Metallurgical Aspects in the Welding of Clad Pipelines—A Global Outlook
Abstract
1. Introduction
2. Production of Clad Pipes
3. Microstructure Evolution
3.1. Solidification
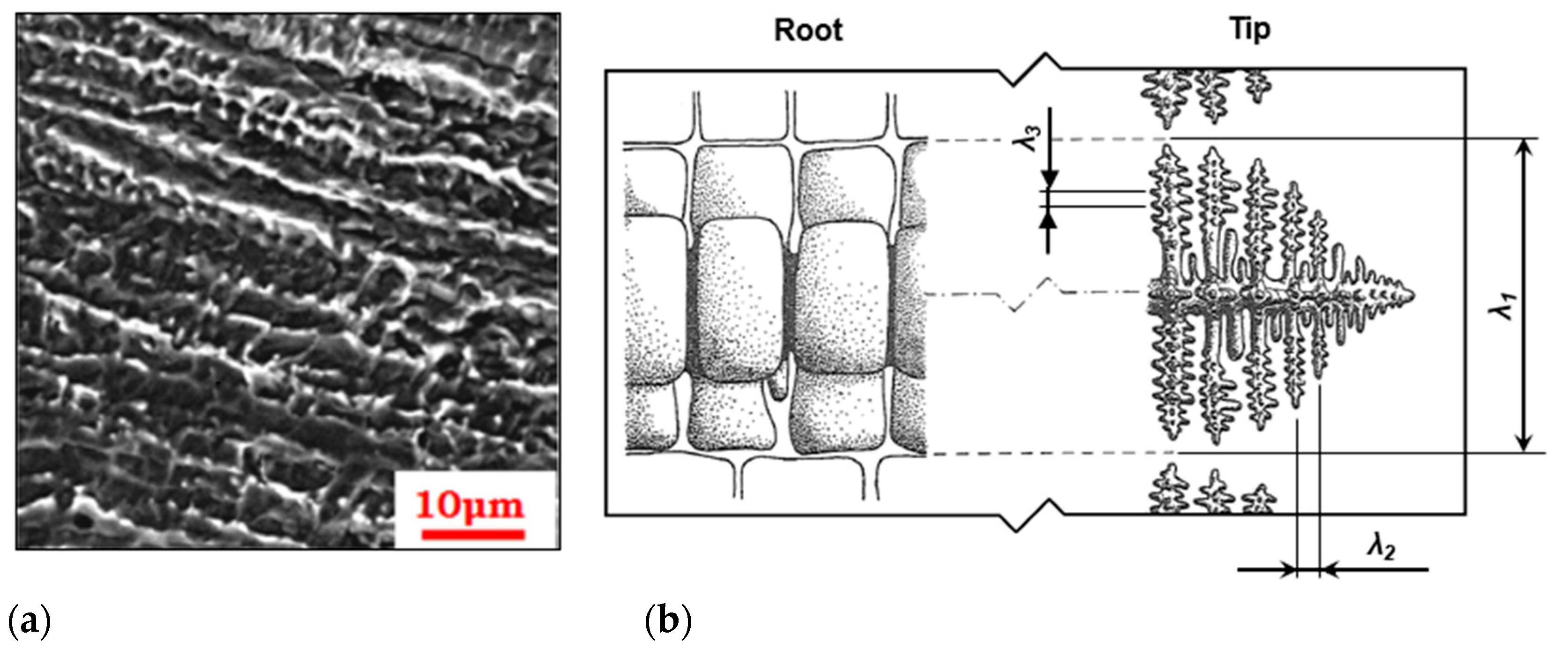
3.1.1. Fundamentals of Solidification Theory
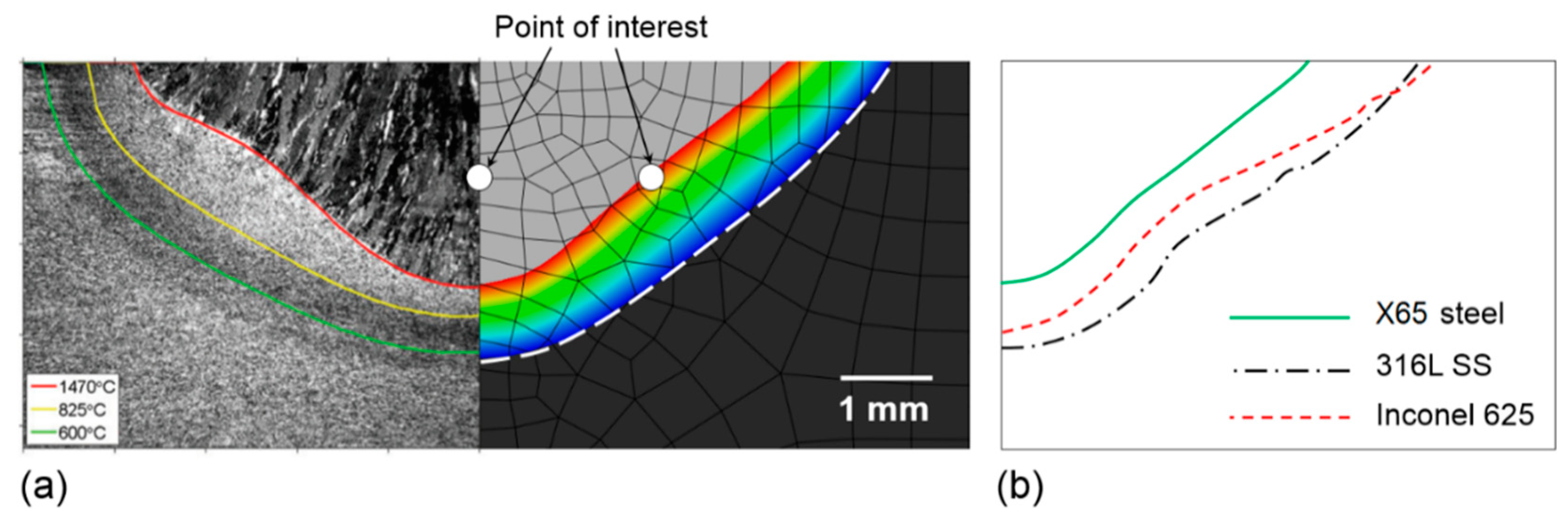
