Mechanical Properties of Tonalite Subjected to Combined Effects of Chemical Corrosion and Freeze-Thaw Cycles
Abstract
:1. Introduction
2. Experimental Setup
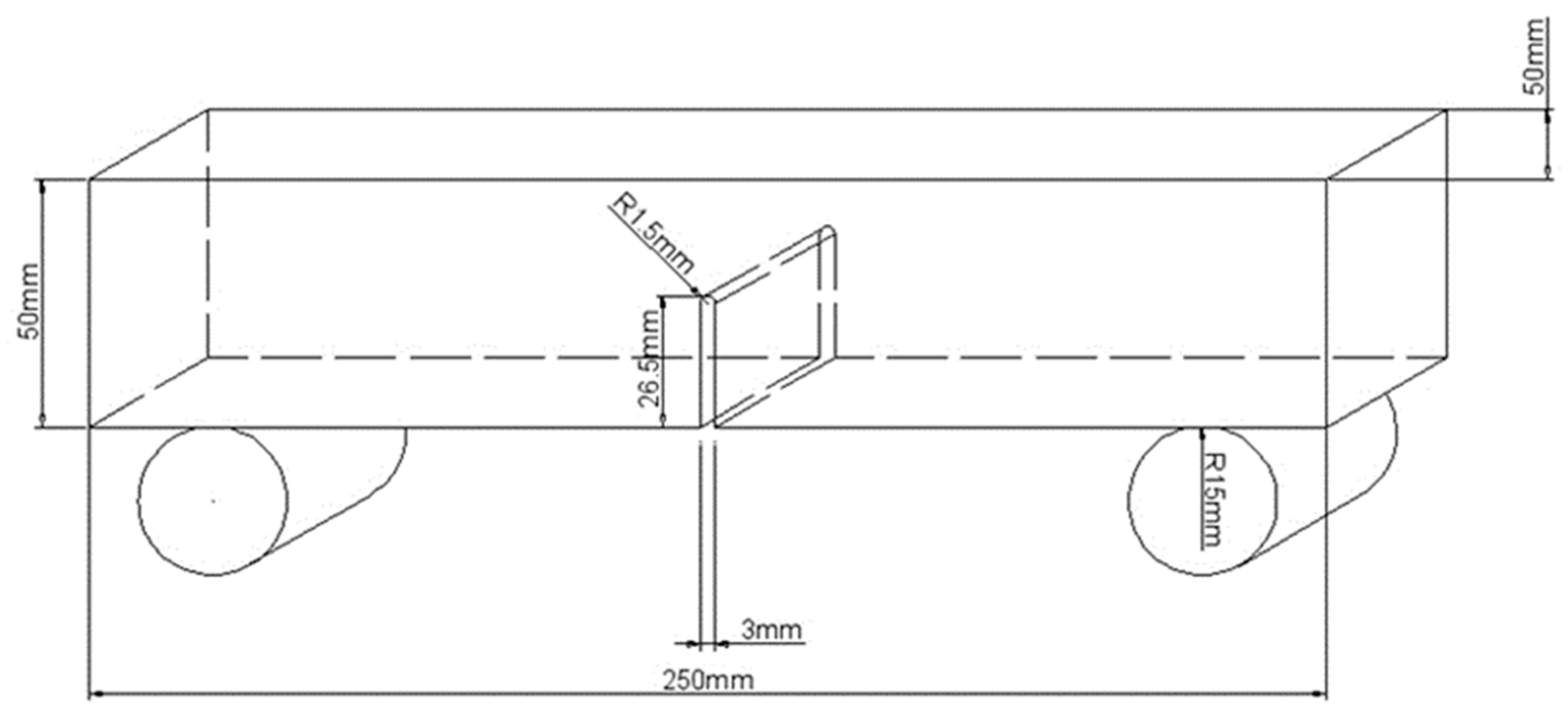
2.1. Specimen Preparation
2.2. Physical Properties
- (1)
- Dry weight, dry density and saturated density determined by saturation and caliper method.
