Non-Thermal Plasma Accelerates Astrocyte Regrowth and Neurite Regeneration Following Physical Trauma In Vitro
Abstract
:1. Introduction
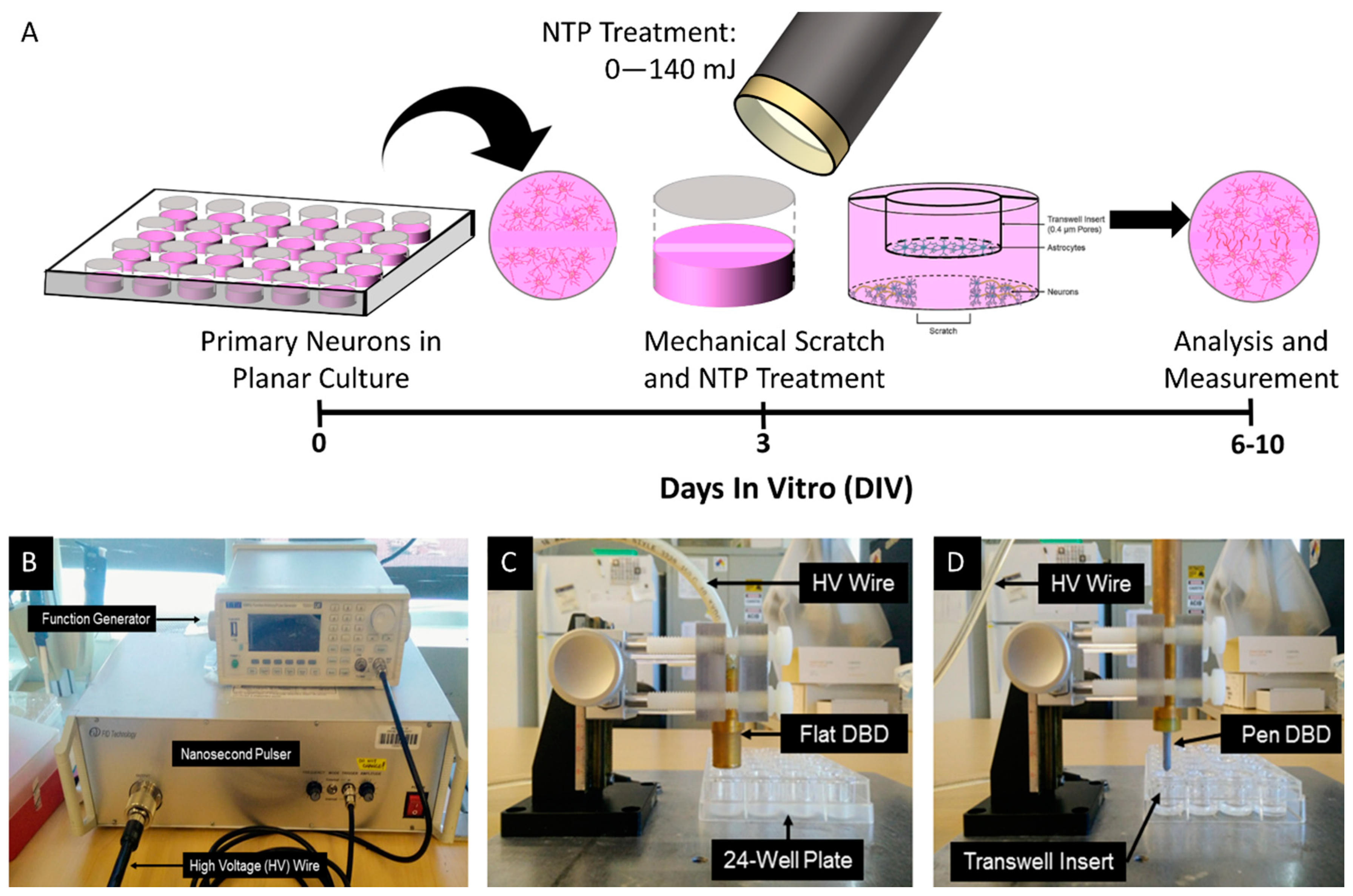
2. Materials and Methods
2.1. Cortical Neuron Harvest and Culture
2.2. Cortical Astrocyte Harvest and Co-Culture
2.3. Scratch Test and NTP Treatment
2.4. Microscopy and Immunocytochemistry
2.5. Image Quantification, Data Analysis, and Statistical Testing
3. Results
3.1. Direct Treatment of Astrocytes with NTP (20–90 mJ) Led to Increased Astrocyte Infiltration
3.2. Neurite Outgrowth was Unaffected by NTP Treatment ≤50 mJ
3.3. The Presence of NTP-Treated Astrocytes Resulted in Increased Neurite Re-Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617. [Google Scholar] [CrossRef] [PubMed]
- Gabella, B.; Hoffman, R.E.; Marine, W.W.; Stallones, L. Urban and rural traumatic brain injuries in Colorado. Ann. Epidemiol. 1997, 7, 207–212. [Google Scholar] [CrossRef]
- Liverman, C.T.; Altevogt, B.M.; Joy, J.E.; Johnson, R.T. Spinal Cord Injury: Progress, Promise, and Priorities; National Academy of Sciences: Washington, DC, USA, 2005. [Google Scholar]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Steinbeck, M.J.; Chernets, N.; Zhang, J.; Kurpad, D.S.; Fridman, G.; Fridman, A.; Freeman, T.A. Skeletal Cell Differentiation Is Enhanced by Atmospheric Dielectric Barrier Discharge Plasma Treatment. PLoS ONE 2013, 8, e82143. [Google Scholar] [CrossRef] [PubMed]
- Chernets, N.; Zhang, J.; Steinbeck, M.J.; Kurpad, D.S.; Koyama, E.; Friedman, G.; Freeman, T.A. Nonthermal atmospheric pressure plasma enhances mouse limb bud survival, growth, and elongation. Tissue Eng. Part A 2014, 21, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Pathak, S.; Castagliuolo, I.; Palù, G.; Brun, P.; Zuin, M.; Cavazzana, R.; Martines, E. Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS ONE 2014, 9, e104397. [Google Scholar] [CrossRef]
- Miller, V.; Lin, A.; Fridman, G.; Dobrynin, D.; Fridman, A. Plasma Stimulation of Migration of Macrophages. Plasma Process. Polym. 2014, 11, 1193–1197. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Friedman, G.; Fridman, A.; Clyne, A.M. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface 2011, rsif20110220. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, I.; Tuchscheerer, N.; Steffens, G.; Simsekyilmaz, S.; Konschalla, S.; Kroh, A.; Simons, D.; Asare, Y.; Schober, A.; Bucala, R.; et al. Differential roles of angiogenic chemokines in endothelial progenitor cell-induced angiogenesis. Basic Res. Cardiol. 