Study of Annealing Temperature Effect on the Photovoltaic Performance of BiOI-Based Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of BiOI and Its Derivation
2.2. Characterization and Photovoltaic Cell Fabrication
3. Results and Discussion
3.1. Structure Analysis
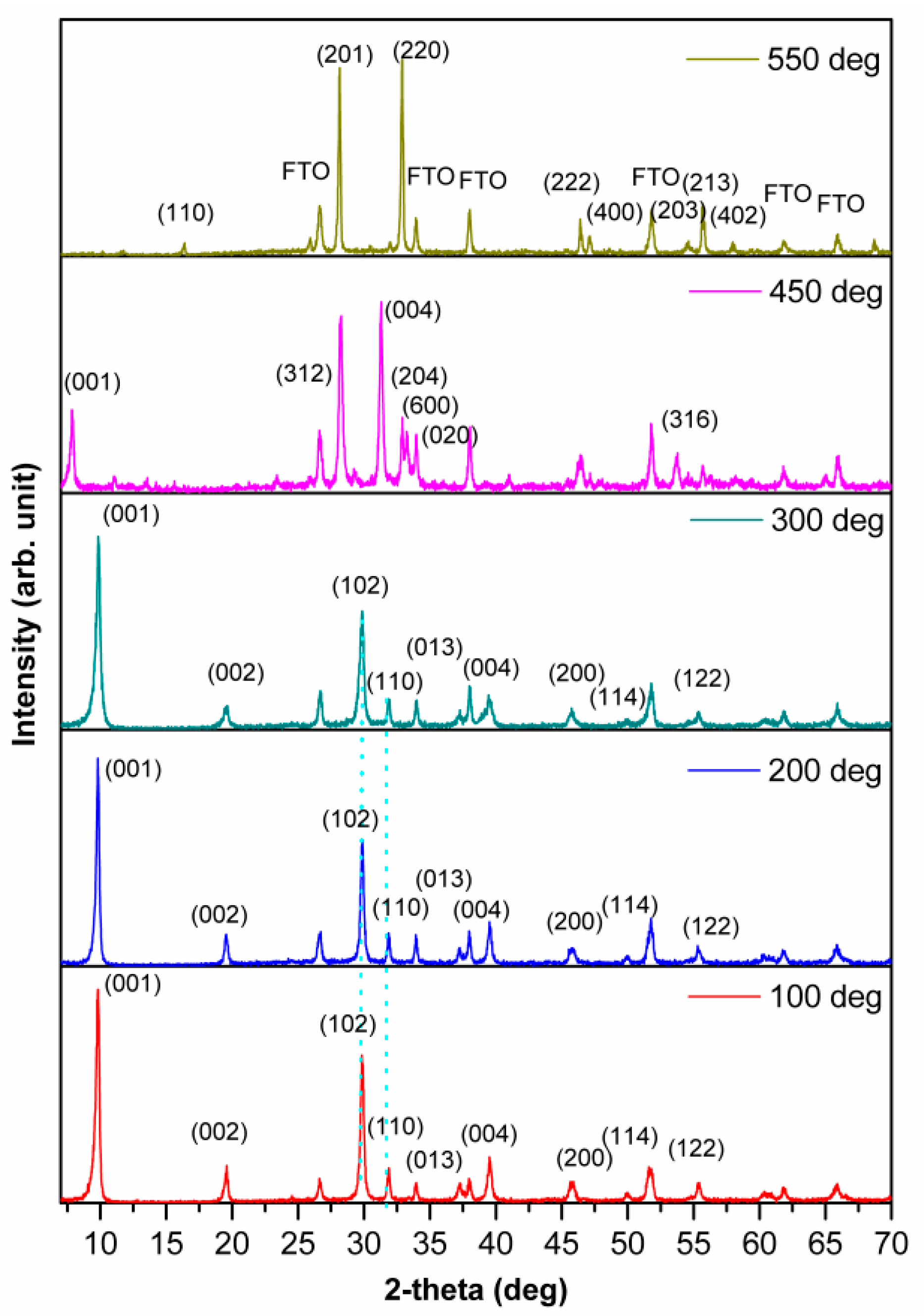
3.1.1. X-Ray Diffraction
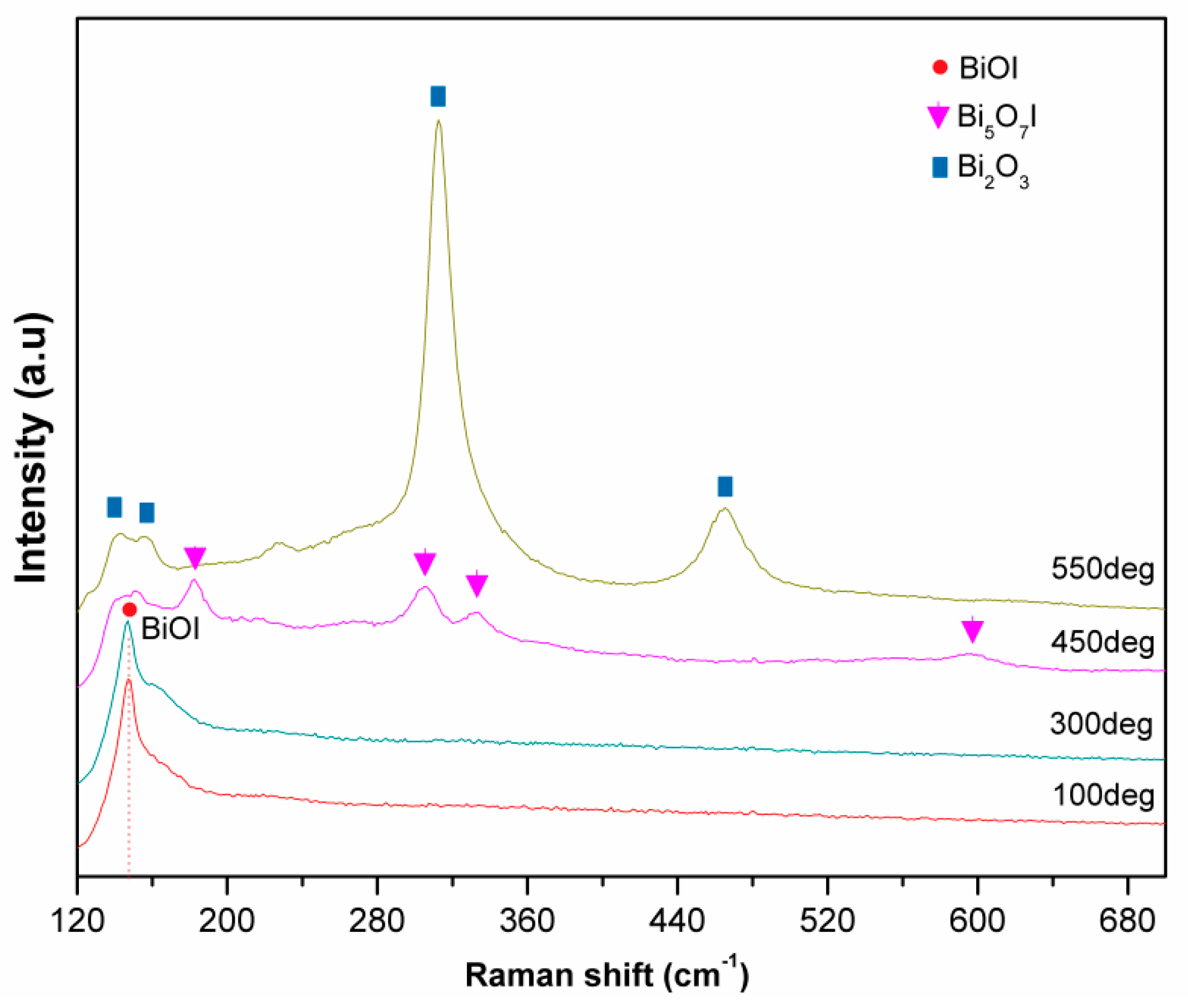
3.1.2. Raman Spectroscopy
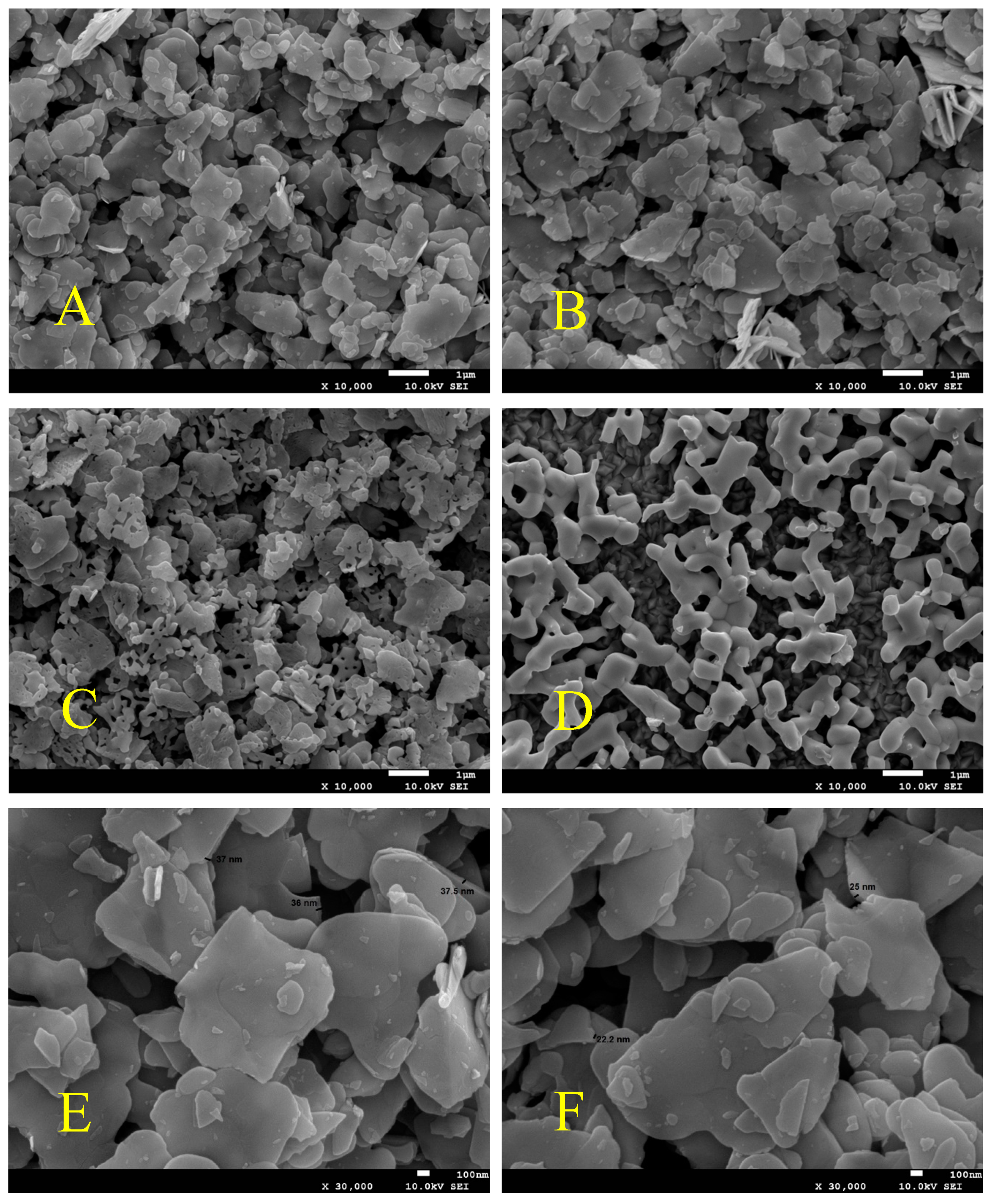
3.2. Morphology Analysis
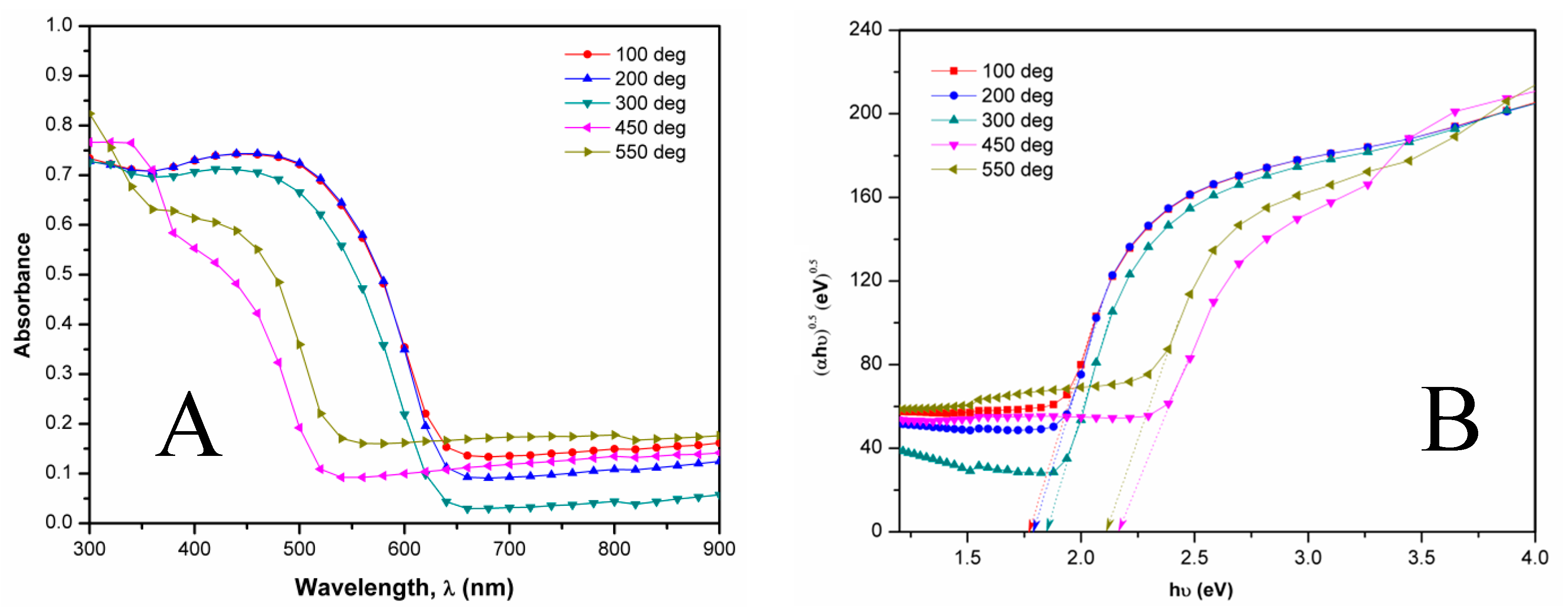
3.3. Optical Study
3.4. Photovoltaic Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, W.W.; Lu, C.S.; Chuang, C.W.; Chen, Y.J.; Fu, J.Y.; Siao, C.W.; Chen, C.C. Synthesis of bismuth oxyiodides and their composites: Characterization, photocatalytic activity, and degradation mechanisms. RSC Adv. 2015, 5, 23450–23463. [Google Scholar] [CrossRef]
- He, R.; Zhang, J.; Yu, J.; Cao, S. Room-temperature synthesis of BiOI with tailorable (001) facets and enhanced photocatalytic activity. J. Colloid Interface Sci. 2016, 478, 201–208. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L. Electronic and band structure tuning of ternary semiconductor photocatalysts by self doping: The case of BiOI. J. Phys. Chem. C 2010, 114, 18198–18206. [Google Scholar] [CrossRef]
- Niu, J.; Dai, P.; Zhang, Q.; Yao, B.; Yu, X. Microwave-assisted solvothermal synthesis of novel hierarchical BiOI/rGO composites for efficient photocatalytic degardation of organic pollutants. Appl. Surf. Sci. 2018, 430, 165–175. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Xie, T.; Wang, D. Low-temperature synthesis and high visible-light-induced photocatalytic activity of BiOI/TiO2 heterostructures. J. Phys. Chem. C 2009, 113, 7371–7378. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.; Luo, B.; Lin, H.; Chen, S. Novel BiOI/BiOBr heterojunction photocatalysts with enhanced visible light photocatalytic properties. Catal. Commun. 2011, 13, 63–68. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, K.; Shen, M.; Wei, R.; Wu, X.; Idrees, F.; Cao, C. Micro and nano hierachical structures of BiOI/activated carbon for efficient visible-light-photocatalytic reactions. Sci. Rep. 2017, 7, 11665. [Google Scholar] [CrossRef]
- Hao, R.; Xiao, X.; Zuo, X.; Nan, J.; Zhang, W. Efficient adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride using mesoporous BiOI microspheres. J. Hazard. Mater. 2012, 209, 137–145. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X. One-Step Synthesis of the Nanostructured AgI/BiOI Composites with Highly Enhanced Visible-Light Photocatalytic Performances. Langmuir 2010, 26, 6618–6624. [Google Scholar] [CrossRef]
- Han, A.; Zhang, H.; Chuah, G.K.; Jaenicke, S. Influence of the halide and exposed facets on the visible-light photoactivity of bismuth oxyhalides for selective aerobic oxidation of primary amines. Appl. Catal. B Environ. 2017, 219, 269–275. [Google Scholar] [CrossRef]
- RHoye, L.Z.; Lee, L.C.; Kurchin, R.C.; Huq, T.N.; Zhang, K.H.L.; Sponseller, M.; Nienhaus, L.; Brandt, R.E.; Jean, J.; Polizzotti, J.A.; et al. Strongly Enhanced Photovoltaic Performance and Defect Physics of Air-Stable Bismuth Oxyiodide (BiOI). Adv. Mater. 2017, 29, 1–10. [Google Scholar]
- Wang, L.; Daoud, W.A. BiOI/TiO2-nanorod array heterojunction solar cell: Growth, charge transport kinetics and photoelectrochemical properties. Appl. Surf. Sci. 2015, 324, 532–537. [Google Scholar] [CrossRef]
