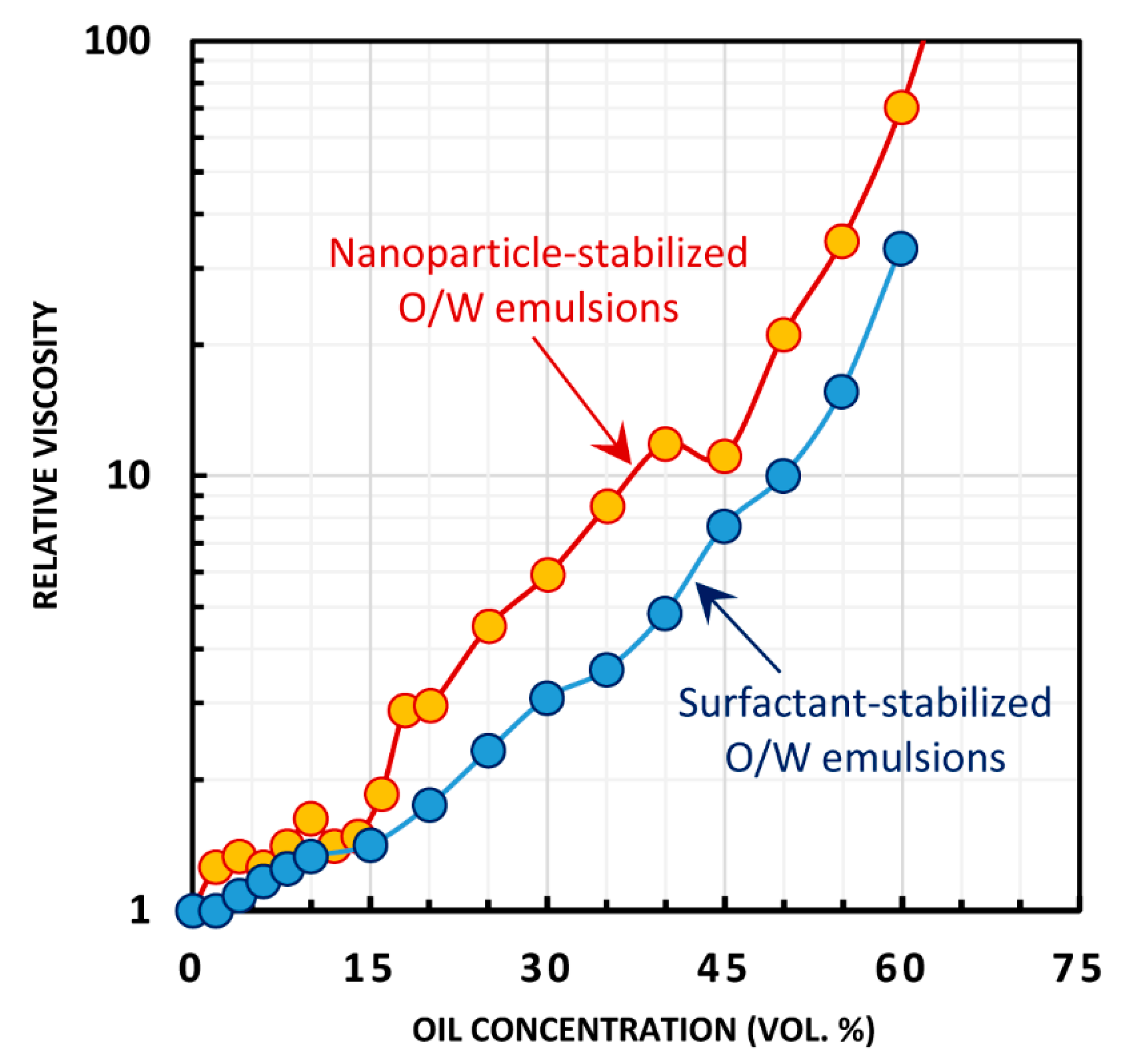
4.1. Rheological Behavior of Emulsions
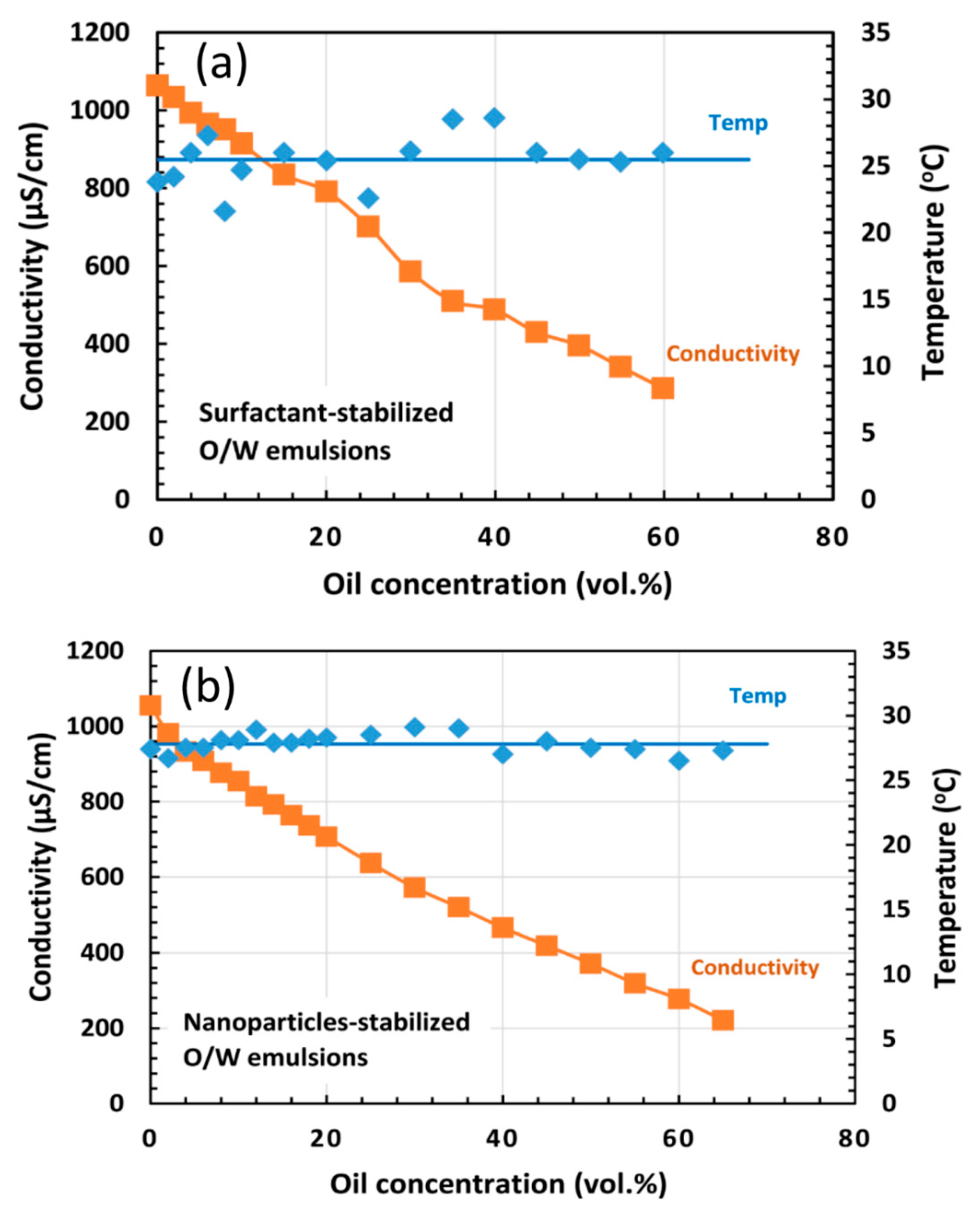
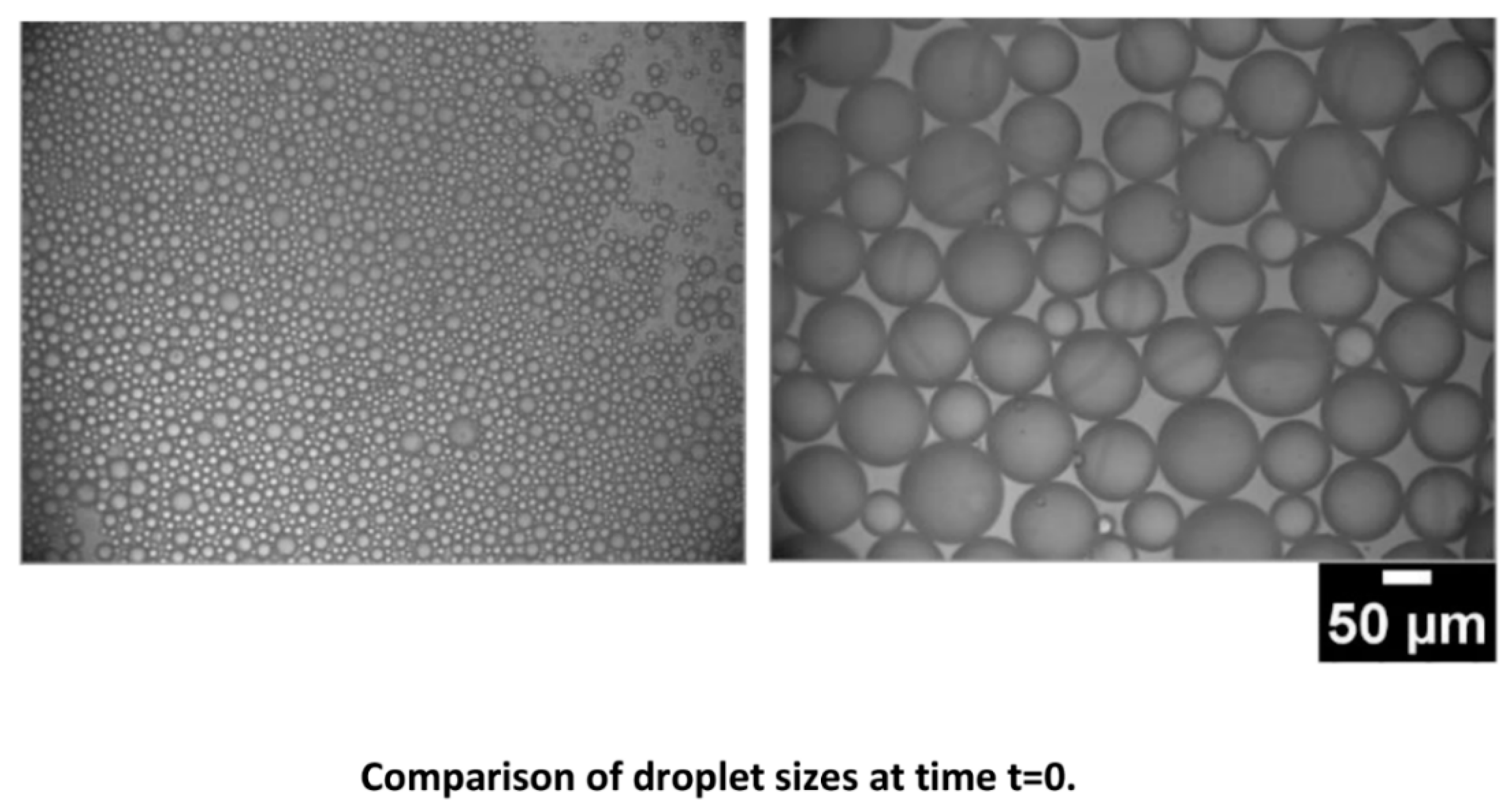
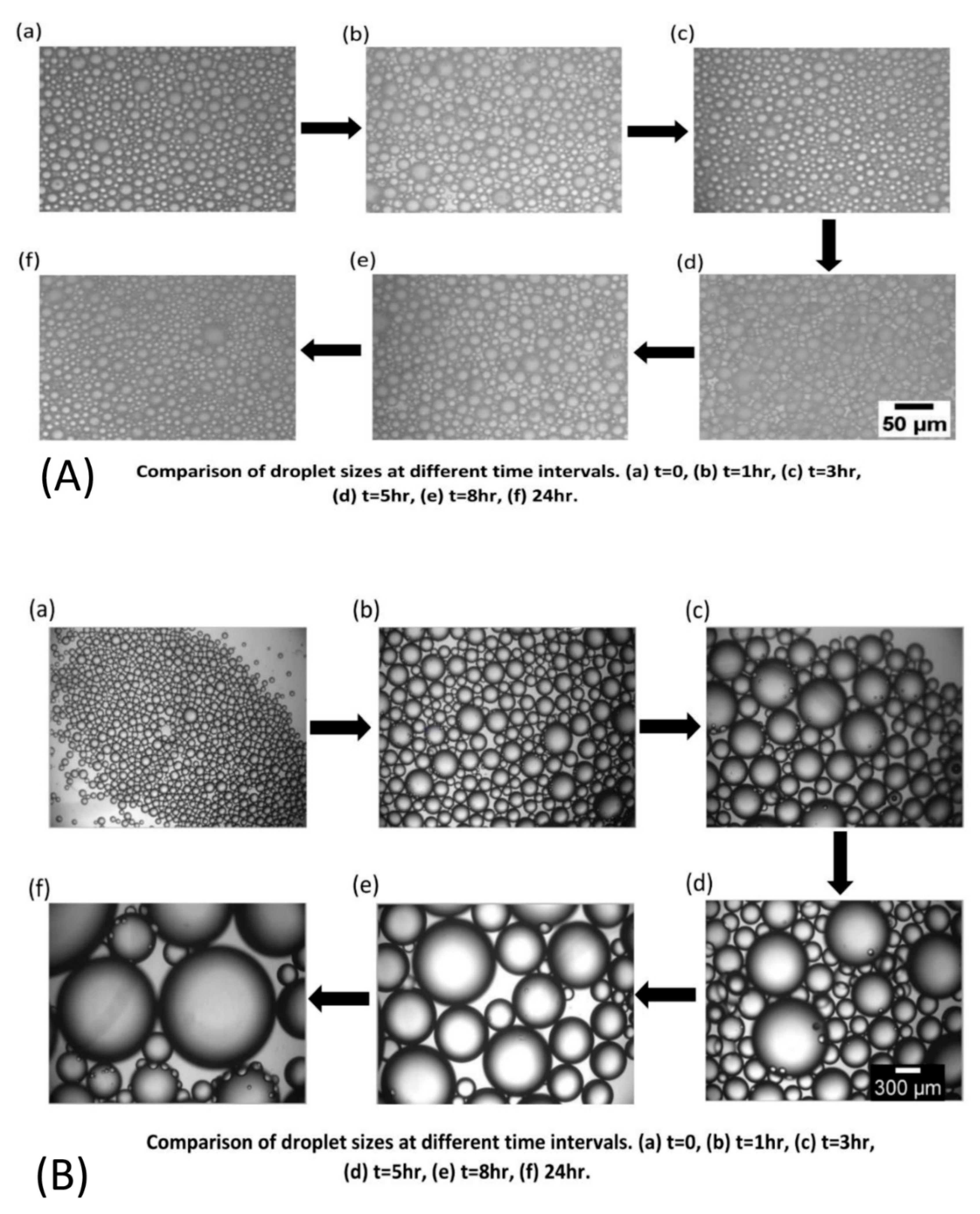
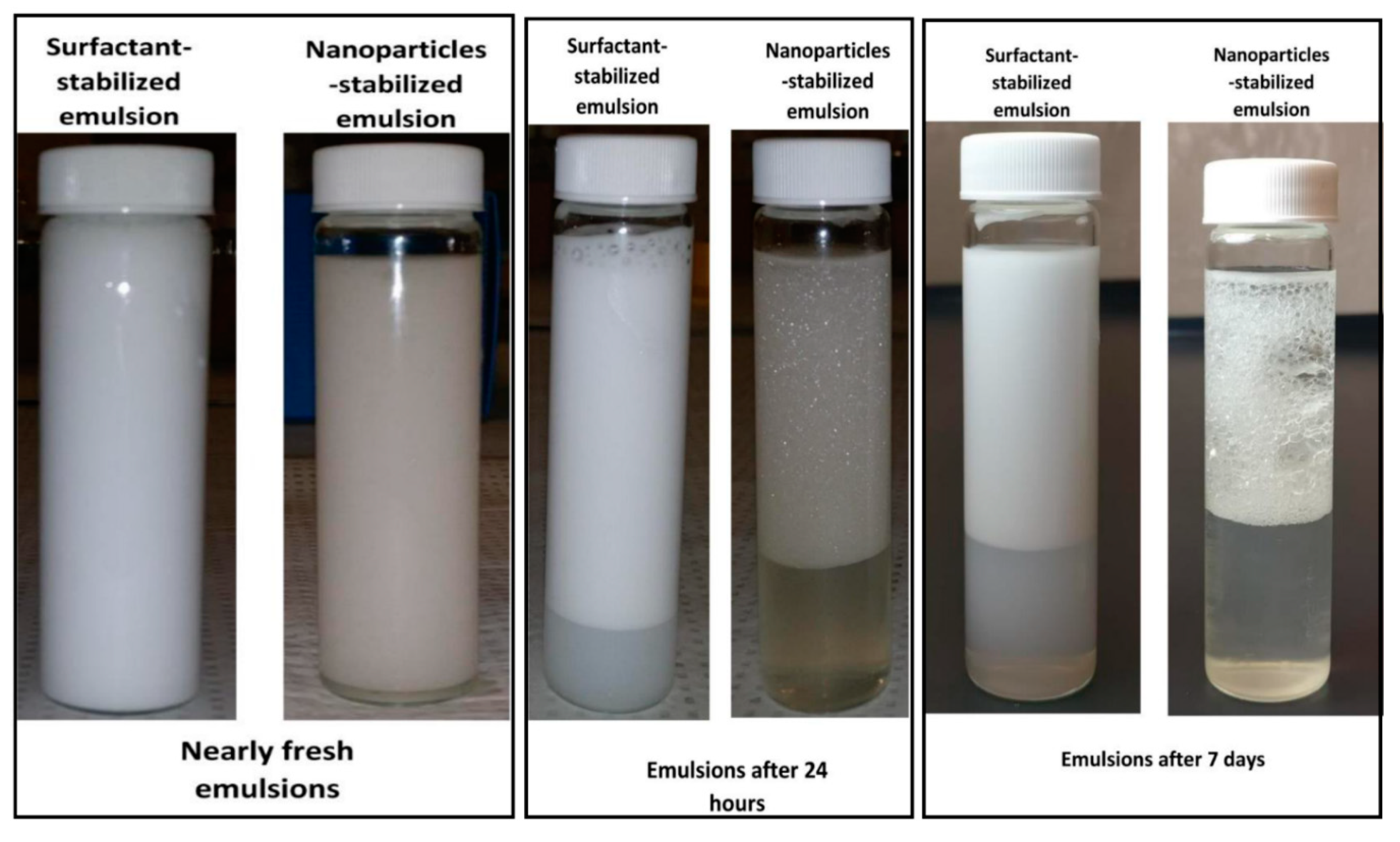
4.2. Emulsion Stability
4.3. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pal, R. Rheology of Particulate Dispersions and Composites; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Becher, P. Emulsions: Theory and Practice; Krieger Publishing Co.: Malabar, FL, USA, 1977. [Google Scholar]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An overview of Pickering emulsions: Solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Torrey, S. Emulsions and Emulsifier Applications—Recent Developments; Noyes Data Corporation: Park Ridge, NJ, USA, 1984. [Google Scholar]