- (2)
- The pore volume values and the porosity were obtained by the water saturation procedure.
2.3. Equipment
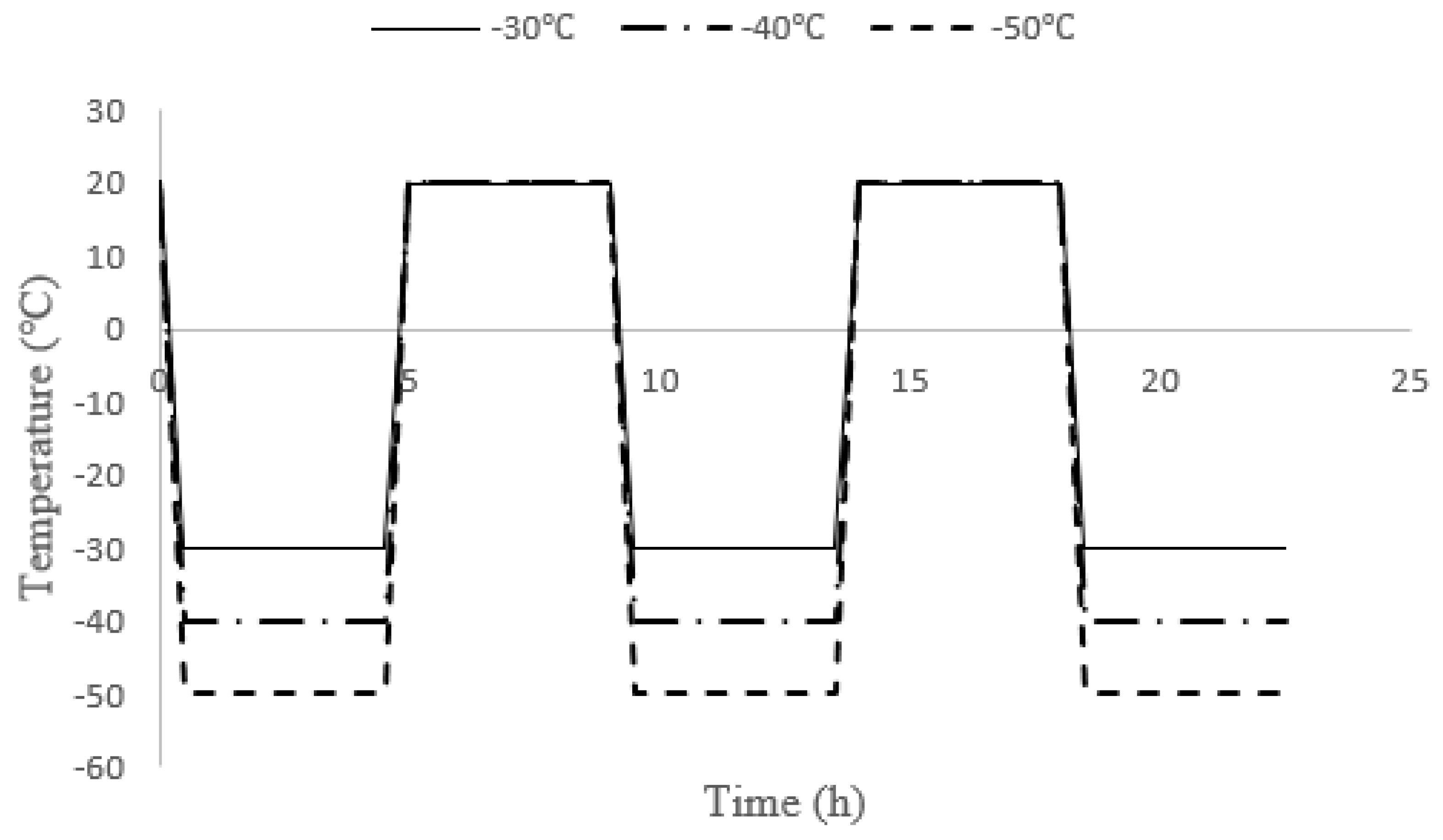
2.4. Test Procedure
3. Results and Analysis
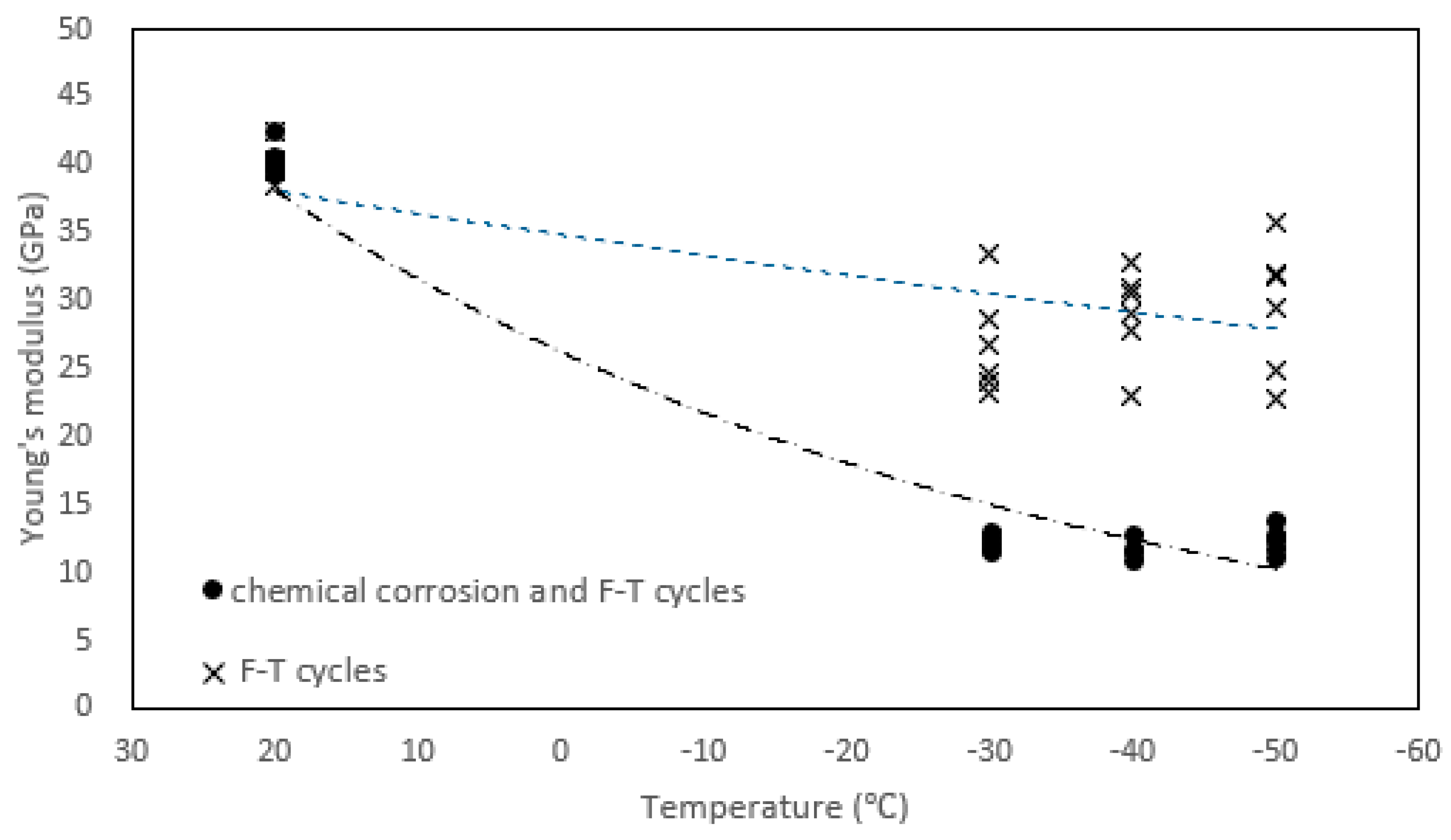
3.1. Young’s Modulus
3.2. Uniaxial Compression Test
