Abstract
Adsorption and desorption of nitrogen (N), phosphorus (P), and potassium (K) soils are controlled by pH, pH buffering capacity, organic matter, and cation exchange capacity (CEC). These factors optimized to improve timely availability of N, P, and K crop use using organic amendments such as chicken litter biochar (CLB). The objective of this study was to determine the effects of CLB on N, P, K sorption and pH buffering capacity of an acid soil. Different rates of CLB were mixed with an acid soil for N, P, and K sorption and pH buffering capacity determination. The CLB increased soil pH and pH buffering capacity, but unlike P and K adsorption, the different rates of CLB significantly increased N adsorption, suggesting that this soil amendment has high affinity for N than P and K. Also, because CLB reduced N, P, and K desorption, it suggests that N in particular will be slowly released with time. The reduced N desorption but higher N adsorption further indicates that N can be temporary fixed by CLB. This work has revealed CLB is more effective controlling soil N availability for timely crop use to avoid losses.
Keywords:
Sorption; acid soils; nutrient release; nutrient availability; soil pH; organic amendments 1. Introduction
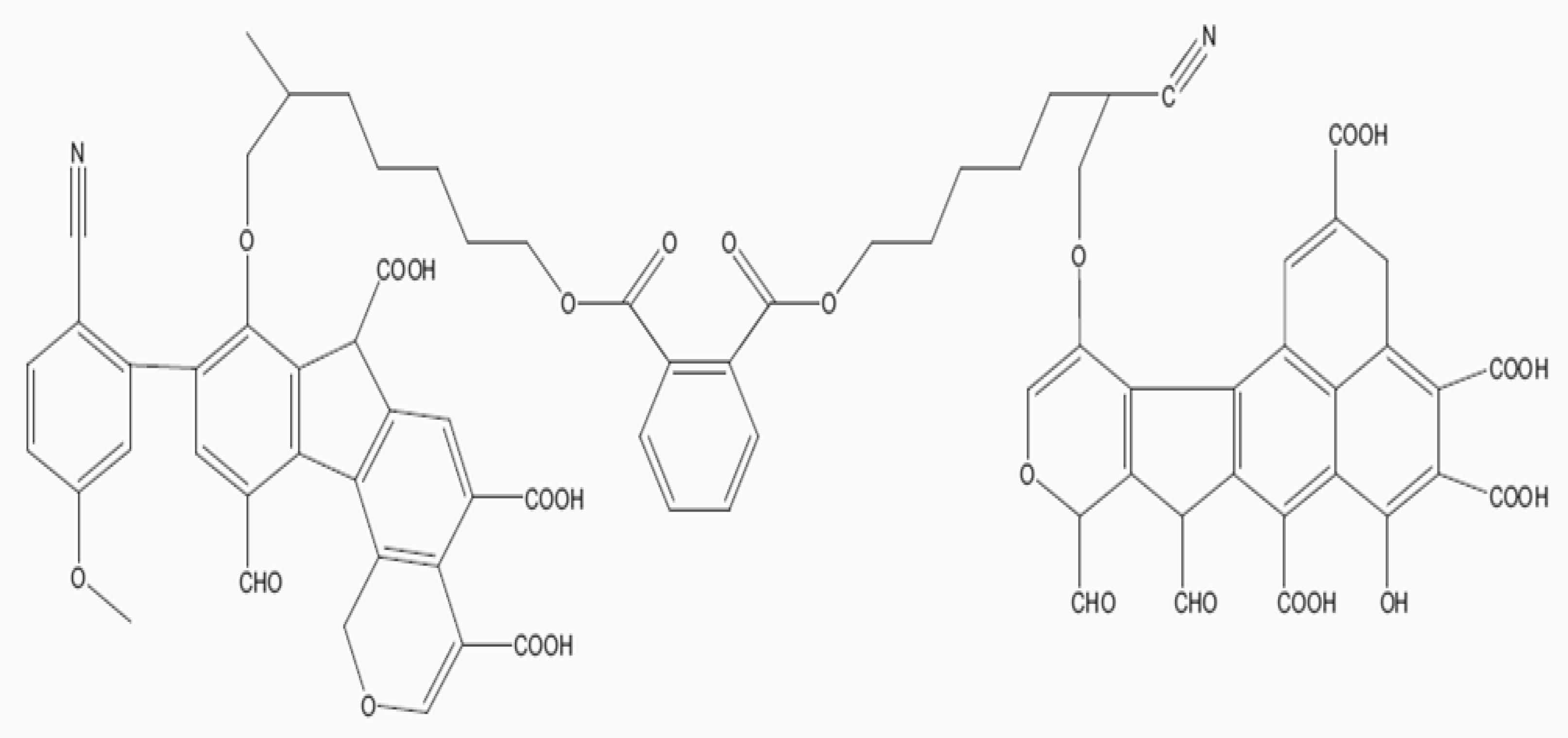
Biochar is a carbon-rich material which is produced through thermal decomposition (pyrolysis) of organic biomass under limited oxygen or no oxygen at relatively low temperature (<700 °C) [1]. Biochars are recalcitrant because of their aromatic and crystalline graphing sheet and this explains why they decompose slower than organic matter [2,3]. Biochars can improve soil and crop productivity because they reside in soils from a few years to thousands of years. As they reside in soils, they are gradually degraded or lost through biotic degradation, abiotic degradation, non-oxidative abiotic degradation, leaching, erosion, and fires. Pyrolytic temperatures produce different honey-comb-like structures because of the original tubular structure of plant cells [4,5]. Moreover, increasing pyrolytic temperature decreases O-H, C=C, C=O stretching, aromatic C=C, C-H deformation modes of alkenes, C-O-C symmetric stretching of cellulose, and hemicellulose [1]. A conceptual model structure of biochar is presented in Figure 1. The functional groups of biochars are reputed to improve soil structure, soil organic matter, cation exchange capacity (CEC), soil pH, and buffering capacity.
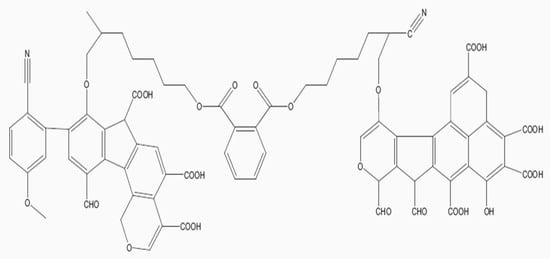
Figure 1.
A conceptual structure of biochar [5].
Biochars adsorb nutrients through electrostatic interaction, ionic exchange, chemical precipitation, and complexation [1]. The pores of biochars do not only serve as habitat for microorganisms but they also stimulate microbial growth because have significant amount of nutrients for microbes to feed on. Their pores also protect microorganisms from their predators and desiccation [3]. It has been reported that biochars can increase N2 fixation in soils [6]. Additionally, biochars are noted for improving soil microbial respiration by creating spaces for soil microbes [7]. This increases soil microbial biodiversity and density [7]. Increasing soil fungi improves soil aggregation through increased fungal hyphae. Moreover, microorganisms which survive in biochars are able to produce polysaccharides and these polysaccharides have been associated with good soil aggregation [2].
Although fertilizers play an important role in crop growth and yield production, their usefulness partly depends on how their nutrients are adsorbed and desorbed. Adsorption is the process by which nutrients in their ionic forms, especially the positive charged ions such as K+, Ca++, Mg++, NH4+ and so on get attached to the negative charged surfaces of minerals such as clinoptilolite zeolite and organic amendments [8]. Functional groups such as carboxyl, phenolic, and alcoholic of organic amendments use to adsorb the aforementioned ions followed by their gradual release (desorption) for timely use by plant. Desorption occurs when adsorbed nutrients are released from the surface of soil organic matter and inorganic minerals [9]. Studies on NH4+ adsorption are essential to enable determination of the ability of soils to respond to N fertilization. Adsorption of NH4+ in soils is important for crop uptake besides minimizing groundwater contamination (for example, eutrophication). Adsorbed nutrients are the most important nutrients because they are available for plant uptake compared with absorbed nutrients. Adsorption of nutrients are significantly controlled by soil pH, temperature, flooding, redox potential, and pH buffering capacity [10]. There is limited information on the effects of soil organic amendments on ammonium, phosphorus, and potassium ions adsorption and desorption [10,11,12,13].