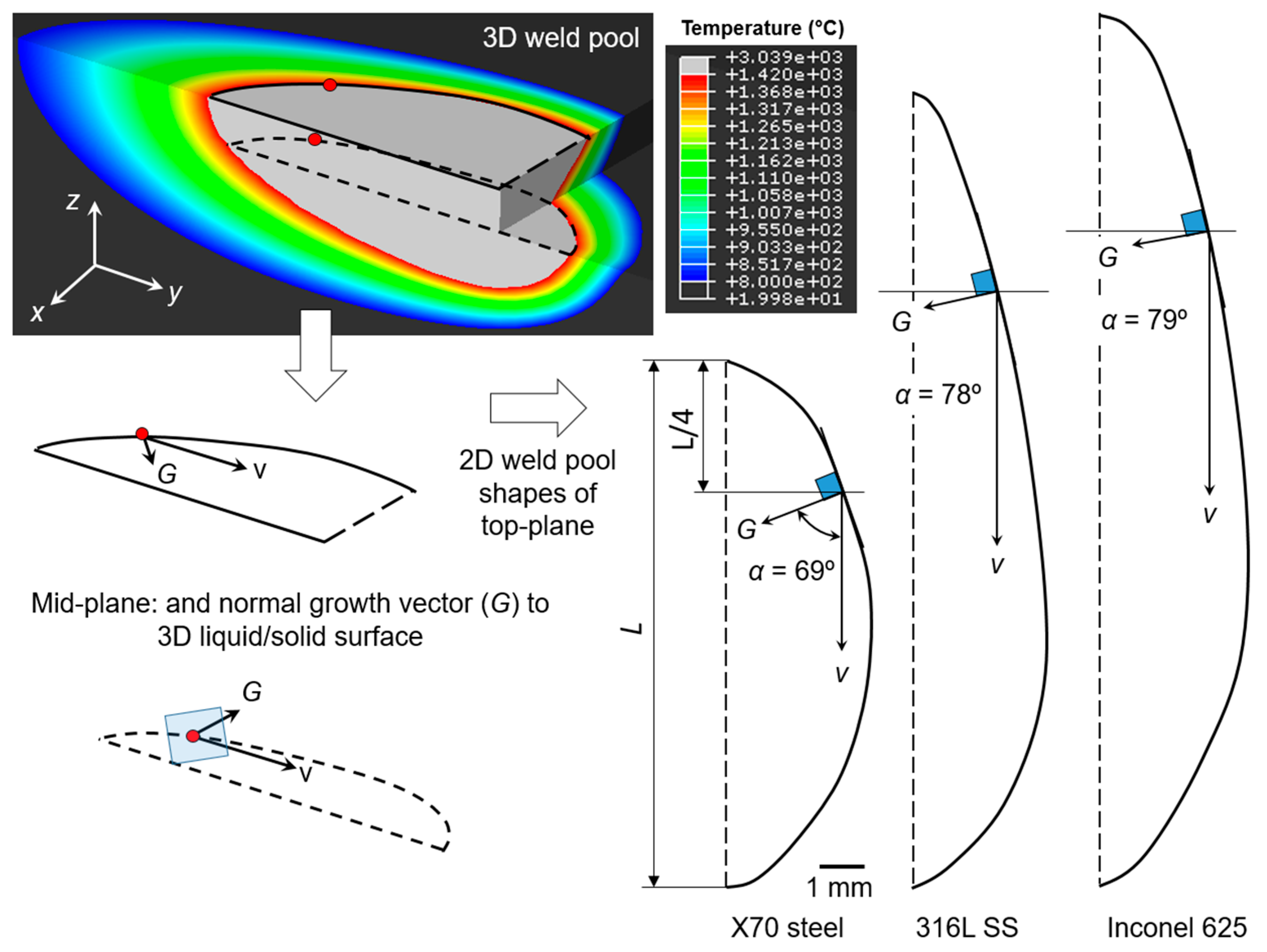
3.1.2. Numerical Simulation of Solidification Parameters
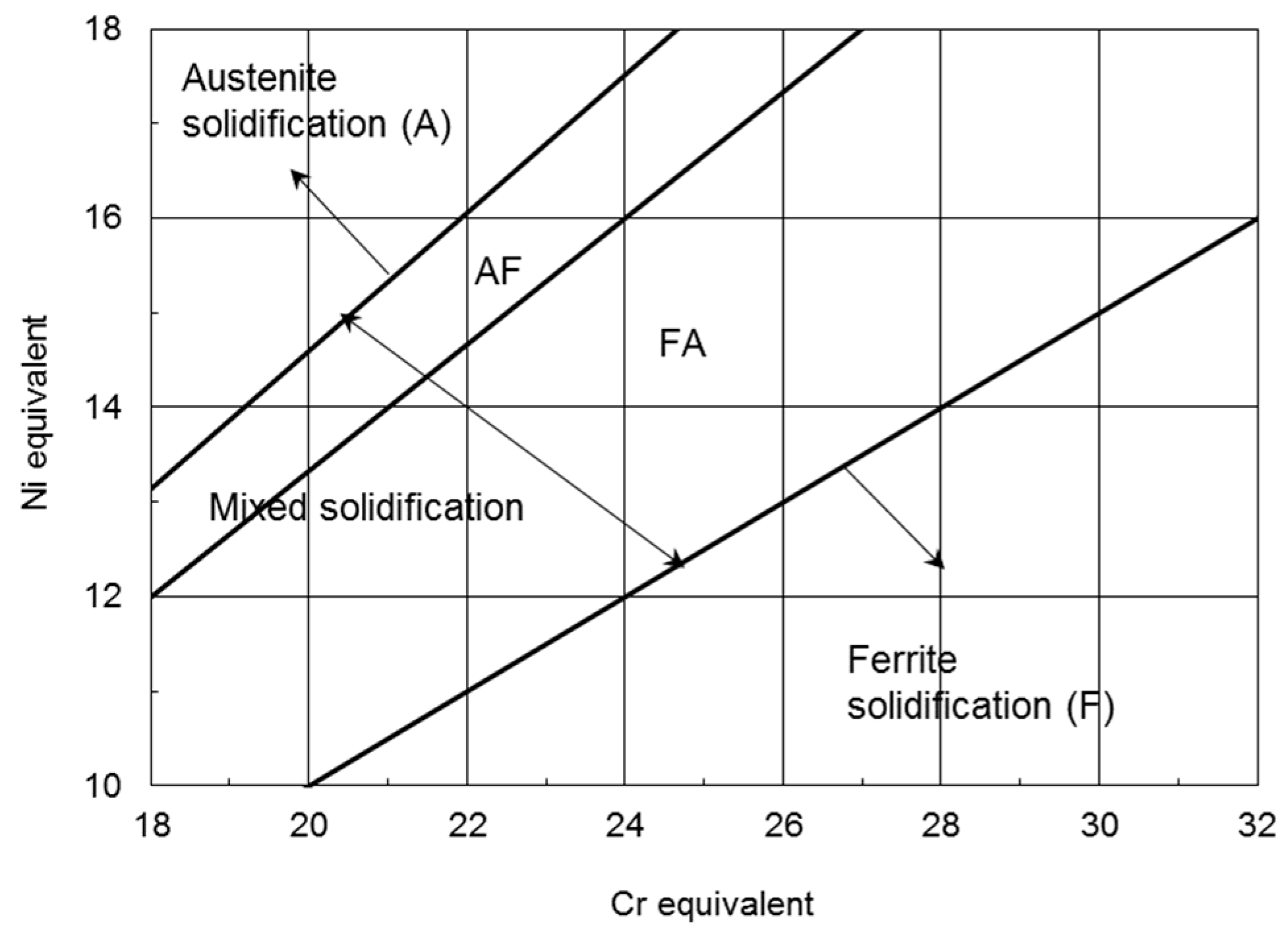