- Sfaelou, S.; Raptis, D.; Dracopoulos, V.; Lianos, P. BiOI solar cells. RSC Adv. 2015, 5, 95813–95816. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, Q.; Liang, J.; Feng, T.; Zhou, X.; Mao, H.; Zhang, W.; Hisaeda, Y.; Song, X.M. Mesoporous TiO2-Based Photoanode Sensitized by BiOI and Investigation of Its Photovoltaic Behavior. Langmuir 2015, 31, 10279–10284. [Google Scholar] [CrossRef]
- Wang, K.; Jia, F.; Zhang, L. Facile construction of low-cost flexible solar cells with p-type BiOI nanoflake arrays fabricated via oriented attachment. Mater. Lett. 2013, 92, 354–357. [Google Scholar] [CrossRef]
- Wang, K.; Jia, F.; Zheng, Z.; Zhang, L. Crossed BiOI flake array solar cells. Electrochem. Commun. 2010, 12, 1764–1767. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Sun, W.; Yuan, C.; Wang, B.; Zhang, W.; Song, X.M. Fe2O3/BiOI-Based Photoanode with n-p Heterogeneous Structure for Photoelectric Conversion. Langmuir 2017, 33, 12065–12071. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, G.; Song, S.; Fan, W.; Pang, M.; Tang, J.; Zhang, H. Room temperature, template-free synthesis of BiOI hierarchical structures: Visible-light photocatalytic and electrochemical hydrogen storage properties. Dalt. Trans. 2010, 39, 3273–3278. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.D. Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity. J. Mater. Chem. 2010, 20, 5866–5870. [Google Scholar] [CrossRef]
- Long, Y.; Han, Q.; Yang, Z.; Ai, Y.; Sun, S.; Wang, Y.; Liang, Q.; Ding, M. A novel solvent-free strategy for the synthesis of bismuth oxyhalides. J. Mater. Chem. A 2018, 6, 13005–13011. [Google Scholar] [CrossRef]