The most common isotherms used in adsorption studies are Langmuir, Freundlich, and Temkin. These isotherms were used in this present study. Langmuir isotherm indicates quantitative formation of a monolayer nutrient (adsorbate) on the outmost surface of the adsorbent and no further adsorption. Therefore, Langmuir is only valid for a monolayer surface adsorption mechanism with fixed number of adsorption sites [14,15]. Freundlich isotherm is an empirical relationship describing adsorption of nutrients to adsorbent surface. It assumes that there are different sites or heterogeneous adsorbent surface with several adsorption energies [10]. Temkin isotherm is used to determine binding energies of the adsorption process. Temkin isotherm assumes that heat of adsorption decreases linearly with increasing adsorbent coverage. The adsorption process occurs with uniform binding energies [16,17].
Among the existing isotherms, the well-fit isotherm is chosen using an error function assessment such as coefficient of determination (R2). This is done to determine fit of isotherm to experimental and experimental equilibrium data. An isotherm is considered well-fit if coefficient of determination (R2) values are greater than 0.9 [12,16]. Although, R2 is the most common error function used to select optimum isotherm in most studies, Chi-square analysis is more appropriate [18]. In this study, because of the high affinity of chicken litter biochar (CLB) for ionic forms of N, P, and K, it is hypothesized that amending acid soil (Typic Paleudults, Bekenu Series) will improve sorption of N, P, and K for optimum crop use. However, there is dearth of information on the N, P, and K sorption characteristics of CLB. The objective of this study was to determine if CLB could be used in acid soils to regulate N, P, and K adsorption and desorption for optimum use of these nutrients by crops.
2. Materials and Methods
2.1. Soil Sampling, Preparation, and Selected Physico-Chemical Analyses
The soil used in this study was Ultisols, Typic Paleudults (Bekenu Series). This soil is fine loamy, siliceous, isohyperthermic, red-yellow to yellow. It has an argillic horizon with fine sandy clay loam texture. The structure is generally weak medium to coarse sub-angular blocky and friable [18]. Although Bekenu Series is less fertile, proper fertilization and management can improve its fertility to support a wide range of crops such as rice, oil palm, rubber, and fruit trees [18]. The soil was sampled at 0 to 25 cm in an uncultivated area of Universiti Putra Malaysia Bintulu Sarawak Campus, Malaysia. Thereafter, the soil samples were air-dried, crushed, and sieved to pass a 2 mm sieve for the adsorption and desorption studies. Soil texture, field capacity, and bulk density were determined using the method described by Tan [19]. pH of the soil was determined in a ratio of 1:2 (soil: distilled water suspension) using a pH meter. Soil total C and N were determined using Leco CHNS Analyzer (LECO Truspec Micro Elemental Analyzer CHNS, New York). Afterwards, the soil C was divided by 0.58 to obtain soil organic matter.
Available P of the soil was extracted using the double acid method [19] followed by the molybdenum blue method [20]. Exchangeable Ca, Mg, and K were extracted using the leaching method [21] after which the contents of these soil cations were determined using Atomic Absorption Spectrophotometry (Analyst 800, Perkin Elmer, Norwalk, CT, USA). Cation exchange capacity was determined using the leaching method [19] followed by steam distillation. The method of Keeney and Nelson [22] was used to extract exchangeable NH4+ and available NO3− after which the concentrations of these soil ions were determined using the steam distillation method. The selected soil properties were comparable to those reported by Paramananthan [18] except CEC, sand, silt, and clay contents. The selected chemical and physical properties of the soil are summarized in Table 1.