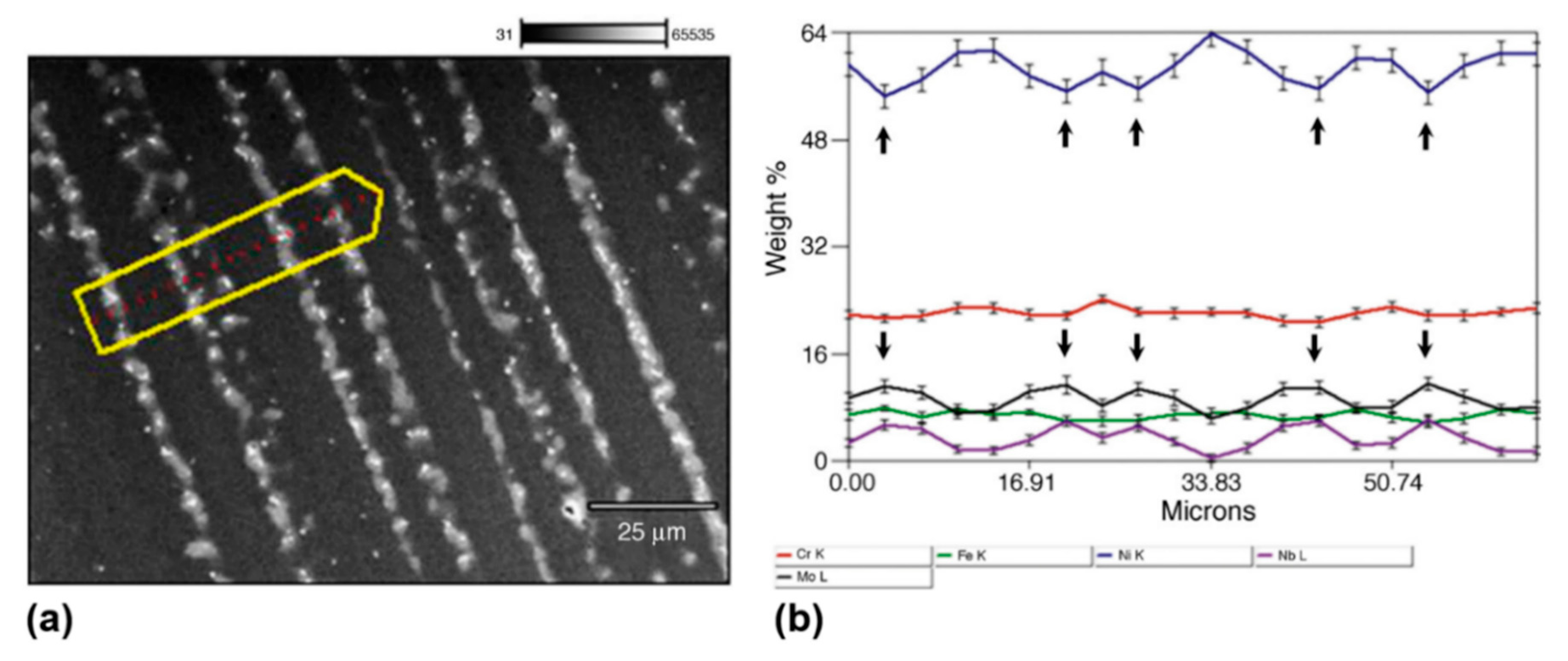
3.2. Segregation in Welds
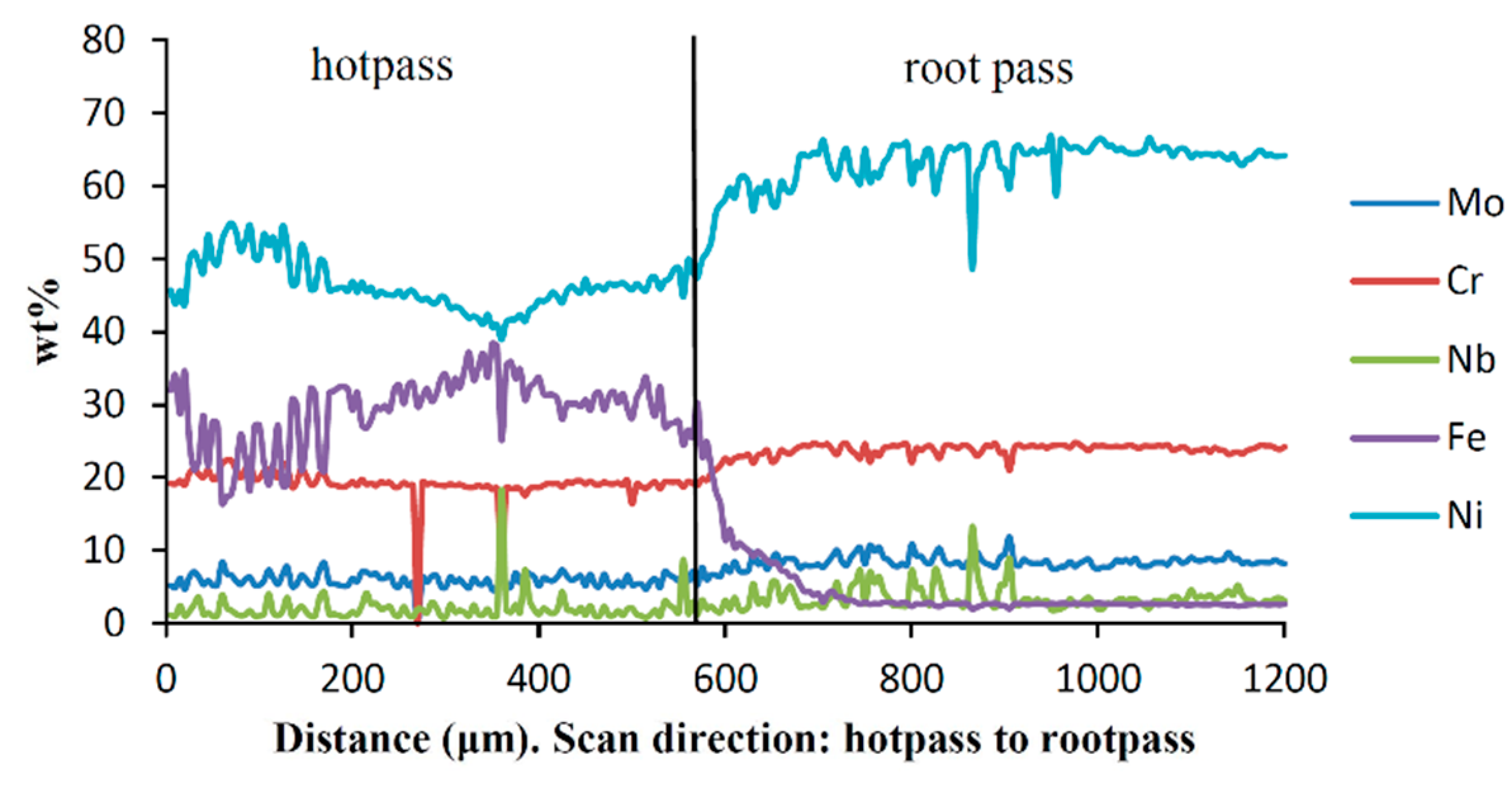
3.3. Weld Metal Microstructures
