Influence of Increasing Tungsten Concentrations and Soil Characteristics on Plant Uptake: Greenhouse Experiments with Zea mays
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Experimental Soils
2.3. Microcosm Experiment
2.4. Tungsten Analysis in Plants and Soil
2.5. Statistical Analysis
3. Results and Discussion
3.1. Biomass Production
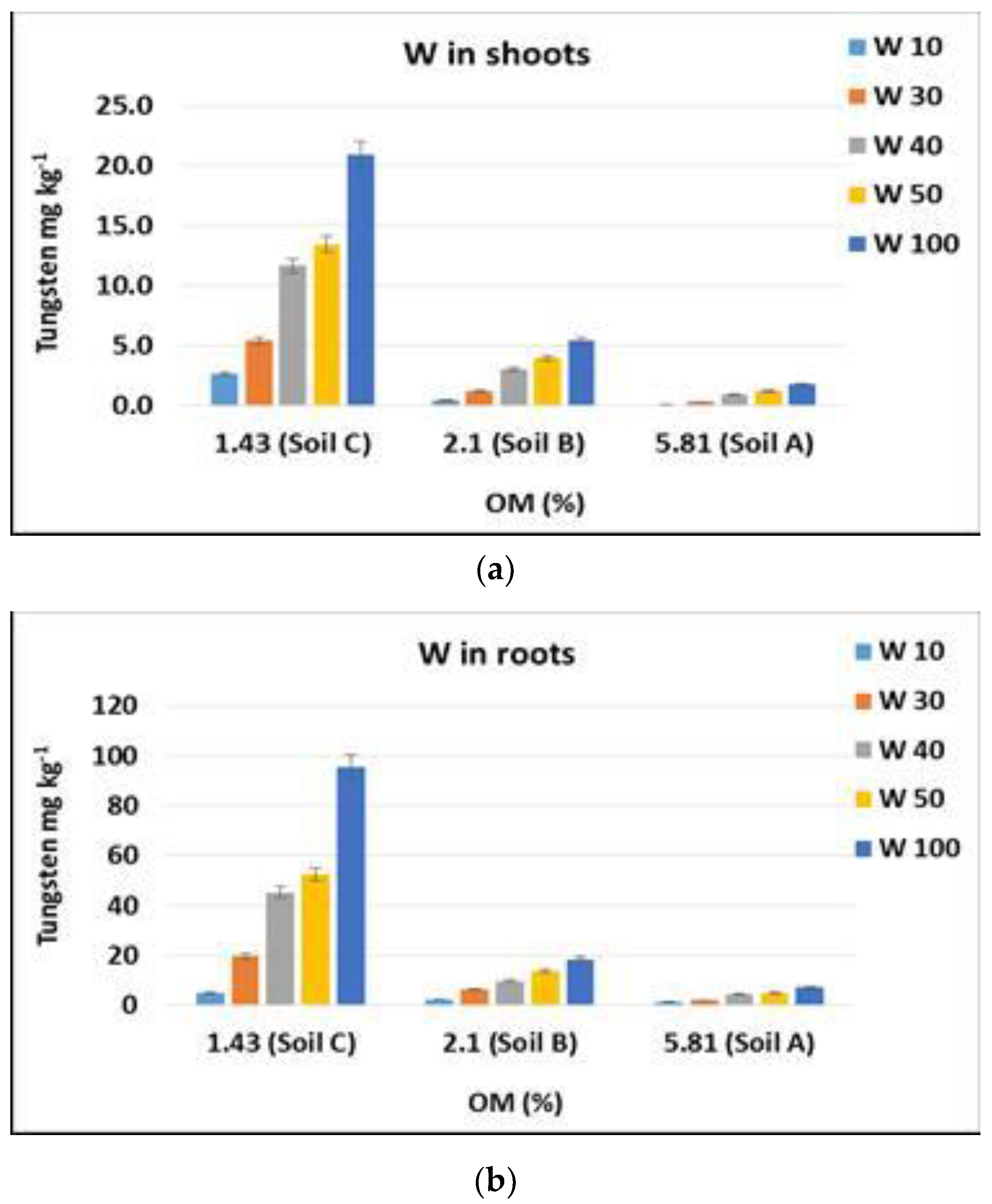
3.2. Tungsten Uptake by Plants
