Feasibility of the Detection of Carrageenan Adulteration in Chicken Meat Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging
Abstract
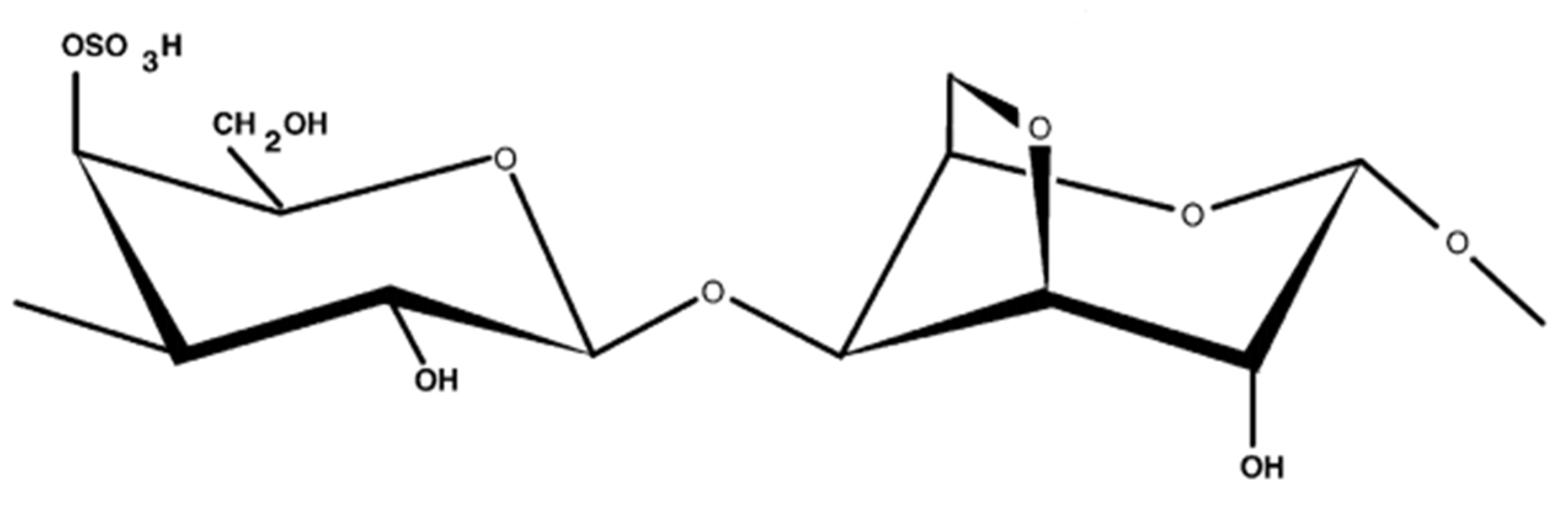
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Hyperspectral Images Acquisition and Calibration
2.3. Image Processing and Spectral Data Extraction
2.4. Establishment and Evaluation of the Models
2.4.1. Data Preprocessing and Models Development
2.4.2. Principal Component Analysis
2.4.3. Optimal Wavelengths Selection
2.4.4. Modeling Performance Evaluation
2.5. Visualization of Adulteration Levels
3. Results and Discussion
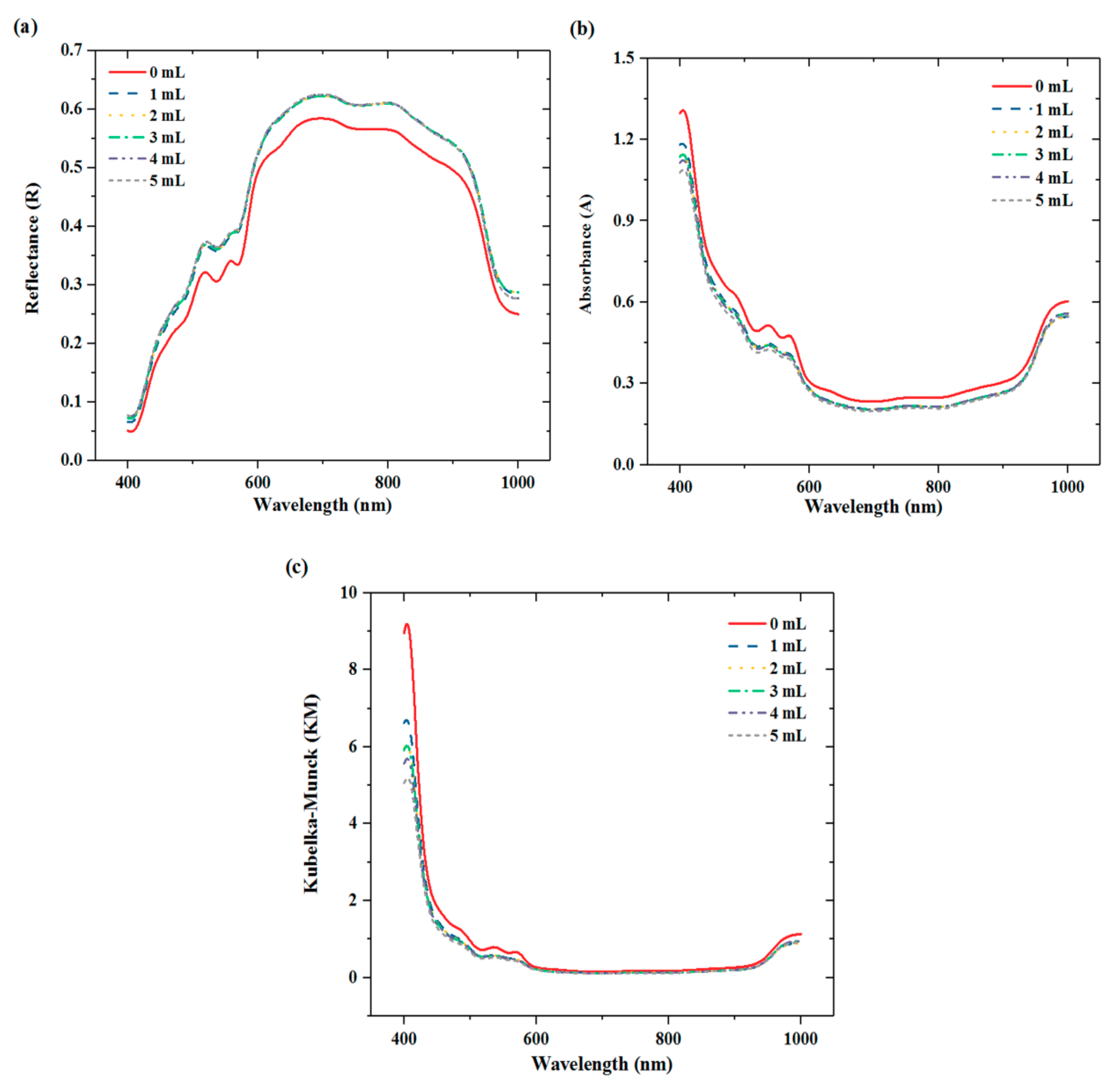
3.1. Spectral Features
3.2. PLSR Models Based on Full Spectra
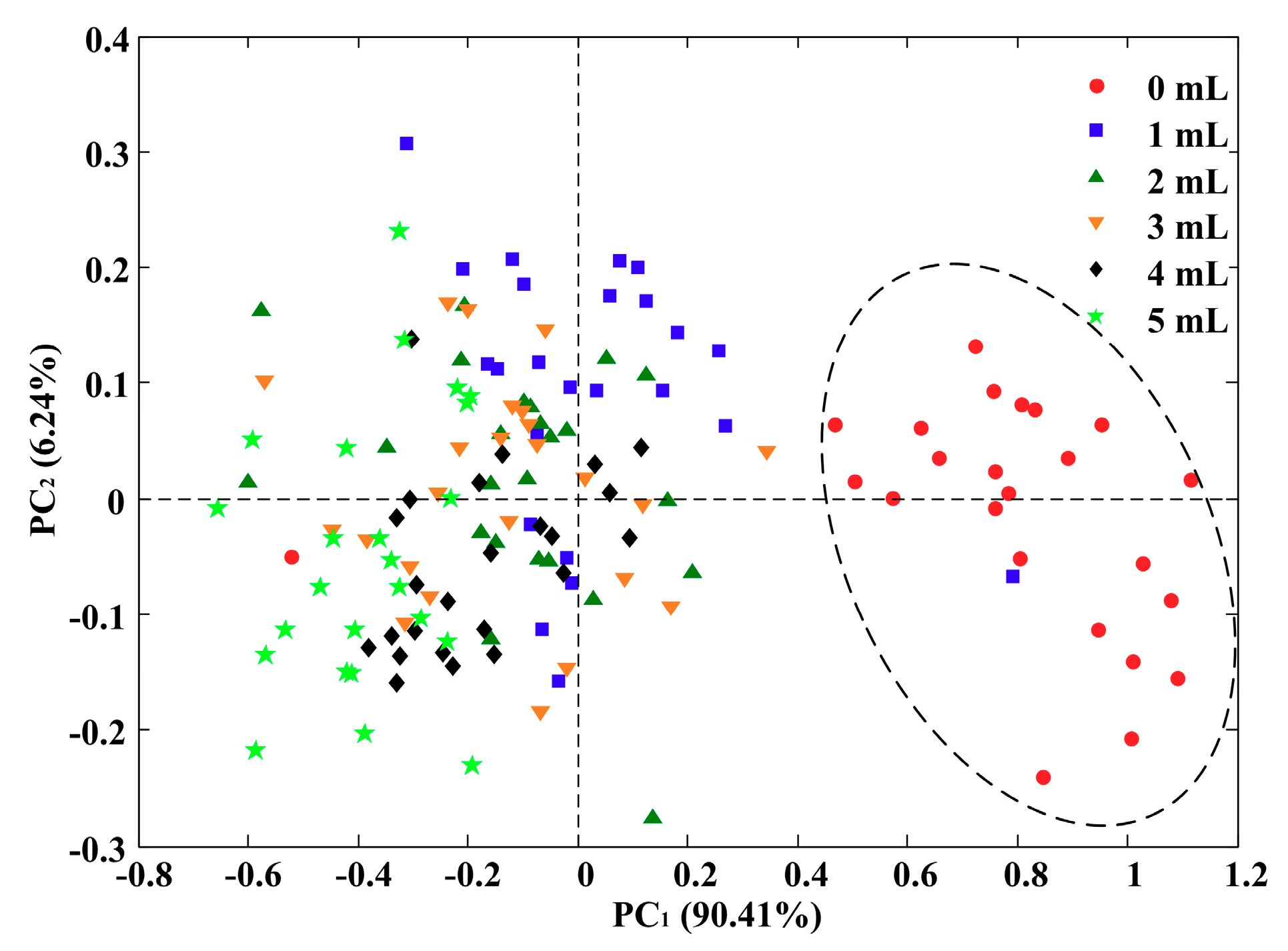
3.3. Principal Component Analysis
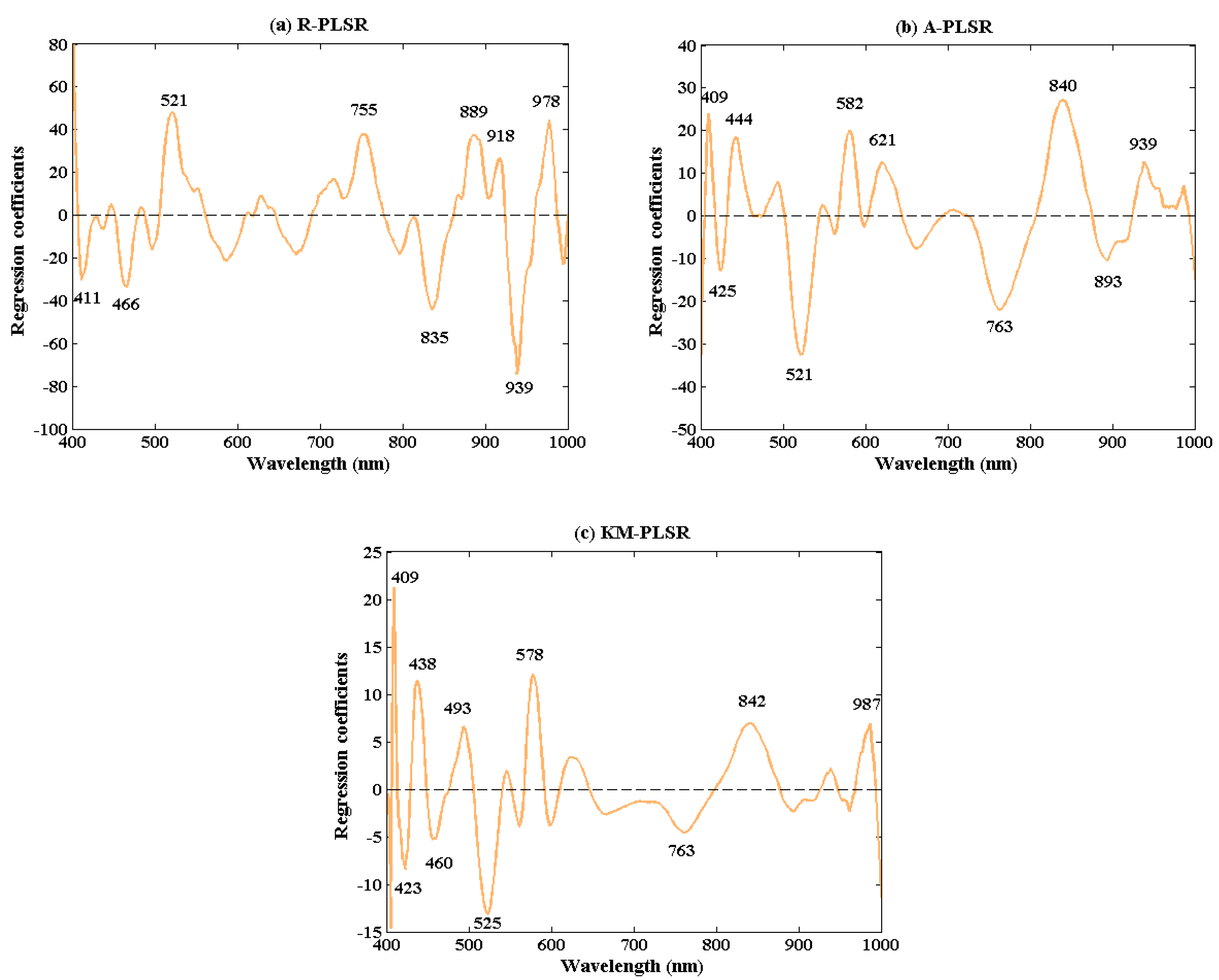
3.4. Wavelengths Selection
3.5. Multispectral Analysis
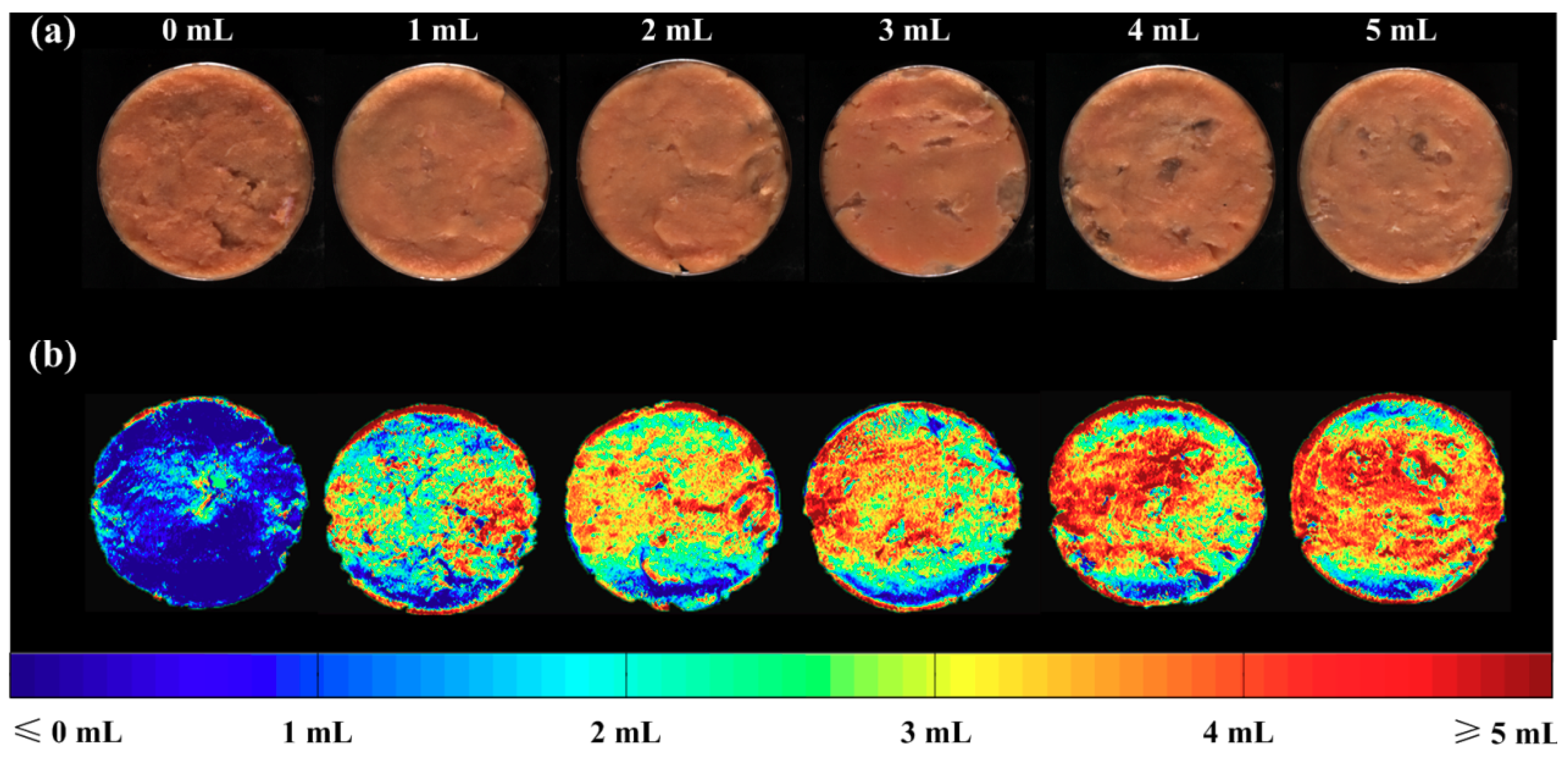
3.6. Visualization of Carrageenan Adulteration Levels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahmati, S.; Julkapli, N.M.; Yehye, W.A.; Wan, J.B. Identification of meat origin in food products—A review. Food Control. 2016, 68, 379–390. [Google Scholar] [CrossRef]
- Ballin, N.Z. Authentication of meat and meat products. Meat Sci. 2010, 86, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.E.; Stewart, H. Changing consumer demands create opportunities for U.S. food system. Food Rev. 2002, 25, 18–23. [Google Scholar]
- Barbut, S. Poultry Products Processing: An Industry Guide; CRC Press, Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2016. [Google Scholar]
- Chicken Seller Injecting Water into Poultry to Profit More. Available online: https://rumble.com/v31p57-chicken-seller-injecting-water-into-poultry-to-profit-more.html (accessed on 14 September 2016).