Table 1.
Selected physical and chemical properties of Bekenu Series.
The CLB used in this study was imported from Australia and its selected chemical properties are listed in Table 2.

Table 2.
Selected chemical properties of chicken litter biochar.
2.2. Nitrogen Adsorption and Desorption Determination
Two grams sample of each treatment was weighed into a 250 mL centrifuge bottle. Each treatment had three replicates. Nitrogen solutions (0, 50, 100, 200, 300, and 400 mg N L−1) were prepared by dissolving ammonium chloride (NH4Cl) in a 0.2 M NaCl solution after which 20 mL of these isonormal N solutions were added to the centrifuge bottles to give 0, 500, 1000, 2000, 3000, and 4000 µg of added N sample−1. Isonormal solutions were used in this adsorption study to preserve constant ionic strength in the mixture (adsorbent and solution) and also to provide competing ions for exchange sites [23]. Two drops of toluene were added to each sample to suppress microbial activities [13]. The samples were shaken 24 h at 180 rpm using an orbital shaker. Afterwards, the samples were centrifuged at 10,000 rpm for 15 min. Nitrogen in the supernatants (equilibrium solutions) was determined using steam distillation method [24]. The amount of N adsorbed per gram soil was calculated as the difference between the initial amount of N added and the amount in the equilibrium solution. The adsorbed N was calculated in µg g−1 soil. The sediments in the centrifuge bottles) were washed with ethanol and centrifuged at 10,000 rpm for 10 min [24]. Thereafter, the ethanol was discarded. A 20 mL of 2 M KCl was added and shaken for 24 h at 180 rpm using an orbital shaker. Afterwards, the samples were centrifuged at 10,000 rpm for 15 min. The supernatants were collected and their N contents were determined using steam distillation method [24].
2.3. Phosphorus Adsorption and Desorption Determination
Phosphorus solutions (0, 50, 100, 200, 300, and 400 mg P L−1) were prepared by dissolving potassium dihydrogen phosphate (KH2PO4) in 0.01 M CaCl2 solution after which 25 mL of these isonormal P solutions were added to the centrifuge bottles to give 0, 652, 1250, 2500, 3750, and 5000 µg of added P sample−1. The supernatants (equilibrium solution) which were collected after centrifugation were analyzed for P using the Blue Method [20]. For the P desorption study, the same samples or the sediments after centrifugation were used. A 20 mL of 0.01 M CaCl2 was added to each sample and shaken for 24 h at 180 rpm using an orbital shaker. Afterwards, the samples were centrifuged at 10,000 rpm for 15 min. The supernatants were collected and their P were determined using the Blue Method [20].
2.4. Potassium Adsorption and Desorption Determination
Potassium solutions (0, 50, 100, 200, 300, and 400 mg K L−1) were prepared by dissolving potassium chloride (KCl) in 0.01 M CaCl2 solution, and 20 mL of after which isonormal K solutions were added to the centrifuge bottles to give 0, 500, 1000, 2000, 3000, and 4000 µg of added K sample−1. The portions were designed based on the justification of the ionic form for example potassium ions (at difference concentration and equilibrium) dissociates and adsorbed on the surface of adsorbent (in the case of the study, using chicken litter biochar). Difference portions were designed to determine relationship between the adsorbate and adsorbent subject to the equilibrium relationship at a given condition known as an isotherm. The supernatants (equilibrium solution) which were collected after centrifugation was analyzed for K using atomic absorption spectrophotometery (AAnalyst 800, Perkin Elmer Instruments, Norwalk, CT, USA). Potassium desorption was carried out on the same samples after the sediments were washed with ethanol and centrifuged at 10,000 rpm for 10 min after which the ethanol was discarded. A 20 mL of 0.01 M CaCl2 was added and shaken for 24 h at 180 rpm using an orbital shaker. Afterwards, the samples were centrifuged at 10,000 rpm for 15 min. Potassium in the supernatants were determined using atomic absorption spectrophotometery (AAnalyst 800, Perkin Elmer Instruments, Norwalk, CT, USA).
2.5. Nitrogen, Phosphorus, and Potassium Adsorption Isotherms
The N, P, and K adsorption data were fitted to Langmuir types 1–4, Freundlich, and Temkin adsorption isotherms (Table 3) [16,25,26].