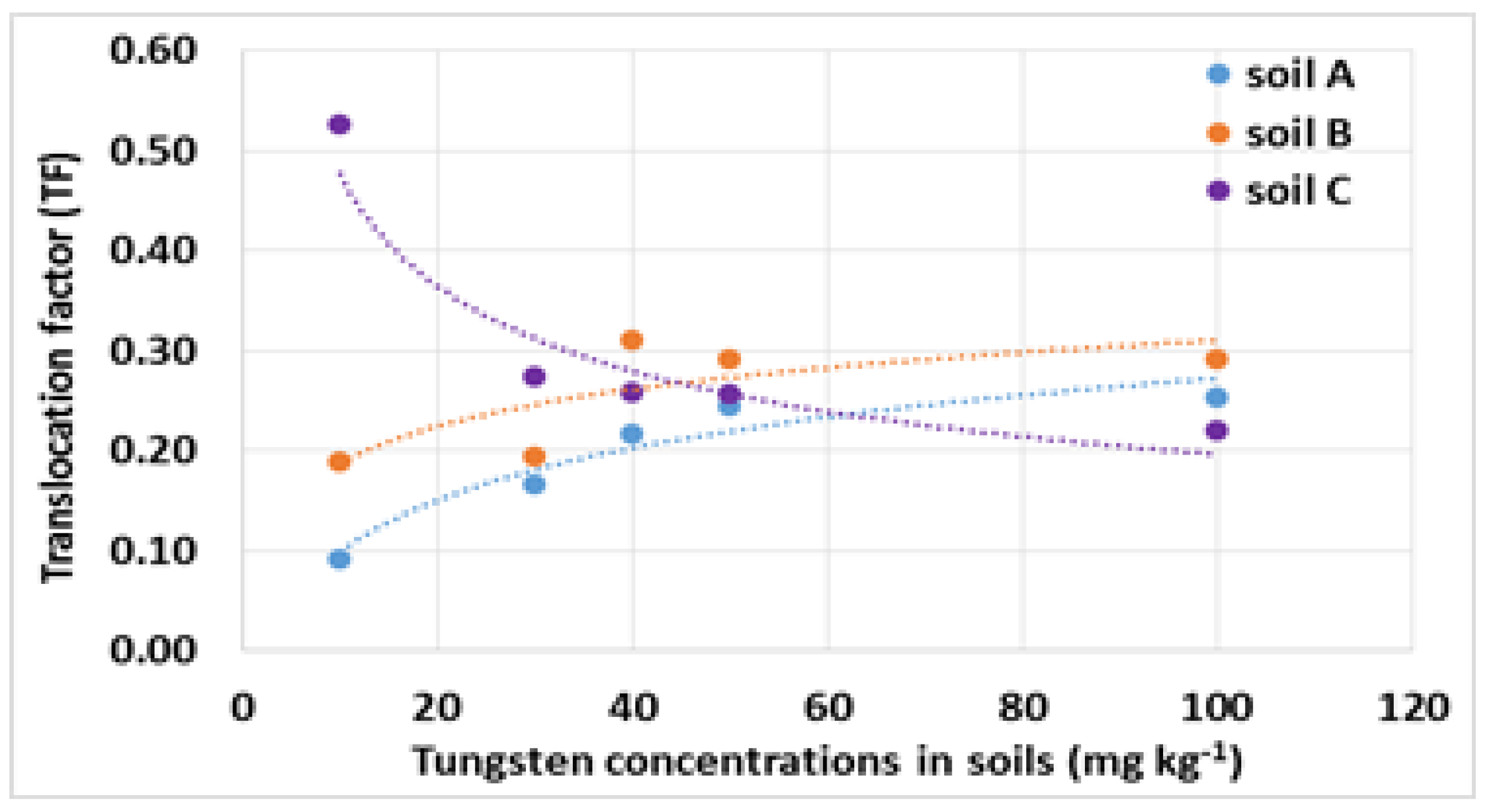
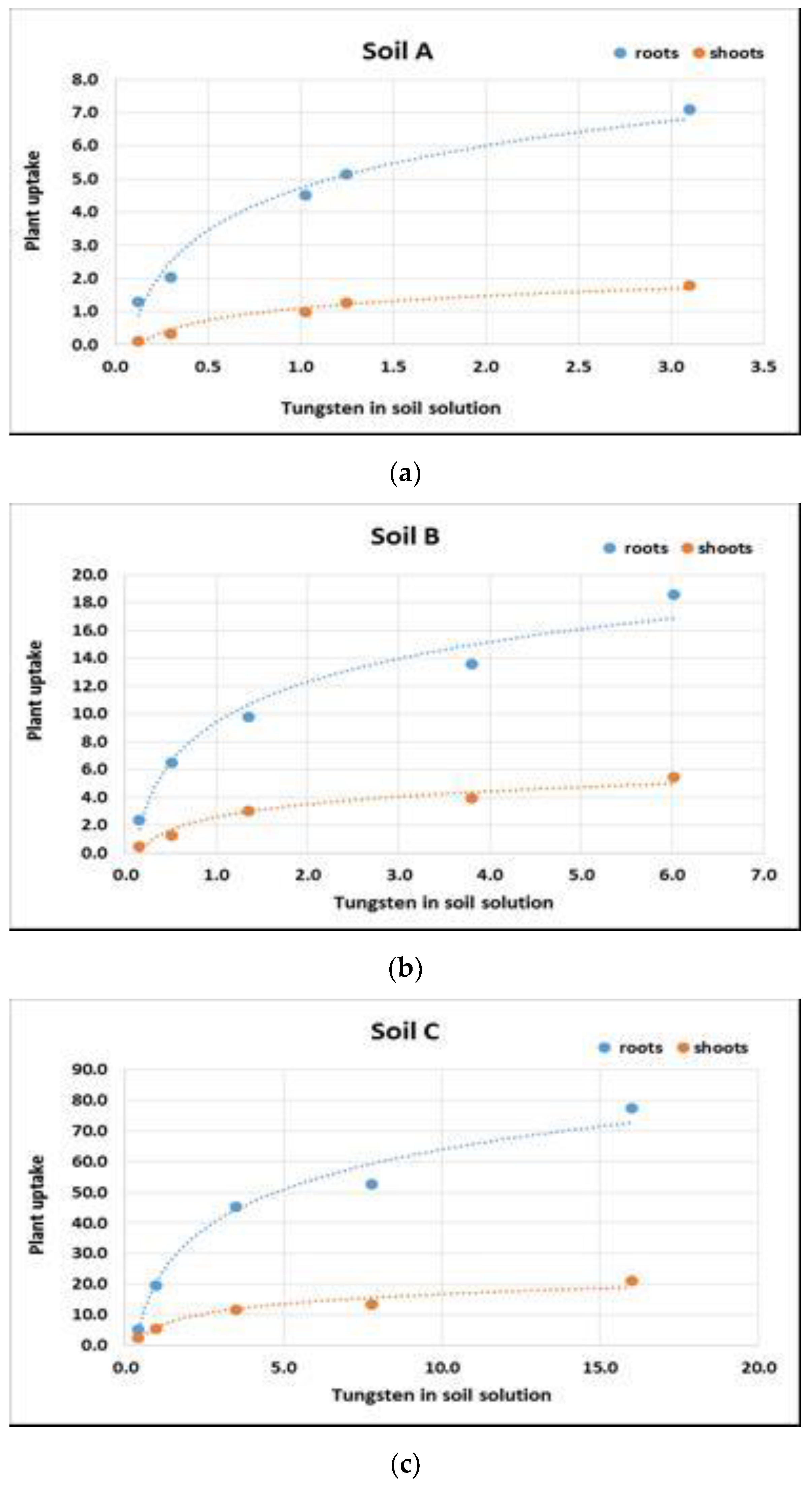
3.3. Influence of Soil Characteristics on Plant Uptake
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koutsospyros, A.D.; Braida, W.J.; Christodoulatos, C.; Dermatas, D.; Strigul, N.S. A review of tungsten: From environmental obscurity to scrutiny. J. Hazard. Mater. 2006, 136, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Koutsospyros, A.D.; Strigul, N.S.; Braida, W.J.; Christodoulatos, C. Tungsten: Environmental pollution and health effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, UK, 2011; pp. 418–426. [Google Scholar]
- Clausen, J.L.; Korte, N. Environmental fate of tungsten from military use. Sci. Tot. Environ. 2009, 407, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Technical Fact Sheet-Tungsten; Environmental Protection Agency: Washington, DC, USA, 2017.