- Meledandri, C.J.; Stolarczyk, J.K.; Ghosh, S.; Brougham, D.F. Nonaqueous Magnetic Nanoparticle Suspensions with Controlled Particle Size and Nuclear Magnetic Resonance Properties. Langmuir 2008, 24, 14159–14165. [Google Scholar] [CrossRef]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-dependent properties of magnetic iron oxidenanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar]
- Effenberger, F.B.; Couto, R.A.; Kiyohara, P.K.; Machado, G.; Masunaga, S.H.; Jardim, R.F.; Rossi, L.M. Economically attractive route for the preparation of high quality magnetic nanoparticles by the thermal decomposition of iron(III) acetylacetonate. Nanotechnology 2017, 28, 115603. [Google Scholar] [CrossRef]
- Hufschmid, R.; Arami, H.; Ferguson, R.M.; Gonzales, M.; Teeman, E.; Brush, L.N.; Browning, N.D.; Krishnan, K.M. Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale 2015, 7, 11142–11154. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.M.; Toumi, M.; Mhiri, T. Begin-Colin, Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Long, M.; Hu, P.; Wu, H.; Chen, Y.; Tan, B.; Cai, W. Understanding the composition and electronic structure dependent photocatalytic performance of bismuth oxyiodides. J. Mater. Chem. A 2015, 3, 5592–5598. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, C.; Hu, R.; Zuo, X.; Nan, J.; Li, L.; Wang, L. Oxygen-rich bismuth oxyhalides: Generalized one-pot synthesis, band structures and visible-light photocatalytic properties. J. Mater. Chem. 2012, 22, 22840. [Google Scholar] [CrossRef]
- Xia, J.; Ji, M.; Di, J.; Wang, B.; Yin, S.; He, M.; Zhang, Q.; Li, H. Improved photocatalytic activity of few-layer Bi4O5I2 nanosheets induced by efficient charge separation and lower valence position. J. Alloy. Compd. 2017, 695, 922–930. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.D. Hierarchical Bi7O9I3 micro/nano-architecture: Facile synthesis, growth mechanism, and high visible light photocatalytic performance. RSC Adv. 2011, 1, 1099–1105. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.; Liu, C.; Xie, T. Preparation and photocatalytic activity of porous Bi5O7I nanosheets. Appl. Surf. Sci. 2014, 319, 265–271. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Lu, J.; Wang, Z.; Xu, B.; Qin, X.; Zhang, X.; Dai, Y. Synergistic effect of crystal and electronic structures on the visible-light-driven photocatalytic performances of Bi2O3 polymorphs. Phys. Chem. Chem. Phys. 2010, 12, 15468–15475. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Cao, S.; Duan, X. Investigation on enhanced photocatalytic degradation of bisphenol A with bismuth oxyiodide catalyst using response surface methodology. RSC Adv. 2018, 8, 5967–5975. [Google Scholar] [CrossRef]
- Ren, X.; Yao, J.; Cai, L.; Li, J.; Cao, X.; Zhang, Y.; Wang, B.; Wei, Y. Band gap engineering of BiOI via oxygen vacancies induced by graphene for improved photocatalysis. New J. Chem. 2019, 43, 1523–1530. [Google Scholar] [CrossRef]
- Chou, S.Y.; Chen, C.C.; Dai, Y.M.; Lin, J.H.; Lee, W.W. Novel synthesis of bismuth oxyiodide/graphitic carbon nitride nanocomposites with enhanced visible-light photocatalytic activity. RSC Adv. 2016, 6, 33478–33491. [Google Scholar] [CrossRef]
- Khedreteralid, M.; Mujahid, M.; Amin, S.; Rawat, R.S.; Nusair, A.; Deen, G.R. Effect of surfactant and heat treatment on morphology, surface area and crystallinity in hydroxyapatite nanocrystals. Ceram. Int. 2013, 39, 39–50. [Google Scholar]
