Study on Improving the Fixation Rate of Impregnated Poplar Wood with Maltodextrin and 1,3-Dimethylol-4,5-Dihydroxyethyleneurea
Abstract
Featured Application
Abstract
1. Introduction
2. Experiment
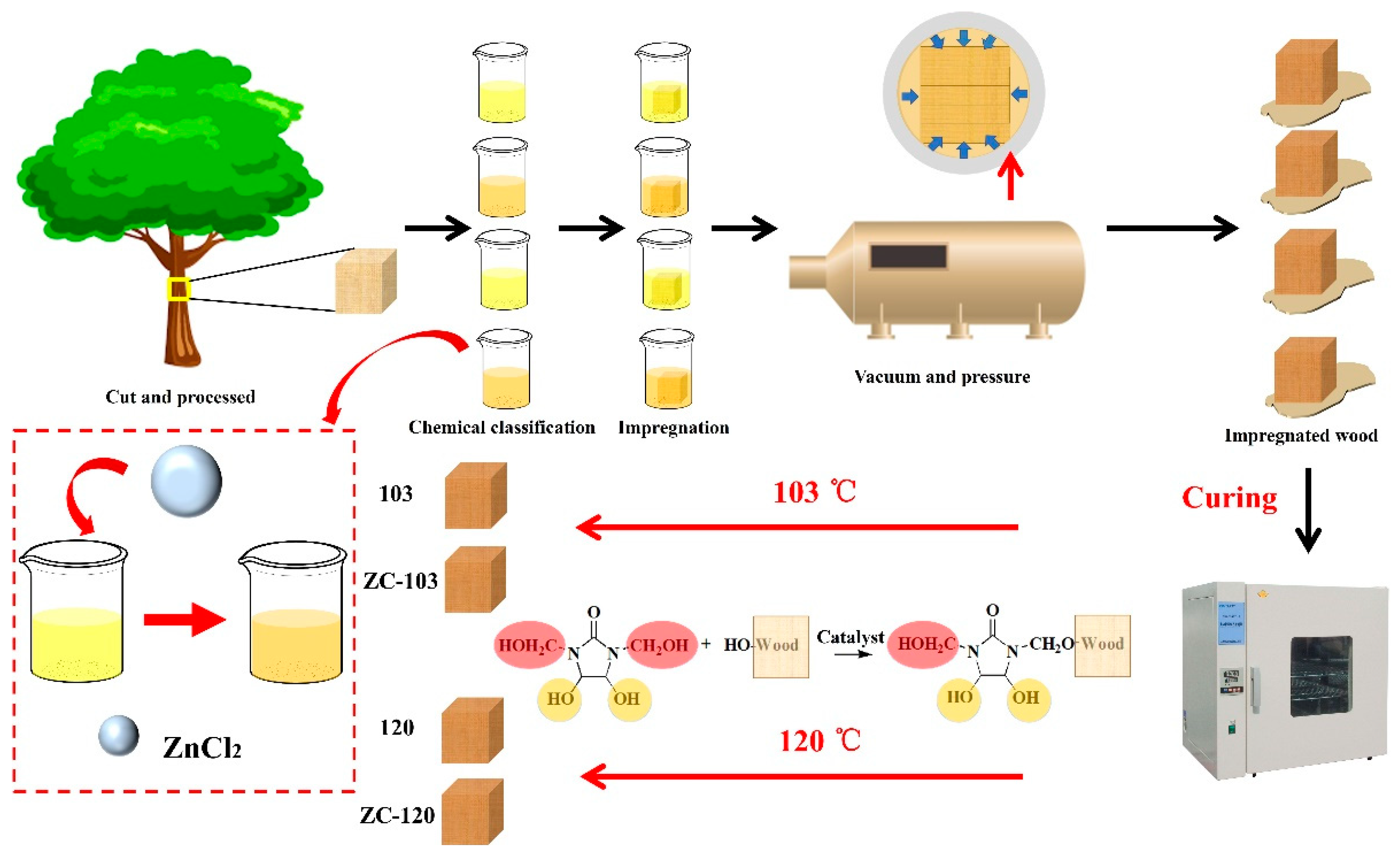
2.1. Materials
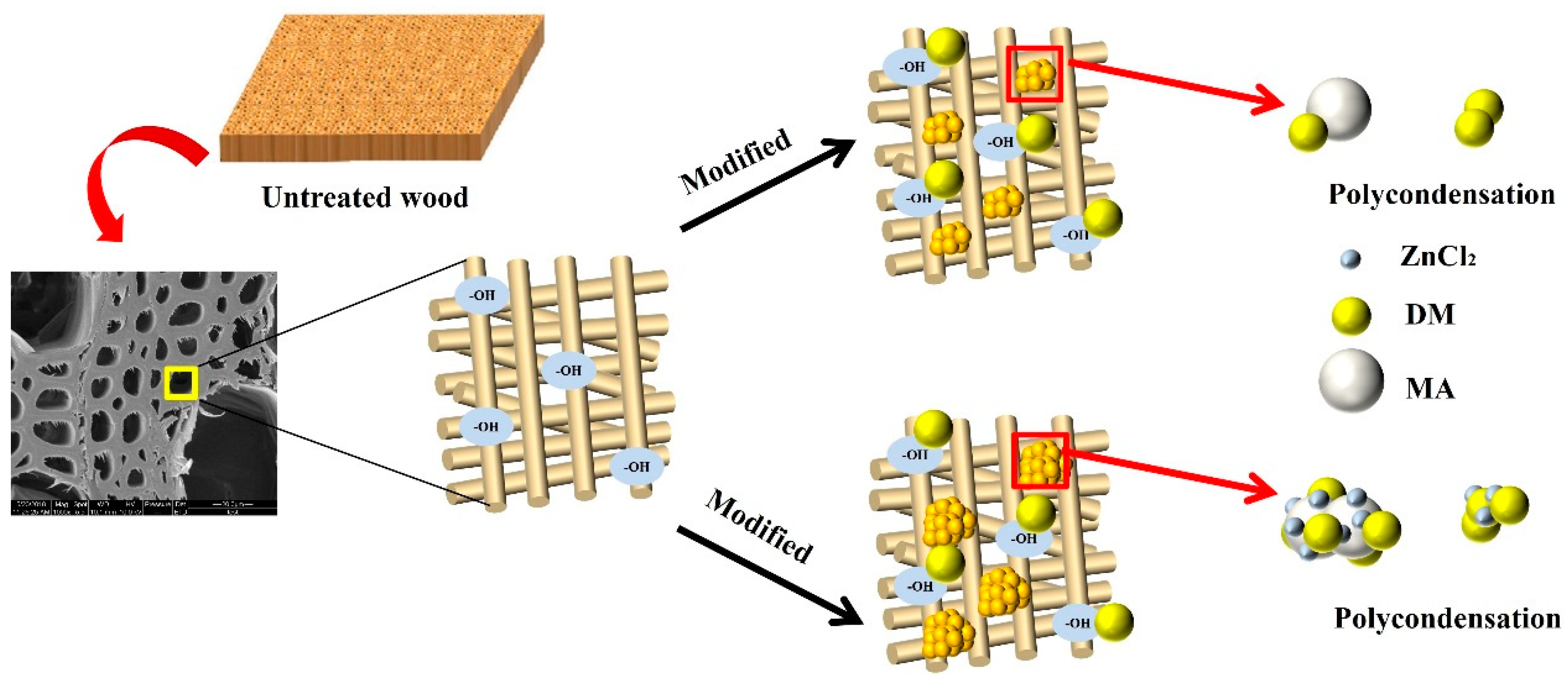
2.2. Preparation of Modified Wood
2.3. Characterization of Weight Percent Gain (WPG) and Weight Leaching Ratio (WLR)
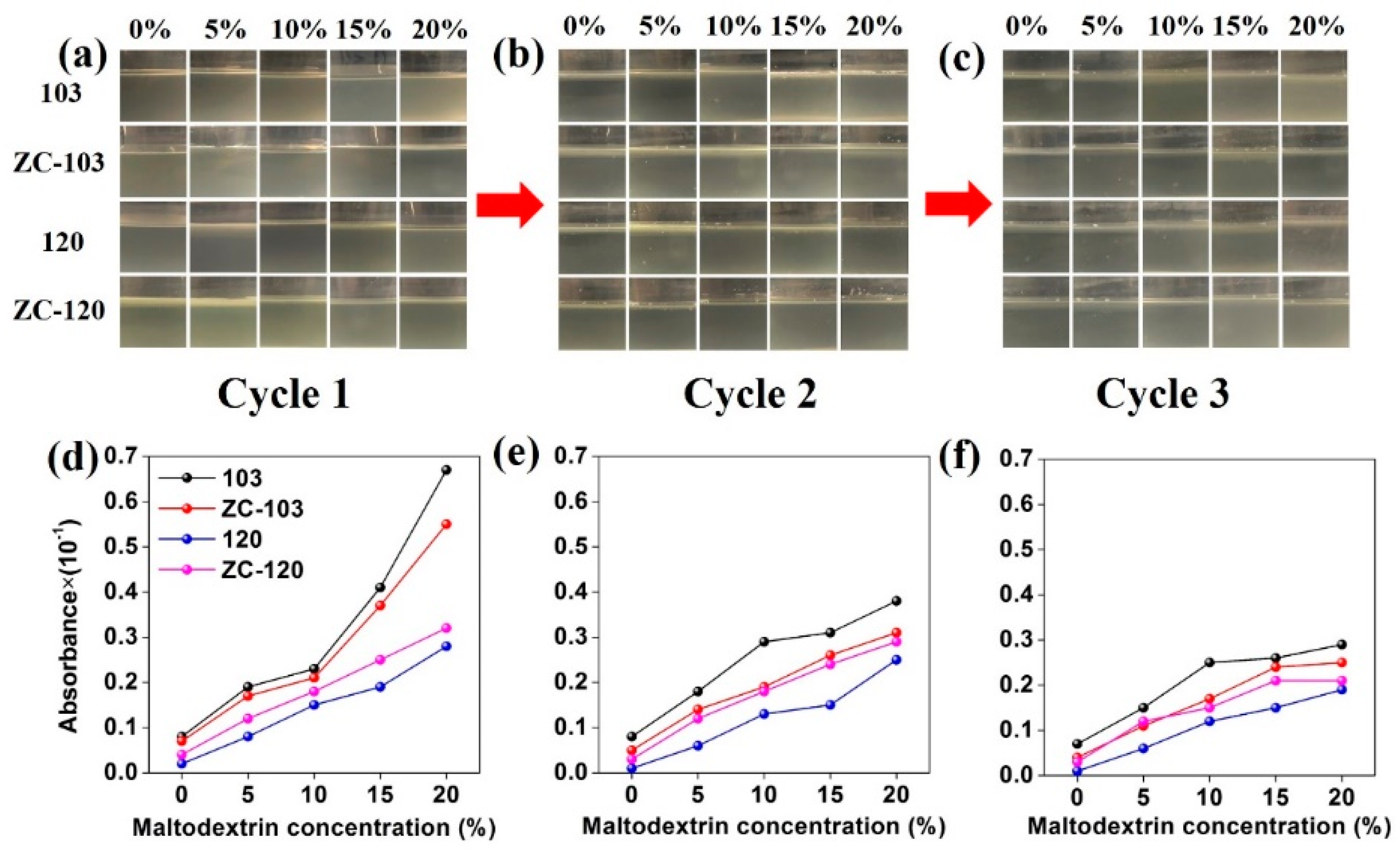
2.4. Leaching Cycle Test
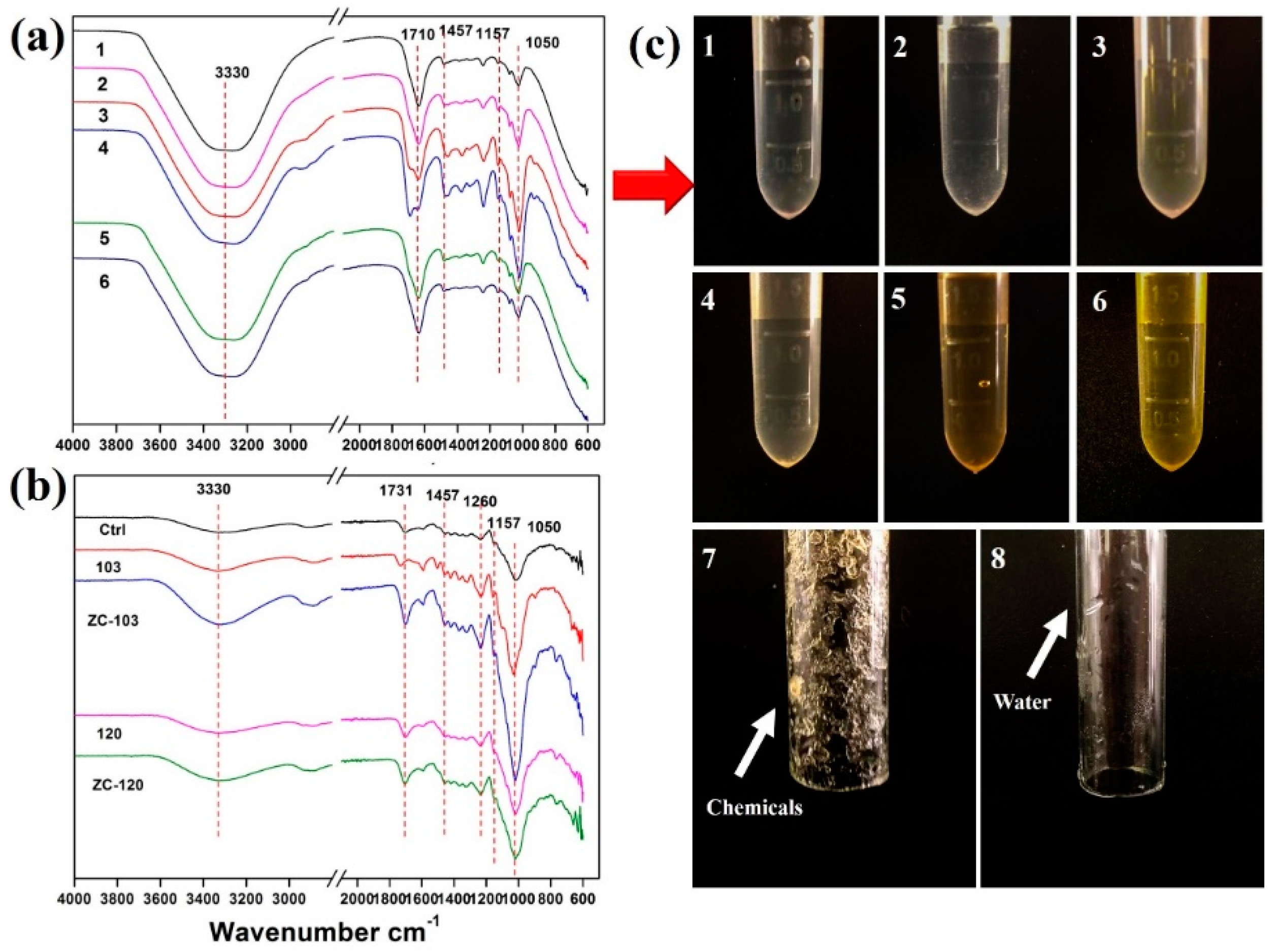
2.5. Fourier-Transform Infrared Spectroscopy
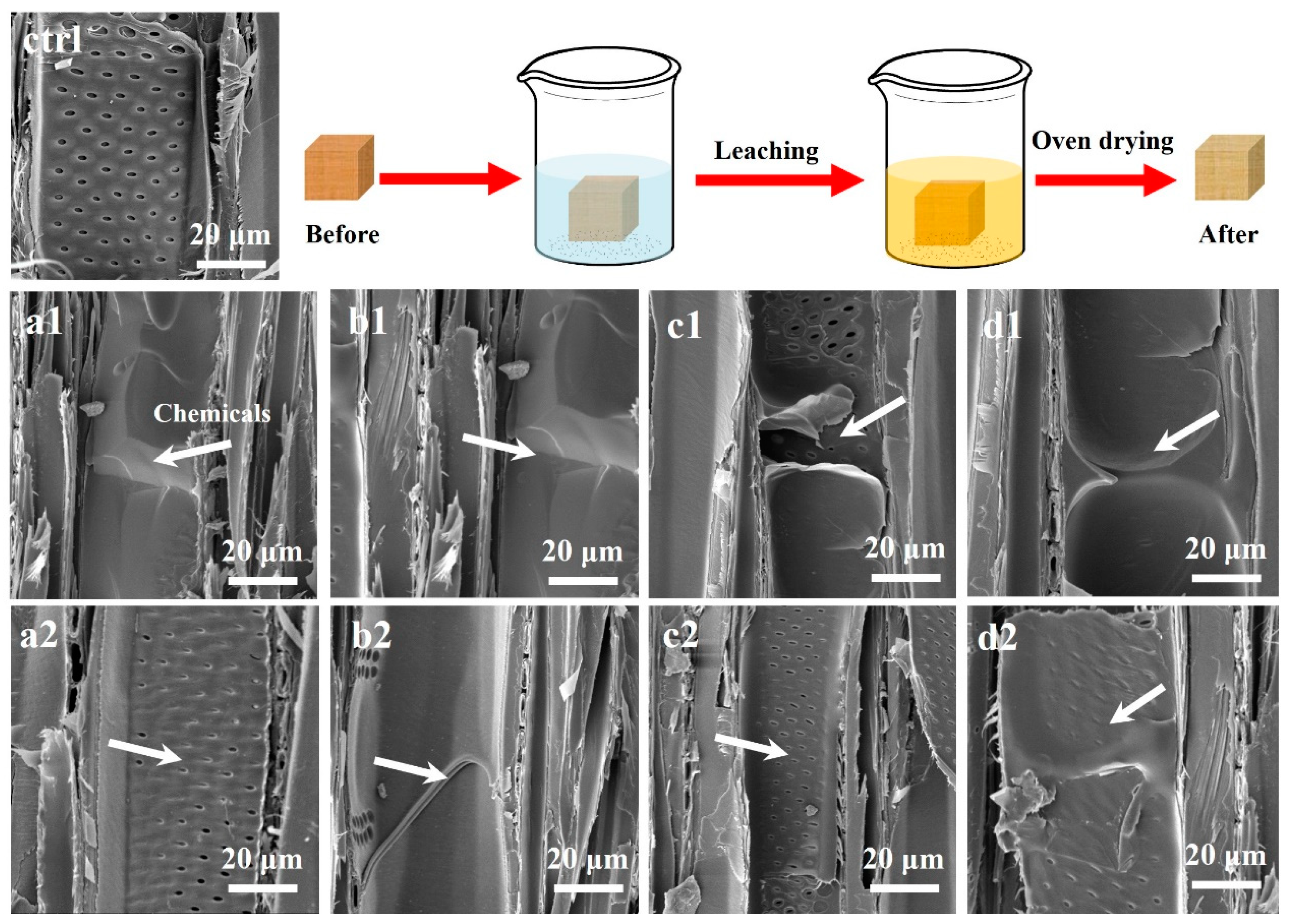
2.6. Morphological Analyses
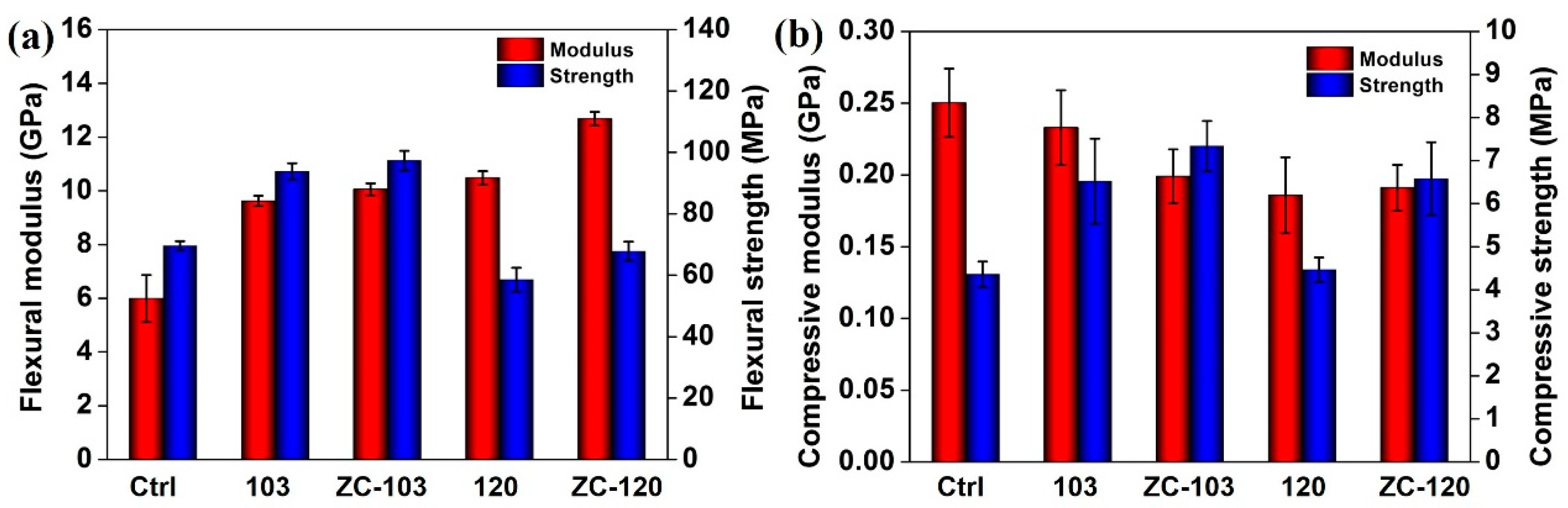
2.7. Mechanical Tests
2.8. Thermal Tests
2.9. Surface Wettability Tests
2.10. Water Absorption Tests
3. Results and Discussion
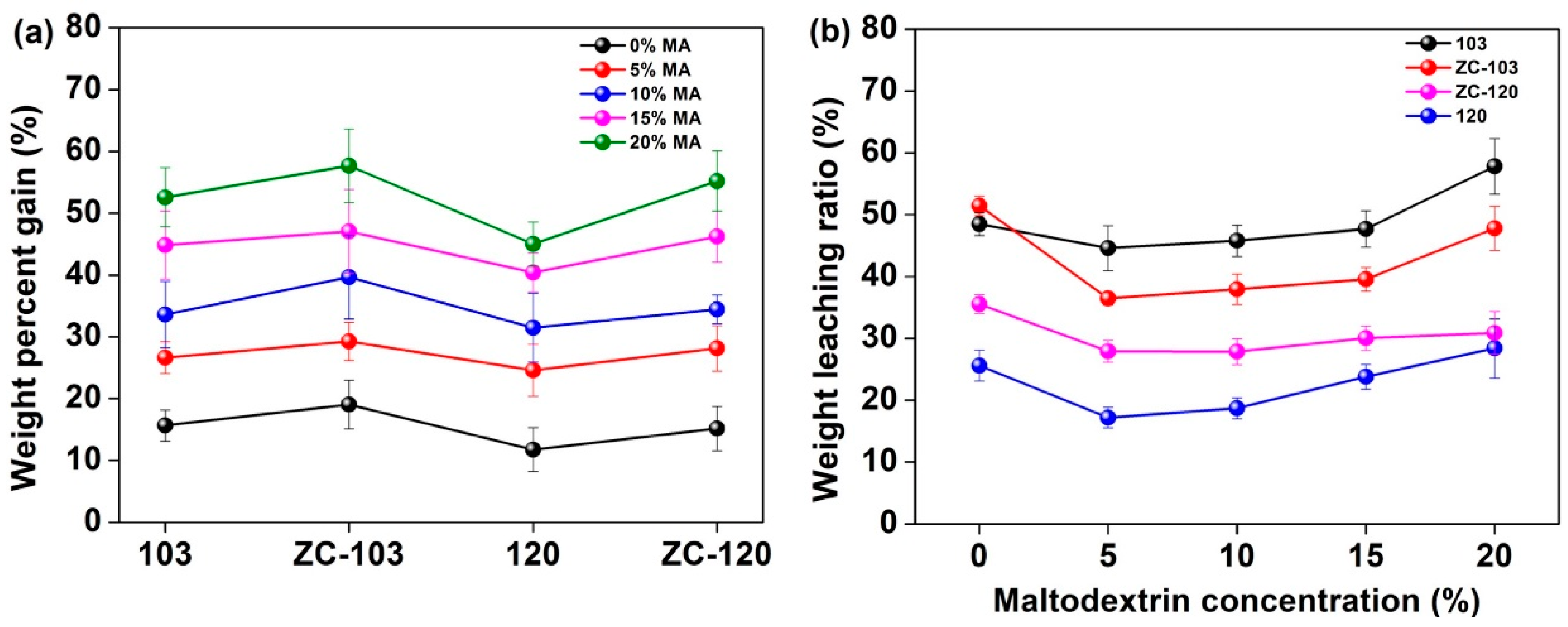
3.1. WPG and WLR Analysis
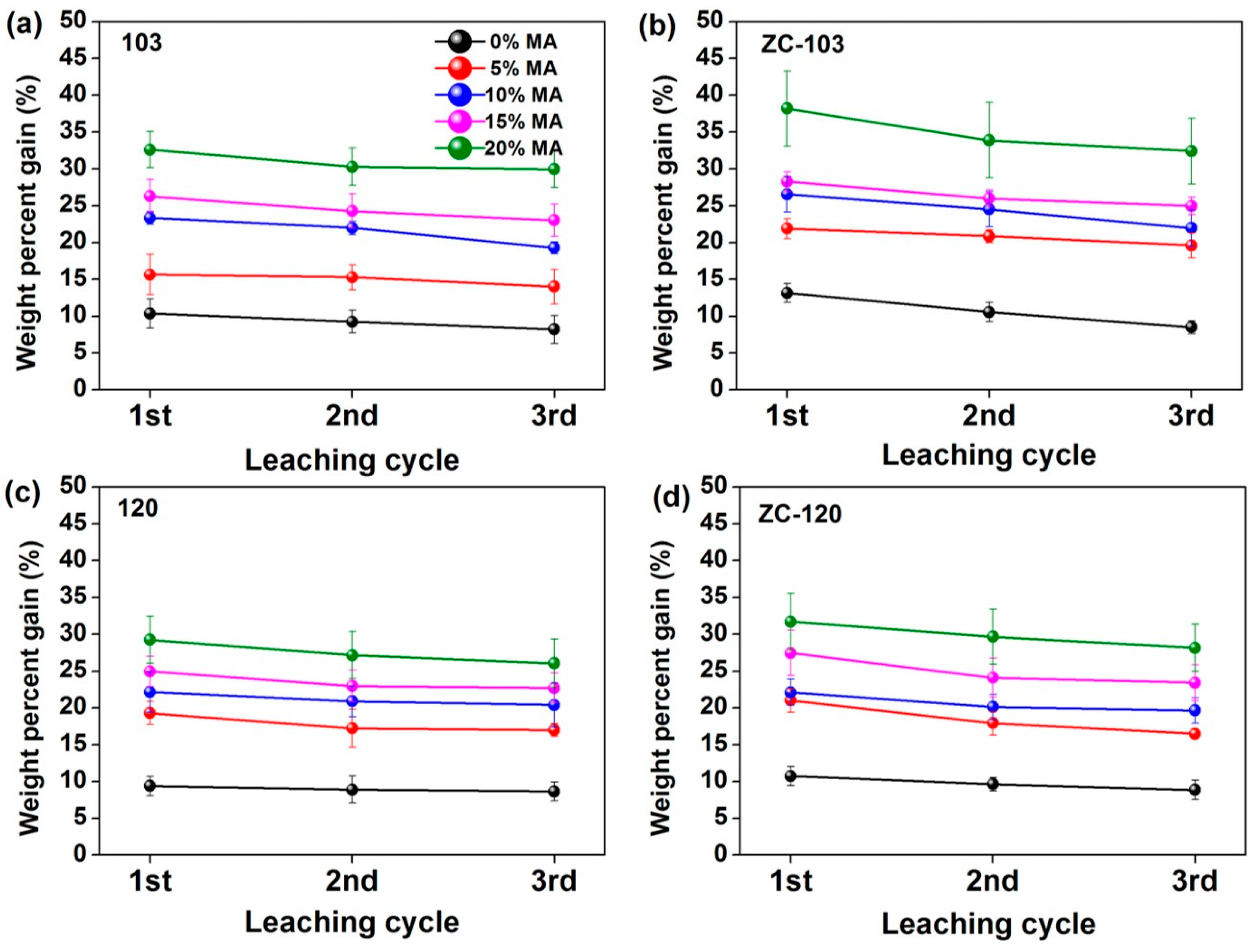
3.2. Effect of Leaching Cycles on WPG
3.3. Fourier-Transform Infrared Spectroscopy
3.4. Morphological Analyses
3.5. Determination of Mechanical Strength
3.6. Thermogravimetric Analysis
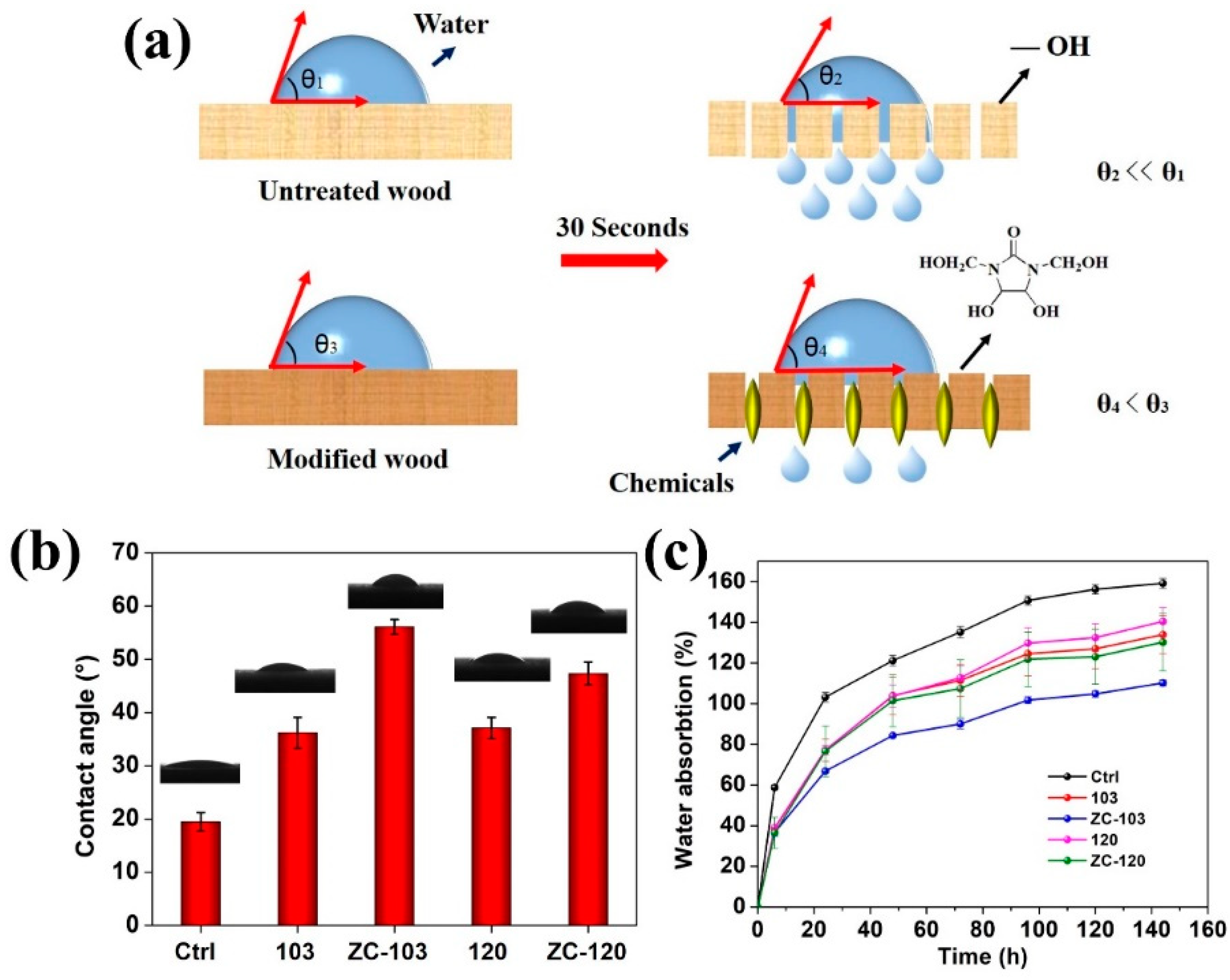
3.7. Determination of Surface Wettability and Water Absorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowyer, J.L.; Shmulsky, R.; Haygreen, J.G. Forest Products and Wood Science: An Introduction; Blackwell Publishing: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kukay, B.; Todd, C.; Jahn, T.; Sannon, J.; Dunlap, L.; White, R.; Dietenberger, M.; Lu, K.; White, R.H.; Fu, F.E. Valuating