Table 3.
List of adsorption isotherms models used in this study and their nonlinear and linear forms.
The linear form of the Langmuir isotherm is reported as:
where: Ce is the equilibrium concentration in liquid phase (mg L−1); qe is the maximum amount of the ions adsorbed at equilibrium (mg g−1); qm is maximum theoretical adsorbed amount at equilibrium (mg g−1); KL is the sorption equilibrium constant (L mg−1).
Ce/qe = Ce/qm + 1/(qmKL)
The linear form of the Freundlich isotherm is reported as:
where: Ce is the equilibrium concentration in liquid phase (mg L−1); qe is the maximum amount of ions adsorbed at equilibrium (mg g−1); KF is the Freundlich adsorption capacity; 1/n is the sorption constant having a value range between 0 and 1.
log (qe) = log (KF) + 1/n log (Ce)
The linear form of the Temkin isotherm is reported as:
where: qe is the amount of ions sorbed (mg L−1); BTln is the equilibrium concentration of adsorbate (mg L−1); KTCe is the parameters.
qe = BTln(KTCe)
2.6. Treatments for Adsorption, Desorption, and pH Buffering Capacity Studies
The treatments evaluated in this study were prepared by mixing chicken litter biochar at different rates with 250 g of soil.
Details of the treatments were as follows:
| Soil | : Soil Alone |
| CLB | : chicken litter biochar alone |
| B1 | : 250 g soil + 20 g chicken litter biochar |
| B2 | : 250 g soil + 40 g chicken litter biochar |
| B3 | : 250 g soil + 60 g chicken litter biochar |
2.7. pH Buffering Capacity of Amendments and Soil Determination
pH buffering capacity of the amendments and soil were determined using titration method [26]. Titration curves were plotted by adding incremental mmol H+ kg−1 samples in water. The ratio of 1:2.5 sample:water (w:v) was used for soil alone, B1, B2, and B3, whereas 1:5 sample:water (w:v) ratio was used for CLB because of the high water absorption capacity of CLB. For each titration, 10 g of soil, B1, B2, and B3 were weighed into 100 mL plastic vials using a digital weighing balance after which 25 mL of distilled water were added. A 5 g of CLB was weighed into a 100 mL plastic vial using a digital weighing balance after which 25 mL of distilled water were added. All the samples were in triplicate. For each sample, 1 mL of 0.05 M CaCl2 was added to minimize variations in ionic strength. A 0.2 mL of toluene was added to inhibit microbial activities [27]. The suspension was shaken for 15 min at 180 rpm using an orbital shaker and equilibrated for seven days at 25 °C because it takes approximately three to six days for the suspension to equilibrate [27,28,29].
At seven days of equilibration, the initial pH of the suspensions was determined using a digital pH meter (SevenEasy pH, Mettler-Toledo GmbH, Switzerland). A 0.1 M NaOH was added to the samples with very low initial pH (pH less than 5.5) using Eppendorf pipette. This was also done to reduce suspension effect [26]. A 0.1 M HCl was used on the samples with slightly acidic to basic initial pH (pH higher than 6). In all of the samples, 1, 2, 3, 4, 6, 8, and 10 mL of 0.1 M HCl or 0.1 M NaOH were used. After addition of 0.1 M HCl or 0.1 M NaOH, the suspensions were stirred thoroughly using a glass rod for 10 s after which pH of the suspensions were determined using a digital pH meter. The quantity of mmol H+ which can change pH by one unit was calculated as the negative reciprocal of slope of the linear regression, where, slope = fitted slope of linear regression line for each sample as given in Formula (1).
2.8. Statistical Analysis
Analysis of variance (ANOVA) was used to detect significant differences between treatments and added N, P, and K, whereas Tukey’s Test was used to compare treatments and added N, P, and K means. PROC REG was used to test linear regression and to as well obtain coefficient of determination (R2) for each linear regression equation. Statistical Analysis System version 9.2 was used for the afore-stated statistical analyses (SAS, 2008). Chi-square analysis (χ2) was done for N, P, and K adsorption isotherm equations to choose the best-fit isotherm. The isotherm model that gave the lowest chi-square value was considered the best best-fit isotherm. Chi-square value was calculated using the following formula:
where, qe is the equilibrium capacity from the experimental data and qe,m is the equilibrium capacity obtained by calculation from model.
3. Results
3.1. Nitrogen, Phosphorus, and Potassium Concentrations in the Solution
Nitrogen, P, and K in the equilibrium solution increased with increasing addition of N, P, and K (Table 4, Table 5 and Table 6). Nitrogen in the equilibrium solution of CLB was the lowest N compared with those in B1, B2, and B3 solutions (Table 4). However, P and K in the equilibrium solutions of CLB were highest compared with those in soil, B1, B2, and B3 solutions (Table 5 and Table 6). Increasing amount of CLB (B1 < B2 < B3) decreased N and K in the equilibrium solutions (Table 4 and Table 6).

Table 4.
Added nitrogen concentration on equilibrium solution nitrogen concentration in different treatments.

Table 5.
Added Phosphorus concentration on equilibrium solution phosphorus concentration in different treatments.

Table 6.
Added potassium concentration on equilibrium solution potassium concentration in different treatments.
There was a linear relationship between N, P, and K concentrations in the equilibrium solution and N, P, and K addition (Table 7). Compared with soil alone, there was marginal N, P, and K increase in the remaining equilibrium solutions of CLB because of the relatively higher N, P, and K of CLB (Table 2). However, increasing rate of the CLB (B1 < B2 < B3) decreased the rate of N, P, and K remaining in the equilibrium solutions (Table 7).

Table 7.
Regression equations and R2 values in relation to nitrogen, phosphorus, and potassium concentrations and nitrogen, phosphorus, and potassium contents remaining in the equilibrium solutions of treatments.
3.2. Nitrogen, Phosphorus, and Potassium Adsorption
Generally, N, P, and K adsorbed increased with increasing rates of N, P, and K (Table 8, Table 9 and Table 10). Unlike K, adsorbed N and P increased with increasing rate of CLB (B1 < B2 < B3) (Table 8, Table 9 and Table 10).