- Yang, J.; Xie, T.; Liu, C.; Xu, L. Facile Fabrication of Dumbbell-Like β-Bi2O3/Graphene Nanocomposites and Their Highly Efficient Photocatalytic Activity. Materials 2018, 11, 1359. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Liu, C.; Lu, Y.; Feng, Q.; Wu, T.; Wang, R. Composite Magnetic Photocatalyst Bi5O7I/MnxZn1−xFe2O4: Hydrothermal-Roasting Preparation and Excellent Photocatalytic Activity. Nanomaterials 2019, 9, 118. [Google Scholar] [CrossRef]
- Park, Y.; Na, Y.; Pradhan, D.; Min, B.K.; Sohn, Y. Adsorption and UV/Visible photocatalytic performance of BiOI for methyl orange, Rhodamine B and methylene blue: Ag and Ti-loading effects. CrystEngComm 2014, 16, 3155–3167. [Google Scholar] [CrossRef]
- Fang, M.; Jia, H.; He, W.; Lei, Y.; Zhang, L.; Zheng, Z. Construction of flexible photoelectrochemical solar cells based on ordered nanostructural BiOI/Bi2S3 heterojunction films. Phys. Chem. Chem. Phys. 2015, 17, 13531–13538. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.J. Room temperature synthesis of Bi4O5I2 and Bi5O7I ultrathin nanosheets with a high visible light photocatalytic performance. Dalt. Trans. 2016, 45, 7720–7727. [Google Scholar] [CrossRef]
- Díaz-Guerra, C.; Almodóvar, P.; Camacho-López, M.; Camacho-López, S.; Piqueras, J. Formation of β-Bi2O3 and δ-Bi2O3 during laser irradiation of Bi films studied in-situ by spatially resolved Raman spectroscopy. J. Alloy. Compd. 2017, 723, 520–526. [Google Scholar]
- Lu, J.; Wu, J.; Xu, W.; Cheng, H.; Qi, X.; Li, Q.; Zhang, Y.; Guan, Y.; Ling, Y.; Zhang, Z. Room temperature synthesis of tetragonal BiOI photocatalyst with surface heterojunction between (0 0 1) facets and (1 1 0) facets. Mater. Lett. 2018, 219, 260–264. [Google Scholar] [CrossRef]