- Yang, S.; Wang, L.; Zhang, H.; Fu, Q. Strengthening market supervision and management, resolutely banning the illegal business phenomenon of selling “gel-injected chicken”. Meat Hyg. 2002, 10, 3–23. (In Chinese) [Google Scholar]
- Yasin, H.; Babji, A.S.; Ismail, H. Optimization and rheological properties of chicken ball as affected by κ-carrageenan, fish gelatin and chicken meat. LWT Food Sci. Technol. 2016, 66, 79–85. [Google Scholar] [CrossRef]
- Gayo, J.; Hale, S.A.; Blanchard, S.M. Quantitative analysis and detection of adulteration in crab meat usingvisible and near-infrared spectroscopy. J. Agric. Food Chem. 2006, 54, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Schmutzler, M.; Beganovic, A.; Böhler, G.; Huck, C.W. Methods for detection of pork adulteration in veal product based on FT-NIR spectroscopy for laboratory, industrial and on-site analysis. Food Control 2015, 57, 258–267. [Google Scholar] [CrossRef]
- Jiang, H.; Zhuang, H.; Sohn, M.; Wang, W. Measurement of soy contents in ground beef using near-infrared spectroscopy. Appl. Sci. 2017, 7, 97. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part I: Fundamentals. Innov. Food Sci. Emerg. Technol. 2013, 19, 1–14. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Sun, D.W.; Elmasry, G.; Allen, P. Fast detection and visualization of minced lamb meat adulteration using NIR hyperspectral imaging and multivariate image analysis. Talanta 2013, 103, 130–136. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S.; Liu, S. Assessment of visible near-infrared hyperspectral imaging as a tool for detection of horsemeat adulteration in minced beef. Food Bioprocess. Technol. 2015, 8, 1054–1062. [Google Scholar] [CrossRef]
