GaN/Ga2O3 Core/Shell Nanowires Growth: Towards High Response Gas Sensors
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Ga2O3-Shell Thickness Evolution and Formation
3.2. Structural Characterization of the Ga2O3-Shell
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Potyrailo, R.A. Multivariable Sensors for Ubiquitous Monitoring of Gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, K.; Shi, T.; Qadir, A.; Wan, X.; Guo, H.; Ali, A.; Xuan, W.; Xu, H.; Gu, Z.; Peng, X.; et al. Designing an Efficient Multimode Environmental Sensor Based on Graphene-Silicon Heterojunction. Adv. Mater. Technol. 2017, 2, 1600262. [Google Scholar] [CrossRef]
- Shankar, P.; Rayappan, J.B.B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases—A review. Sci. Lett. J. 2015, 4, 126. [Google Scholar]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yamazaki, T.; Shen, Y.; Kikuta, T.; Nakatani, N.; Li, Y. O2 and CO sensing of Ga2O3 multiple nanowire gas sensors. Sens. Actuators B Chem. 2008, 129, 666–670. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Electron mobility in single- and polycrystalline Ga2O3. J. Appl. Phys. 1993, 74, 300–305. [Google Scholar] [CrossRef]
- Mazeina, L.; Picard, Y.N.; Maximenko, S.I.; Perkins, F.K.; Glaser, E.R.; Twigg, M.E.; Freitas, J.A.; Prokes, S.M. Growth of Sn-Doped β- Ga2O3 Nanowires and Ga2O3−SnO2 Heterostructures for Gas Sensing Applications. Cryst. Growth Des. 2009, 9, 4471–4479. [Google Scholar] [CrossRef]
- Rsan, N.B.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter 2003, 15, R813–R839. [Google Scholar]
- Hahn, S.; Barsan, N.; Weimar, U.; Ejakov, S.; Visser, J.; Soltis, R. CO sensing with SnO2 thick film sensors: Role of oxygen and water vapour. Thin Solid Films 2003, 436, 17–24. [Google Scholar] [CrossRef]
- Huang, J.; Wan, Q. Gas Sensors Based on Semiconducting Metal Oxide One-Dimensional Nanostructures. Sensors 2009, 9, 9903–9924. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, F.; Tarancón, A.; Casals, O.; Arbiol, J.; Romanorodriguez, A.; Morante, J. High response and stability in CO and humidity measures using a single SnO2 nanowire. Sens. Actuators B Chem. 2007, 121, 3–17. [Google Scholar] [CrossRef]
- Savage, N.O.; Akbar, S.A.; Dutta, P.K. Titanium dioxide based high temperature carbon monoxide selective sensor. Sens. Actuators B Chem. 2001, 72, 239–248. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Oon, H.S.; Cheong, K.Y. Effect of Oxidation Time on Thermally Grown Oxide on GaN. J. Mater. Eng. Perf. 2013, 22, 1341–1347. [Google Scholar] [CrossRef]
- Korbutowicz, R.; Prazmowska, J. Wet Thermal Oxidation of GaAs and GaN. In Semiconductor Technologies; Grym, J., Ed.; InTech: London, UK, 2010. [Google Scholar]
- Hoefera, U.; Frank, J.; Fleischer, M. High temperature Ga2O3-gas sensor and SnO2-gas sensors: A comparison. Sens. Actuators B 2011, 78, 6. [Google Scholar] [CrossRef]
- Ogita, M.; Saika, N.; Nakanishi, Y.; Hatanaka, Y. Ga2O3 thin films for high-temperature gas sensors. Appl. Surf. Sci. 1999, 142, 188–191. [Google Scholar] [CrossRef]
- Schwebel, T.; Fleischer, M.; Meixner, H.; Kohl, C.-D. CO-Sensor for domestic use based on high temperature stable Ga2O3 thin films. Sens. Actuators B Chem. 1998, 49, 46–51. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and Metal Oxide Nanoparticles in Chemiresistors: Does the Nanoscale Matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef]
- Wal, R.V.; Hunter, G.; Xu, J.; Kulis, M.; Berger, G.; Ticich, T. Metal-oxide nanostructure and gas-sensing performance. Sens. Actuators B Chem. 2009, 138, 113–119. [Google Scholar]
- Jimerez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Choi, Y.-K. Chemical sensors based on nanostructured materials. Sens. Actuators B Chem. 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Comini, C. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, P. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wong, C.K.; Yuan, C.A.; Zhang, G. Nanowire-based gas sensors. Sens. Actuators B 2013, 177, 178. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Yang, Y.; Zacharias, M. Nanowire-based sensors. Small 2010, 6, 1705–1722. [Google Scholar] [CrossRef] [PubMed]