- Du, Y.; Huang, R.; Song, R.; Ma, L.B.; Liu, C.; Li, C.R.; Cao, Z.X. Effect of oxygen inclusion on microstructure and thermal stability of copper nitride thin films. J. Mater. Res. 2007, 22, 3052–3057. [Google Scholar] [CrossRef][Green Version]
- Brugnoli, E.; Björkman, O. Chloroplast movements in leaves: Influence on chlorophyll fluorescence and measurements of light-induced absorbance changes related to ΔpH and zeaxanthin formation. Photosynth. Res. 1992, 32, 23–35. [Google Scholar] [CrossRef]
- Ma, F.Q.; Yao, J.W.; Zhang, Y.F.; Wei, Y. Unique band structure enhanced visible light photocatalytic activity of phosphorus-doped BiOI hierarchical microspheres. RSC Adv. 2017, 7, 36288–36296. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhang, L.; Zhou, L.; Yin, W.; Shang, M. Visible light-induced efficient contaminant removal by Bi5O7I. Environ. Sci. Technol. 2009, 43, 2005–2010. [Google Scholar] [CrossRef]
- Schlesinger, M.; Weber, M.; Schulze, S.; Hietschold, M.; Mehring, M. Metastable β-Bi2O3 Nanoparticles with Potential for Photocatalytic Water Purification Using Visible Light Irradiation. ChemistryOpen 2013, 2, 146–155. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Balogun, M.S.; Liu, W.; Tong, Y.; Lu, X.; Ji, H. Oxygen Vacancy Induced Bismuth Oxyiodide with Remarkably Increased Visible-Light Absorption and Superior Photocatalytic Performance. ACS Appl. Mater. Interfaces 2014, 6, 22920–22927. [Google Scholar] [CrossRef]
- Wang, Z.S.; Cui, Y.; Hara, K.; Dan-oh, Y.; Kasada, C.; Shinpo, A. Shinpo, A High-Light-Harvesting-Efficiency Coumarin Dye for Stable Dye-Sensitized Solar Cells. Adv. Mater. 2007, 19, 1138–1141. [Google Scholar] [CrossRef]
- Putri, A.A.; Kato, S.; Kishi, N.; Soga, T. Angle dependence of synthesized BiOI prepared by dip coating and its effect on the photovoltaic performance. Jpn. J. Appl. Phys. 2019, 58, SAAD09. [Google Scholar] [CrossRef]