- Ropodi, A.I.; Panagou, E.Z.; Nychas, G.J.E. Multispectral imaging (MSI): A promising method for the detection of minced beef adulteration with horsemeat. Food Control 2017, 73, 57–63. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Hyperspectral imaging in tandem with multivariate analysis and image processing for non-invasive detection and visualization of pork adulteration in minced beef. Anal. Methods 2015, 7, 7496–7502. [Google Scholar] [CrossRef]
- Ropodi, A.I.; Pavlidis, D.E.; Mohareb, F.; Panagou, E.Z.; Nychas, G.J.E. Multispectral image analysis approach to detect adulteration of beef and pork in raw meats. Food Res. Int. 2015, 67, 12–18. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Rapid and non-destructive detection of chicken adulteration in minced beef using visible near-infrared hyperspectral imaging and machine learning. J. Food Eng. 2016, 170, 8–15. [Google Scholar] [CrossRef]
- Wu, D.; Shi, H.; He, Y.; Yu, X.; Bao, Y. Potential of hyperspectral imaging and multivariate analysis for rapid and non-invasive detection of gelatin adulteration in prawn. J. Food Eng. 2013, 119, 680–686. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Sun, D.W. Determination of total viable count (TVC) in chicken breast fillets by near-infrared hyperspectral imaging and spectroscopic transforms. Talanta 2013, 105, 244–249. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Sun, D.W. Inspection of harmful microbial contamination occurred in edible salmon flesh using imaging technology. J. Food Eng. 2015, 150, 82–89. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, W.; Zhuang, H.; Yoon, S.Y.; Yang, Y.; Zhao, X. Hyperspectral imaging for a rapid detection and visualization of duck meat adulteration in beef. Food Anal. Methods 2019, 12, 2205–2215. [Google Scholar] [CrossRef]