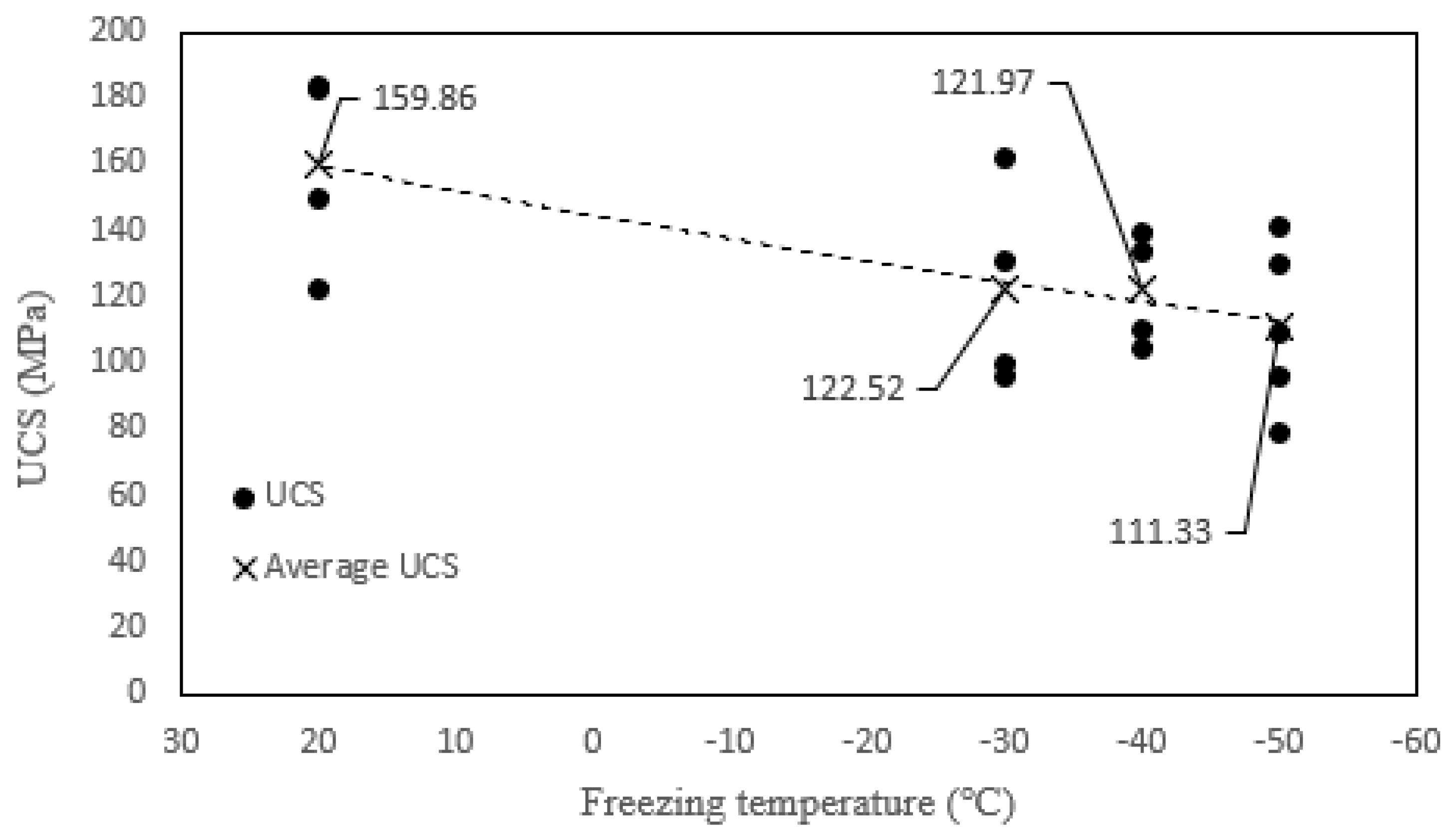
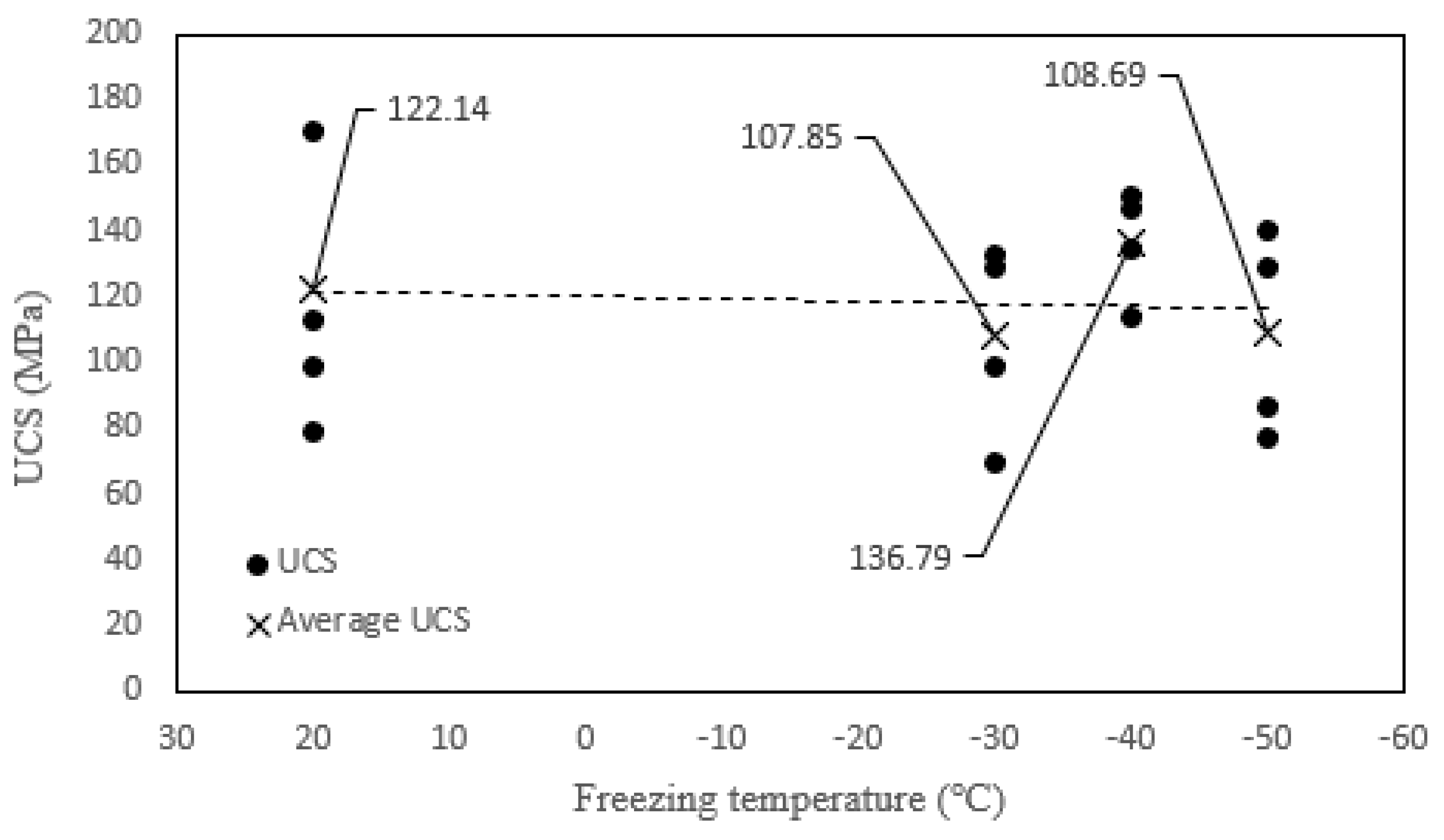
3.2.1. Uniaxial Compressive Strength
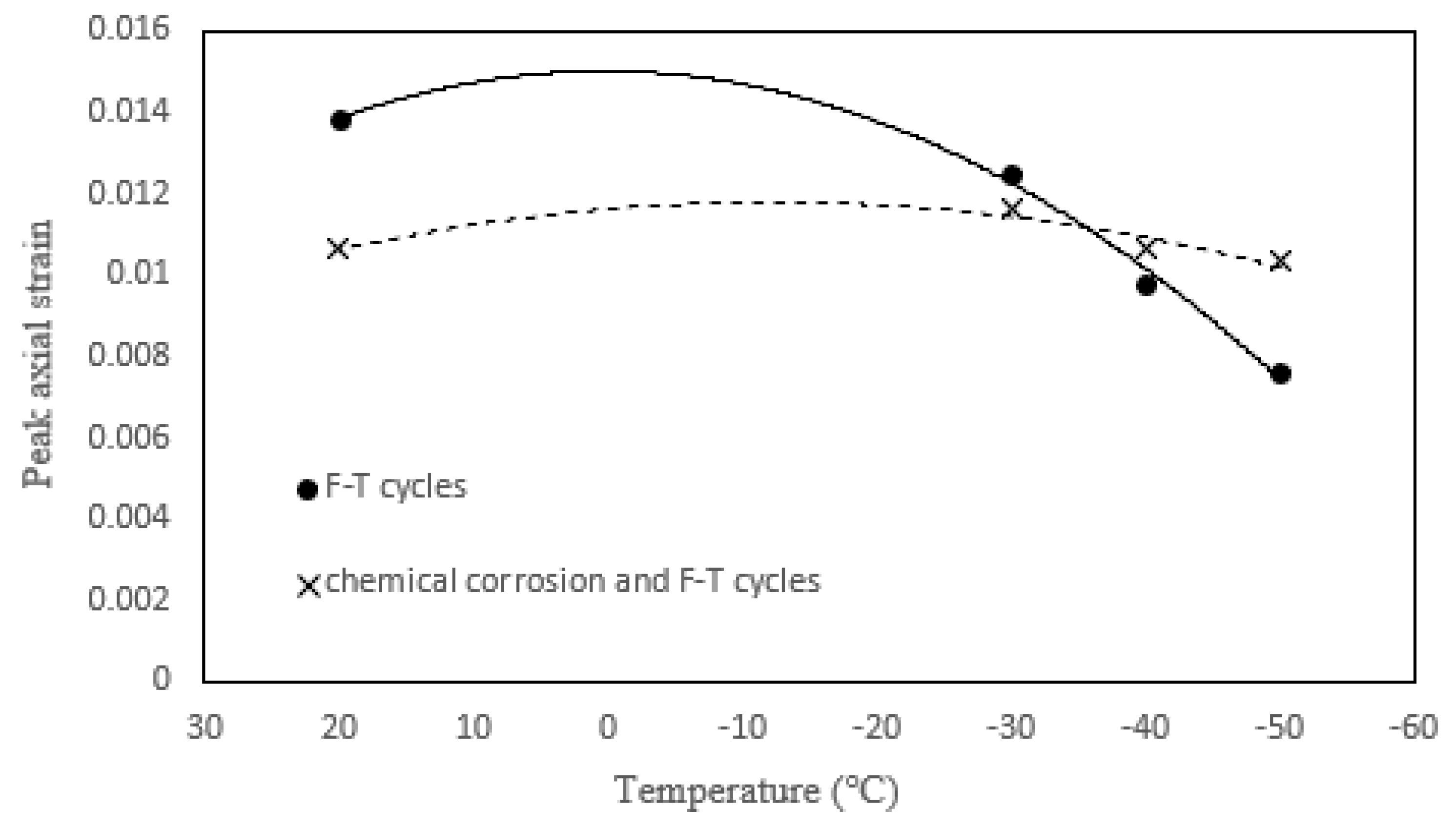
3.2.2. Peak Strain
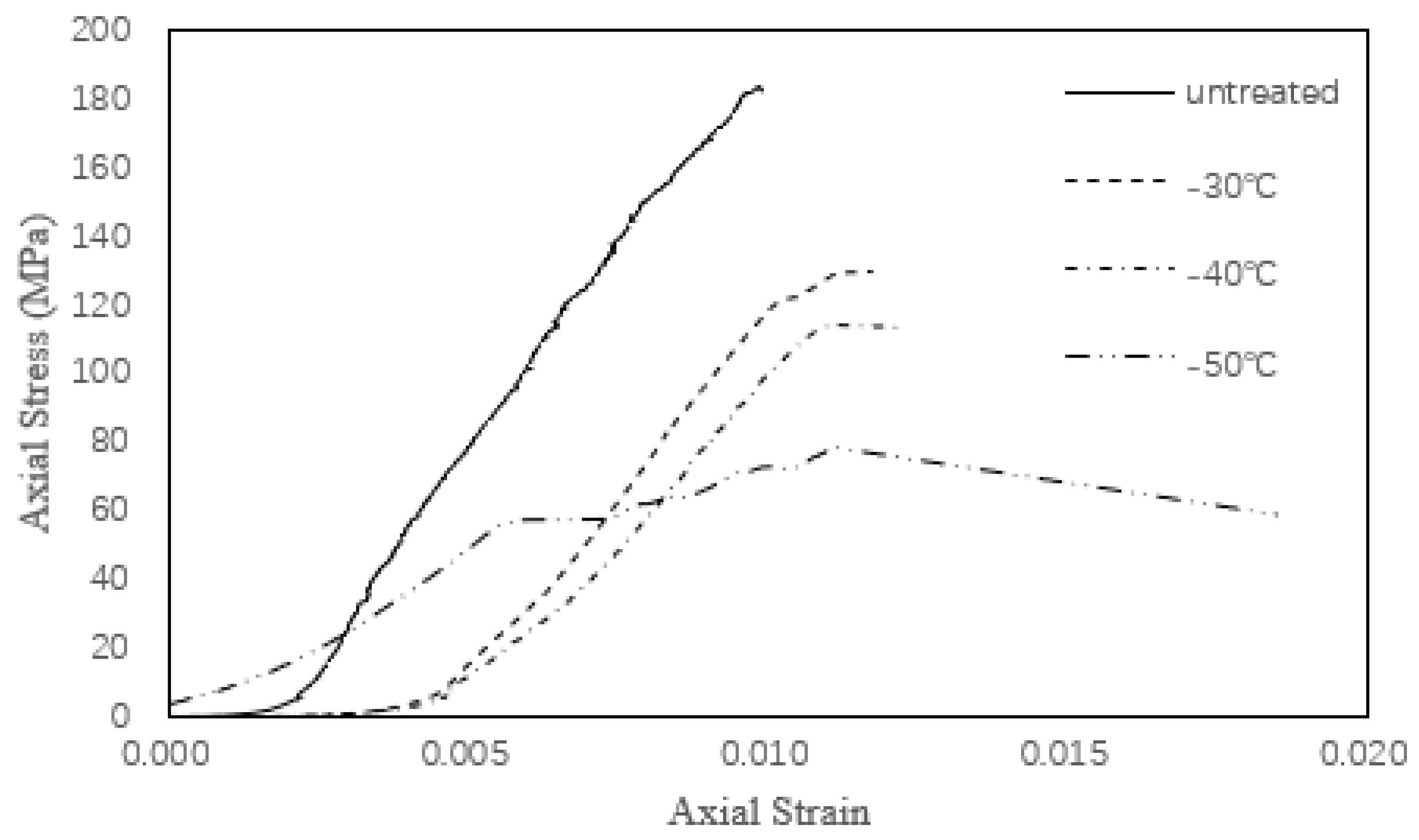
3.2.3. The Compressive Stress-Strain Curves for the G4
3.3. Three-Point Bending Test