- Kaiser, B.N.; Gridley, K.L.; Ngaire Brady, J.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Van der Voet, G.B.; Todorov, T.I.; Centeno, J.A.; Jonas, W.; Ives, J.; Mullick, F.G. Metals and Health: A Clinical Toxicological Perspective on Tungsten and Review of the Literature. Mil. Med. 2007, 172, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.D.R.; Lemaire, M.; Young, Y.K.; Eustache, J.H.; Guilbert, C.; Molina, M.F.; Mann, K.K. In vivo tungsten exposure alters B-cell development and increases DNA damage in murine bone marrow. Toxicol. Sci. 2013, 131, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Laulicht, F.; Brocato, J.; Cartularo, L.; Vaughan, J.; Wu, F.; Kluz, T.; Sun, H.; Oksuz, B.A.; Shen, S.; Peana, M.; et al. Tungsten-induced carcinogenesis in human bronchial epithelial cells. Toxicol. Appl. Pharm. 2015, 288, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Strigul, N. Does speciation matter for tungsten ecotoxicology? Ecotoxicol. Environ. Saf. 2010, 73, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.R.; Ridenour, G.; Speakman, R.J.; Witten, M.L. Elevated tungsten and cobalt in airborne particulates in Fallon, Nevada: Possible implications for the childhood leukemia cluster. Appl. Geochem. 2006, 21, 152–165. [Google Scholar] [CrossRef]
- Sheppard, P.R.; Speakman, R.J.; Ridenour, G.; Witten, M.L. Using lichen chemistry to assess airborne tungsten and cobalt in Fallon, Nevada. Environ. Monit. Assess. 2007, 130, 511–518. [Google Scholar] [CrossRef]
- Thomas, V.G.; Roberts, M.J.; Harrison, P.T. Assessment of the environmental toxicity and carcinogenicity of tungsten-based shot. Ecotoxicol. Environ. Saf. 2009, 72, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Ringelberg, D.B.; Reynolds, C.M.; Winfield, L.E.; Inouye, L.S.; Johnson, D.R.; Bednar, A.J. Tungsten effects on microbial community structure and activity in a soil. J. Environ. Qual. 2009, 38, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Strigul, N.; Koutsospyros, A.D.; Arienti, P.; Christodoulatos, C.; Dermatas, D.; Braida, W.J. Effects of tungsten on environmental systems. Chemosphere 2005, 61, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Senesi, N.; Padovano, G.; Brunetti, G. Scandium, titanium, tungsten and zirconium content in commercial inorganic fertilizers and their contribution to soil. Environ. Technol. Lett. 1988, 9, 1011–1020. [Google Scholar] [CrossRef]
- Wik, A.; Lycken, J.; Dave, G. Sediment quality assessment of road runoff detention systems in Sweden and the potential contribution of tire wear. Water Air Soil Pollut. 2008, 194, 301–314. [Google Scholar] [CrossRef]
- Bamford, J.E.; Butler, A.D.; Heim, K.E.; Pittinger, C.A.; Lemus, R.; Staveley, J.P.; Lee, K.B.; Venezia, C.; Pardus, M.J. Toxicity of sodium tungstate to earthworm, oat, radish and lettuce. Environ. Toxicol. Chem. 2011, 30, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.D.S.; Eleftheriou, E.P.; Rost, T.L. Effects of sodium tungstate on the ultrastructure and growth of pea (Pisum sativum) and cotton (Gossypium hirsutum) seedlings. Environ. Exp. Bot. 2008, 63, 416–425. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Panteris, E.; Eleftheriou, E.P. Tungsten affects the cortical microtubules of Pisum sativum root cells: Experiments on tungsten-molybdenum antagonism. Plant Biol. 2010, 12, 114–124. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Panteris, E.; Eleftheriou, E.P. The fatal effect of tungsten on Pisum sativum L. root cells: Indications for endoplasmic reticulum stress-induced programmed cell death. Planta 2011, 234, 21–34. [Google Scholar] [CrossRef]