- Devan, R.S.; Patil, R.A.; Lin, J.-H.; Ma, Y.-R. One-Dimensional Metal-Oxide Nanostructures: Recent Developments in Synthesis, Characterization, and Applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Kolmakov, A.; Zhang, Y.; Cheng, G.; Moskovits, M. Detection of CO and O2 Using Tin Oxide Nanowire Sensors. Adv. Mater. 2003, 15, 997–1000. [Google Scholar] [CrossRef]
- Reddeppa, M.; Park, B.-G.; Chinh, N.D.; Kim, D.; Oh, J.-E.; Kim, T.G.; Kim, M.-D.; Maddaka, R. A novel low-temperature resistive NO gas sensor based on InGaN/GaN multi-quantum well-embedded p-i-n GaN nanorods. Dalton Trans. 2019, 48, 1367–1375. [Google Scholar] [CrossRef]
- Reddeppa, M.; Park, B.-G.; Kim, M.-D.; Peta, K.R.; Chinh, N.D.; Kim, D.; Kim, S.-G.; Murali, G. H2, H2S gas sensing properties of rGO/GaN nanorods at room temperature: Effect of UV illumination. Sens. Actuators B Chem. 2018, 264, 353–362. [Google Scholar] [CrossRef]
- Reddeppa, M.; Mitta, S.B.; Park, B.-G.; Kim, S.-G.; Park, S.H.; Kim, M.-D. DNA-CTMA functionalized GaN surfaces for NO2 gas sensor at room temperature under UV illumination. Org. Electron. 2019, 65, 334–340. [Google Scholar] [CrossRef]
- Yang, P.; Yan, R.; Fardy, M. Semiconductor Nanowire: What’s Next? Nano Lett. 2010, 10, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Cao, G. Nanostructures and Nanomaterials; Imperial Collage Press: London, UK, 2004. [Google Scholar]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Willinger, M.-G.; Neri, G.; Rauwel, E.; Bonavita, A.; Micali, G.; Pinna, N. Vanadium Oxide Sensing Layer Grown on Carbon Nanotubes by a New Atomic Layer Deposition Process. Nano Lett. 2008, 8, 4201–4204. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-G.; Kim, W.-S.; Kim, D.-H.; Hong, S.-H. Fabrication of Ga2O3/SnO2 core–shell nanowires and their ethanol gas sensing properties. J. Mater. Res. 2011, 26, 2322–2327. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Chen, J.; Xu, H.; Shen, X.; Yuan, A. Co3O4/ZnO nanocomposites for gas-sensing applications. Appl. Surf. Sci. 2013, 265, 379–384. [Google Scholar] [CrossRef]
- Lai, H.-Y.; Chen, C.-H. Highly sensitive room-temperature CO gas sensors: Pt and Pd nanoparticle-decorated In2O3 flower-like nanobundles. J. Mater. Chem. 2012, 22, 13204. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H.; Park, S.Y.; Lee, C. Synthesis and CO gas sensing properties of surface-nitridated Ga2O3 nanowires. RSC Adv. 2014, 4, 63402–63407. [Google Scholar] [CrossRef]
- Prasad, R.M.; Lauterbach, S.; Kleebe, H.-J.; Merdrignac-Conanec, O.; Barsan, N.; Weimar, U.; Gurlo, A. Response of Gallium Nitride Chemiresistors to Carbon Monoxide is Due to Oxygen Contamination. ACS Sensors 2017, 2, 713–717. [Google Scholar] [CrossRef]
- Galopin, E.; Gogneau, N.; Travers, L.; Harmand, J.-C.; Largeau, L.; Glas, F. N-Polar GaN Nanowires Seeded by Al Droplets on Si(111). Cryst. Growth Des. 2012, 12, 2724–2729. [Google Scholar]
- Brubaker, M.D.; Levin, I.; Davydov, A.V.; Rourke, D.M.; Sanford, N.A.; Bright, V.M.; Bertness, K.A. GaN based nanorods for solid state lighting. J. Appl. Phys. 2011, 110, 053506. [Google Scholar] [CrossRef]
- Songmuang, R.; Landré, O.; Daudin, B. Molecular beam epitaxy growth and optical properties of AlN nanowires. Appl. Phys. Lett. 2007, 91, 251902. [Google Scholar] [CrossRef]
- Bertness, K.; Roshko, A.; Mansfield, L.; Harvey, T.; Sanford, N. Nucleation conditions for catalyst-free GaN nanowires. J. Cryst. Growth 2007, 300, 94–99. [Google Scholar] [CrossRef]
- Largeau, L.; Dheeraj, D.L.; Tchernycheva, M.; Cirlin, G.E.; Harmand, J.C. Facet and in-plane crystallographic orientations of GaN nanowires grown on Si(111). Nanotechnology 2008, 19, 155704. [Google Scholar] [CrossRef] [PubMed]
- Auzelle, T.; Haas, B.; Minj, A.; Bougerol, C.; Rouvière, J.-L.; Cros, A.; Colchero, J.; Daudin, B. The influence of AlN buffer over the polarity and the nucleation of self-organized GaN nanowires. J. Appl. Phys. 2015, 117, 245303. [Google Scholar] [CrossRef]
- Parameters of Semiconductor. Available online: http://www.ioffe.ru/SVA/NSM/Semicond (accessed on 19 August 2019).
- Gallium(III) Oxide. Available online: http://www.sigmaaldrich.com/catalog/product/aldrich/215066 (accessed on 19 August 2019).