- Lissant, K.J. Emulsions and Emulsion Technology; Marcel Dekker: New York, NY, USA, 1974. [Google Scholar]
- Friberg, S. Food Emulsions; Marcel Dekker: New York, NY, USA, 1976. [Google Scholar]
- Pal, R. Rheology of simple and multiple emulsions. Curr. Opin. Colloid Interface Sci. 2011, 16, 41–60. [Google Scholar] [CrossRef]
- Pal, R. Effect of droplet size on the rheology of emulsions. AIChE J. 1996, 42, 3181–3190. [Google Scholar] [CrossRef]
- Pal, R. Techniques for measuring the composition (oil and water content) of emulsions-a state of the art review. Colloids Surf. A Physicochem. Eng. Asp. 1994, 84, 141–193. [Google Scholar] [CrossRef]
- Bains, U. In-Situ Continuous Monitoring of Catastrophic Phase Inversion and Viscosity of Pickering Emulsions. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2018. [Google Scholar]
- Whorlow, R.W. Rheological Techniques; Ellis Horwood Ltd.: Chichester, UK, 1980. [Google Scholar]
- Ogunlaja, S.B.; Pal, R.; Sarikhani, K. Effect of starch nanoparticles on phase inversion of Pickering emulsions. Can. J. Chem. Eng. 2018, 96, 1089–1097. [Google Scholar] [CrossRef]
- Pal, R. Viscosity and storage/loss moduli for mixtures of fine and coarse emulsions. Chem. Eng. J. 1997, 67, 37–44. [Google Scholar] [CrossRef]
- Pal, R. Scaling of the relative viscosity of emulsions. J. Rheol. 1997, 41, 141–150. [Google Scholar] [CrossRef]