- Pratas, J.; Prasad, M.N.V.; Freitas, H.; Conde, L. Plants growing in abandoned mines of Portugal are useful for exploration of arsenic, antimony, tungsten and mine reclamation. J. Geochem. Explor. 2005, 85, 99–107. [Google Scholar] [CrossRef]
- Wilson, B.; Pyatt, F.B. Bioavailability of tungsten in the vicinity of an abandoned mine in the English Lake District and some potential health implications. Sci. Total Environ. 2006, 370, 401–408. [Google Scholar] [CrossRef]
- Wilson, B.; Pyatt, F.B. Bioavailability of tungsten and associated metals in calcareous soils in the vicinity of an ancient metalliferous mine in the Corbieres area, Southwestern France. J. Toxicol. Environ. Heal. A 2009, 72, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, R.; Cheng, H.; Wang, J.; Shao, X. Tungsten Distribution in Soil and Rice in the Vicinity of the World’s Largest and Longest-Operating Tungsten Mine in China. PLoS ONE 2014, 9, e91981. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Heilmeier, H.; Hartung, W. Abscisic acid relations of plants grown on tungsten enriched substrates. Plant Soil 2007, 301, 37–49. [Google Scholar] [CrossRef]
- Johnson, D.R.; Inouye, L.S.; Bednar, A.J.; Clarke, J.U.; Winfield, L.E.; Boyd, R.E.; Ang, C.Y. Tungsten bioavailability and toxicity in sunflowers (Helianthus annuus). Land Contam. Reclamat. 2009, 17, 141–151. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America Book Series; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America book series; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis. Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America Book Series; Soil Science Society of America Inc.: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Oburger, E.; Vergara Cid, C.; Preiner, J.; Hu, J.; Hann, S.; Wanek, W.; Richter, A. pH-Dependent Bioavailability, Speciation, and Phytotoxicity of Tungsten (W) in Soil Affect Growth and Molybdoenzyme Activity of Nodulated Soybeans. Environ. Sci. Technol. 2018, 52, 6146–6156. [Google Scholar] [CrossRef] [PubMed]
- Poykio, R.; Torvela, H.; Peramaki, P.; Kuokkanen, T.; Ronkkomaki, H. Comparison of dissolution methods for multielement analysis of some plant materials used as bioindicator of sulphur and heavy metal deposition determined by ICP-AES and ICPMS. Analusis 2000, 28, 850–854. [Google Scholar] [CrossRef]
- Dermatas, D.; Braida, W.; Christodoulatos, C.; Strigul, N.; Panikov, N.; Los, M.; Larson, S. Solubility, sorption, and soil respiration effects of tungsten and tungsten alloys. Environ. Forensics 2004, 5, 5–13. [Google Scholar] [CrossRef]
- Bednar, A.J.; Jones, W.T.; Chappell, M.A.; Johnson, D.R.; Ringelberg, D.B. A modified acid digestion procedure for extraction of tungsten from soil. Talanta 2010, 80, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Aery, N.C. Effect of tungsten on growth, biochemical constituents, molybdenum and tungsten contents in wheat. Plant Soil Environ. 2011, 57, 519–525. [Google Scholar] [CrossRef]