Fire-Damaged Components of Historic Covered Bridges; United States Department of Agriculture, Forest Service, Forest Products: Washington, DC, USA, 2016. [Google Scholar]
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood modification technologies-a review. iForest-Biogeosciences For. 2017, 10, 895. [Google Scholar] [CrossRef]
- Matsuda, H. Chemical Modification of Solid Wood, Chemical Modification of Lignocellulosic Materials; Routledge: Abingdon, UK, 2017; pp. 159–183. [Google Scholar]
- Hill, C.A. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Wang, C.; Yang, Z.; Wang, X.; Yu, Q. New research progress of functional wood. J. For. Eng. 2019, 4, 10–18. [Google Scholar]
- Sobczak, L.; Brüggemann, O.; Putz, R. Polyolefin composites with natural fibers and wood-modification of the fiber/filler–matrix interaction. J. Appl. Polym. Sci. 2013, 127, 1–17. [Google Scholar] [CrossRef]
- Ibrahim, N.; El-Sayed, Z.; Fahmy, H.; Hassabo, A.; Abo-Shosha, M. Perfume finishing of cotton/polyester fabric cross-linked with DMDHEU in presence of softeners. Res. J. Text. Appar. 2013, 17, 58–63. [Google Scholar] [CrossRef]
- Xie, Y.; Fu, Q.; Wang, Q.; Xiao, Z.; Militz, H. Effects of chemical modification on the mechanical properties of wood. Eur. J. Wood Wood Prod. 2013, 71, 401–416. [Google Scholar] [CrossRef]
- Gérardin, P. New alternatives for wood preservation based on thermal and chemical modification of wood—A review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef]
