Preparation and Removal Properties of Cimetidine from Aqueous Solution by Waste Bricks Incorporated with Different Iron Oxides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. The Preparation of Adsorbents
2.3. The Characterization of Waste Bricks
2.4. The Adsorption Experiments of Cimetidine onto Bricks
2.5. Statistical Analyses
3. Results and Discussion
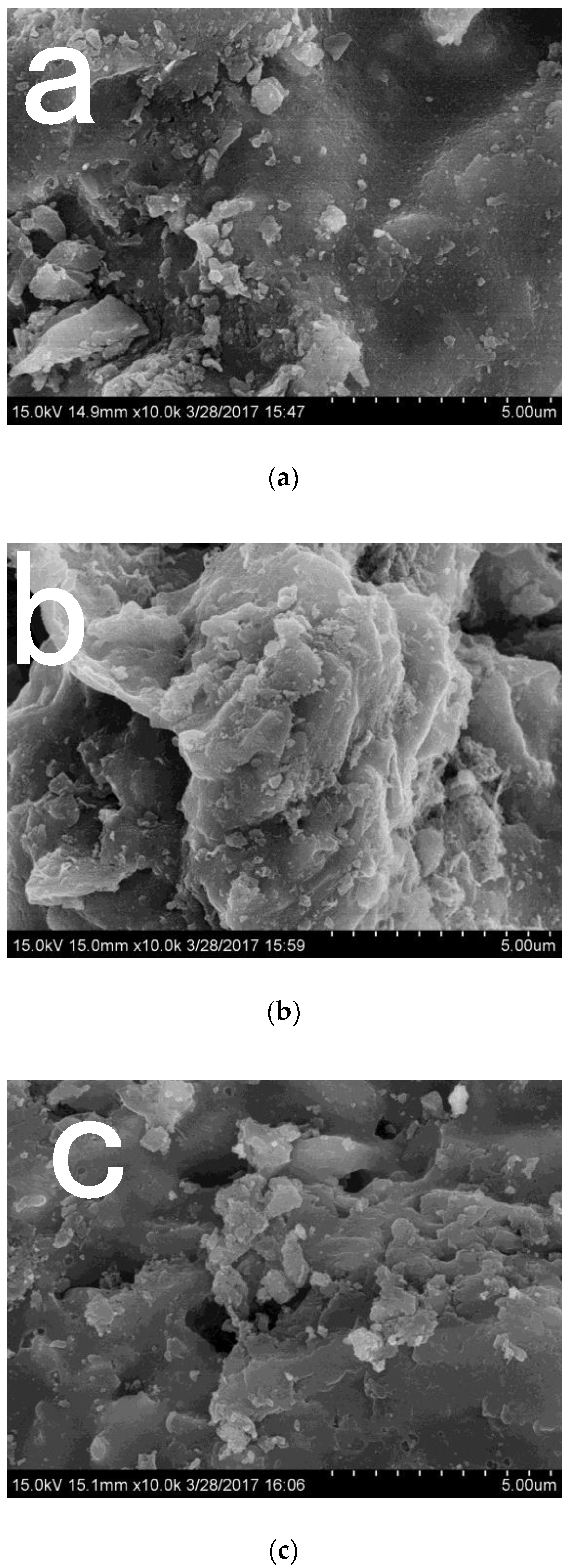
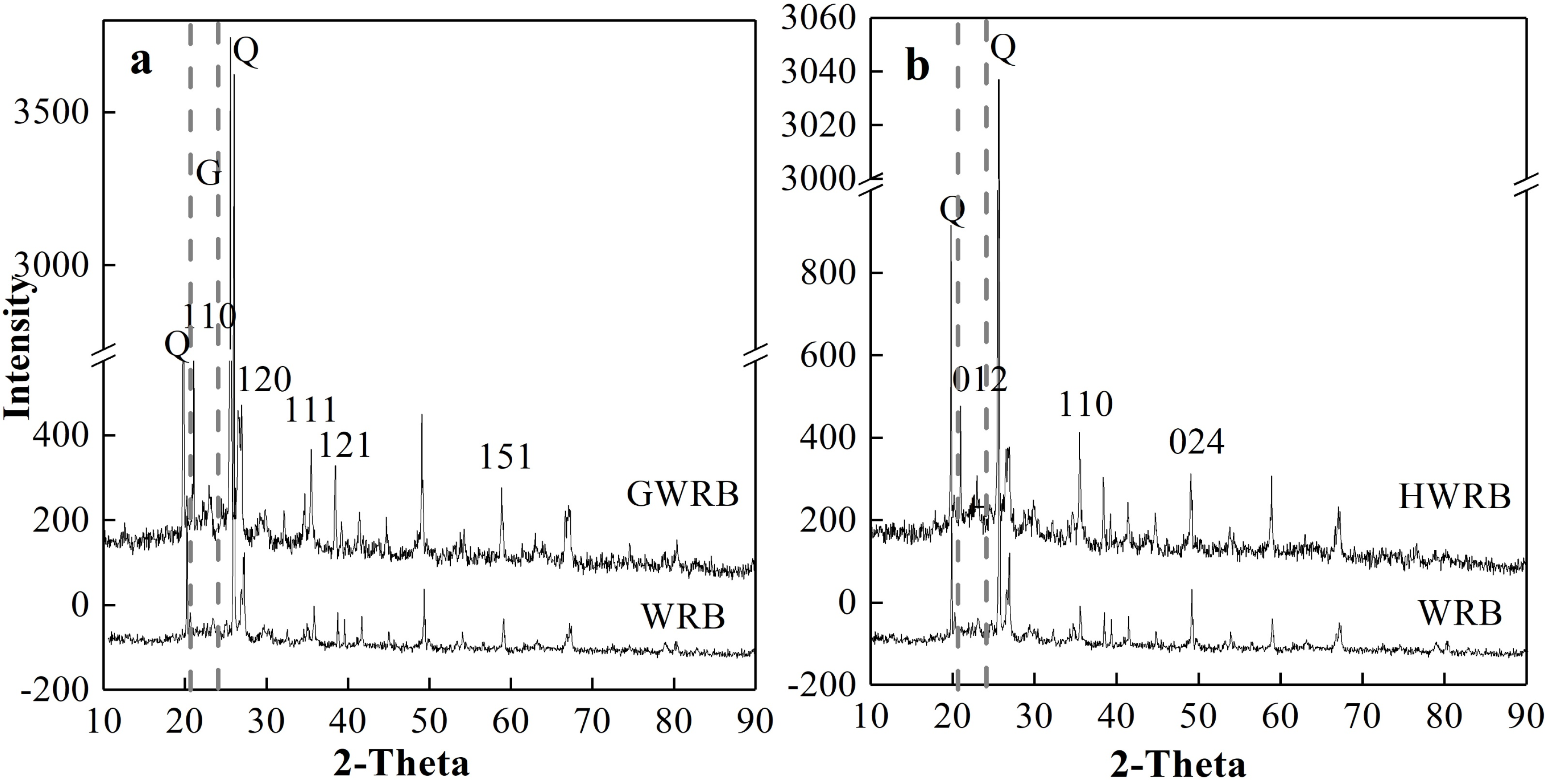
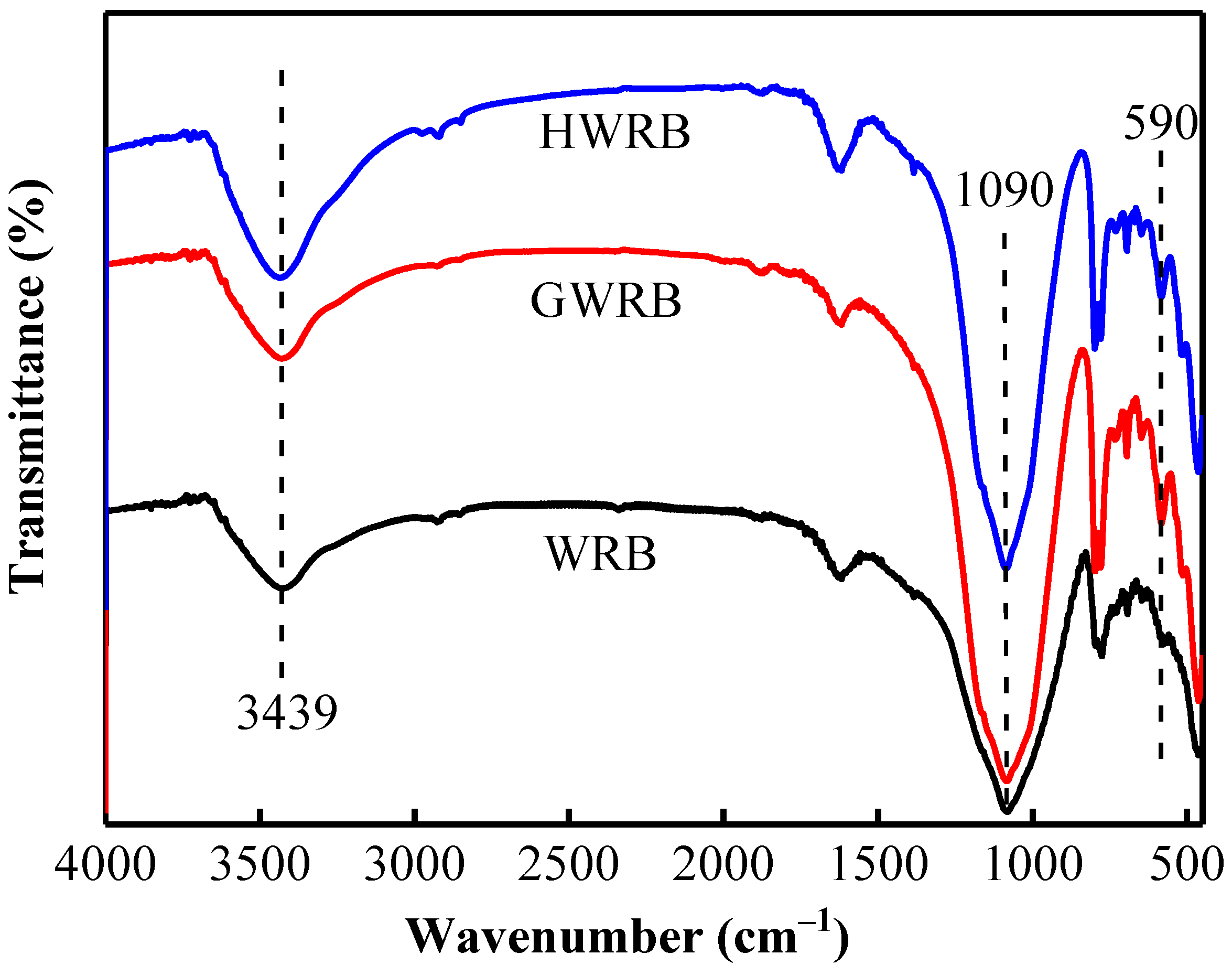
3.1. The Physicochemical Characterization of WRB, GWRB, and HWRB
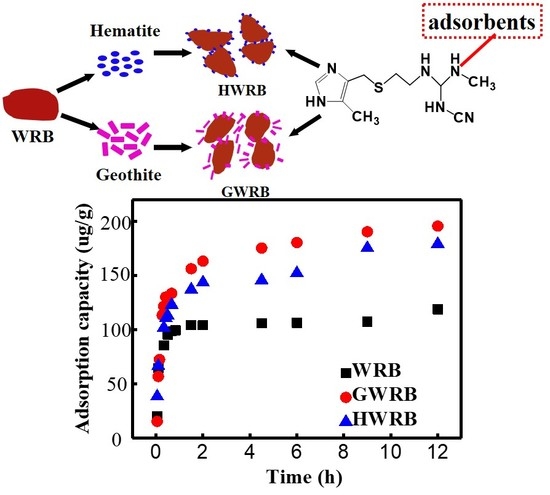
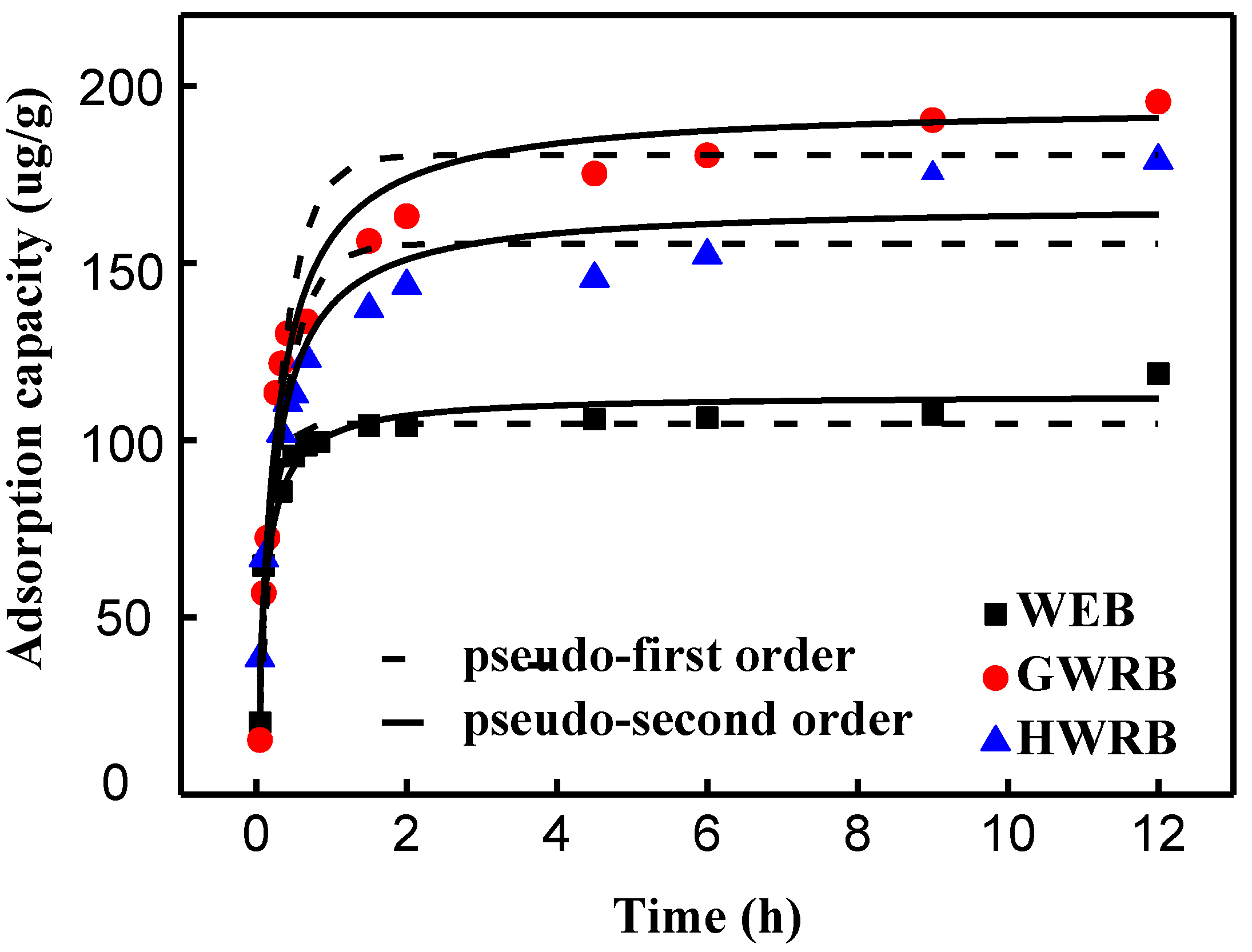
3.2. The Adsorption Kinetics of Cimetidine onto WRB, GWRB, and HWR
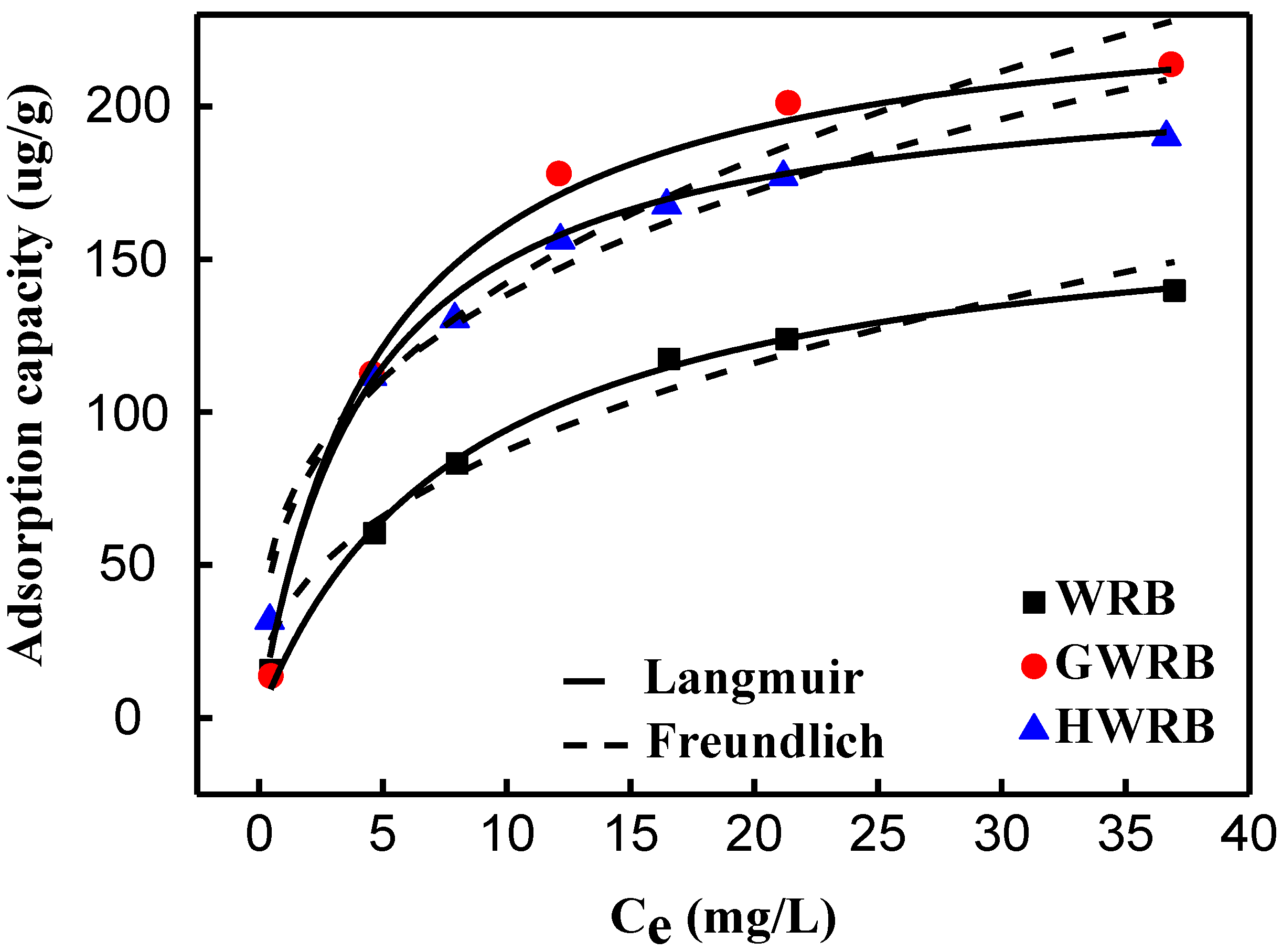
3.3. The Adsorption Isotherms of Cimetidine onto WRB, GWRB, and HWR
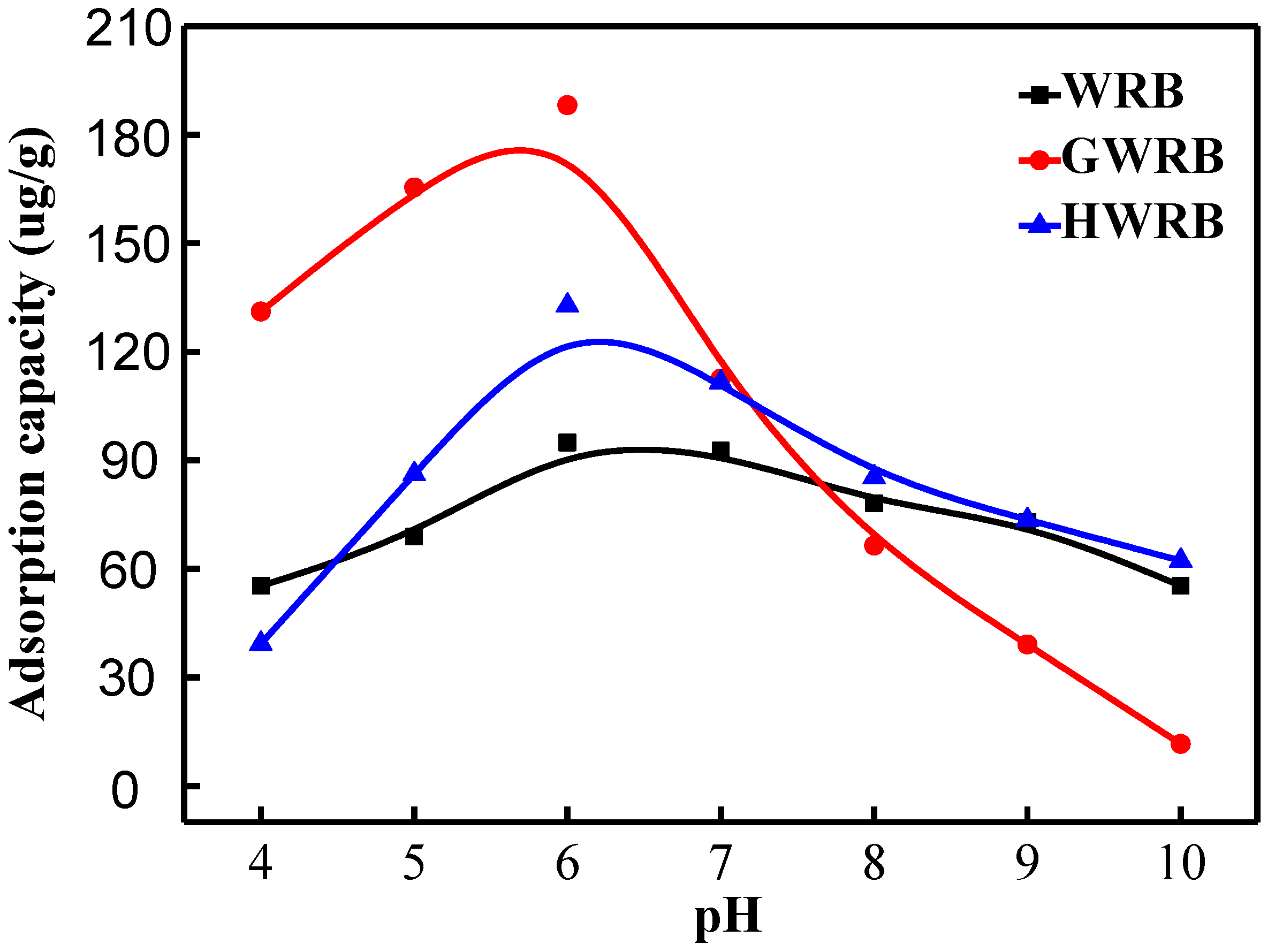
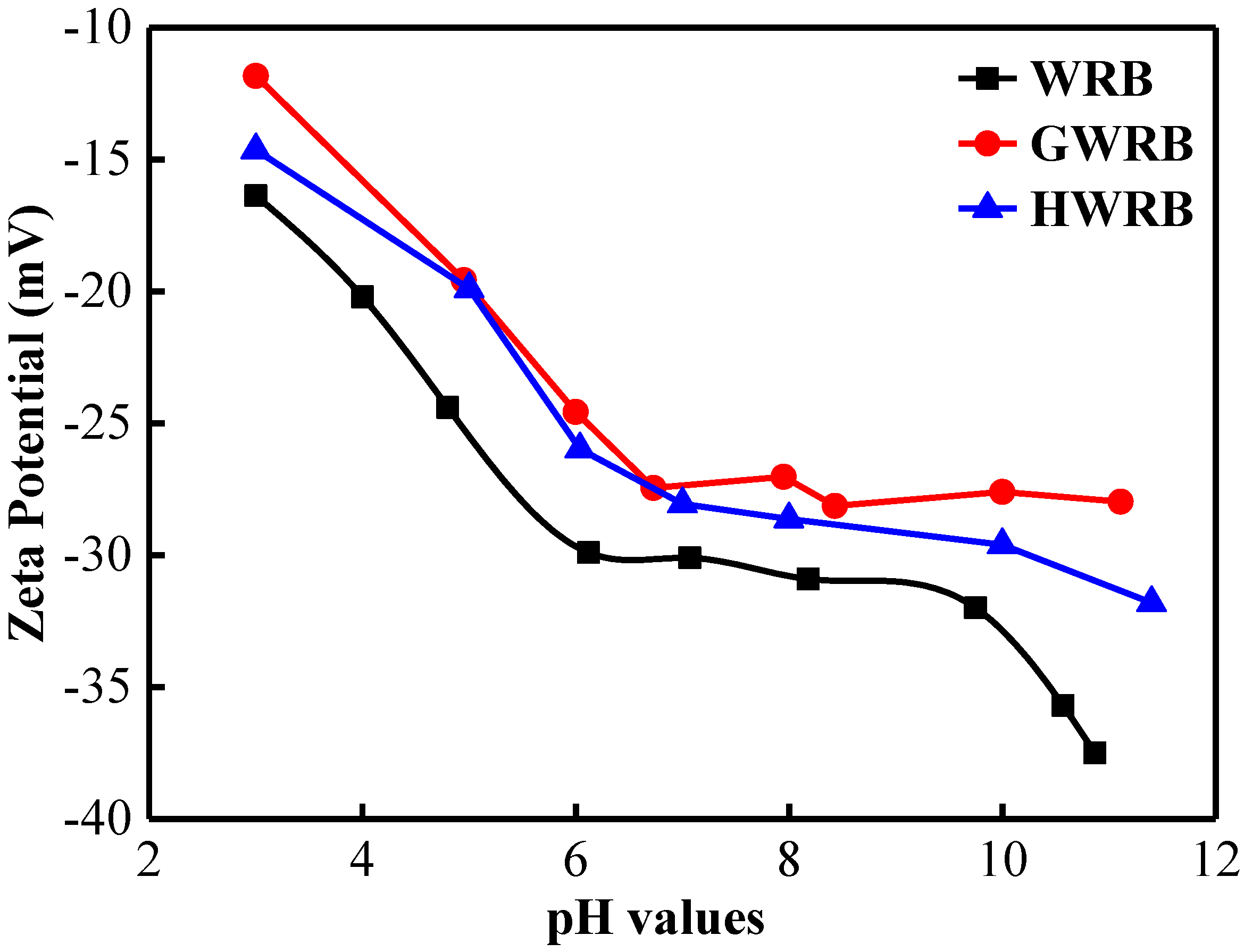
3.4. The Effect of pH on the Adsorption of Cimetidine
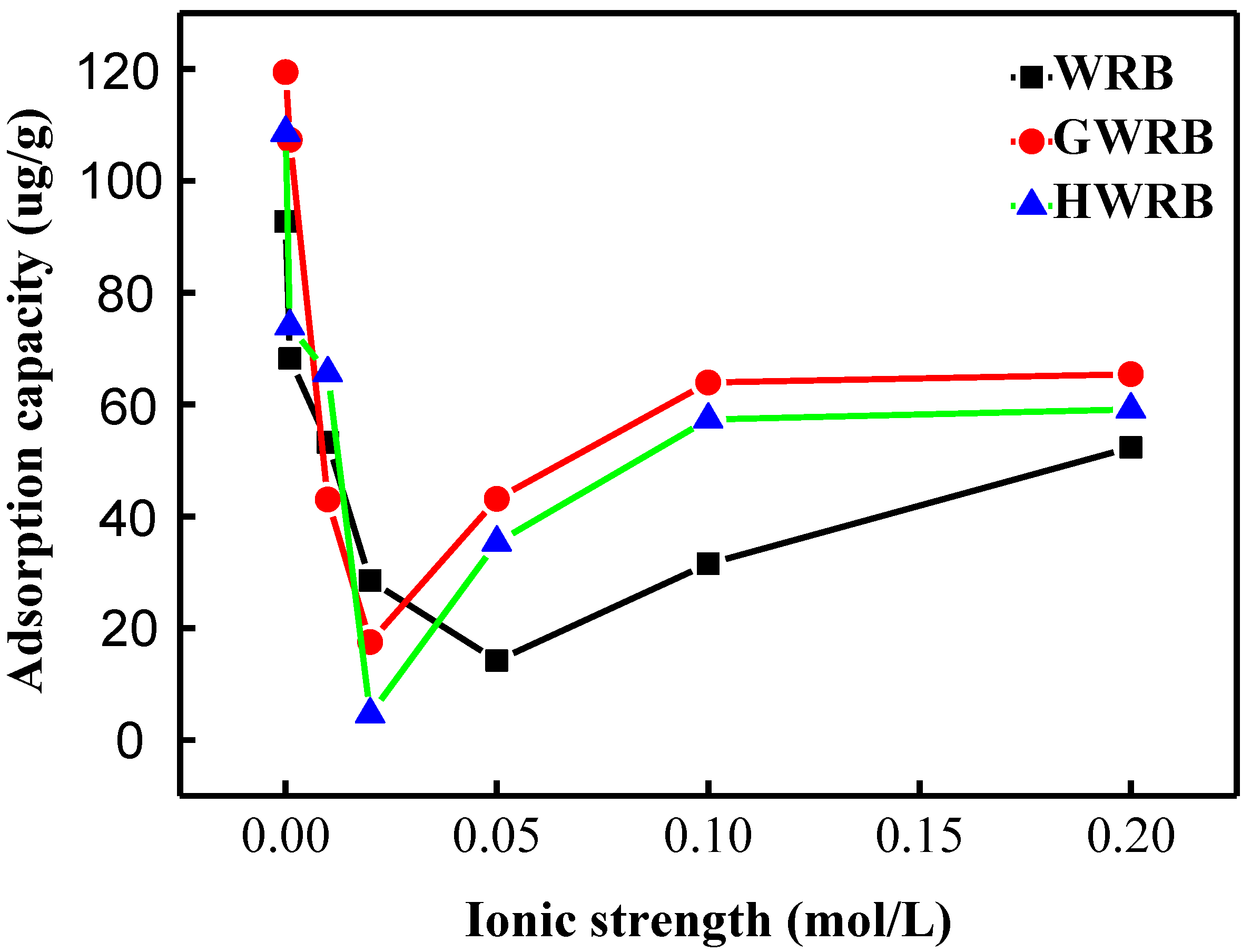
3.5. Effect of Ionic Strength on the Adsorption of Cimetidine onto Bricks
4. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fairbairn, D.J.; Arnold, W.A.; Barber, B.L.; Kaufenberg, E.F.; Koskinen, W.C.; Novak, P.J.; Rice, P.J.; Swackhamer, D.L. Contaminants of Emerging Concern: Mass Balance and Comparison of Wastewater Effluent and Upstream Sources in a Mixed-Use Watershed. Environ. Sci. Technol. 