- Kumar, A.; Aery, N.C. Effect of tungsten on growth, dry-matter production, and biochemical constituents of cowpea. Commun. Soil Sci. Plan. 2012, 43, 1098–1107. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Panteris, E.; Eleftheriou, E.P. Tungsten toxicity in plants. Plants 2012, 1, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Clemente, R.; Nicholas, W.L. Tungsten. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer Science and Business Media: Dordrecht, The Netherlands, 2013; pp. 559–564. [Google Scholar]
- Semhi, K.; Boutin, R.; Nallusamy, S.; Al Busaidi, W.; Al Hamdi, A.; Al Dhafri, K.; Al Busaidi, A. Impact of a Variable Tungsten Pollution on the Elemental Uptake of Two Plant Species. Water Air Soil Pollut. 2018, 229, 294. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci. Total Environ. 2008, 398, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P. Modelling molybdate and tungstate adsorption to ferrihydrite. Chem. Geol. 2003, 200, 105–115. [Google Scholar] [CrossRef]
- Bednar, A.J.; Boyd, R.E.; Jones, W.T.; McGrath, C.J.; Johnson, D.R.; Chappell, M.A.; Ringelberg, D.B. Investigations of tungsten mobility in soil using column tests. Chemosphere 2009, 75, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Sen Tuna, G.; Braida, W. Evaluation of the adsorption of mono- and poly- tungstates onto different types of clay minerals and Pahokee peat. Soil Sediment Contamin. 2014, 23, 838–849. [Google Scholar] [CrossRef]
- Thomas Arrigo, L.K.; Mikutta, C.; Byrne, J.; Kappler, A.; Kretzschmar, R. Iron(II)-catalyzed iron atom exchange and mineralogical changes in Iron-rich organic freshwater flocs: An Iron isotope tracer study. Environ. Sci. Technol. 2017, 51, 6897–6907. [Google Scholar] [CrossRef]
- Karimian, N.; Johnston, S.G.; Burton, E.D. Antimony and arsenic behavior during Fe(II)-induced transformation of Jarosite. Environ. Sci. Technol. 2017, 51, 4259–4268. [Google Scholar] [CrossRef]
- Karimian, N.; Johnston, S.G.; Burton, E.D. Antimony and arsenic partitioning during Fe2+-induced transformation of jarosite under acidic conditions. Chemosphere 2018, 195, 515–523. [Google Scholar] [CrossRef]
- Karimian, N.; Burton, E.D.; Johnston, S.G.; Hockmann, K.; Choppala, G. Humic acid impacts antimony partitioning and speciation during iron(II)-induced ferrihydrite transformation. Sci. Total Environ. 2019, 683, 399–410. [Google Scholar] [CrossRef]
- Krauss, M.; Wilcke, W.; Kobza, J.; Zech, W. Predicting heavy metals transfer from soil to plant: Potential use of Freundlich-type functions. J. Plant Nutr. Soil Sci. 2002, 165, 3–8. [Google Scholar] [CrossRef]
- Han, F.X.; Su, Y.; Monts, D.L.; Waggoner, C.A.; Plodinec, M.J. Binding, distribution, and plant uptake of mercury in a soil from Oak Ridge, Tennessee, USA. Sci. Total Environ. 2006, 368, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Kalis, E.J.J.; Temminghoff, E.J.M.; Visser, A.; Van Riemsdijk, W.H. Metal uptake by Lolium perenne in contaminated soils using a four-step approach. Environ. Toxicol. Chem. 2007, 26, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Blanco Rodriguez, P.; Vera Tomé, F.; Lozano, J.C. About the assumption of linearity in soil-to-plant transfer factors for uranium and thorium isotopes and 226Ra. Sci. Total Environ. 2002, 284, 167–175. [Google Scholar] [CrossRef]