Table 8.
Nitrogen addition to nitrogen adsorption based on different treatments.

Table 9.
Phosphorus addition to phosphorus adsorption based on different treatments.

Table 10.
Potassium addition to potassium adsorption based on different treatments.
3.3. Nitrogen Adsorption Isotherms
The experimental data on Langmuir N adsorption isotherms 1, 2, and 3 showed negative slopes or intercepts (Table 11). This suggests that N adsorption in this present study does not follow Langmuir approach [30].

Table 11.
Regression equations, Coefficient of determination (R2), and Chi-square analysis (χ2) for the fit of the Langmuir type 1, 2, and 3 isotherms to the nitrogen adsorption data of the treatments.
Nitrogen adsorption followed Freundlich approach because of significant coefficient of determination (R2) and lower χ2 values compared with Langmuir (type 4) and Temkin adsorption equations (Table 12).

Table 12.
Regression equations, Coefficient of determination (R2), and Chi-square analysis (χ2) for the fit of the Langmuir type 4, Freundlich, and Temkin isotherms to the nitrogen adsorption data of the treatments.
Higher KF values of CLB, B1, B2, and B3 (Table 13) indicate that the CLB has higher N adsorption capacity than soil alone. This is possible because of higher CEC of the CLB (Table 2).

Table 13.
Freundlich adsorption capacity (KF) and Freundlich adsorption isotherm constant related to adsorption condition (1/n) for nitrogen adsorption of different treatments.
3.4. Adsorption Isotherms of Phosphorus
For CLB, the negative intercept or slope and insignificant R2 of the Langmuir regression equations (types 1, 2, 3, and 4) suggest that the P adsorption data did not fit Langmuir isotherms (Table 14 and Table 15). Phosphorus adsorption data on soil alone, B1, B2, and B3 fit Langmuir type 2 isotherm. This was because of their significant coefficient of determination (R2) with lower χ2 values compared with those of Freundlich and Temkin (Table 14 and Table 15). Soil alone showed the highest P adsorption bonding energy constant (KL) (Table 16).

Table 14.
Regression equations, Coefficient of determination (R2), and Chi-square analysis (χ2) for the fit of the Langmuir type 1, 2, and 3 isotherms to the phosphorus adsorption data of the treatments.

Table 15.
Regression equations, Coefficient of determination (R2), and Chi-square analysis (χ2) for the fit of the Langmuir type 4, Freundlich, and Temkin isotherms to the phosphorus adsorption data of the treatments.

Table 16.
Variables from Langmuir and Freundlich isotherms for phosphorus adsorption of different treatments.
3.5. Potassium Adsorption Isotherms
Based on the significant coefficient of determination (R2) and lower χ2 values, K adsorption data on soil alone, B1, and B2 fit Freundlich (Table 17) whereas K adsorption data on CLB and B3 fit Langmuir type 4 isotherm. Chicken litter biochar only showed lower Langmuir bonding energy constant (KL) compared with that of B3 (Table 17 and Table 18). The maximum adsorption capacity (qm) of B3 was higher than with CLB (Table 19).

Table 17.
Regression equations, Coefficient of determination (R2), and Chi-square analysis (χ2) for the fit of the Langmuir type 1, 2, and 3 isotherms to the potassium adsorption data of the treatments.

Table 18.
Regression equations, Coefficient of determination (R2), and Chi-square analysis (χ2) for the fit of the Langmuir type 4, Freundlich, and Temkin isotherms to the potassium adsorption data of the treatments.

Table 19.
Variables from Langmuir and Freundlich isotherms for potassium adsorption of different treatments.
3.6. Nitrogen, Phosphorus, and Potassium Desorption
There was a linear relationship between N and P desorption and addition of N and P (Table 20). Nitrogen and P desorption increased with increasing rate of CLB (B1 < B2 < B3) increased N and P desorption rates but the opposite was true for K desorption (Table 20).