3.3.1. Root Pass Welding with Ni-Based Wire and Stainless Steel Clad
3.3.2. Root Pass Welding with Stainless Steel Wire and Stainless Steel Clad
3.3.3. Root Pass Welding with Ni-Based Filler Wire and Inconel 625 Clad
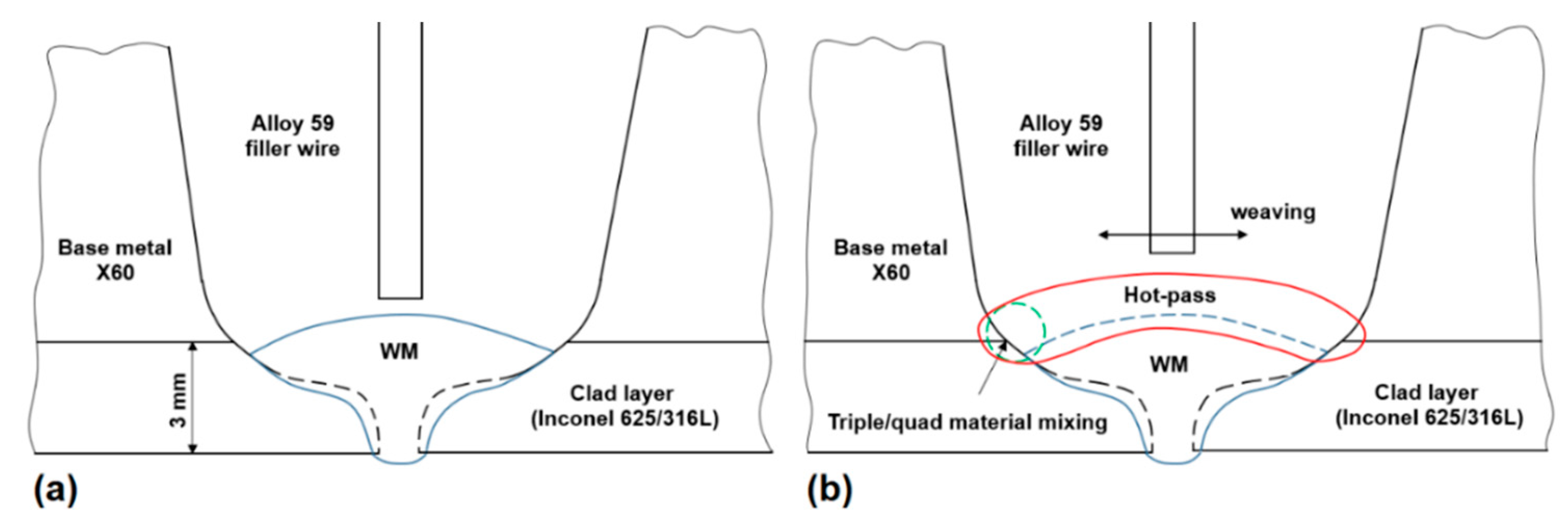
3.3.4. Hot-Pass Welding