- Tuovinen, T.S.; Roivainen, P.; Makkonen, S.; Kolehmainen, M.; Holopainen, T.; Juutilainen, J. Soil-to-plant transfer of elements is not linear: Results for five elements relevant to radioactive waste in five boreal forest species. Sci. Total Environ. 2011, 410, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fantke, P.; Charles, R.; de Alencastro, L.F.; Friedrich, R.; Jolliet, O. Plant uptake of pesticides and human health: Dynamic modeling of residues in wheat and ingestion intake. Chemosphere 2011, 85, 1639–1647. [Google Scholar] [CrossRef]
- Trapp, S.; Eggen, T. Simulation of the plant uptake of organophosphates and other emerging pollutants for greenhouse experiments and field conditions. Environ. Sci. Pollut. Res. 2013, 20, 4018–4029. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Escudey, M.; Chang, A.C.; Chen, W.; Arancibia-Miranda, N. Trace element uptake dynamics for maize (Zea mays L.) grown under field conditions. Plant Soil 2013, 370, 471–483. [Google Scholar] [CrossRef]
- Liang, Z.; Ding, Q.; Wei, D.; Li, J.; Chen, S.; Ma, Y. Major controlling factors and predictions for cadmium transfer from the soil into spinach plants. Ecotoxicol. Environ. Saf. 2013, 93, 180–185. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Applicability of a Freundlich-Like Model for Plant Uptake at an Industrial Contaminated Site with a High Variable Arsenic Concentration. Environments 2017, 4, 67. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F. Tungstate adsorption onto Italian soils with different characteristics. Environ. Monit. Assess. 2017, 189, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Bostick, B.C.; Sun, J.; Landis, J.D.; Clausen, J.L. Tungsten Speciation and Solubility in Munitions-Impacted Soils. Environ. Sci. Technol. 2018, 52, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Cruywagen, J.J. Protonation, oligomerization, and condensation reactions of vanadate (V), molybdate (VI). In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press Inc.: San Diego, CA, USA, 2000; pp. 127–182. [Google Scholar]
- Davantès, A.; Costa, D.; Lefèvre, G. Infrared study of (poly)tungstate ions in solution and sorbed into layered double hydroxides: Vibrational calculations and in situ analysis. J. Phys. Chem. C 2015, 119, 12356–12364. [Google Scholar] [CrossRef]
- Karimian, N.; Johnston, S.G.; Burton, E.D. Iron and sulfur cycling in acid sulfate soil wetlands under dynamic redox conditions: A review. Chemosphere 2018, 197, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Vero, S.E.; Hettiarachchi, G.M.; Johannesson, K. Tungsten Contamination of Soils and Sediments: Current State of Science. Curr. Pollut. Rep. 2017, 3, 55–64. [Google Scholar] [CrossRef]
- USEPA. Emerging Contaminant-Tungsten. Technical Fact Sheet; Environmental Protection Agency: Washington, DC, USA, 2008.
- Karimian, N.; Johnston, S.G.; Burton, E.D. Acidity generation accompanying iron and sulfur transformations during drought simulation of freshwater re-flooded acid sulphate soils. Geoderma 2017, 285, 117–131. [Google Scholar] [CrossRef]