- Mamiński, M.Ł.; Witek, S.; Szymona, K.; Parzuchowski, P. Novel adhesive system based on 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU) and hyperbranched polyglycerols. Eur. J. Wood Wood Prod. 2013, 71, 267–275. [Google Scholar] [CrossRef][Green Version]
- Adamopoulos, S.; Xie, Y.; Militz, H. Distribution of blue stain in untreated and DMDHEU treated Scots pine sapwood panels after six years of outdoor weathering. Eur. J. Wood Wood Prod. 2011, 69, 333–336. [Google Scholar] [CrossRef][Green Version]
- Lionetto, F.; del Sole, R.; Cannoletta, D.; Vasapollo, G.; Maffezzoli, A. Monitoring wood degradation during weathering by cellulose crystallinity. Materials 2012, 5, 1910–1922. [Google Scholar] [CrossRef]
- Kutzli, I.; Gibis, M.; Baier, S.K.; Weiss, J. Fabrication and characterization of food-grade fibers from mixtures of maltodextrin and whey protein isolate using needleless electrospinning. J. Appl. Polym. Sci. 2018, 135, 46328. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Jin, D.; Yan, R.; Wang, H.; Wu, Q. Amphiphilic Maltodextrins Modified with Polyurethane Microparticles. J. Biobased Mater. Bioenergy 2012, 6, 538–543. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, Y.G. Introduction on a Kind of Environmental Biological Materials-Dextrins, Advanced Materials Research. Trans. Tech. Publ. 2013, 671–674, 1889–1892. [Google Scholar]