2013, 108, 310. [Google Scholar] [CrossRef] [PubMed]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, V.; Lin, A.; Kako, F.; Gabunia, K.; Kelemen, S.; Brettschneider, J.; Fridman, G.; Fridman, A.; Autieri, M. Microsecond-pulsed dielectric barrier discharge plasma stimulation of tissue macrophages for treatment of peripheral vascular disease. Phys. Plasmas (1994 Present) 2015, 22, 122005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shubayev, V.I.; Myers, R.R. Matrix metalloproteinase-9 promotes nerve growth factor-induced neurite elongation but not new sprout formation in vitro. J. Neurosci. Res. 2004, 77, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Mao, X.O.; Greenberg, D.A. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J. Neurobiol. 2006, 66, 236–242. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhao, S.; Mao, X.; Lu, X.; He, G.; Yang, G.; Chen, M.; Ishaq, M.; Ostrikov, K. Selective neuronal differentiation of neural stem cells induced by nanosecond microplasma agitation. Stem Cell Res. 2014, 12, 387–399. [Google Scholar] [CrossRef]
- Yan, X.; Qiao, Y.; Ouyang, J.; Jia, M.; Li, J.; Yuan, F. Protective effect of atmospheric pressure plasma on oxidative stress-induced neuronal injuries: An in vitro study. J. Phys. D Appl. Phys. 2017, 50, 095401. [Google Scholar] [CrossRef]
- Zhao, S.; Han, R.; Li, Y.; Lu, C.; Chen, X.; Xiong, Z.; Mao, X. Investigation of the mechanism of enhanced and directed differentiation of neural stem cells by an atmospheric plasma jet: A gene-level study. J. Appl. Phys. 2019, 125, 163301. [Google Scholar] [CrossRef]
- Dowell, J.A.; Johnson, J.A.; Li, L. Identification of astrocyte secreted proteins with a combination of shotgun proteomics and bioinformatics. J. Proteome Res. 2009, 8, 4135–4143. [Google Scholar] [CrossRef]
- Cullen, D.K.; Gilroy, M.E.; Irons, H.R.; Laplaca, M.C. Synapse-to-neuron ratio is inversely related to neuronal density in mature neuronal cultures. Brain Res. 2010, 1359, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiyar, K.S.; Winter, C.C.; Struzyna, L.A.; Harris, J.P.; Cullen, D.K. Mechanical elongation of astrocyte processes to create living scaffolds for nervous system regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 2737–2751. [Google Scholar] [CrossRef] [PubMed]
- Laplaca, M.C.; Vernekar, V.N.; Shoemaker, J.T.; Cullen, D.K. Three-dimensional neuronal cultures. In Methods in Bioengineering: 3D Tissue Engineering; Artech House: Norwood, MA, USA, 2010; pp. 187–204. [Google Scholar]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed DBD Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Lin, A.; Fridman, A.; Wende, K.; Weltmann, K.; Miller, V. A Comparison of Floating-Electrode DBD and kINPen Jet: Plasma Parameters to Achieve Similar Growth Reduction in Colon Cancer Cells Under Standardized Conditions. Plasma Chem. Plasma Process. 2018, 38, 1–12. [Google Scholar] [CrossRef]