3.4. Factors Influencing the Mechanical Properties of Tonalite under the Combined Effect of Chemical Corrosion and Freeze-Thaw Cycles
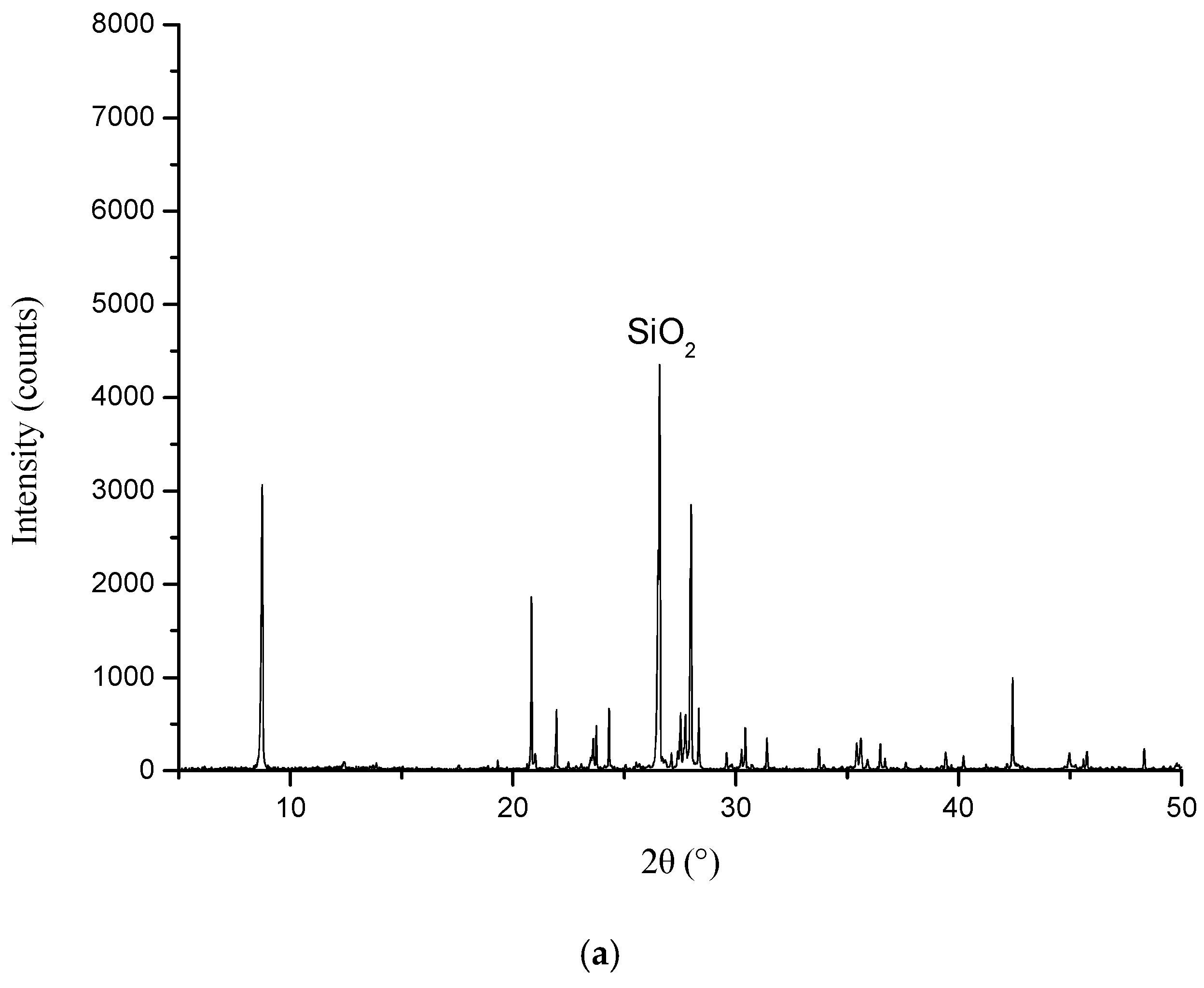
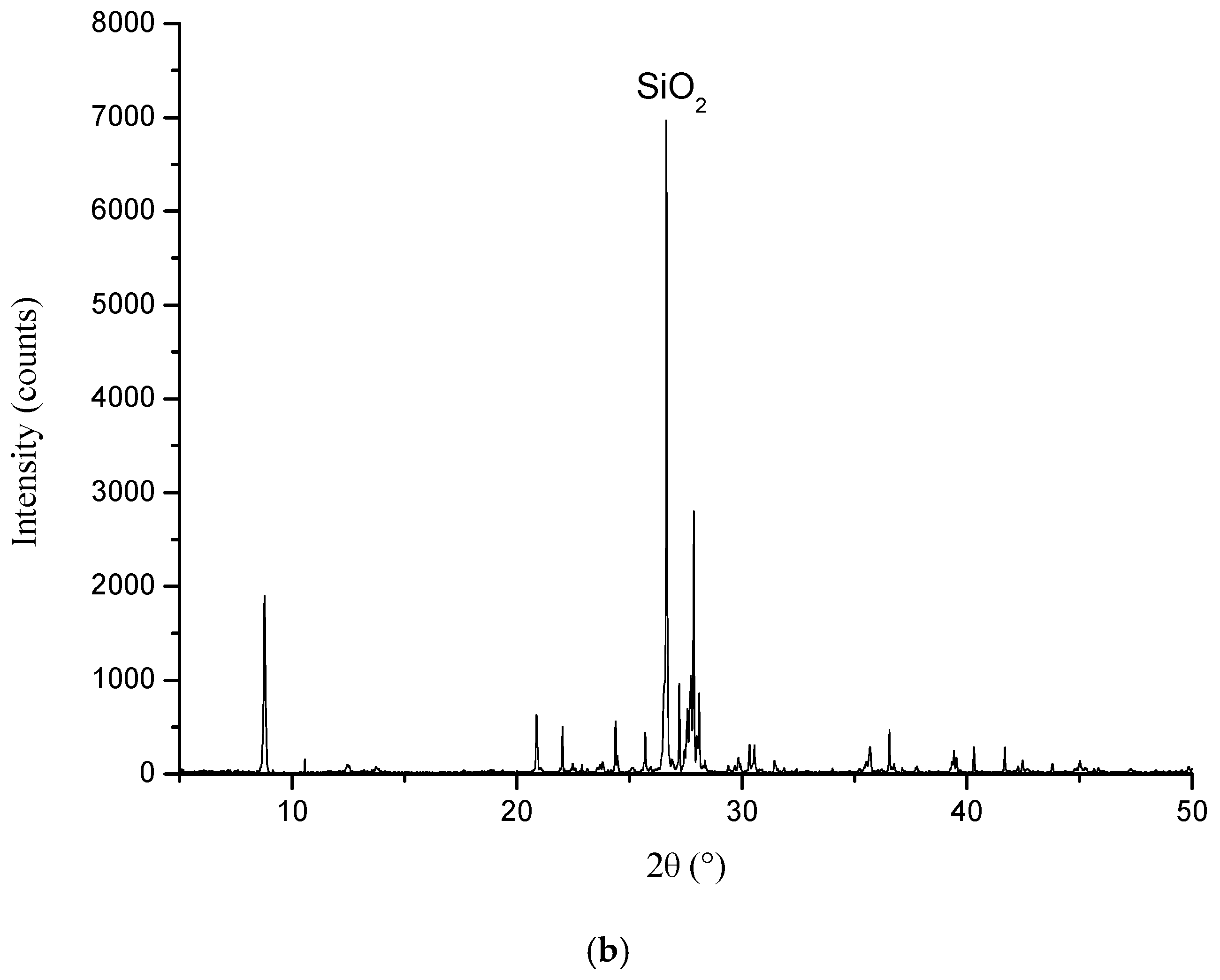
3.5. XRD Analysis
3.6. SEM Observation
4. Discussion and Conclusions
- (1)
- The Young’s modulus of several tonalite specimens subjected to the combined effect of chemical corrosion and freeze-thaw weathering is far less than that found for the specimens subjected only to either chemical corrosion or freeze-thaw weathering. Based on Figure 3, nitric acid solution amplifies the damage caused by freeze-thaw cycles.