- Yamada, T.; Ito, J.; Asahara, R.; Watanabe, K.; Nozaki, M.; Nakazawa, S.; Anda, Y.; Ishida, M.; Ueda, T.; Yoshigoe, A.; et al. Comprehensive study on initial thermal oxidation of GaN(0001) surface and subsequent oxide growth in dry oxygen ambient. J. Appl. Phys. 2017, 121, 035303. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, R.; Xu, X.F.; Zhou, Y.G.; Chen, Z.Z.; Xie, S.Y.; Li, W.P.; Zheng, Y.D. The oxidation of gallium nitride epilayers in dry oxygen. Appl. Phys. A 2000, 71, 191. [Google Scholar] [CrossRef]
- Wolter, S.D.; Mohney, S.E.; Venugopalan, H.; Wickenden, A.E.; Koleske, D.D. Kinetic Study of the Oxidation of Gallium Nitride in Dry Air. J. Electrochem. Soc. 1998, 145, 629–632. [Google Scholar] [CrossRef]
- Zhou, Y.; Ahyi, C.; Isaacs-Smith, T.; Bozack, M.; Tin, C.-C.; Williams, J.; Park, M.; Cheng, A.-J.; Park, J.-H.; Kim, D.-J.; et al. Formation, etching and electrical characterization of a thermally grown gallium oxide on the Ga-face of a bulk GaN substrate. Solid State Electr. 2008, 52, 756–764. [Google Scholar] [CrossRef]