- Lin, A.; Gorbanev, Y.; de Backer, J.; van Loenhout, J.; van Boxem, W.; Lemière, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Chernets, N.; Han, J.; Alicea, Y.; Dobrynin, D.; Fridman, G.; Freeman, T.A.; Fridman, A.; Miller, V. Non-Equilibrium Dielectric Barrier Discharge Treatment of Mesenchymal Stem Cells: Charges and Reactive Oxygen Species Play the Major Role in Cell Death. Plasma Process. Polym. 2015, 12, 1117–1127. [Google Scholar] [CrossRef]
- Ayan, H.; Staack, D.; Fridman, G.; Gutsol, A.; Mukhin, Y.; Starikovskii, A.; Fridman, A.; Friedman, G. Application of nanosecond-pulsed dielectric barrier discharge for biomedical treatment of topographically non-uniform surfaces. J. Phys. D Appl. Phys. 2009, 42. [Google Scholar] [CrossRef]
- Ayan, H.; Fridman, G.; Gutsol, A.F.; Vasilets, V.N.; Fridman, A.; Friedman, G. Nanosecond-pulsed uniform dielectric-barrier discharge. IEEE Trans. Plasma Sci. 2008, 36, 5. [Google Scholar] [CrossRef]
- Liu, C.; Dobrynin, D.; Fridman, A. Uniform and non-uniform modes of nanosecond-pulsed dielectric barrier discharge in atmospheric air: Fast imaging and spectroscopic measurements of electric field. J. Phys. D Appl. Phys. 2014, 47, 252003. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Fridman, G.; Fridman, A.A.; Miller, V. Immune Cells Enhance Selectivity of Nanosecond-Pulsed DBD Plasma Against Tumor Cells. Plasma Med. 2017, 7, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, P.; Shrivastav, R.; Wang, M.; Lin, A.; Fridman, G.; Fridman, A.; Han, L.H.; Miller, V. Nanosecond pulsed dielectric barrier discharge induced anti-tumor effects propagate through the depth of tissue via intracellular signaling. Plasma Med. 2017, 7, 283–297. [Google Scholar] [CrossRef]
- Rybnikova, E.; Samoilov, M. Current insights into the molecular mechanisms of hypoxic pre- and postconditioning using hypobaric hypoxia. Front. Neurosci. 2015, 9, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Shin, J.E.; Ewan, E.E.; Oh, Y.M.; Pita-Thomas, W.; Cavalli, V. Activating Injury-Responsive Genes with Hypoxia Enhances Axon Regeneration through Neuronal HIF-1alpha. Neuron 2015, 88, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Horiba, M.; Kamiya, T.; Hara, H.; Adachi, T. Cytoprotective effects of mild plasma-activated medium against oxidative stress in human skin fibroblasts. Sci. Rep. 2017, 7, 42208. [Google Scholar] [CrossRef] [PubMed]