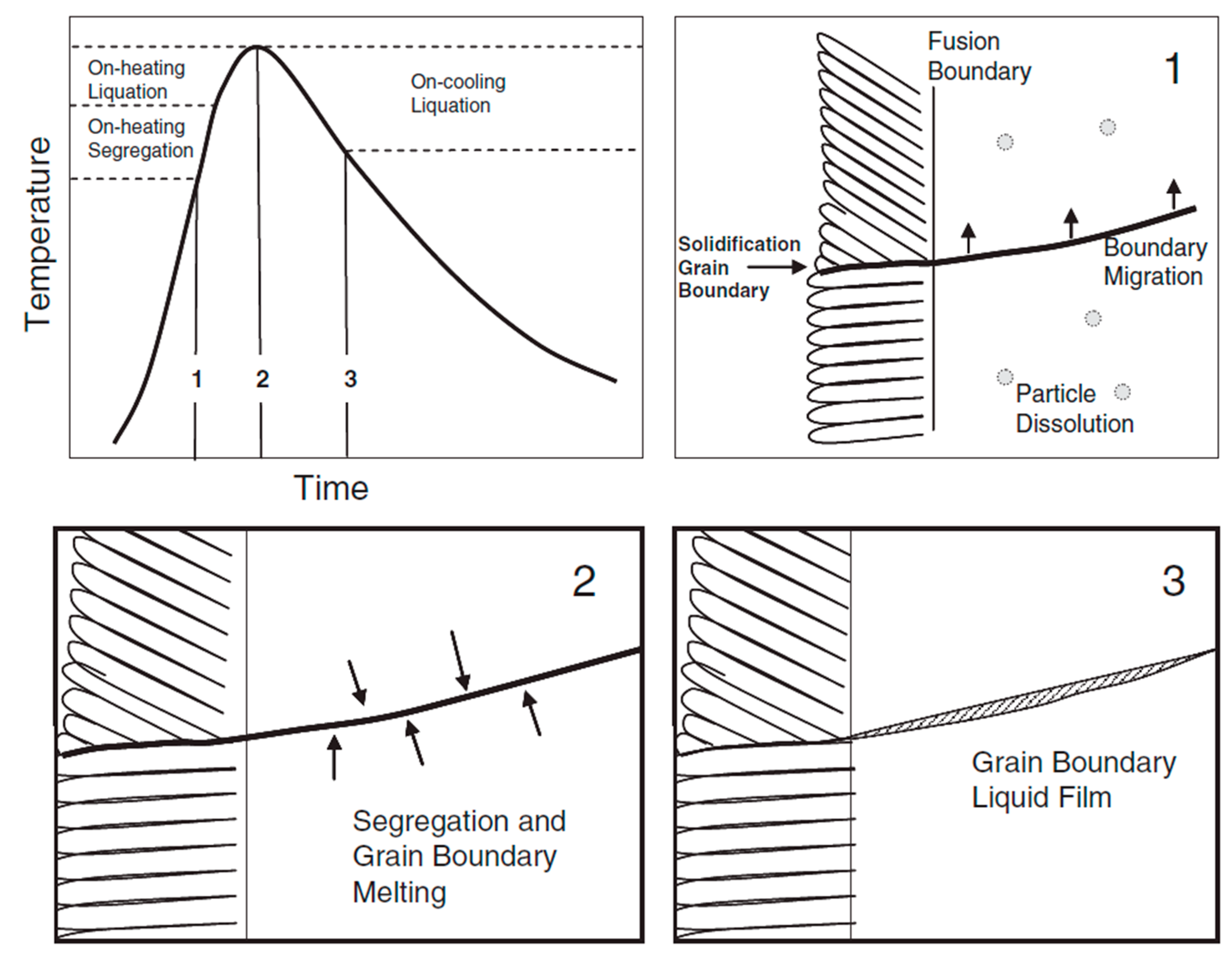
3.4. Heat-Affected Zone
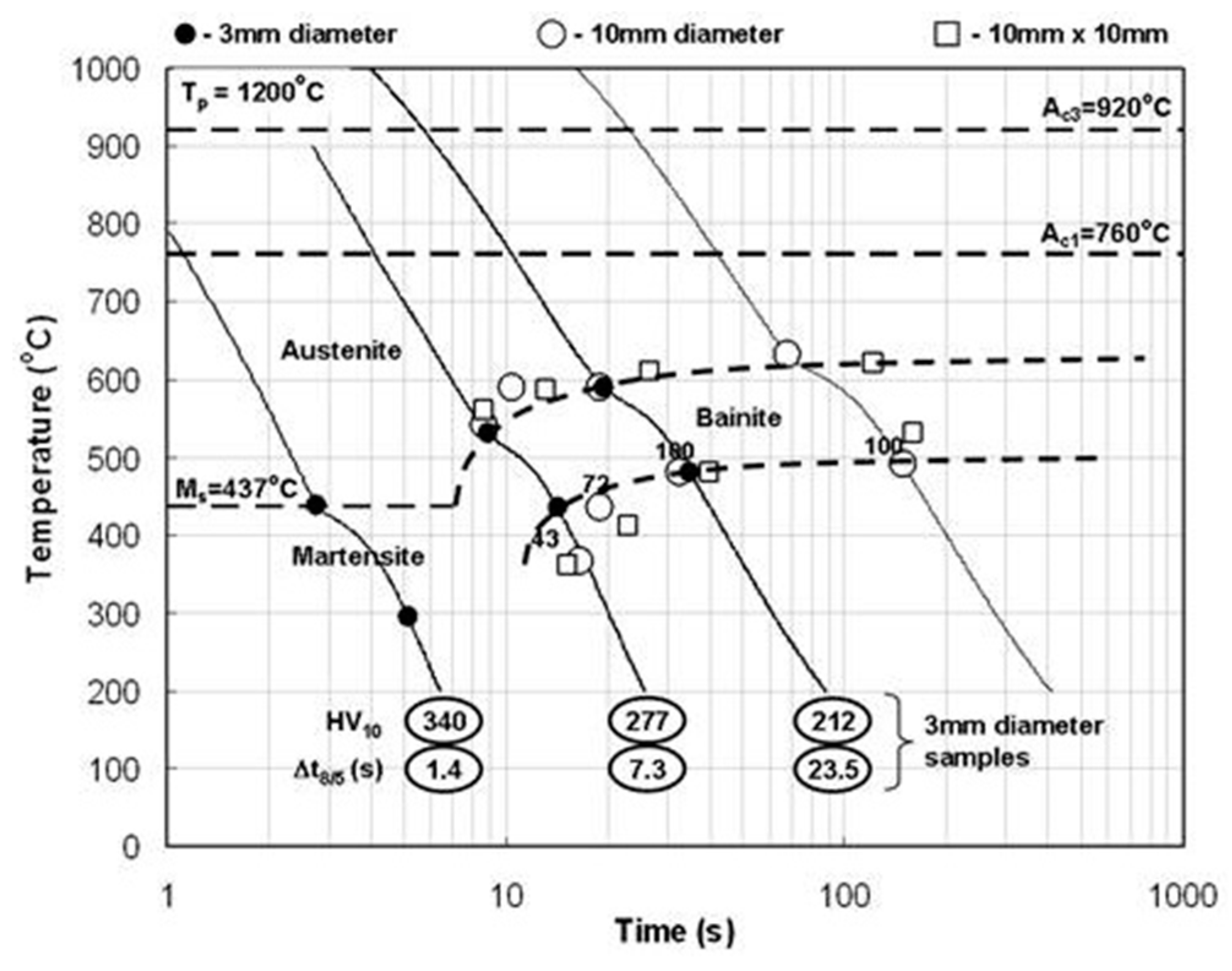
3.4.1. High-Strength Low-Alloy Steel HAZ