- Cai, M.; Fu, Z.; Cai, Y.; Li, Z.; Xu, C.; Xu, C.; Li, S. Effect of Impregnation with Maltodextrin and 1,3-Dimethylol-4, 5-Dihydroxyethyleneurea on Poplar Wood. Forests 2018, 9, 676. [Google Scholar] [CrossRef]
- Cai, M.; Fu, Z.; Cai, Y.; Zhang, Y.; Cai, J.; Xu, C. Effect of Impregnation with Maltodextrin and 1,3-Dimethylol-4, 5-Dihydroxyethyleneurea on the Conventional Drying Characteristics of Poplar Wood. Appl. Sci. 2019, 9, 473. [Google Scholar] [CrossRef]
- Sun, F.; Peng, X.; Liu, L. Study on modification of poplar lumber by 2D resin. China For. Sci. Technol. 2015, 29, 98–101. [Google Scholar]
- He, X.; Xiao, Z.; Feng, X.; Sui, S.; Wang, Q.; Xie, Y. Modification of poplar wood with glucose crosslinked with citric acid and 1,3-dimethylol-4,5-dihydroxy ethyleneurea. Holzforschung 2016, 70, 47–53. [Google Scholar] [CrossRef]
- Che, W.; Xiao, Z.; Han, G.; Zheng, Z.; Xie, Y. Radiata pine wood treatment with a dispersion of aqueous styrene/acrylic acid copolymer. Holzforschung 2018, 72, 387–396. [Google Scholar] [CrossRef]
- Yuan, J.; Hu, Y.; Li, L.; Cheng, F. The mechanical strength change of wood modified with DMDHEU. BioResources 2013, 8, 1076–1088. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, Z.; Sui, S.; Wang, Q.; Xie, Y. Esterification of wood with citric acid: The catalytic effects of sodium hypophosphite (SHP). Holzforschung 2014, 68, 427–433. [Google Scholar] [CrossRef]