- Jeurgens, L.P.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Growth kinetics and mechanisms of aluminum-oxide films formed by thermal oxidation of aluminum. J. Appl. Phys. 2002, 92, 1649–1656. [Google Scholar] [CrossRef]
- Krzeminski, C.; Larrieu, G.; Penaud, J.; Lampin, E.; Dubois, E. Silicon dry oxidation kinetics at low temperature in thenanometric range: Modeling and experiment. J. App. Phys. 2007, 101, 064908. [Google Scholar] [CrossRef]
- Gerlach, G.; Maser, K. A Self-Consistent Model for Thermal Oxidation of Silicon at Low Oxide Thickness. Adv. Condens. Matter Phys. 2016, 2016, 1–13. [Google Scholar] [CrossRef][Green Version]
- Korbutowicz, R.; Zakrzewski, A.; Rac-Rumijowska, O.; Stafiniak, A.; Vincze, A. Oxidation rates of aluminium nitride thin films: Effect of composition of the atmosphere. J. Mater. Sci. Mater. Electron. 2017, 28, 13937–13949. [Google Scholar] [CrossRef]
- Popova, I.; Zhukov, V.; Yates, J., Jr. Role of surface Al2O3 clusters on the kinetics of Al(111) oxidation. J. Appl. Phys. 2000, 87, 8143–8147. [Google Scholar] [CrossRef]