- Pal, R. Shear viscosity behavior of emulsions of two immiscible liquids. J. Colloid Interface Sci. 2000, 225, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Pal, R. Relative viscosity of non-Newtonian concentrated emulsions of non-colloidal droplets. Ind. Eng. Chem. Res. 2000, 39, 4933–4943. [Google Scholar] [CrossRef]
- Parkinson, C.; Matsumoto, S.; Sherman, P. The influence of particle-size distribution on the apparent viscosity of non-Newtonian dispersed systems. J. Colloid Interface Sci. 1970, 33, 150–160. [Google Scholar] [CrossRef]
- Chong, J.S.; Christiansen, E.B.; Baer, A.D. Rheology of concentrated suspensions. J. Appl. Poly. Sci. 1971, 15, 2007–2021. [Google Scholar] [CrossRef]
- Hoffman, R.L. Factors affecting the viscosity of unimodal and multimodal colloidal dispersions. J. Rheol. 1992, 36, 947–965. [Google Scholar] [CrossRef]
- Rodriguez, B.E.; Kaler, E.W.; Wolfe, M.S. Binary mixtures of monodisperse latex dispersions: Viscosity. Langmuir 1992, 8, 2382–2389. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Ventura, G.; Taddeucci, J. The effect of particle size on the rheology of liquid-solid mixtures with application to lava flows: Results from analogue experiments. Geochem. Geophys. Geosyst. 2013, 14, 2661–2669. [Google Scholar] [CrossRef]
- Pal, R. A simple model for the viscosity of Pickering emulsions. Fluids 2018, 3, 2. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bains, U.; Pal, R. In-Situ Continuous Monitoring of the Viscosity of Surfactant-Stabilized and Nanoparticles-Stabilized Pickering Emulsions. Appl. Sci. 2019, 9, 4044. https://doi.org/10.3390/app9194044
Bains U, Pal R. In-Situ Continuous Monitoring of the Viscosity of Surfactant-Stabilized and Nanoparticles-Stabilized Pickering Emulsions. Applied Sciences. 2019; 9(19):4044. https://doi.org/10.3390/app9194044
Chicago/Turabian StyleBains, Upinder, and Rajinder Pal. 2019. "In-Situ Continuous Monitoring of the Viscosity of Surfactant-Stabilized and Nanoparticles-Stabilized Pickering Emulsions" Applied Sciences 9, no. 19: 4044. https://doi.org/10.3390/app9194044
APA StyleBains, U., & Pal, R. (2019). In-Situ Continuous Monitoring of the Viscosity of Surfactant-Stabilized and Nanoparticles-Stabilized Pickering Emulsions. Applied Sciences, 9(19), 4044. https://doi.org/10.3390/app9194044