- Hill, C.A.; Mallon, S. The chemical modification of Scots pine with succinic anhydride or octenyl succinic anhydride. I. Dimensional stabilization. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 1998, 52, 427–433. [Google Scholar]
- Xiao, Z.; Xie, Y.; Militz, H.; Mai, C. Effect of glutaraldehyde on water related properties of solid wood. Holzforschung 2010, 64, 483–488. [Google Scholar] [CrossRef]
- Emmerich, L.; Bollmus, S.; Militz, H. Wood modification with DMDHEU (1.3-dimethylol-4.5-dihydroxyethyleneurea)–State of the art, recent research activities and future perspectives. Wood Mater. Sci. Eng. 2019, 14, 3–18. [Google Scholar] [CrossRef]
- Kretschmann, D. Mechanical Properties of Wood. In Wood Handbook: Wood as an Engineering Material, Centennial ed.; General Technical Report FPL; United States Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010; pp. 5.1–5.46. [Google Scholar]
- Xie, Y.; Xiao, Z.; Grüneberg, T.; Militz, H.; Hill, C.A.; Steuernagel, L.; Mai, C. Effects of chemical modification of wood particles with glutaraldehyde and 1,3-dimethylol-4, 5-dihydroxyethyleneurea on properties of the resulting polypropylene composites. Compos. Sci. Technol. 2010, 70, 2003–2011. [Google Scholar] [CrossRef]
- Dieste, A.; Krause, A.; Bollmus, S.; Militz, H. Gluing ability of plywood produced with DMDHEU-modified veneers of Fagus sp., Betula sp., and Picea sp. Int. J. Adhes. Adhes. 2009, 29, 206–209. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, D.; Gotama, J.; Bateman, S. Wood fiber reinforced polyethylene and polypropylene composites with high modulus and impact strength. J. Thermoplast. Compos. Mater. 2008, 21, 195–208. [Google Scholar] [CrossRef]
