Evaluation of Biochar Nitrate Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
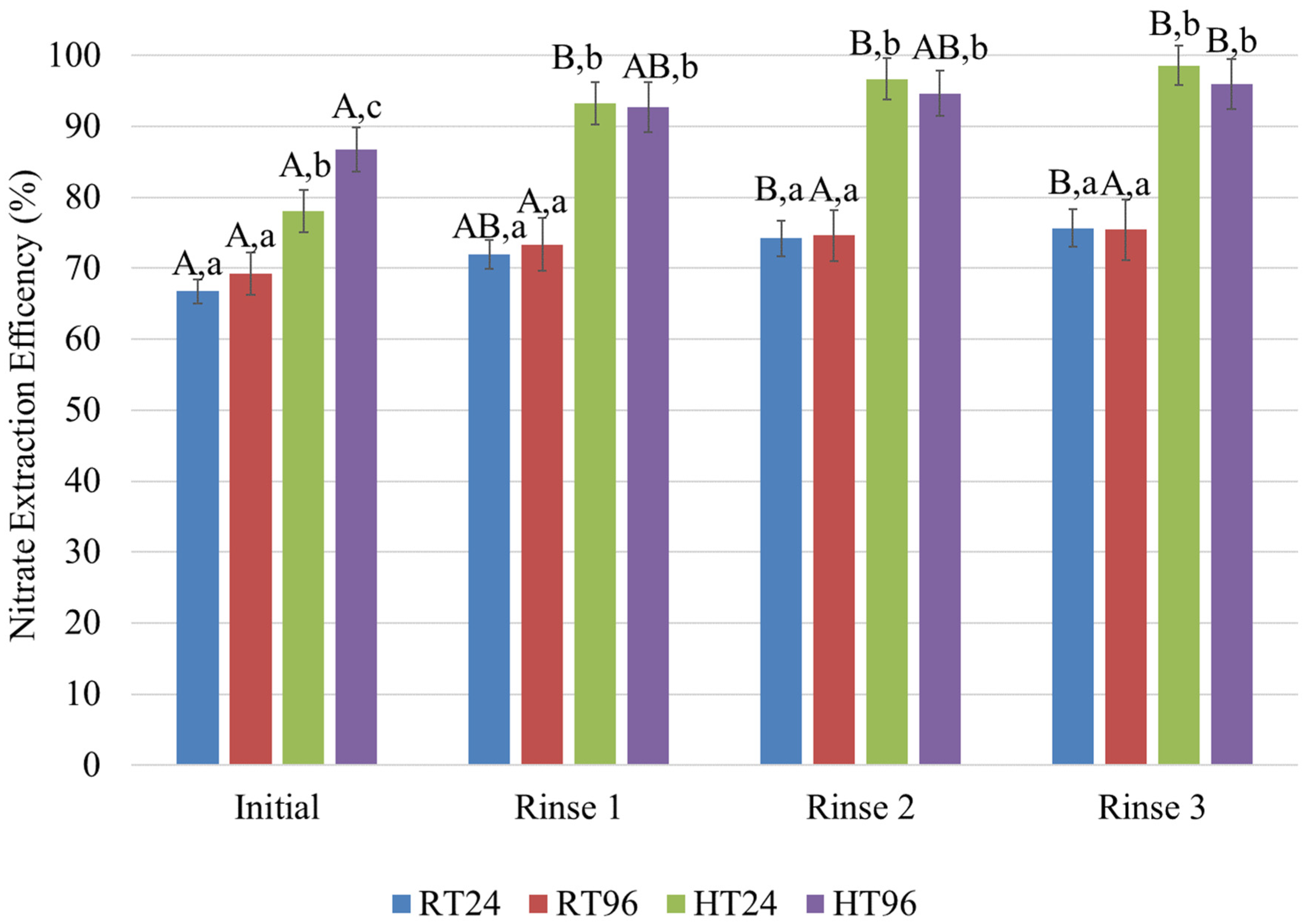
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science, Technology, and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 1–14. [Google Scholar]
- Ippolito, J.A.; Spoka, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Environmental Management: Science, Technology, and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 139–163. [Google Scholar]
- Chia, C.; Downie, A.; Munroe, P. Characteristics of biochar: Physical and structural properties. In Biochar for Environmental Management: Science, Technology, and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 89–109. [Google Scholar]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Sorrenti, G.; Panzacchi, P.; George, E.; Tonon, G. Biochar Reduces Short-Term Nitrate Leaching from A Horizon in an Apple Orchard. J. Environ. Qual. 2012, 42, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Bradley, A.; Larson, R.A.; Runge, T. Effect of wood biochar in manure-applied sand columns on leachate quality. J. Environ. Qual. 2015, 44, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.R.; Larson, R.A.; Runge, T. Nitrate sorption to biochar following chemical oxidation. Sci. Total Environ. 2019, 669, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Stephen, J. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Kammann, C.I.; Schmidt, H.; Kappler, A.; Behrens, S. Nitrate capture and slow release in biochar amended compost and soil. PLoS ONE 2017, 12, e0171214. [Google Scholar] [CrossRef] [PubMed]
- Keeney, D.R.; Nelson, D.W. Nitrogen in organic forms. In Methods of Soil Analysis. Part 2, 2nd ed.; Page, A.L., Ed.; Agron. Monogr. 9. ASA and SSSA: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Haider, G.; Steffens, D.; Müller, C.; Kammann, C.I. Standard Extraction Methods May Underestimate Nitrate Stocks Captured by Field-Aged Biochar. J. Environ. Qual. 2016, 45, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- USEPA. Clean Water Act Analytical Methods: Approved General-Purpose Methods. Available online: http://water.epa.gov/scitech/methods/cwa/methods_index (accessed on 12 June 2019).
- Conte, P.; Hanke, U.M.; Marsala, V.; Cimoò, G.; Alonzo, G.; Glaser, B. Mechanisms of water interaction with pore systems of hydrochar and pyrochar from poplar forestry waste. J. Agric. Food Chem. 2014, 62, 4917–4923. [Google Scholar] [CrossRef] [PubMed]
- SAS. Base SAS® 9.4 Procedures Guide: Statistical Procedures, 2nd ed.; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
| Treatment | Temperature (°C) | Duration (h) |
|---|---|---|
| RT24 | 20 | 24 |
| RT96 | 20 | 96 |
| HT24 | 50 | 24 |
| HT96 | 50 | 96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, J.; Sanford, J.; Larson, R. Evaluation of Biochar Nitrate Extraction Methods. Appl. Sci. 2019, 9, 3514. https://doi.org/10.3390/app9173514
Walsh J, Sanford J, Larson R. Evaluation of Biochar Nitrate Extraction Methods. Applied Sciences. 2019; 9(17):3514. https://doi.org/10.3390/app9173514
Chicago/Turabian StyleWalsh, Jenna, Joseph Sanford, and Rebecca Larson. 2019. "Evaluation of Biochar Nitrate Extraction Methods" Applied Sciences 9, no. 17: 3514. https://doi.org/10.3390/app9173514
APA StyleWalsh, J., Sanford, J., & Larson, R. (2019). Evaluation of Biochar Nitrate Extraction Methods. Applied Sciences, 9(17), 3514. https://doi.org/10.3390/app9173514