2016, 50, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, B.; Yin, L.; Zhang, Y.; Deng, S.; Huang, J.; Wang, Y.; Yu, G. Characterization of pharmaceutically active compounds in Beijing, China: Occurrence pattern, spatiotemporal distribution and its environmental implication. J. Hazard. Mater. 2017, 323, 147–155. [Google Scholar] [CrossRef]
- Hosseinifard, S.M.; Ahmadpour, A.; Amiri, B.M.; Mansour, M.R.; Ebrahimpour, A. Immunomodulatory effect of cimetidine in common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2013, 39, 1505–1511. [Google Scholar] [CrossRef]
- Shemenski, J.; Shemenski, D.J. Cimetidine as a novel adjunctive treatment for early stage Lyme disease. Med. Hypotheses 2019, 128, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, Y.; Park, J.; Park, C.K.; Kim, M.; Kim, H.S.; Kim, P. Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Sci. Total Environ. 2008, 405, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalez, R.; Rodríguez-Mozaz, S.; Gros, M.; Pérez-Cánovas, E.; Barceló, D.; León, V.M. Input of pharmaceuticals through coastal surface watercourses into a Mediterranean lagoon (Mar Menor, SE Spain): Sources and seasonal variations. Sci. Total Environ. 2014, 490, 59–72. [Google Scholar] [CrossRef]
- Reis, E.O.; Foureaux, A.F.S.; Rodrigues, J.S.; Moreira, V.R.; Lebron, Y.A.; Santos, L.V.; Amaral, M.C.; Lange, L.C. Occurrence, removal and seasonal variation of pharmaceuticals in Brasilian drinking water treatment plants. Environ. Pollut. 2019, 250, 773–781. [Google Scholar] [CrossRef]