- Brossmann, U.; Würschum, R.; Södervall, U.; Schaefer, H.-E. Oxygen diffusion in ultrafine grained monoclinic ZrO2. J. Appl. Phys. 1999, 85, 7646–7654. [Google Scholar] [CrossRef]
- Opila, E.; Jacobson, N.; Humphrey, D.; Yoshio, T.; Oda, K. High Temperature Corrosion and Materials Chemistry; Hou, P.Y., Mc Nallan, M., Oltra, R., Opila, E.J., Shores, D.A., Eds.; The Electrochemical Society, Inc.: Pennington, NJ, USA, 1998. [Google Scholar]
- Thomas, S.M.; Jennings, M.R.; Sharma, Y.K.; Fisher, C.A.; Mawby, P.A. Impact of the Oxidation Temperature on the Interface Trap Density in 4H-SiC MOS Capacitors. Mater. Sci. Forum 2014, 778, 599–602. [Google Scholar] [CrossRef]
- Li, Y.; Tokizono, T.; Liao, M.; Zhong, M.; Koide, Y.; Yamada, I.; Delaunay, J.-J. Efficient Assembly of Bridged β- Ga2O3 Nanowires for Solar-Blind Photodetection. Adv. Funct. Mater. 2010, 20, 3972–3978. [Google Scholar] [CrossRef]
- Hanada, K.; Moribayashi, T.; Koshi, K.; Sasaki, K.; Kuramata, A.; Ueda, O.; Kasu, M. Origins of etch pits in β-Ga2O3 (010) single crystals. Jpn. J. Appl. Phys. 2016, 55, 1202BG. [Google Scholar] [CrossRef]