- Wang, W.; Heitschmidt, G.W.; Ni, X.; Windham, W.R.; Hawkins, S.; Chu, X. Identification of aflatoxin B1 on maize kernel surfaces using hyperspectral imaging. Food Control 2014, 42, 78–86. [Google Scholar] [CrossRef]
- Menesatti, P.; Zanella, A.; D’Andrea, S.; Costa, C.; Paglia, G.; Pallottino, F. Supervised multivariate analysis of hyper-spectral NIR images to evaluate the starch index of apples. Food Bioprocess. Technol. 2009, 2, 308–314. [Google Scholar] [CrossRef]
- Qin, J.; Burks, T.F.; Kim, M.S.; Chao, K.; Ritenour, M.A. Citrus canker detection using hyperspectral reflectance imaging and PCA-based image classification method. Sens. Instrum. Food Qual. 2008, 2, 168–177. [Google Scholar] [CrossRef]
- He, H.J.; Wu, D.; Sun, D.W. Potential of hyperspectral imaging combined with chemometric analysis for assessing and visualising tenderness distribution in raw farmed salmon fillets. J. Food Eng. 2014, 126, 156–164. [Google Scholar] [CrossRef]
- Alexandrakis, D.; Downey, G.; Scannell, A.G.M. Rapid non-destructive detection of spoilage of intact chicken breast muscle using near-infrared and Fourier transform mid-infrared spectroscopy and multivariate statistics. Food Bioprocess. Technol. 2012, 5, 338–347. [Google Scholar] [CrossRef]
- Kapper, C.; Klont, R.E.; Verdonk, J.; Urlings, H. Prediction of pork quality with near infrared spectroscopy (NIRS): 1. Feasibility and robustness of NIRS measurements at laboratory scale. Meat Sci. 2012, 91, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Chen, Y.R. Two-dimensional correlation spectroscopy study of visible and near-infrared spectral variations of chicken meats in cold storage. Appl. Spectrosc. 