- D’Alessio, M.; Onanong, S.; Snow, D.D.; Ray, C. Occurrence and removal of pharmaceutical compounds and steroids at four wastewater treatment plants in Hawai’i and their environmental fate. Sci. Total Environ. 2018, 631, 1360–1370. [Google Scholar] [CrossRef]
- García-García, M.; Liarte, S.; Gómez-González, N.E.; García-Alcázar, A.; Pérez-Sánchez, J.; Meseguer, J.; Mulero, V.; García-Ayala, A.; Chaves-Pozo, E. Cimetidine disrupts the renewal of testicular cells and the steroidogenesis in a hermaphrodite fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 189, 44–53. [Google Scholar] [CrossRef]
- Wang, Z.; Lege, S.; Obst, M.; Roehler, S.; Burkhardt, C.J.; Zwiener, C. Application and characterization of electroactive membranes based on carbon nanotubes and zerovalent iron nanoparticles. Water Res. 2017, 108, 78–85. [Google Scholar]
- Kumari, P.; Bahadur, N.; Dumée, L.F. Photo-catalytic membrane reactors for the remediation of persistent organic pollutants—A review. Sep. Purif. Technol. 2020, 230, 115878. [Google Scholar] [CrossRef]
- Bodhipaksha, L.C.; Sharpless, C.M.; Chin, Y.-P.; Mackay, A.A. Role of effluent organic matter in the photochemical degradation of compounds of wastewater origin. Water Res. 2017, 110, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Tomul, F.; Arslan, Y.; Başoğlu, F.T.; Babuçcuoğlu, Y.; Tran, H.N. Efficient removal of anti-inflammatory from solution by Fe-containing activated carbon: Adsorption kinetics, isotherms, and thermodynamics. J. Environ. Manag. 2019, 238, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, J.; Shen, Y.; Shi, X. Enhanced phosphorus removal in intermittently aerated constructed wetlands filled with various construction wastes. Environ. Sci. Pollut. Res. 2017, 24, 22524–22534. [Google Scholar]