- Morassi, M.; Largeau, L.; Oehler, F.; Song, H.-G.; Travers, L.; Julien, F.H.; Harmand, J.-C.; Cho, Y.-H.; Glas, F.; Tchernycheva, M.; et al. Morphology Tailoring and Growth Mechanism of Indium-Rich InGaN/GaN Axial Nanowire Heterostructures by Plasma-Assisted Molecular Beam Epitaxy. Cryst. Growth Des. 2018, 18, 2545–2554. [Google Scholar] [CrossRef]
- Lee, C.-T.; Yan, J.-T. Sensing mechanisms of Pt/β-Ga2O3/GaN hydrogen sensor diodes. Sens. Actuators B Chem. 2010, 147, 723–729. [Google Scholar] [CrossRef]
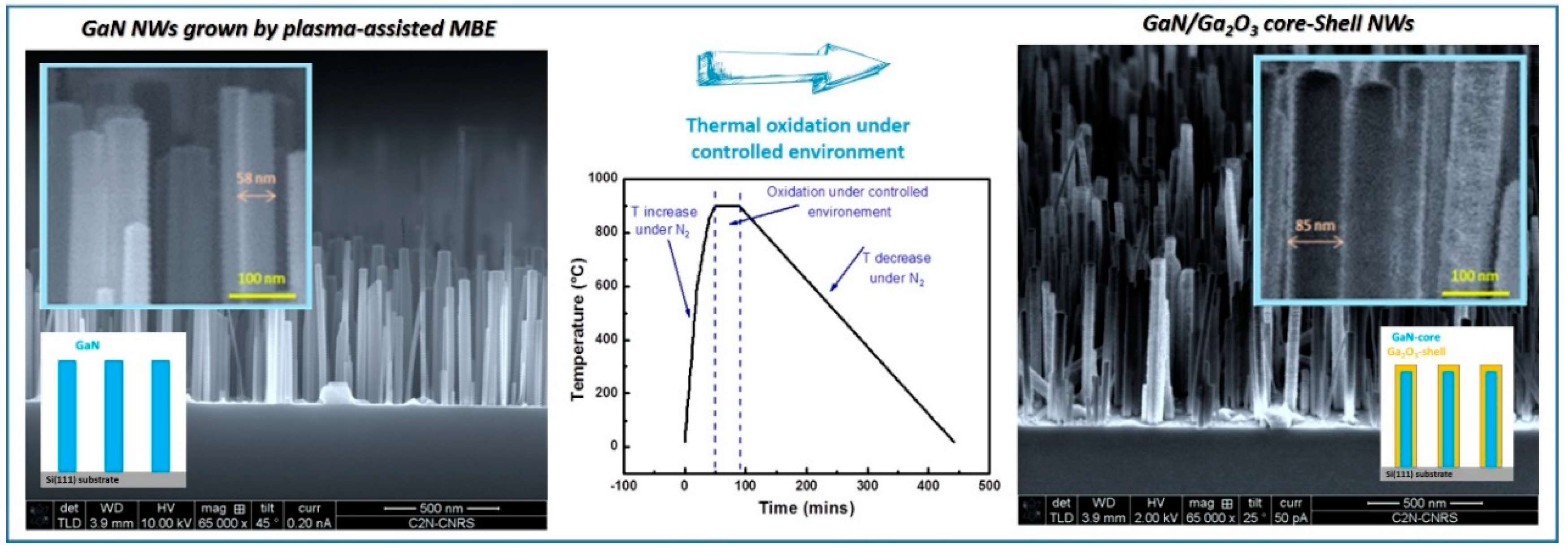
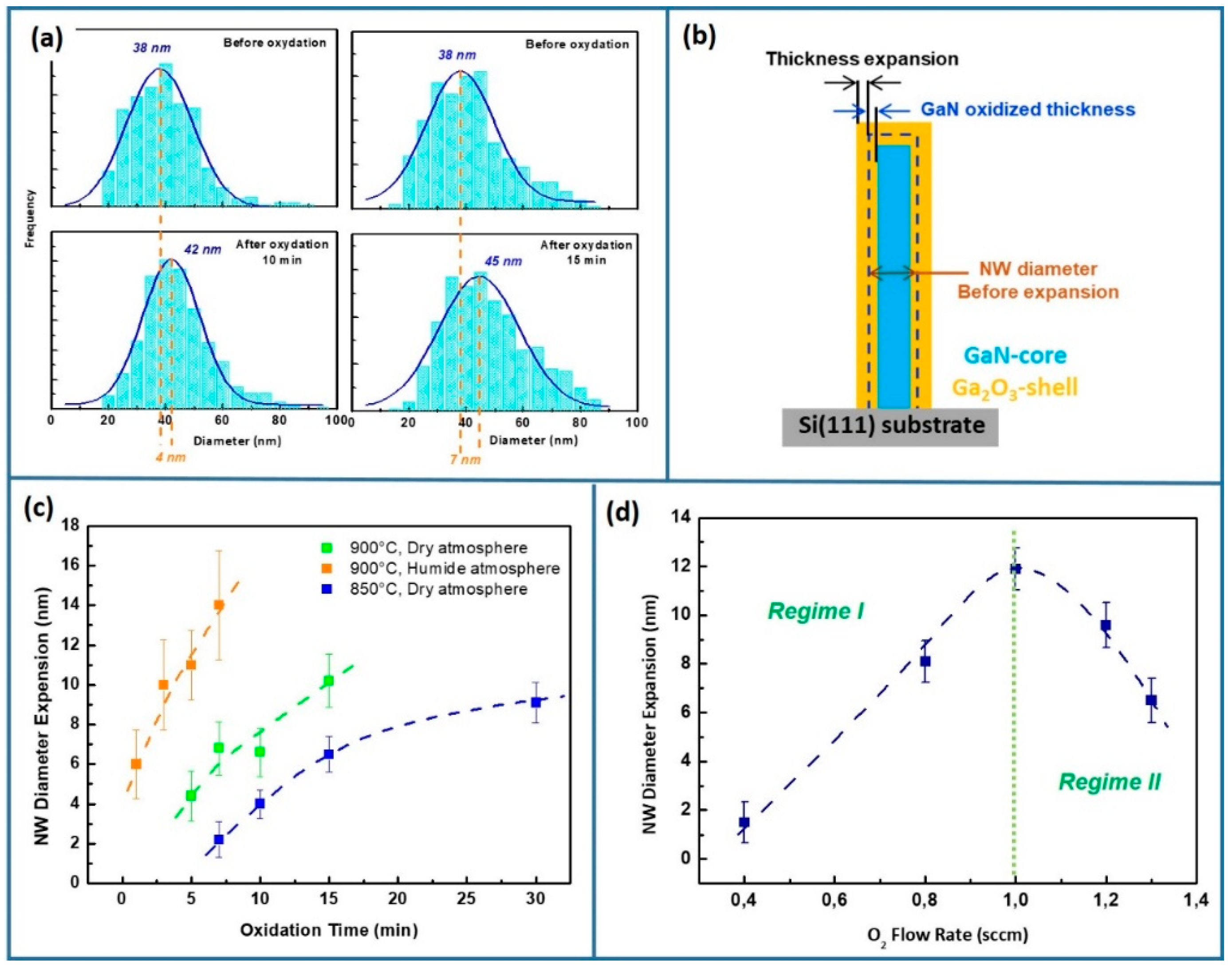
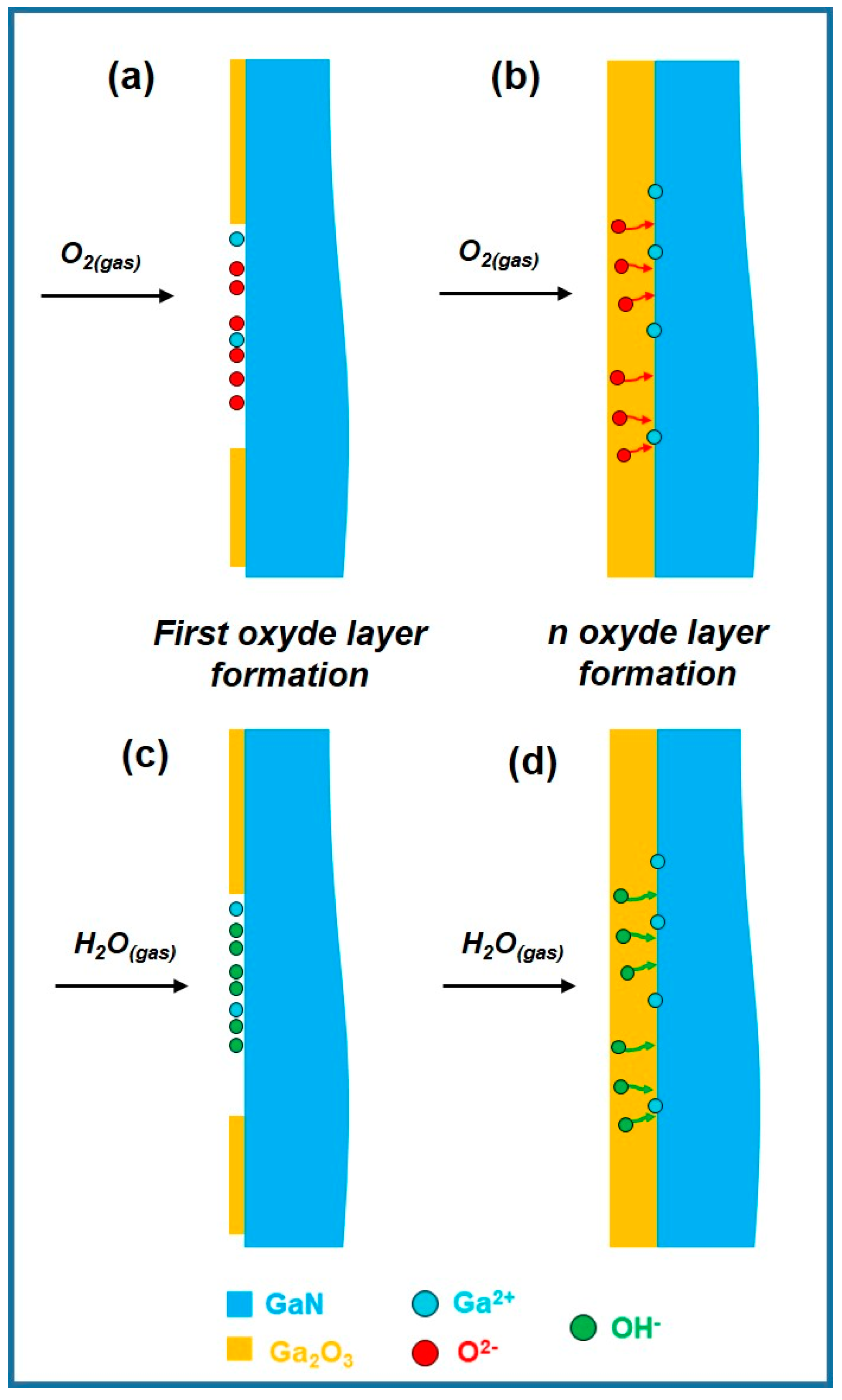
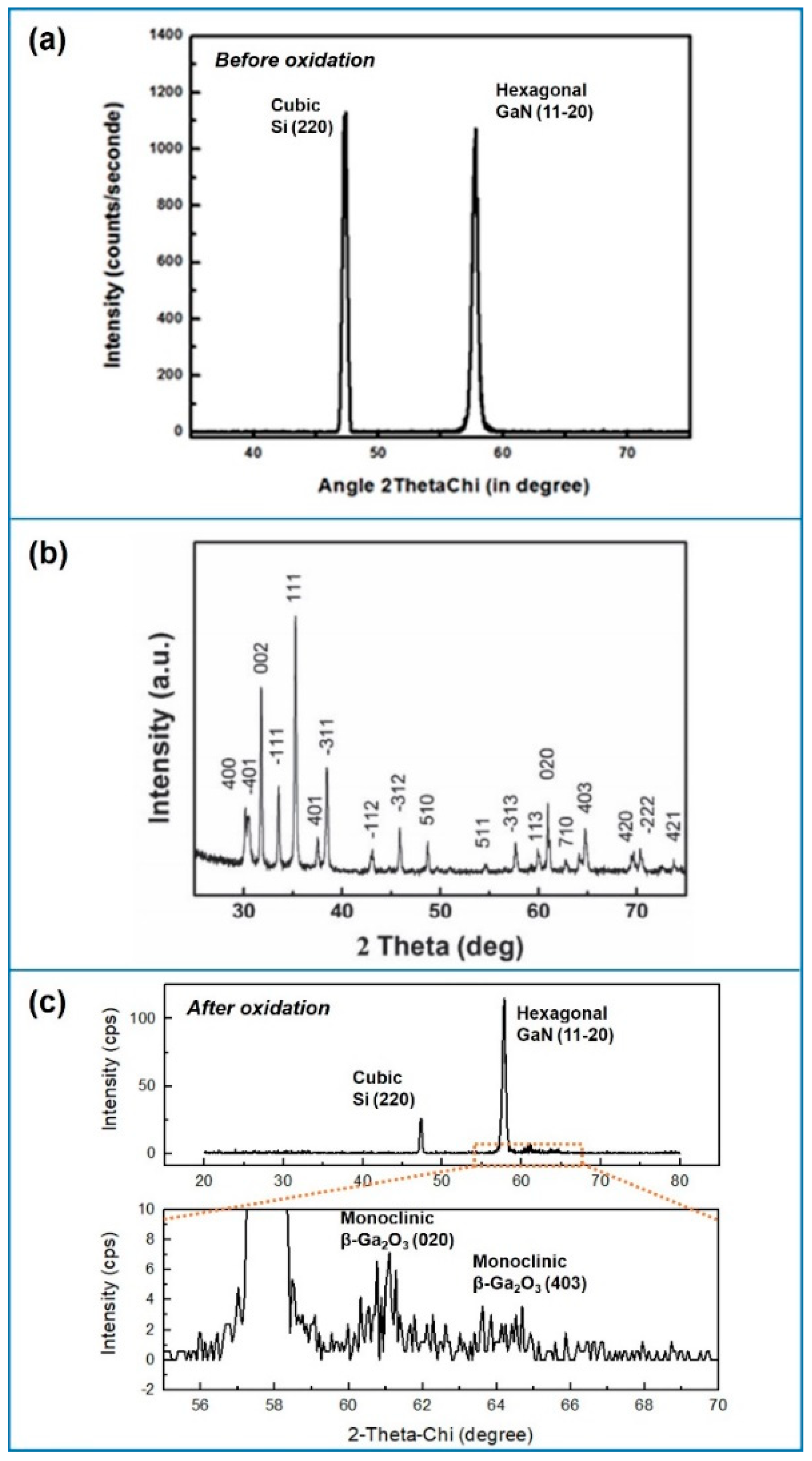
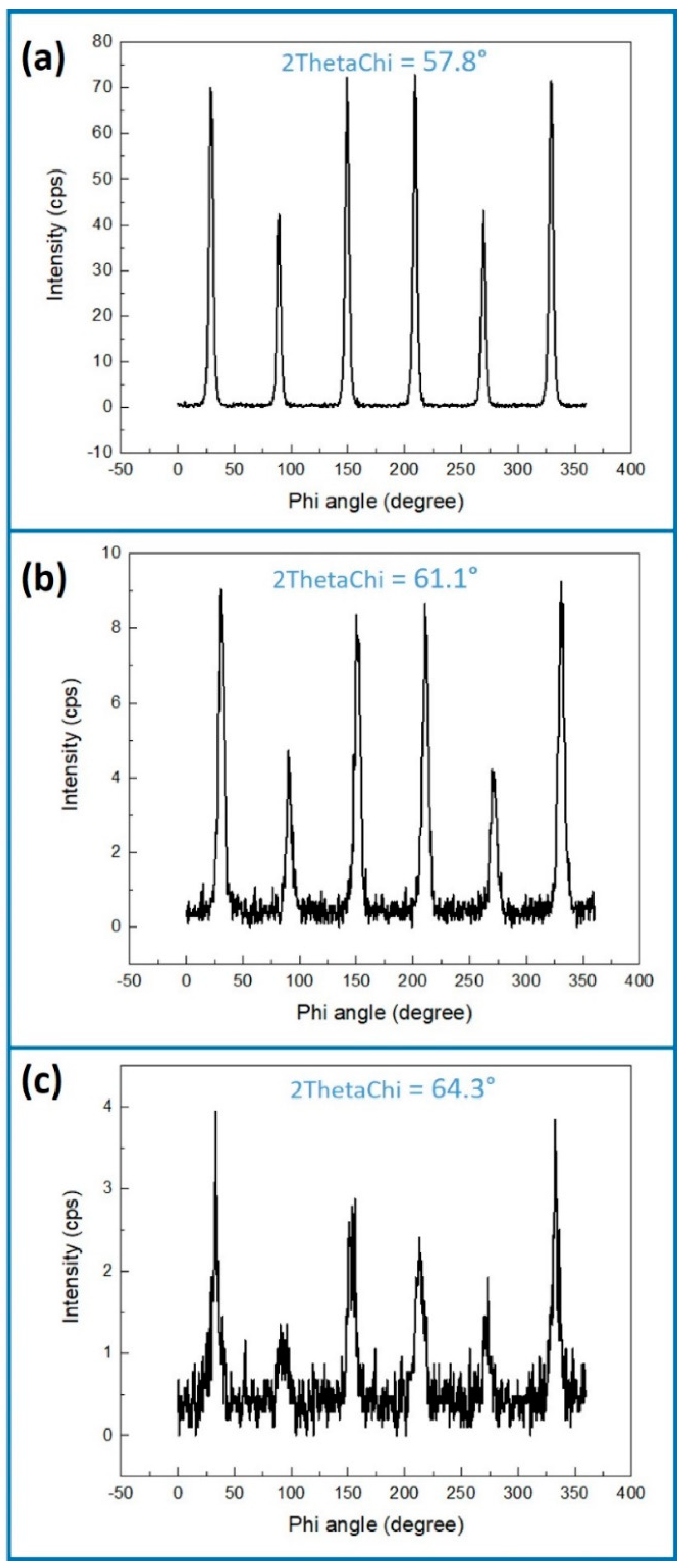
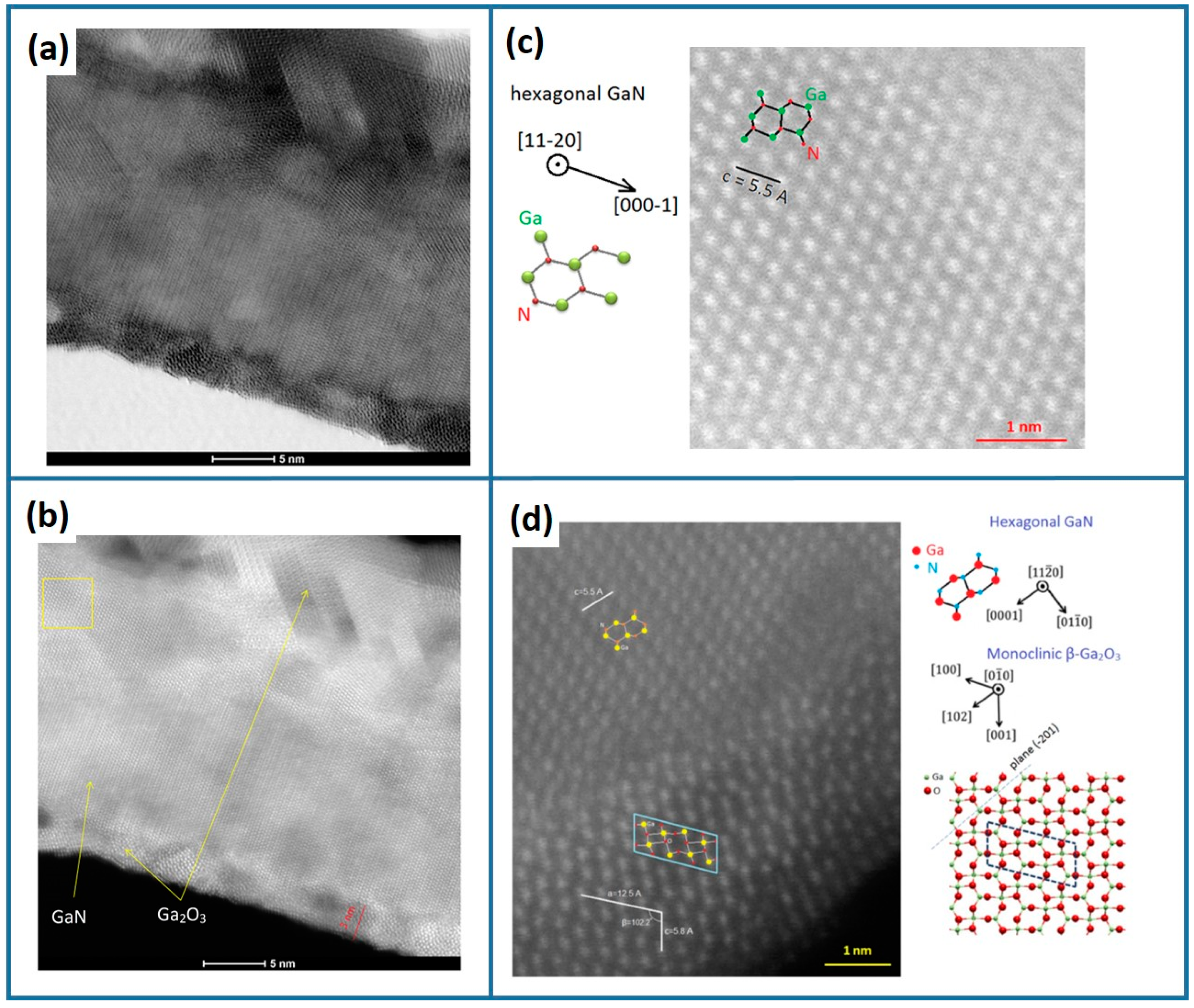
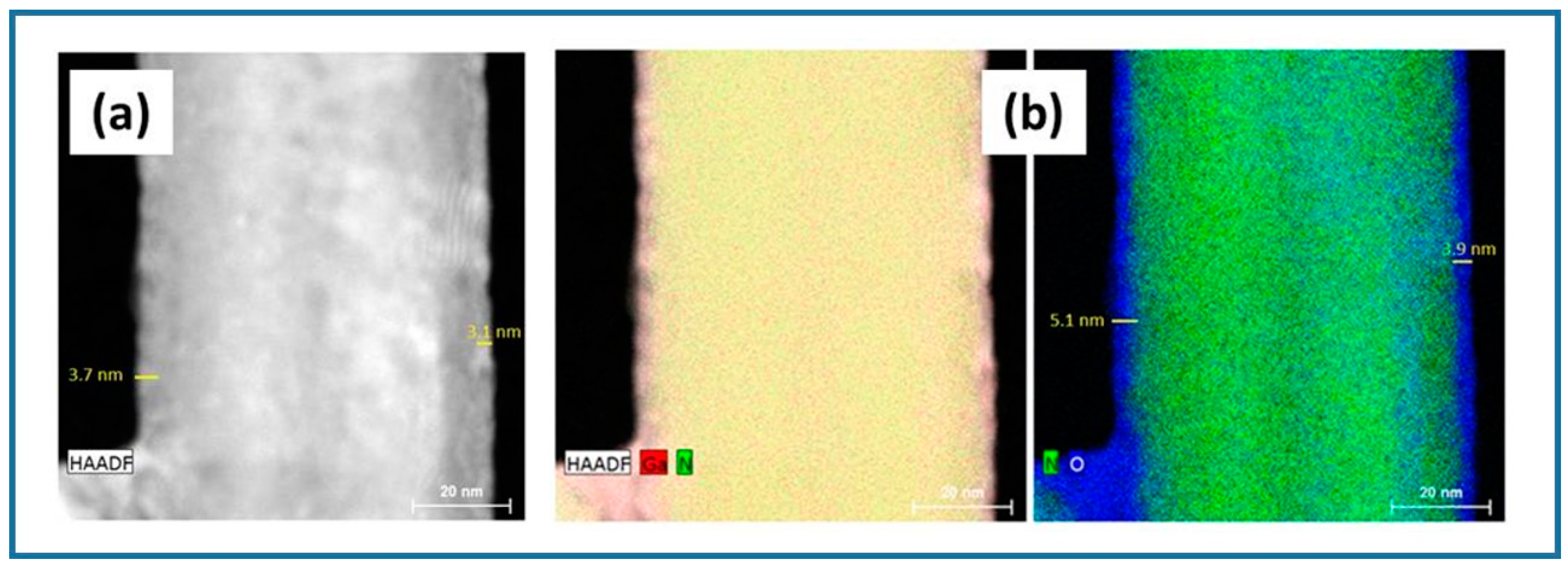
| Factor | Temperature (°C) | O2 Flux (sccm) | N2 Flux (sccm) | Time (min) | |
|---|---|---|---|---|---|
| Series | |||||
| 1 | 900 | 1.3 dry atmosphere | 5 | 5–15 | |
| 2 | 900 | 1.3 humid atmosphere | 5 | 3–15 | |
| 3 | 850 | 1.3 dry atmosphere | 5 | 7–30 | |
| 4 | 850 | 0.4–1.3 dry atmosphere | 5 | 15 | |
| Si (220) 1 | GaN (11-20) | Ga2O3 (020) | Ga2O3 (403) | |