- Soccalingame, L.; Bourmaud, A.; Perrin, D.; Bénézet, J.C.; Bergeret, A. Reprocessing of wood flour reinforced polypropylene composites: Impact of particle size and coupling agent on composite and particle properties. Polym. Degrad. Stab. 2015, 113, 72–85. [Google Scholar] [CrossRef]
- Liu, R.; Morrell, J.J.; Yan, L. Thermogravimetric analysis studies of thermally-treated glycerol impregnated poplar wood. BioResources 2018, 13, 1563–1575. [Google Scholar] [CrossRef]
- Azizi, K.; Moraveji, M.K.; Najafabadi, H.A. Characteristics and kinetics study of simultaneous pyrolysis of microalgae Chlorella vulgaris, wood and polypropylene through TGA[J]. Bioresour. Technol. 2017, 243, 481–491. [Google Scholar] [CrossRef]
- Anwar, Z.; Kausar, A.; Rafique, I.; Muhammad, B. Advances in epoxy/graphene nanoplatelet composite with enhanced physical properties: A review. Polym. Plast. Technol. Eng. 2016, 55, 643–662. [Google Scholar] [CrossRef]
- Ahmed, A.; Afolabi, E.A.; Garba, M.U.; Musa, U.; Alhassan, M.; Ishaq, K. Effect of particle size on thermal decomposition and devolatilization kinetics of melon seed shell. Chem. Eng. Commun. 2019, 206, 1228–1240. [Google Scholar] [CrossRef]