- Saca, N.; Dimache, A.; Radu, L.R.; Iancu, I. Leaching behavior of some demolition wastes. J. Mater. Cycles Waste Manag. 2017, 19, 623–630. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Yang, L.; Huang, T. Adsorption characteristics of construction waste for heavy metals from urban stormwater runoff. Chin. J. Chem. Eng. 2015, 23, 1542–1550. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L.; Al-Faze, R.; Al-Sehimi, A. Removal enhancement of basic blue 41 by brick waste from an aqueous solution. Arab. J. Chem. 2015, 8, 333–342. [Google Scholar] [CrossRef]
- Allahdin, O.; Wartel, M.; Recourt, P.; Revel, B.; Ouddane, B.; Billon, G.; Mabingui, J.; Boughriet, A. Adsorption capacity of iron oxyhydroxide-coated brick for cationic metals and nature of ion–surface interactions. Appl. Clay Sci. 2014, 90, 141–149. [Google Scholar] [CrossRef]
- Allaoui, A.; Hattab, Z.; Zerdoum, R.; Djellabi, R.; Berredjem, Y.; Bessashia, W.; Guerfi, K. Adsorption of hexavalent chromium by crushed brick: Effect of operating parameters and modeling study. Desalin. Water Treat. 2018, 131, 291–304. [Google Scholar] [CrossRef]