- Giulian, D.; Woodward, J.; Young, D.G.; Krebs, J.F.; Lachman, L.B. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J. Neurosci. 1988, 8, 2485–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Asher, R.A.; Morgenstern, D.A.; Fidler, P.S.; Adcock, K.H.; Oohira, A.; Braistead, J.E.; Levine, J.M.; Margolis, R.U.; Rogers, J.H.; Fawcett, J.W. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J. Neurosci. 2000, 20, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Lagord, C.; Berry, M.; Logan, A. Expression of TGFbeta2 but not TGFbeta1 correlates with the deposition of scar tissue in the lesioned spinal cord. Mol. Cell. Neurosci. 2002, 20, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kang, S.K.; Park, S.M.; Jung, H.Y.; Choi, B.H.; Sim, J.Y.; Lee, J.K. Characterization and effects of Ar/Air microwave plasma on wound healing. Plasma Process. Polym. 2015, 12, 1423–1434. [Google Scholar] [CrossRef]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.F.; Morfill, G.E.; Bosserhoff, A.K.; Karrer, S. Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS ONE 2015, 10, e0120041. [Google Scholar] [CrossRef] [PubMed]
- Fathollah, S.; Mirpour, S.; Mansouri, P.; Dehpour, A.R.; Ghoranneviss, M.; Rahimi, N.; Naraghi, Z.S.; Chalangari, R.; Chalangari, K.M. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci. Rep. 2016, 6, 19144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, V.W.; Moumdjian, R.; Yong, F.P.; Ruijs, T.C.; Freedman, M.S.; Cashman, N.; Antel, J.P. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proc. Natl. Acad. Sci. USA 1991, 88, 7016–7020. [Google Scholar] [CrossRef] [PubMed]
- DiProspero, N.A.; Meiners, S.; Geller, H.M. Inflammatory cytokines interact to modulate extracellular matrix and astrocytic support of neurite outgrowth. Exp. Neurol. 1997, 148, 628–639. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katiyar, K.S.; Lin, A.; Fridman, A.; Keating, C.E.; Cullen, D.K.; Miller, V. Non-Thermal Plasma Accelerates Astrocyte Regrowth and Neurite Regeneration Following Physical Trauma In Vitro. Appl. Sci. 2019, 9, 3747. https://doi.org/10.3390/app9183747
Katiyar KS, Lin A, Fridman A, Keating CE, Cullen DK, Miller V. Non-Thermal Plasma Accelerates Astrocyte Regrowth and Neurite Regeneration Following Physical Trauma In Vitro. Applied Sciences. 2019; 9(18):3747. https://doi.org/10.3390/app9183747
Chicago/Turabian StyleKatiyar, Kritika S., Abraham Lin, Alexander Fridman, Carolyn E. Keating, D. Kacy Cullen, and Vandana Miller. 2019. "Non-Thermal Plasma Accelerates Astrocyte Regrowth and Neurite Regeneration Following Physical Trauma In Vitro" Applied Sciences 9, no. 18: 3747. https://doi.org/10.3390/app9183747
APA StyleKatiyar, K. S., Lin, A., Fridman, A., Keating, C. E., Cullen, D. K., & Miller, V. (2019). Non-Thermal Plasma Accelerates Astrocyte Regrowth and Neurite Regeneration Following Physical Trauma In Vitro. Applied Sciences, 9(18), 3747. https://doi.org/10.3390/app9183747