Table 20.
Regression equations and R2 values relating added nitrogen, phosphorus, and potassium as well as desorbed nitrogen, phosphorus, and potassium in treatments.
3.7. Soil Buffering Capacity
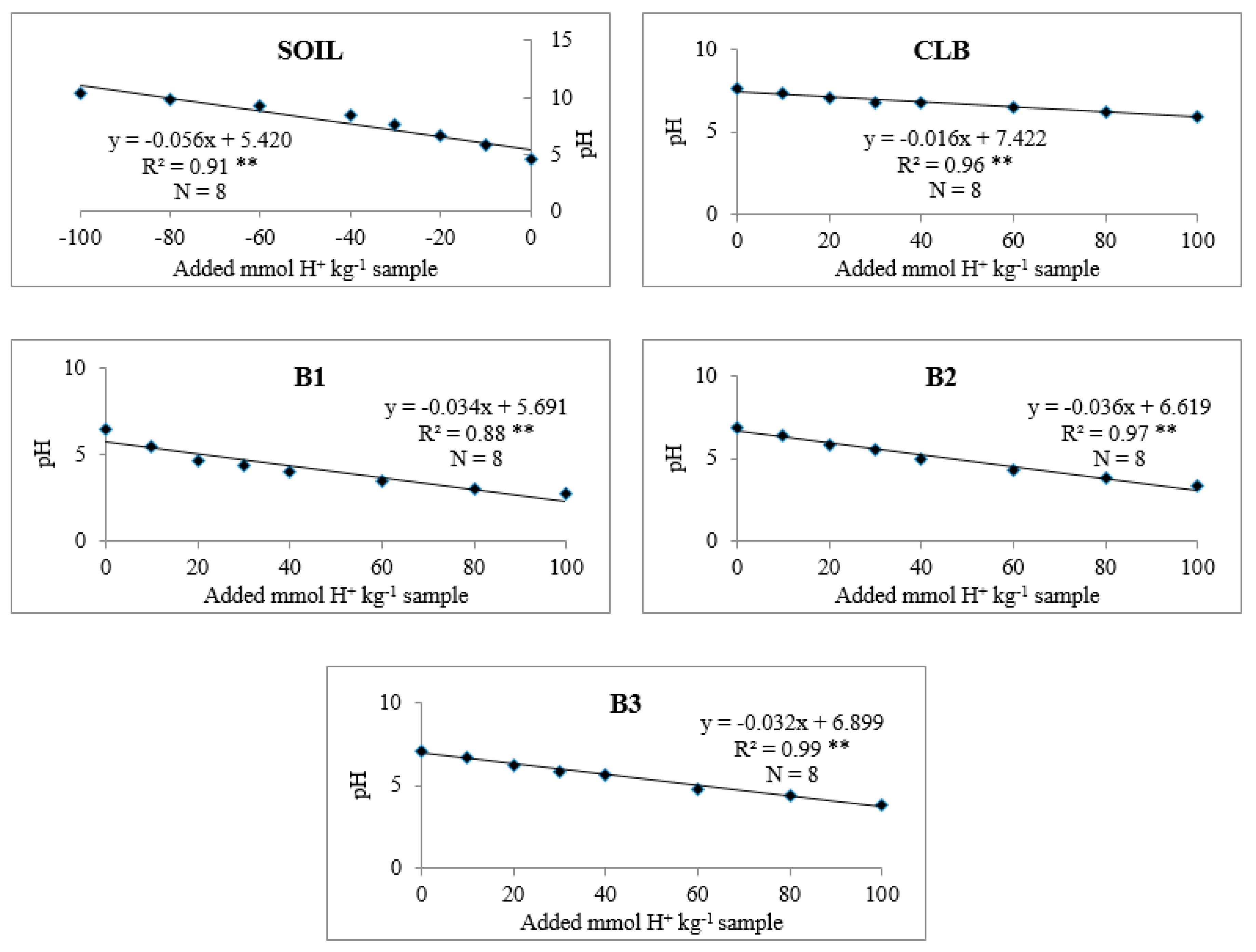
The effects of soil alone, CLB, and B1, B2, and B3 on soil pH buffering capacity are shown in Figure 2 and Table 21. Regardless of treatment, there was a negative linear relationship between soil pH and added mmol H+. Soil alone showed the lowest soil pH and pH buffering capacity (Figure 2).
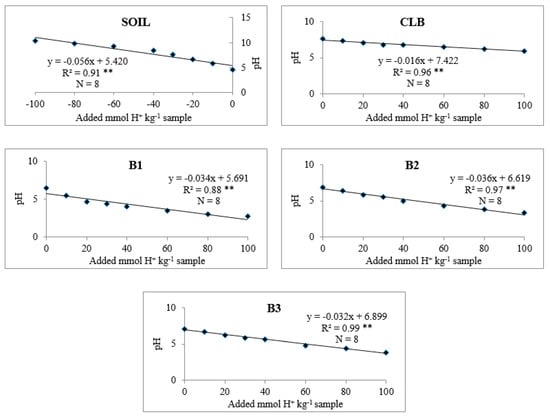
Figure 2.
Linear regression between the added mmol H+ kg−1 sample and the pH of suspension show coefficients of determination (R2) > 0.73 and ** significant at P = 0.01.