- Rathore, V.K.; Mondal, P. Stabilization of arsenic and fluoride bearing spent adsorbent in clay bricks: Preparation, characterization and leaching studies. J. Environ. Manag. 2017, 200, 160–169. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Abboudi, M.; Hassani, H.O.; Rakass, S.; Ibrahim, S.M.; Al Wadaani, F. Waste Bricks Applied as Removal Agent of Basic Blue 41 from Aqueous Solutions: Base Treatment and Their Regeneration Efficiency. Appl. Sci. 2019, 9, 1237. [Google Scholar] [CrossRef]
- Shi, J.; Xing, C.; Chen, Y.; Xu, Z.; Du, Q.; Cui, Y. Pb2+ adsorption on TiO2@HF-waste building bricks: Kinetics, thermodynamics, and mechanisms. Water Environ. Res. 2019, 91, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Pintor, A.M.; Vieira, B.R.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Arsenate and arsenite adsorption onto iron-coated cork granulates. Sci. Total Environ. 2018, 642, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory Preparation and Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Li, W.; Wang, L.; Liu, F.; Liang, X.; Feng, X.; Tan, W.; Zheng, L.; Yin, H. Effects of Al3+ doping on the structure and properties of goethite and its adsorption behavior towards phosphate. J. Environ. Sci. 2016, 45, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, Z.; Chai, X.; Weng, Z.; Ding, R.; Dong, L. Fabrication of hematite (α-Fe2O3) nanoparticles using electrochemical deposition. Appl. Surf. Sci. 2016, 368, 303–308. [Google Scholar] [CrossRef]