2000, 54, 1458–1470. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W. Application of visible and near infrared hyperspectral imaging for non-invasively measuring distribution of water-holding capacity in salmon flesh. Talanta 2013, 116, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Bowker, B.; Hawkins, S.; Zhuang, H. Measurement of water-holding capacity in raw and freeze-dried broiler breast meat with visible and near-infrared spectroscopy. Poult. Sci. 2014, 93, 1834–1841. [Google Scholar] [CrossRef]
- Yousefi, B.; Sojasi, S.; Castanedo, C.I.; Maldague, X.P.; Beaudoin, G.; Chamberland, M. Comparison assessment of low rank sparse-PCA based-clustering/classification for automatic mineral identification in long wave infrared hyperspectral imagery. Infrared Phys. Technol. 2018, 93, 103–111. [Google Scholar] [CrossRef]
- Shah, C.A.; Arora, M.K.; Varshney, P.K. Unsupervised classification of hyperspectral data: An ICA mixture model based approach. Int. J. Remote Sens. 2004, 25, 481–487. [Google Scholar] [CrossRef]
- Buddenbaum, H.; Schlerf, M.; Hill, J. Classification of coniferous tree species and age classes using hyperspectral data and geostatistical methods. Int. J. Remote Sens. 2005, 26, 5453–5465. [Google Scholar] [CrossRef]
- Jiang, H.; Yoon, S.C.; Zhuang, H.; Wang, W.; Li, Y.; Yang, Y. Integration of spectral and textural features of visible and near-infrared hyperspectral imaging for differentiating between normal and white striping broiler breast meat. Spectrochim. Acta Part A 2019, 213, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Kamruzzaman, M.; Sun, D.W. Selection of feature wavelengths for developing multispectral imaging systems for quality, safety and authenticity of muscle foods-a review. Trends Food Sci. Technol. 2015, 45, 86–104. [Google Scholar] [CrossRef]