- (2)
- The peak strain of tonalite specimens subjected to the combined effect decreases with decreasing freezing temperature. Moreover, the peak strain of tonalite specimens subjected to the coupled effect is the highest. While the UCS of tonalite specimens submitted to the coupled effect decreases with decreasing freezing temperature, the values are not significantly different at each temperature. It can be shown that the nitric acid solution has a softening effect on the tonalite specimen.
- (3)
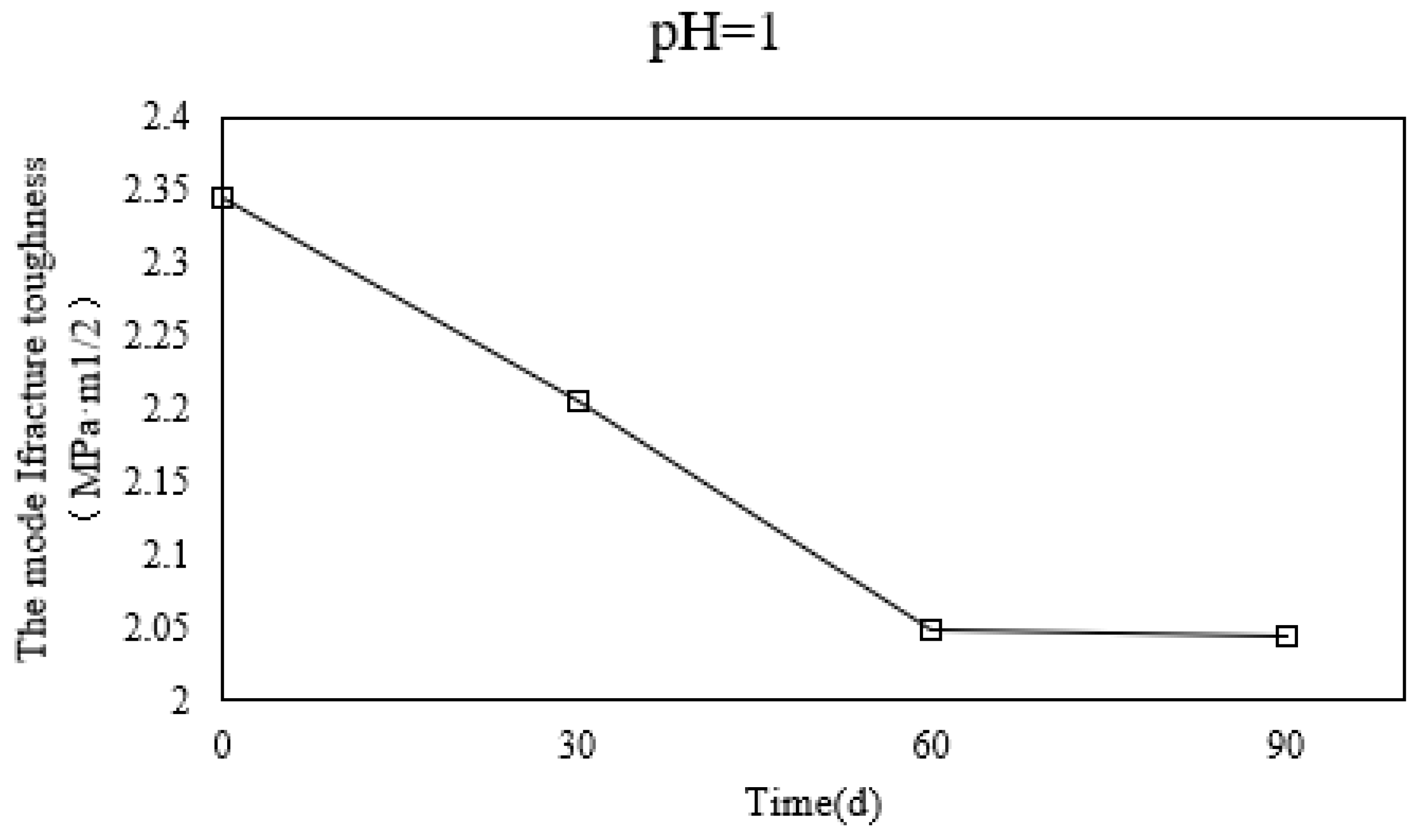
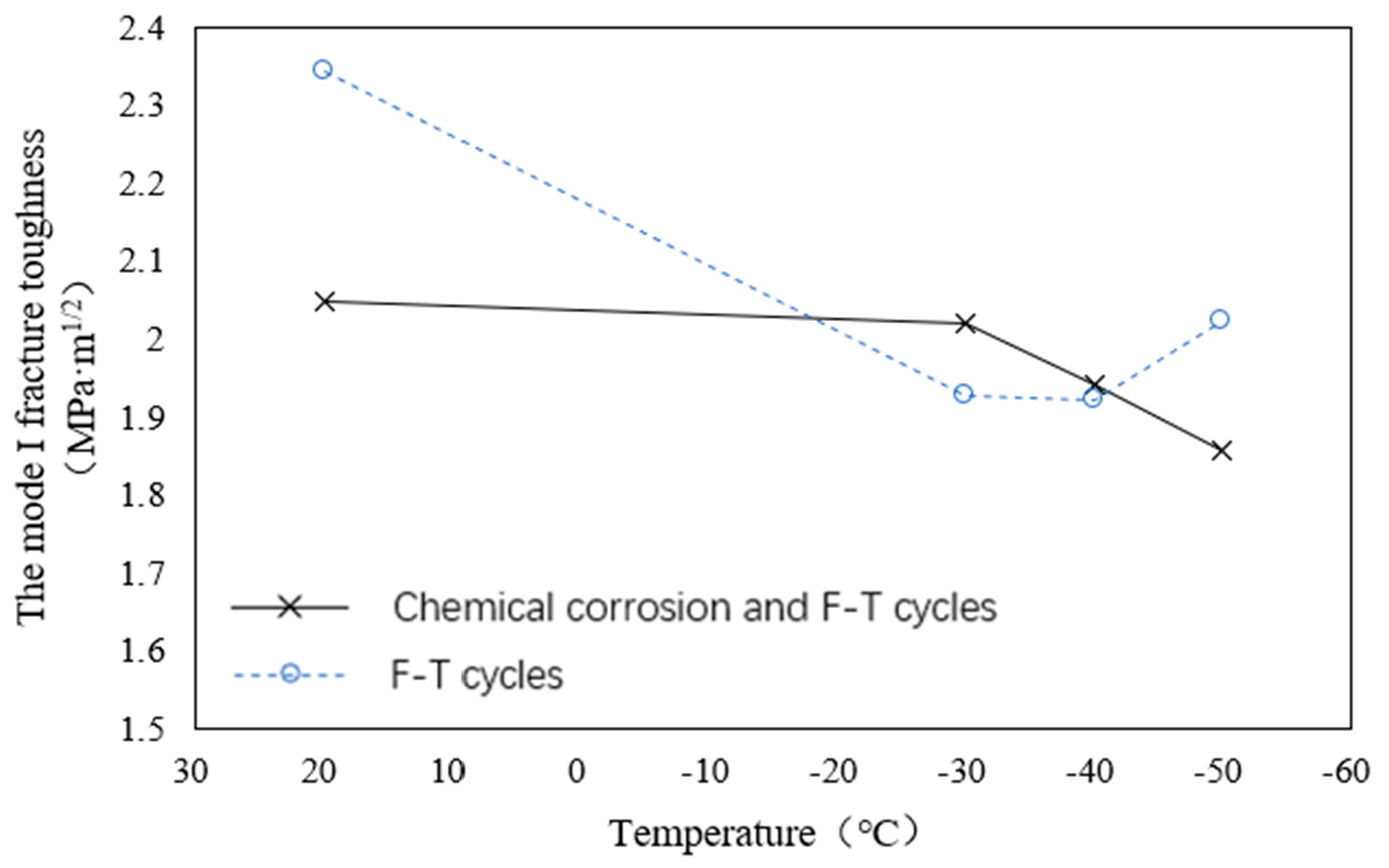
- The KIC of tonalite specimens decreases with the increase of soaking time in HNO3 (pH = 1), but the decline is almost the same from 60 days to 90 days. It can also be seen that the KIC of G4 declined less than the KIC of G3 compared with the control group.
- (4)
- Based on the results of SEM, it was verified that colloidal particles are generated when tonalite specimens are subjected to nitric acid. Following the combined effect, a number of pores and cracks will be filled by colloidal particles. This process enhances the cohesion of the tonalite specimen and decreases the damage that results from freeze-thaw weathering.
- (5)
- The main failure mode in tonalite specimens is intercrystalline crack when the specimens are subjected to both chemical corrosion and freeze-thaw weathering.
Author Contributions
Funding
Conflicts of Interest
References
- Ni, J.; Chen, Y.L.; Wang, P.; Wang, S.R.; Peng, B.; Azzam, R. Effect of chemical erosion and freeze-thaw cycling on the physical and mechanical characteristic of tonalites. Bull. Eng. Geol. Environ. 2017, 76, 169–179. [Google Scholar] [CrossRef]
- Ozbek, A. Investigation of the effects of wetting-drying and freezing-thawing cycles on some physical and mechanical properties of selected ignimbrites. Bull. Eng. Geol. Environ. 2014, 73, 595–609. [Google Scholar] [CrossRef]
- Ozcelik, Y.; Careddu, N.; Yilmazkaya, E. The effects of freeze-thaw cycles on the gloss values of polished stone surfaces. Cold Reg. Sci. Technol. 2012, 82, 49–55. [Google Scholar] [CrossRef]
- Muneo, H. Micromechanical analysis on deterioration due to freezing and thawing in porous brittle materials. Int. J. Eng. Sci. 1998, 36, 511–522. [Google Scholar]
- Beier, N.A.; Sego, D.C. Cyclic freeze-thaw to enhance the stability of coal tailings. Cold Reg. Sci. Technol. 2009, 55, 278–285. [Google Scholar] [CrossRef]
- Park, C.; Synn, J.H.; Shin, D.S. Experimental study on the thermal characteristics of rock at low temperatures. Int. J. Rock Mech. Min. Sci. 2004, 41, 81–86. [Google Scholar] [CrossRef]
- Bellanger, M.; Homand, F.; Remy, J.M. Water behaviour in limestones as a function of pores structure: Application to frost resistance of some Lorraine limestones. Eng. Geol. 1993, 36, 99–108. [Google Scholar] [CrossRef]
- Saad, A.; Guédon, S.; Martineau, F. Microstructural weathering of sedimentary rocks by freeze-thaw cycles: Experimental study of state and transfer parameters. Comptes Rendus Geosci. 2010, 34, 197–203. [Google Scholar] [CrossRef]
- Altindag, R.; Alyildiz, I.S.; Onargan, T. Mechanical property degradation of ignimbrite subjected to recurrent freeze-thaw cycles. Int. J. Rock Mech. Min. Sci. 2004, 41, 1023–1028. [Google Scholar] [CrossRef]
- Karaca, Z.; Deliormanli, A.H.; Elci, H.; Pamukcu, C. Effect of freeze-thaw process on the abrasion loss value of stones. Int. J. Rock Mech. Min. Sci. 2010, 47, 1207–1211. [Google Scholar] [CrossRef]