- Yan, Z.; Yuan, J.; Zhu, G.; Zou, Y.; Chen, C.; Yang, S.; Yao, S. A new strategy based on cholesterol-functionalized iron oxide magnetic nanoparticles for determination of polycyclic aromatic hydrocarbons by high-performance liquid chromatography with cholesterol column. Anal. Chim. Acta 2013, 780, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xiong, D.; Zhao, T.; He, H.; Pan, X. Adsorptive removal of PPCPs by biomorphic HAP templated from cotton. Water Sci. Technol. 2016, 74, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Attia, T.M.S.; Hu, X.L.; Yin, D.Q. Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Chemosphere 2013, 93, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hou, X.; Zhang, X.; Li, H. The synergistic adsorption of pyrene and copper onto Fe(III) functionalized mesoporous silica from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 39–45. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, S.; Jing, R.; Shao, Y.; Liu, X.; Lv, F.; Hu, X.; Zhang, Q.; Meng, Z.; Liu, A. Competitive adsorption of antibiotic tetracycline and ciprofloxacin on montmorillonite. Appl. Clay Sci. 2019, 180, 105175. [Google Scholar] [CrossRef]
- Zhang, Z.; Lan, H.; Liu, H.; Qu, J. Removal of tetracycline antibiotics from aqueous solution by amino-Fe (III) functionalized SBA15. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 133–138. [Google Scholar] [CrossRef]
- Miao, J.; Wang, F.; Chen, Y.; Zhu, Y.; Zhou, Y.; Zhang, S. The adsorption performance of tetracyclines on magnetic graphene oxide: A novel antibiotics absorbent. Appl. Surf. Sci. 2019, 475, 549–558. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, Y.; Han, R.; Wei, W. Enhanced tetracycline adsorption onto hydroxyapatite by Fe(III) incorporation. J. Mol. Liq. 2017, 247, 171–181. [Google Scholar] [CrossRef]