- Moghaddam, T.M.; Razavi, S.M.A.; Taghizadeh, M. Applications of hyperspectral imaging in grains and nuts quality and safety assessment: A review. J. Food Meas. Charact. 2013, 7, 129–140. [Google Scholar] [CrossRef]
- Achata, E.M.; Inguglia, E.S.; Esquerre, C.A.; Tiwari, B.K.; O’Donnell, C.P. Evaluation of Vis-NIR hyperspectral imaging as a process analytical tool to classify brined pork samples and predict brining salt concentration. J. Food Eng. 2019, 246, 134–140. [Google Scholar] [CrossRef]
- Singh, C.B.; Jayas, D.S.; Paliwal, J.; White, N.D.G. Detection of insect-damaged wheat kernels using near-infrared hyperspectral imaging. J. Stored Prod. Res. 2009, 45, 151–158. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xiao, H.; Gu, X.; Pan, L.; Tu, K. Hyperspectral imaging detection of decayed honey peaches based on their chlorophyll content. Food Chem. 2017, 235, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yoon, S.C.; Zhuang, H.; Wang, W.; Lawrence, K.C.; Yang, Y. Tenderness classification of fresh broiler breast fillets using visible and near-infrared hyperspectral imaging. Meat Sci. 2018, 139, 82–90. [Google Scholar] [CrossRef]
- Barbin, D.; Elmasry, G.; Sun, D.W.; Allen, P. Near-infrared hyperspectral imaging for grading and classification of pork. Meat Sci. 2012, 90, 259–268. [Google Scholar] [CrossRef]
- Jiang, H.; Yoon, S.C.; Zhuang, H.; Wang, W.; Li, Y.; Lu, C.; Li, N. Non-destructive assessment of final color and pH attributes of broiler breast fillets using visible and near-infrared hyperspectral imaging: A preliminary study. Infrared Phys. Technol. 2018, 92, 309–317. [Google Scholar] [CrossRef]