- Seto, M. Freeze-thaw cycles on rock surfaces below the timberline in a montane zone: Field measurements in Kobugahara, Northern Ashio Mountains, Central Japan. Catena 2010, 82, 218–226. [Google Scholar] [CrossRef]
- Park, J.; Hyun, C.; Park, H.D. Changes in microstructure and physical properties of rocks caused by artificial freeze-thaw action. Bull. Eng. Geol. Environ. 2015, 74, 555–565. [Google Scholar] [CrossRef]
- Zhou, K.P.; Li, B.; Li, J.L.; Deng, H.W.; Bin, F. Microscopic damage and dynamic mechanical properties of rock under freeze-thaw environment. Trans. Nonferrous Met. Soc. China 2015, 25, 1254–1261. [Google Scholar] [CrossRef]
- Khanlari, G.H.; Abdilor, Y. The influence of wet-dry, freeze-thaw and heat-cool cycles on physical and mechanical properties of upper red sandstones, central part of Iran. Bull. Eng. Geol. Environ. 2015, 74, 1287–1300. [Google Scholar] [CrossRef]
- Takarli, M.; Prince, W.; Siddique, R. Damage in tonalite under heating/cooling cycles and water freeze–thaw condition. Int. J. Rock Mech. Min. Sci. 2008, 45, 1164–1175. [Google Scholar] [CrossRef]
- Momeni, A.; Abdilor, Y.; Khanlari, G.R.; Heidari, M.; Sepahi, A.A. The effect of freeze–thaw cycles on physical and mechanical properties of granitoid hard rocks. Bull. Eng. Geol. Environ. 2016, 75, 1649–1656. [Google Scholar] [CrossRef]
- Chen, Y.L.; Ni, J.; Jiang, L.H.; Liu, M.L.; Wang, P.; Azzam, R. Experimental study on mechanical properties of tonalite after freeze-thaw cycling. Environ. Earth Sci. 2014, 71, 3349–3354. [Google Scholar] [CrossRef]
- Tan, X.J.; Chen, W.Z.; Yang, J.P.; Cao, J.J. Laboratory investigations on the mechanical properties degradation of tonalite under freeze-thaw cycles. Cold Reg. Sci. Technol. 2011, 68, 130–138. [Google Scholar] [CrossRef]
- Gao, Y.H.; Feng, X.T.; Zhang, X.W.; Feng, G.L.; Jiang, Q.Q.; Li, S. Characteristic Stress Levels and Brittle Fracturing of Hard Rocks Subjected to True Triaxial Compression with Low Minimum Principal Stress. Rock Mech. Rock Eng. 2018, 51, 3681–3697. [Google Scholar] [CrossRef]
- Tang, L.S.; Zhang, P.C.; Wang, S.J. Testing study on effects of chemical action of aqueous solution on crack propagation in rock. Chin. J. Rock Mech. Eng. 2002, 21, 822–827. [Google Scholar]
- Ding, W.X.; Feng, X.T. Testing study on mechanical effect for limestone under chemical erosion. Chin. J. Rock Mech. Eng. 2004, 23, 3571–3576. [Google Scholar]
- Sheng, J.C.; Li, F.; Yao, D.; Huang, Q.; Song, H.; Zhan, M. Experimental study of seepage properties in rocks fracture under coupled hydro-mechano-chemical process. Chin. J. Rock Mech. Eng. 2012, 31, 1016–1025. [Google Scholar]
- Liu, Y.S. Micromechanical properties of surrounding rock in deep roadways under chemical corrosion. Chin. J. Geotech. Eng. 2003, 35, 350–353. [Google Scholar]
- Qiao, L.P.; Liu, J.; Feng, X.T. Study on damage mechanism of sandstone under hydro-physico-chemical effects. Chin. J. Rock Mech. Eng. 2007, 26, 2117–2124. [Google Scholar]
- Li, P.; Liu, J.; Li, G.H.; Zhu, J.B.; Liu, S.G. Experimental study for shear strength characteristics of sandstone under water-rock interaction effects. Rock Soil Mech. 2011, 32, 380–386. [Google Scholar]
- Dunning, J.; Douglas, B.; Miller, M.; McDonald, S. The role of the chemical environment in frictional deformation: Stress corrosion cracking and comminution. Pure Appl. Geophys. 1994, 143, 151–178. [Google Scholar] [CrossRef]