- Qin, X.; Liu, F.; Wang, G.; Weng, L.; Li, L. Adsorption of levofloxacin onto goethite: Effects of pH, calcium and phosphate. Colloids Surf. B Biointerfaces 2014, 116, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Brigante, M.; Avena, M. Biotemplated synthesis of mesoporous silica for doxycycline removal. Effect of pH, temperature, ionic strength and Ca2+ concentration on the adsorption behaviour. Microporous Mesoporous Mater. 2016, 225, 534–542. [Google Scholar] [CrossRef]
- Behera, S.K.; Oh, S.-Y.; Park, H.-S. Sorption of triclosan onto activated carbon, kaolinite and montmorillonite: Effects of pH, ionic strength, and humic acid. J. Hazard. Mater. 2010, 179, 684–691. [Google Scholar] [CrossRef] [PubMed]
| Compound | Structure | Mol. wt. | Solubility | pKa |
|---|---|---|---|---|
| Cimetidine (C10H16N6S) | 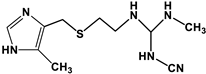 | 252.34 g/mol | 5 g/L | 6.8 |
| Sorbents | Chemical Analysis (Weight %, X-ray Fluorescence (XRF)) | Specific Surface Area (m2/g) | Fe Content (mg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| Si2O | Al2O3 | Fe2O3 | CaO | MgO | Others | |||
| WRB | 70.37 | 15.87 | 4.12 | 1.33 | 2.03 | 5.69 | 17.46 | - |
| GWRB | 71.22 | 15.47 | 5.17 | 1.73 | 1.77 | 6.32 | 8.20 | 4.28 |
| HWRB | 69.58 | 15.32 | 5.20 | 1.56 | 1.95 | 7.54 | 2.20 | 3.41 |
| Adsorbents | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| K1 | qe | R2 | K2 | qe | R2 | |