- Li, W.; Chen, C.; Su, H.; Du, Q. Local binary patterns and extreme learning machine for hyperspectral imagery classification. IEEE Trans. Geosci. Electron. 2015, 53, 3681–3693. [Google Scholar] [CrossRef]
- Chen, Y.; Nasrabadi, N.M.; Tran, T.D. Hyperspectral image classification using dictionary-based sparse representation. IEEE Trans. Geosci. Electron. 2011, 49, 3973–3985. [Google Scholar] [CrossRef]
- Chen, Y.; Nasrabadi, N.M.; Tran, T.D. Sparse representation for target detection in hyperspectral imagery. IEEE J. Sel. Top. Signal. Process. 2011, 5, 629–640. [Google Scholar] [CrossRef]
| Model | Preprocessings | LVs | Calibration | Cross-validation | Prediction | RPD | |||
|---|---|---|---|---|---|---|---|---|---|
| Rc2 | RMSEC | Rcv2 | RMSECV | Rp2 | RMSEP | ||||
| R-PLSR | None | 12 | 0.93 | 0.46 | 0.89 | 0.57 | 0.87 | 0.68 | 4.37 |
| MSC | 14 | 0.94 | 0.40 | 0.90 | 0.54 | 0.86 | 0.66 | 4.51 | |
| SNV | 15 | 0.95 | 0.37 | 0.91 | 0.52 | 0.86 | 0.64 | 4.66 | |
| SNV + Detrend | 14 | 0.94 | 0.40 | 0.90 | 0.54 | 0.86 | 0.65 | 4.56 | |
| Der1 | 17 | 0.97 | 0.28 | 0.93 | 0.45 | 0.90 | 0.56 | 5.32 | |
| Der2 | 13 | 0.98 | 0.25 | 0.93 | 0.44 | 0.90 | 0.57 | 5.30 | |
| A-PLSR | None | 16 | 0.96 | 0.35 | 0.92 | 0.49 | 0.91 | 0.52 | 5.70 |
| MSC | 16 | 0.97 | 0.32 | 0.93 | 0.46 | 0.92 | 0.48 | 6.17 | |
| SNV | 16 | 0.97 | 0.32 | 0.93 | 0.47 | 0.92 | 0.48 | 6.18 | |
| SNV + Detrend | 16 | 0.96 | 0.32 | 0.93 | 0.47 | 0.92 | 0.49 | 6.11 | |
| Der1 | 14 | 0.96 | 0.32 | 0.93 | 0.45 | 0.91 | 0.53 | 5.67 | |
| Der2 | 14 | 0.97 | 0.29 | 0.92 | 0.49 | 0.93 | 0.49 | 6.11 | |
| KM-PLSR | None | 15 | 0.96 | 0.34 | 0.92 | 0.49 | 0.89 | 0.64 | 4.64 |
| MSC | 15 | 0.96 | 0.34 | 0.92 | 0.49 | 0.88 | 0.64 | 4.64 | |
| SNV | 15 | 0.96 | 0.32 | 0.93 | 0.46 | 0.88 | 0.70 | 4.26 | |