Table 21.
Effects of treatments on initial suspension pH and pH buffering capacity.
4. Discussion
4.1. Concentrations of Nitrogen, Phosphorus, and Potassium in Equilibrium Solution
The higher P and K of the equilibrium solution of CLB were because of the initial contents of these nutrients in the CLB (Table 2). The lower N of the CLB was due to N loss during pyrolysis [31]. However, the decreasing contents of N, P, and K in the equilibrium solution with increasing rate of CLB (B1, B2, and B3) suggest that addition of this organic amendment increased N, P, and K adsorption. The increasing or similar amounts of P or K remaining in the equilibrium solution with increasing amount of CLB indicate that addition of CLB to the acid soil used in this present study did not maximize adsorption of these nutrients.
4.2. Adsorption of Nitrogen, Phosphorus, and Potassium
The CLB adsorbed more N, P, and K than with soil alone because of the higher CEC of the organic amendment (Table 2). The increase in N, P, and K adsorption with increasing rate of the CLB, the lowest rate of the CLB is enough adsorb significant amounts of P and K because the adsorption of these ions with higher amounts of the CLB were similar to the lower CLB rate.
4.3. Adsorption Isotherms of Nitrogen, Phosphorus, and Potassium
The fact that N adsorption followed only Freundlich isotherm (Table 11 and Table 12) suggests that N adsorption in all of the treatments occurred on the heterogeneous adsorbent surfaces of the samples [10]. Moreover, 1/n > 1 in all of the samples suggests that the N adsorption is an unfavorable adsorption reaction [25]. In terms of P adsorption isotherms, soil alone showed the highest bonding energy constant (KL) (Table 16) because P fixation by exchangeable Al3+ and Fe2+. This reaction transforms for example, orthophosphates or soluble P into insoluble P [32]. Moreover, highly weathered tropical soils tend adsorb P ions because of their anion exchange capacity [33]. On the other hand, B1, B2, and B3 showed lower KL compared with soil alone because of the high CEC of the CLB (Table 2). This suggests that the negative charges of the CLB might have repelled phosphate ions [34].
Maximum adsorption capacity (qm) is the maximum mass adsorbed at saturation conditions per mass unit of adsorbent in monolayer manners where, high qm requires less P saturation maximum mass adsorbed at saturation conditions per mass unit of adsorbent [35]. Thus, the higher maximum adsorption capacities (qm) of B1, B2, and B3 compared with soil alone suggest that B1, B2, and B3 required less P to saturate the adsorbent because of the highly negative charged exchange sites of the chicken litter biochar repelled P from being adsorbed. Furthermore, the relatively higher P of the chicken litter biochar (Table 2) reduced P requirement from other sources to saturate the adsorbent. Langmuir P adsorption isotherm on soil alone, B1, B2, and B3 indicates that P was adsorbed by formation of a monolayer on the outermost surface of the adsorbent [12,13]. Phosphorus adsorption was consistent with Freundlich isotherm because of lower χ2 value compared with Temkin isotherm. This finding suggests that P adsorption occurred on the heterogeneous adsorbent surface of CLB and this is inconsistent with Langmuir monolayer formation assumption [10]. Maximum buffering capacity (MBC) is defined as the ability of an adsorbent to replenish ions in for example, soil solution [35]. This explains why soil alone showed the highest release P into the soil solution compared with B1, B2, and B3.
In terms of K, the lower Langmuir bonding energy constants (KL) of B1, B2, and B3 (Table 17) was due to the initial K content of the chicken litter biochar (Table 2) which might have saturated the adsorption sites with K. The higher maximum adsorption capacity (qm) of B3 compared with CLB suggests that the soil with B3 required less K to saturate the adsorbent [35] because of lower exchange sites to adsorb K (Table 19). The soils with B3 showed higher maximum buffering capacity (MBC) compared with CLB because soils with low CEC can easily release K into soil solution (Table 19). According to Salarirad and Behnamfard [25], 1/n > 1 of B1 and B2 indicate unfavorable K adsorption.
4.4. Desorption of Nitrogen, Phosphorus, and Potassium
The higher N and P desorption rates of CLB relative to soil alone (Table 20) suggest that the biochar chicken litter is able to temporary retain these ions (Table 2). The decreasing K desorption with increasing rate of CLB was consistent with the preceding finding where it was noticed that higher amendment rate lowered adsorption capacity.
4.5. Soil Buffering Capacity of Bekenu Series Soil
The lowest soil pH and pH buffering capacity (Figure 2) in soil alone compared with soil with CLB was due to the lower organic matter and CEC of the soil (Table 1). pH buffering capacity of the soil used in this study is typical of Ultisols and Oxisols (9 to 27 mmol kg−1 pH−1) [27]. Because the CLB was higher in organic matter and CEC, pH buffering capacity of the soil with this organic amendment was higher than that of soil alone (Table 21 and Figure 2) [35]. pH buffering capacities of soil alone and the soils with amendments B1, B2, and B3 were within the standard range of 10 to 100 mmol H+ kg−1 pH−1 [26].
5. Conclusions
Chicken litter biochar increased soil pH, and pH buffering capacity but unlike P and K adsorption, the different rates of CLB significantly increased N adsorption, suggesting that this soil amendment has high affinity for N than P and K. Also, because CLB reduced N, P, and K desorption, it suggests that N in particular will be slowly released with time. The reduced N desorption but higher N adsorption further indicates that N can be temporary fixed by CLB. This work has revealed that CLB is more effective in controlling soil N availability for timely crop use to avoid losses than P and K.
Author Contributions
P.P. was responsible for conceptualization, investigation, writing, and original draft preparation as well as for data analysis and visualization. O.H.A. was responsible for supervising, funding acquisition, project administration, experimental methodology, editing, and reviewing. O.L. was responsible for data arrangement and editing second draft. N.M.A.M. was also involve in funding acquisition and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundamental Research Grant Scheme (FRGS), Project Code: 07-01-16-1876FR (FRGS/1/2016/WAB01/UPM/02/2) and Translational Research Grant (TRG), Vote Number 5526500 from the Ministry of Higher Education Malaysia.