| WRB | 6.50 | 104.7 | 0.91 | 80.43 | 11.3 | 0.93 |
| HWRB | 3.12 | 155.4 | 0.86 | 29.08 | 16.6 | 0.95 |
| GWRB | 3.13 | 180.5 | 0.93 | 21.30 | 19.5 | 0.97 |
| Adsorbents | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL | qmax | R2 | Kf | 1/n | R2 | |
| WRB | 0.12 | 172.1 | 0.99 | 34.2 | 0.41 | 0.96 |
| HWRB | 0.20 | 240.3 | 0.99 | 61.8 | 0.36 | 0.87 |
| GWRB | 0.23 | 214.1 | 0.98 | 66.7 | 0.32 | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liu, F.; Xu, C.; Chen, H.; Tan, C.; Zhang, X. Preparation and Removal Properties of Cimetidine from Aqueous Solution by Waste Bricks Incorporated with Different Iron Oxides. Appl. Sci. 2019, 9, 3346. https://doi.org/10.3390/app9163346
Zhang Z, Liu F, Xu C, Chen H, Tan C, Zhang X. Preparation and Removal Properties of Cimetidine from Aqueous Solution by Waste Bricks Incorporated with Different Iron Oxides. Applied Sciences. 2019; 9(16):3346. https://doi.org/10.3390/app9163346
Chicago/Turabian StyleZhang, Ziyang, Fangyuan Liu, Chunya Xu, Hongrui Chen, Chaohong Tan, and Xiaoran Zhang. 2019. "Preparation and Removal Properties of Cimetidine from Aqueous Solution by Waste Bricks Incorporated with Different Iron Oxides" Applied Sciences 9, no. 16: 3346. https://doi.org/10.3390/app9163346
APA StyleZhang, Z., Liu, F., Xu, C., Chen, H., Tan, C., & Zhang, X. (2019). Preparation and Removal Properties of Cimetidine from Aqueous Solution by Waste Bricks Incorporated with Different Iron Oxides. Applied Sciences, 9(16), 3346. https://doi.org/10.3390/app9163346