| SNV + Detrend | 15 | 0.96 | 0.33 | 0.93 | 0.47 | 0.88 | 0.65 | 4.60 | |
| Der1 | 15 | 0.96 | 0.35 | 0.92 | 0.49 | 0.89 | 0.66 | 4.50 | |
| Der2 | 14 | 0.95 | 0.37 | 0.89 | 0.57 | 0.89 | 0.59 | 5.04 | |
| Model | Wavelengths (nm) | Calibration | Cross-validation | Prediction | RPD | |||
|---|---|---|---|---|---|---|---|---|
| Rc2 | RMSEC | Rcv2 | RMSECV | Rp2 | RMSEP | |||
| RC-R-PLSR | 411, 466, 521, 755, 835, 889, 918, 939, 978 | 0.89 | 0.56 | 0.87 | 0.61 | 0.85 | 0.99 | 3.02 |
| RC-A-PLSR | 409, 425, 444, 521, 582, 621, 763, 840, 893, 939 | 0.89 | 0.55 | 0.87 | 0.62 | 0.85 | 0.93 | 3.20 |
| RC-KM-PLSR | 409, 423, 438, 460, 493, 525, 578, 763, 842, 987 | 0.89 | 0.57 | 0.85 | 0.65 | 0.84 | 0.99 | 3.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, H.; Wang, W. Feasibility of the Detection of Carrageenan Adulteration in Chicken Meat Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging. Appl. Sci. 2019, 9, 3926. https://doi.org/10.3390/app9183926
Zhang Y, Jiang H, Wang W. Feasibility of the Detection of Carrageenan Adulteration in Chicken Meat Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging. Applied Sciences. 2019; 9(18):3926. https://doi.org/10.3390/app9183926
Chicago/Turabian StyleZhang, Yue, Hongzhe Jiang, and Wei Wang. 2019. "Feasibility of the Detection of Carrageenan Adulteration in Chicken Meat Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging" Applied Sciences 9, no. 18: 3926. https://doi.org/10.3390/app9183926
APA StyleZhang, Y., Jiang, H., & Wang, W. (2019). Feasibility of the Detection of Carrageenan Adulteration in Chicken Meat Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging. Applied Sciences, 9(18), 3926. https://doi.org/10.3390/app9183926