- Ning, L.; Yunming, Z.; Bo, S.; Gunter, S. A chemical damage model of sandstone in acid solution. Int. J. Rock Mech. Min. Sci. 2003, 40, 243–249. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, P.; Zhang, X.W.; Du, X. Experimental research on mechanical properties of tonalite in chemical dissolution under freeze-thaw cycles. Chin. J. Geotech. Eng. 2014, 36, 2226–2235. [Google Scholar]
- Fang, X.Y.; Xu, J.X.; Wang, P.X. Compressive failure characteristics of yellow sandstone subjected to the coupling effects of chemical corrosion and repeated freezing and thawing. Eng. Geol. 2018, 233, 160–171. [Google Scholar] [CrossRef]
- Han, T.L.; Shi, J.P.; Chen, Y.S.; Li, Z.H.; Li, C.L. Laboratory investigation on the mechanical propertyies of sandstone immersed in different chemical corrosion under freeze-thaw cycles. Chin. J. Solid Mech. 2017, 38, 503–520. [Google Scholar]
- Han, T.L.; Shi, J.P.; Chen, Y.S. Laboratory investigations on the mechanical properties degradation of sandstone under the combined action between water chemical corrosion and freezing and thawing cycles. J. Hydraul. Eng. 2016, 47, 644–655. [Google Scholar]
- Liu, S.; Chen, Y.; Xi, D.U.; Huang, J.; Nie, D. Study on influence factor of freezing-thawing damage of white sandstone in acid erosion area. J. Water Res. Water Eng. 2014, 25, 127–131. [Google Scholar]
- Li, X.; Chen, Y.; Xu, P.; Zhong, M.; Wang, Q. Experiment on mechanical property of triaxial compression of white sandstone after effect of chemical solution and freeze-thaw cycle. J. Water Res. Water Eng. 2015, 26, 212–218. [Google Scholar]
- Ding, W.; Xu, T.; Wang, H.Y.; Chen, J.P. Experimental study of mechanical property of limestone under coupled chemical solution and freezing-thawing process. Chin. J. Rock Mech. Eng. 2015, 34, 979–985. [Google Scholar]
- Liu, N. Analysis on Freeze-Thaw Cycling and Mechanical Experiment and Thermo-Hydro-Mechanics Coupling of Rock. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2010. [Google Scholar]



| Dry Weight (g) | Dry Density (g/cm3) | Saturated Density (g/cm3) | Saturation Moisture Content (%) | Porosity (%) |
|---|---|---|---|---|
| 538.13 | 2.74 | 2.744 | 0.083 | 0.23 |
| Group | Method |
|---|---|
| G1 | No treatment (1 group) |
| G2 | Immersed in HNO3 (pH = 1) for 60 days (1 group) |
| G3 | Subjected to 30 freeze-thaw cycles at −30 °C, −40 °C and −50 °C (3 groups) |
| G4 | Immersed in HNO3 (pH = 1) for 60 days, then subjected to 30 freeze-thaw cycles at −30 °C, −40 °C and −50 °C (3 groups) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Chen, Y.; Wang, S.; Javadi, A.; Du, X.; Azzam, R. Mechanical Properties of Tonalite Subjected to Combined Effects of Chemical Corrosion and Freeze-Thaw Cycles. Appl. Sci. 2019, 9, 3890. https://doi.org/10.3390/app9183890
Xu C, Chen Y, Wang S, Javadi A, Du X, Azzam R. Mechanical Properties of Tonalite Subjected to Combined Effects of Chemical Corrosion and Freeze-Thaw Cycles. Applied Sciences. 2019; 9(18):3890. https://doi.org/10.3390/app9183890
Chicago/Turabian StyleXu, Chao, Youliang Chen, Suran Wang, Akbar Javadi, Xi Du, and Rafig Azzam. 2019. "Mechanical Properties of Tonalite Subjected to Combined Effects of Chemical Corrosion and Freeze-Thaw Cycles" Applied Sciences 9, no. 18: 3890. https://doi.org/10.3390/app9183890
APA StyleXu, C., Chen, Y., Wang, S., Javadi, A., Du, X., & Azzam, R. (2019). Mechanical Properties of Tonalite Subjected to Combined Effects of Chemical Corrosion and Freeze-Thaw Cycles. Applied Sciences, 9(18), 3890. https://doi.org/10.3390/app9183890