- Putri, A.A.; Kato, S.; Kishi, N.; Soga, T. Relevance of precursor molarity in the prepared bismuth oxyiodide films by successive ionic layer adsorption and reaction for solar cell application. J. Sci. Adv. Mater. Devices 2019, 4, 116–124. [Google Scholar] [CrossRef]

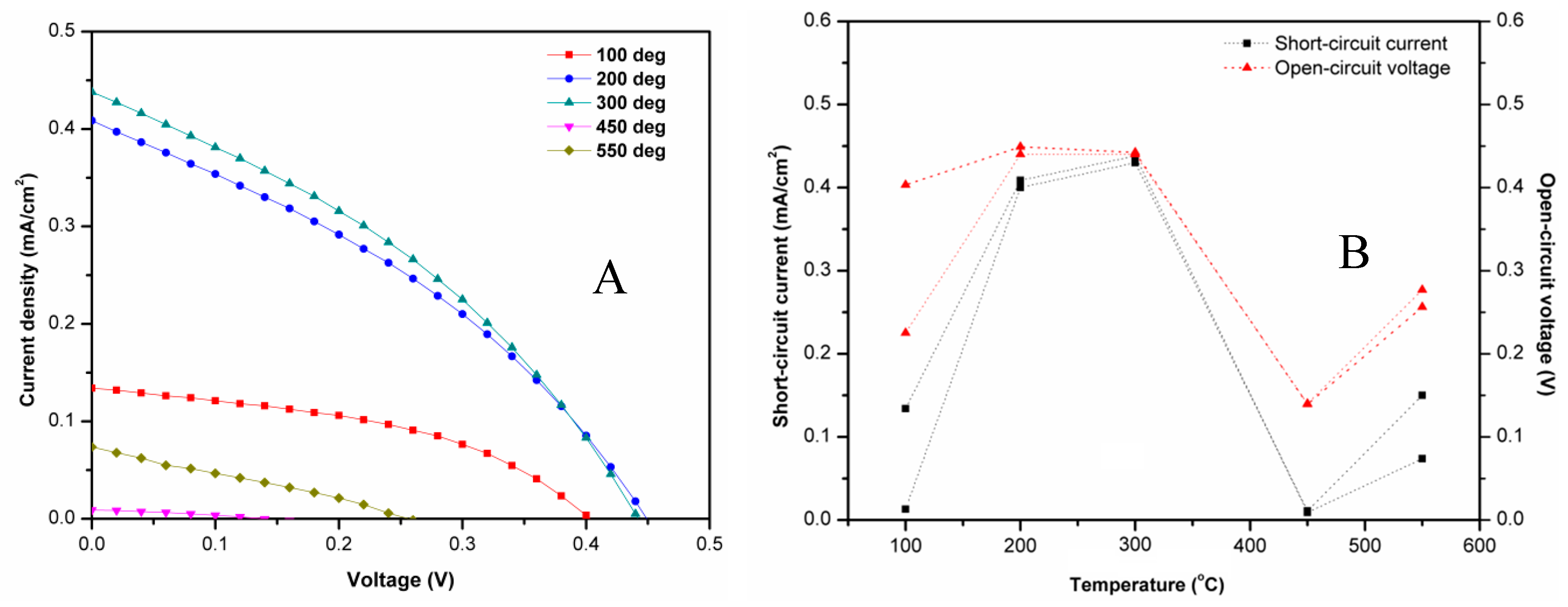
| Temperature (°C) | Jsc (mA/cm2) | Voc (V) | Fill Factor | Efficiency (%) | Rsh (103 × Ω cm2) | Rs (103 × Ω cm2) |
|---|---|---|---|---|---|---|
| 100 | 0.133 | 0.403 | 0.440 | 0.023 | 9.183 | 0.170 |
| 200 | 0.408 | 0.449 | 0.349 | 0.064 | 11.49 | 0.156 |
| 300 | 0.438 | 0.442 | 0.357 | 0.069 | 10.82 | 0.148 |
| 450 | 0.009 | 0.139 | 0.320 | 0.0004 | 7.898 | 0.162 |
| 550 | 0.073 | 0.256 | 0.276 | 0.0052 | 9.247 | 0.112 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putri, A.A.; Kato, S.; Kishi, N.; Soga, T. Study of Annealing Temperature Effect on the Photovoltaic Performance of BiOI-Based Materials. Appl. Sci. 2019, 9, 3342. https://doi.org/10.3390/app9163342
Putri AA, Kato S, Kishi N, Soga T. Study of Annealing Temperature Effect on the Photovoltaic Performance of BiOI-Based Materials. Applied Sciences. 2019; 9(16):3342. https://doi.org/10.3390/app9163342
Chicago/Turabian StylePutri, Anissa A., Shinya Kato, Naoki Kishi, and Tetsuo Soga. 2019. "Study of Annealing Temperature Effect on the Photovoltaic Performance of BiOI-Based Materials" Applied Sciences 9, no. 16: 3342. https://doi.org/10.3390/app9163342
APA StylePutri, A. A., Kato, S., Kishi, N., & Soga, T. (2019). Study of Annealing Temperature Effect on the Photovoltaic Performance of BiOI-Based Materials. Applied Sciences, 9(16), 3342. https://doi.org/10.3390/app9163342