|---|---|---|---|---|
| T = 900 °C, N2/O2 = 5/1.3, Time = 7 min, Shell thickness = 6.9 | 47.3516° | 57.8236° | 61.0465° | 64.6155° |
| T = 850 °C, N2/O2 = 5/1.3, Time = 15 min, Shell thickness = 6.5 | 47.3277° | 57.8005° | 61.0825° | 64.0635° |
| T = 850 °C, N2/O2 = 5/0.8, Time = 15 min, Shell thickness = 8.1 | 47.3262° | 57.8005° | 61.0400° | 64.4000° |
| Pure GaN NWs | 47.3211° | 57.7507° | ||
| Theory 2ThetaChi | 47.3010° | 57.7742° | 60.8969° | 64.6319° |
| a of Si | a of GaN | b of Ga2O3 | |
|---|---|---|---|
| T = 900 °C, N2/O2 = 5/1.3, Time = 7 min, Shell thickness = 6.9 | 5.426 Å | 3.186 Å | 3.033 Å |
| T = 850 °C, N2/O2 = 5/1.3, Time = 15 min, Shell thickness = 6.5 | 5.428 Å | 3.188 Å | 3.031 Å |
| T = 850 °C, N2/O2 = 5/0.8, Time = 15 min, Shell thickness = 8.1 | 5.428 Å | 3.188 Å | 3.034 Å |
| Pure GaN NWs | 5.429 Å | 3.190 Å | |
| Theory parameter | 5.431 Å | 3.189 Å | 3.040 Å |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, Q.C.; Largeau, L.; Morassi, M.; Jegenyes, N.; Mauguin, O.; Travers, L.; Lafosse, X.; Dupuis, C.; Harmand, J.-C.; Tchernycheva, M.; et al. GaN/Ga2O3 Core/Shell Nanowires Growth: Towards High Response Gas Sensors. Appl. Sci. 2019, 9, 3528. https://doi.org/10.3390/app9173528