- O’Connor, G.A.; Brobst, R.B.; Chaney, R.L.; Kincaid, R.L.; Mcdowell, L.R.; Pierzynski, G.M.; Rubin, A.; Riper, G.V. A modified risk assessment to establish molybdenum standards for land application of biosolids. J. Environ. Qual. 2001, 30, 1490–1507. [Google Scholar] [CrossRef]


| Soil | pH | OM (%) | Clay (%) | Silt (%) | Sand (%) | Total W (mg kg−1) |
|---|---|---|---|---|---|---|
| A | 4.50 | 5.81 | 7.10 | 24.8 | 67.6 | 0.38 |
| B | 5.80 | 2.10 | 12.5 | 28.8 | 59.3 | 0.44 |
| C | 7.40 | 1.43 | 7.65 | 14.0 | 78.3 | 0.29 |
| W in soil (mg kg−1) | Shoot | Root | ||||
|---|---|---|---|---|---|---|
| Soil A | Soil B | Soil C | Soil A | Soil B | Soil C | |
| CT | 283 ± 13.2a | 279 ± 10.4a | 285 ± 13.8a | 118 ± 7.2a | 116 ± 12.4a | 126 ± 9.0a |
| 10 | 281 ± 14.6a | 275 ± 14.7a | 295 ± 19.3a | 115 ± 9.1a | 114 ± 10.1a | 124 ± 8.3a |
| 30 | 276 ± 15.1a | 257 ± 14.1a | 278 ± 15.4a | 123 ± 4.3a | 117 ± 7.1a | 118 ± 7.6a |
| 40 | 263 ± 11.2a | 263 ± 12.9a | 251 ± 16.1a | 129 ± 8.6a | 121 ± 8.6a | 122 ± 8.1a |
| 50 | 284 ± 18.6a | 259 ± 10.1a | 245 ± 18.5a | 110 ± 9.6a | 125 ± 5.9a | 119 ± 7.4a |
| 100 | 251 ± 16.3a | 269 ± 16.5a | 269 ± 13.6a | 119 ± 5.1a | 116 ± 4.9a | 115 ± 5.9a |
| W in Soil (mg kg−1) | Shoot | Root | ||||
|---|---|---|---|---|---|---|
| Soil A | Soil B | Soil C | Soil A | Soil B | Soil C | |
| 10 | 0.12 ± 0.02a | 0.45 ± 0.04b | 2.69 ± 0.02c | 1.30 ± 0.07a | 2.38 ± 0.6b | 5.10 ± 0.4c |
| 30 | 0.34 ± 0.08a | 1.26 ± 0.3b | 5.42 ± 0.4c | 2.04 ± 0.3a | 6.50 ± 0.9b | 19.8 ± 1.1c |
| 40 | 0.98 ± 0.07a | 3.05 ± 0.5b | 11.7 ± 0.8c | 4.51 ± 0.4a | 9.80 ± 2.3b | 45.3 ± 3.8c |
| 50 | 1.26 ± 0.08a | 3.98 ± 0.3b | 13.5 ± 0.6c | 5.15 ± 0.8a | 13.6 ± 2.1b | 52.6 ± 3.0c |
| 100 | 1.80 ± 0.04a | 5.43 ± 0.6b | 21.0 ± 1.3 c | 7.10 ± 0.9a | 18.6 ± 4.2b | 95.5 ± 6.1c |
| Soil | Shoots | Roots | ||||
|---|---|---|---|---|---|---|
| Log K | 1/n | R2 | Log K | 1/n | R2 | |
| A | −2.17 | 1.26 | 0.922 | −0.718 | 0.791 | 0.896 |
| B | −1.48 | 1.15 | 0.932 | −0.5357 | 0.933 | 0.937 |
| C | −0.522 | 0.936 | 0.942 | −0.4886 | 1.24 | 0.944 |
| Soil | Shoots | Roots | ||||
|---|---|---|---|---|---|---|
| Log K | 1/n | R2 | Log K | 1/n | R2 | |
| A | −0.066 | 0.863 | 0.970 | 0.619 | 0.553 | 0.988 |
| B | 0.260 | 0.670 | 0.961 | 0.874 | 0.528 | 0.959 |
| C | 0.6992 | 0.534 | 0.973 | 1.14 | 0.685 | 0.968 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzzelli, G.; Pedron, F. Influence of Increasing Tungsten Concentrations and Soil Characteristics on Plant Uptake: Greenhouse Experiments with Zea mays. Appl. Sci. 2019, 9, 3998. https://doi.org/10.3390/app9193998
Petruzzelli G, Pedron F. Influence of Increasing Tungsten Concentrations and Soil Characteristics on Plant Uptake: Greenhouse Experiments with Zea mays. Applied Sciences. 2019; 9(19):3998. https://doi.org/10.3390/app9193998
Chicago/Turabian StylePetruzzelli, Gianniantonio, and Francesca Pedron. 2019. "Influence of Increasing Tungsten Concentrations and Soil Characteristics on Plant Uptake: Greenhouse Experiments with Zea mays" Applied Sciences 9, no. 19: 3998. https://doi.org/10.3390/app9193998
APA StylePetruzzelli, G., & Pedron, F. (2019). Influence of Increasing Tungsten Concentrations and Soil Characteristics on Plant Uptake: Greenhouse Experiments with Zea mays. Applied Sciences, 9(19), 3998. https://doi.org/10.3390/app9193998