- Hanif, M.P.M.; Rozyanty, A.R.; Hajar, M.D.S.; Tan, S.J.; Supri, A.G. Entylene Vinyl Acetate/Carbonized Wood Fiber Composites: The Effect of Zinc Chloride Content on Tensile Properties, Electrical Properties and Thermal Degradation, Proceeding of the IOP Conference Series: Materials Science and Engineering, Sarawak, Malaysia, 15–17 August 2018; IOP Publishing: Bristol, UK, 2018; p. 012037. [Google Scholar]
- Espinach, F.; Boufi, S.; Delgado-Aguilar, M.; Julián, F.; Mutjé, P.; Méndez, J. Composites from poly (lactic acid) and bleached chemical fibres: Thermal properties. Compos. Part B Eng. 2018, 134, 169–176. [Google Scholar] [CrossRef]
- Ziglio, A.C.; Sardela, M.R.; Gonçalves, D. Wettability, surface free energy and cellulose crystallinity for pine wood (Pinus sp.) modified with chili pepper extracts as natural preservatives. Cellulose 2018, 25, 6151–6160. [Google Scholar] [CrossRef]
- Darmawan, W.; Nandika, D.; Noviyanti, E.; Alipraja, I.; Lumongga, D.; Gardner, D.; Gérardin, P. Wettability and bonding quality of exterior coatings on jabon and sengon wood surfaces. J. Coat. Technol. Res. 2018, 15, 95–104. [Google Scholar] [CrossRef]
- Petrič, M.; Oven, P. Determination of wettability of wood and its significance in wood science and technology: A critical review. Rev. Adhes. Adhes. 2015, 3, 121–187. [Google Scholar] [CrossRef]


| Methods | 103 (%) | ZC-103 (%) | 120 (%) | ZC-120 (%) |
|---|---|---|---|---|
| 10% DM | 8.21 ± 1.88 | 8.49 ± 0.87 | 8.65 ± 1.27 | 8.85 ± 1.28 |
| 10% DM + 5% MA | 14.03 ± 2.38 | 19.61 ± 1.67 | 16.99 ± 0.85 | 16.48 ± 0.600 |
| 10% DM + 10% MA | 19.28 ± 0.83 | 21.97 ± 2.49 | 20.39 ± 2.99 | 19.65 ± 1.72 |