Bui QC, Largeau L, Morassi M, Jegenyes N, Mauguin O, Travers L, Lafosse X, Dupuis C, Harmand J-C, Tchernycheva M, et al. GaN/Ga2O3 Core/Shell Nanowires Growth: Towards High Response Gas Sensors. Applied Sciences. 2019; 9(17):3528. https://doi.org/10.3390/app9173528
Chicago/Turabian StyleBui, Quang Chieu, Ludovic Largeau, Martina Morassi, Nikoletta Jegenyes, Olivia Mauguin, Laurent Travers, Xavier Lafosse, Christophe Dupuis, Jean-Christophe Harmand, Maria Tchernycheva, and et al. 2019. "GaN/Ga2O3 Core/Shell Nanowires Growth: Towards High Response Gas Sensors" Applied Sciences 9, no. 17: 3528. https://doi.org/10.3390/app9173528
APA StyleBui, Q. C., Largeau, L., Morassi, M., Jegenyes, N., Mauguin, O., Travers, L., Lafosse, X., Dupuis, C., Harmand, J.-C., Tchernycheva, M., & Gogneau, N. (2019). GaN/Ga2O3 Core/Shell Nanowires Growth: Towards High Response Gas Sensors. Applied Sciences, 9(17), 3528. https://doi.org/10.3390/app9173528