Acknowledgments
The authors gratefully acknowledge the financial support of Fundamental Research Grant Scheme (FRGS), Project Code: 07-01-16-1876FR (FRGS/1/2016/WAB01/UPM/02/2) and Translational Research Grant (TRG), Vote Number 5526500 from the Ministry of Higher Education Malaysia and Universiti Putra Malaysia for the collaborative research. Appreciation also goes our administrative and technical support staff for providing materials used for the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nartey, O.D.; Zhao, B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Adv. Mater. Sci. Eng. 2014, 2014, 715398. [Google Scholar] [CrossRef]
- Aslam, Z.; Khalid, M.; Aon, M. Impact of biochar on soil physical properties. J. Agric. Sci. 2014, 4, 280–284. [Google Scholar]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Gurwick, N.P.; Kelly, C.; Elias, P. The Scientific Basis for Biochar as a Climate Change Mitigation Strategy: Does It Measure up? 2012. Available online: http://www.ucsusa.org/sites/default/files/legacy/assets/documents/global_warming/Biochar-Climate-Change-Mitigation-Strategy-Does-It-Measure-Up (accessed on 15 September 2019).
- Zhao, N.; Lv, Y.; Yang, X. A new 3D conceptual structure modeling of biochars by molecular mechanic and molecular dynamic simulation. J. Soils Sediments 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Muller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Reddy, S.B.N. Biocharculture: Biochar for Environment and Development, 1st ed.; Meta Meta: Hertogenbosch, The Netherlands, 2014. [Google Scholar]
- Ahmed, M.F.; Kennedy, I.R.; Choudhury, T.M.; Kecskes, M.L.; Deaker, R. Phosphorus adsorption in some Australian soils and influence of bacteria on the desorption of phosphorus. Commun. Soil Sci. Plant Anal. 2008, 39, 1269–1294. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Nik, A.M. Soil pH buffering capacity and nitrogen availability following rice straw compost application in a tropical acid soil. Compost Sci. Util. 2018, 26, 1–15. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin—Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Hoseini, Y.; Taleshmikaiel, R.D. Comparison of phosphorus adsorption isotherms in soil and its relation to soil properties. Int. J. Agric. Res. Rev. 2013, 3, 163–171. [Google Scholar]
- Okeola, F.O.; Odebunmi, E.O. Freundlich and Langmuir Isotherms parameters for adsorption of methylene blue by activated carbon derived from agrowastes. Adv. Nat. Appl. Sci. 2010, 4, 281–288. [Google Scholar]
- Dada, A.O.; Ojediran, J.O.; Olalekan, A.P. Sorption of Pb2+ from aqueous solution unto modified rice husk: Isotherms studies. Adv. Phys. Chem. 2013, 2013, 842425. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Kwiatkowska-Malina, J.; Malina, G.; Kasela, T. Removal of lead and benzene from groundwater by zeolite and brown coal: Isotherm and kinetic studies. In Proceedings of the 4th International Conference on Environmental Pollution and Remediation, Prague, Czech Republic, 11–13 August 2014; pp. 1–7. [Google Scholar]
- Alfaro-Cuevas-Villanueva, R.; Hidalgo-Vazquez, A.R.; Cortes Penagos, C.D.J.; Cortes-Martinez, R. Thermodynamic, kinetic, and equilibrium parameters for the removal of lead and cadmium from aqueous solutions with calcium alginate beads. Sci. World J. 2014, 2014, 647512. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, B.; Das, A. Linearised and non-linearised isotherm models optimization analysis by error functions and statistical means. J. Environ. Health Sci. Eng. 2014, 12, 92. [Google Scholar] [CrossRef]
- Paramananthan, S. Soils of Malaysia: Their Characteristics and Identification Vol. 1; Academy of Sciences: Kuala Lumpur, Malaysia, 2000. [Google Scholar]
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Murphy, J.; Riley, J. A modified single solution for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen-inorganic forms. In Methods of Soil Analysis Part 2; Page, A.L., Keeney, D.R., Baker, D.E., Miller, R.H., Ellis, R.J., Rhoades, J.D., Eds.; ASA and SSSA: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Kithome, M.; Paul, J.W.; Lavkulich, L.M.; Bomke, A.A. Kinetics of ammonium adsorption and desorption by the natural zeolite clinoptilolite. Soil Sci. Soc. Am. J. 1998, 62, 622–629. [Google Scholar] [CrossRef]
- Chowdhury, S.; Misra, R.; Kushwaha, P.; Das, P. Optimum sorption isotherm by linear and nonlinear methods for safranin onto alkali-treated rice husk. Bioremediat. J. 2011, 15, 77–89. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis, Part 2; Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.E., Dinauer, R.D., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Salarirad, M.M.; Behnamfard, A. Modelling of equilibrium data for free cyanide adsorption onto activated carbon by linear and non-linear regression methods. In Proceedings of the 2011 International Conference on Environment and Industrial Innovation IPCBEE, Singapore, 4–5 June 2011; Volume 12, pp. 79–84. [Google Scholar]
- Rowell, D.L. Soil Science: Methods and Applications; Longman Scientific & Technical: Essex, UK, 1994. [Google Scholar]
- Xu, R.; Zhao, A.; Yuan, J.; Jiang, J. pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J. Soils Sediments 2012, 12, 494–502. [Google Scholar] [CrossRef]
- Kissel, D.E.; Sonon, L.S.; Cabrera, M.L. Rapid measurement of soil pH buffering capacity. Soil Sci. Soc. Am. J. 2012, 76, 694–699. [Google Scholar] [CrossRef]
- Costello, R.C.; Sullivan, D.M. Determining the pH buffering capacity of compost via titration with dilute sulfuric acid. Waste Biomass Valorization 2014, 5, 505–513. [Google Scholar] [CrossRef]
- Kiurski, J.; Adamovic, S.; Oros, I.; Krstic, J.; Kovacevic, I. Adsorption feasibility in the Cr (total) ions removal from waste printing developer. Glob. NEST J. 2012, 14, 18–23. [Google Scholar]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Chan, K.Y.; Xu, Z.H. Biochar: Nutrient properties and their enhancement. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 67–84. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Onyango, M.S.; Masukume, M.; Ochieng, A.; Otieno, F. Functionalized natural zeolite and its potential for treating drinking water containing excess amount of nitrate. Water SA 2010, 36, 655–662. [Google Scholar] [CrossRef]
- Mehdi, S.M.; Obaid-ur-rehman Ranjha, A.M.; Sarfraz, M. Adsorption capacities and availability of phosphorus in soil solution for rice wheat cropping system. World Appl. Sci. J. 2007, 2, 244–265. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).