| 10% DM + 15% MA | 23.03 ± 2.19 | 24.97 ± 1.21 | 22.69 ± 2.00 | 23.42 ± 2.45 |
| 10% DM + 20% MA | 29.95 ± 2.49 | 32.43 ± 4.47 | 26.07 ± 3.29 | 28.15 ± 3.19 |
| Methods | Tonset (°C) | Tmax (°C) | Tend (°C) | Residual Mass (%) |
|---|---|---|---|---|
| Ctrl | 221.36 | 348.21 | 494.23 | 14.72 |
| 103 | 203.68 | 332.42 | 495.49 | 23.25 |
| ZC-103 | 187.80 | 323.96 | 495.34 | 27.65 |
| 120 | 210.85 | 337.45 | 495.50 | 19.82 |
| ZC-120 | 205.75 | 325.01 | 495.40 | 26.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Fu, Z.; Cai, Y.; Zhang, Y. Study on Improving the Fixation Rate of Impregnated Poplar Wood with Maltodextrin and 1,3-Dimethylol-4,5-Dihydroxyethyleneurea. Appl. Sci. 2019, 9, 3237. https://doi.org/10.3390/app9163237
Cai M, Fu Z, Cai Y, Zhang Y. Study on Improving the Fixation Rate of Impregnated Poplar Wood with Maltodextrin and 1,3-Dimethylol-4,5-Dihydroxyethyleneurea. Applied Sciences. 2019; 9(16):3237. https://doi.org/10.3390/app9163237
Chicago/Turabian StyleCai, Mingzhen, Zongying Fu, Yingchun Cai, and Yue Zhang. 2019. "Study on Improving the Fixation Rate of Impregnated Poplar Wood with Maltodextrin and 1,3-Dimethylol-4,5-Dihydroxyethyleneurea" Applied Sciences 9, no. 16: 3237. https://doi.org/10.3390/app9163237
APA StyleCai, M., Fu, Z., Cai, Y., & Zhang, Y. (2019). Study on Improving the Fixation Rate of Impregnated Poplar Wood with Maltodextrin and 1,3-Dimethylol-4,5-Dihydroxyethyleneurea. Applied Sciences, 9(16), 3237. https://doi.org/10.3390/app9163237