3.4.2. HAZ of Welded 316L Stainless Steel Clad
3.4.3. HAZ of Ni-Based Superalloy Clad
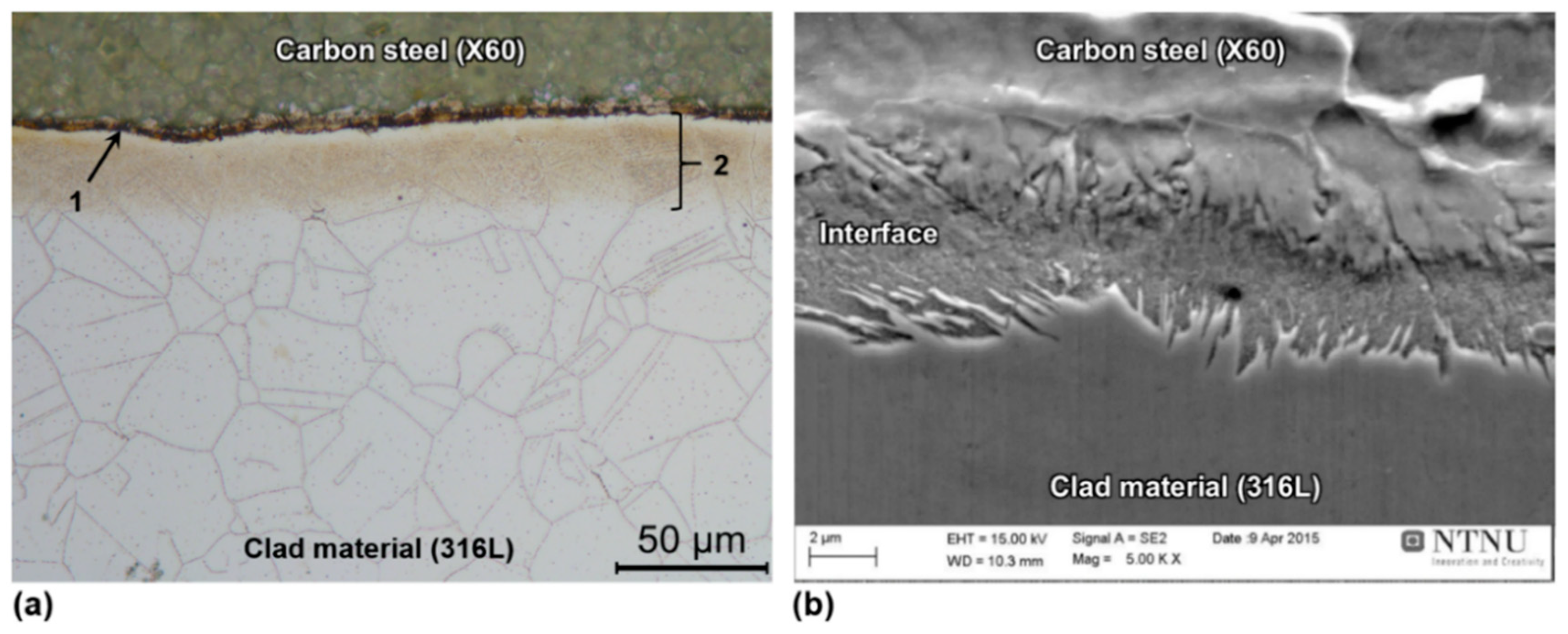
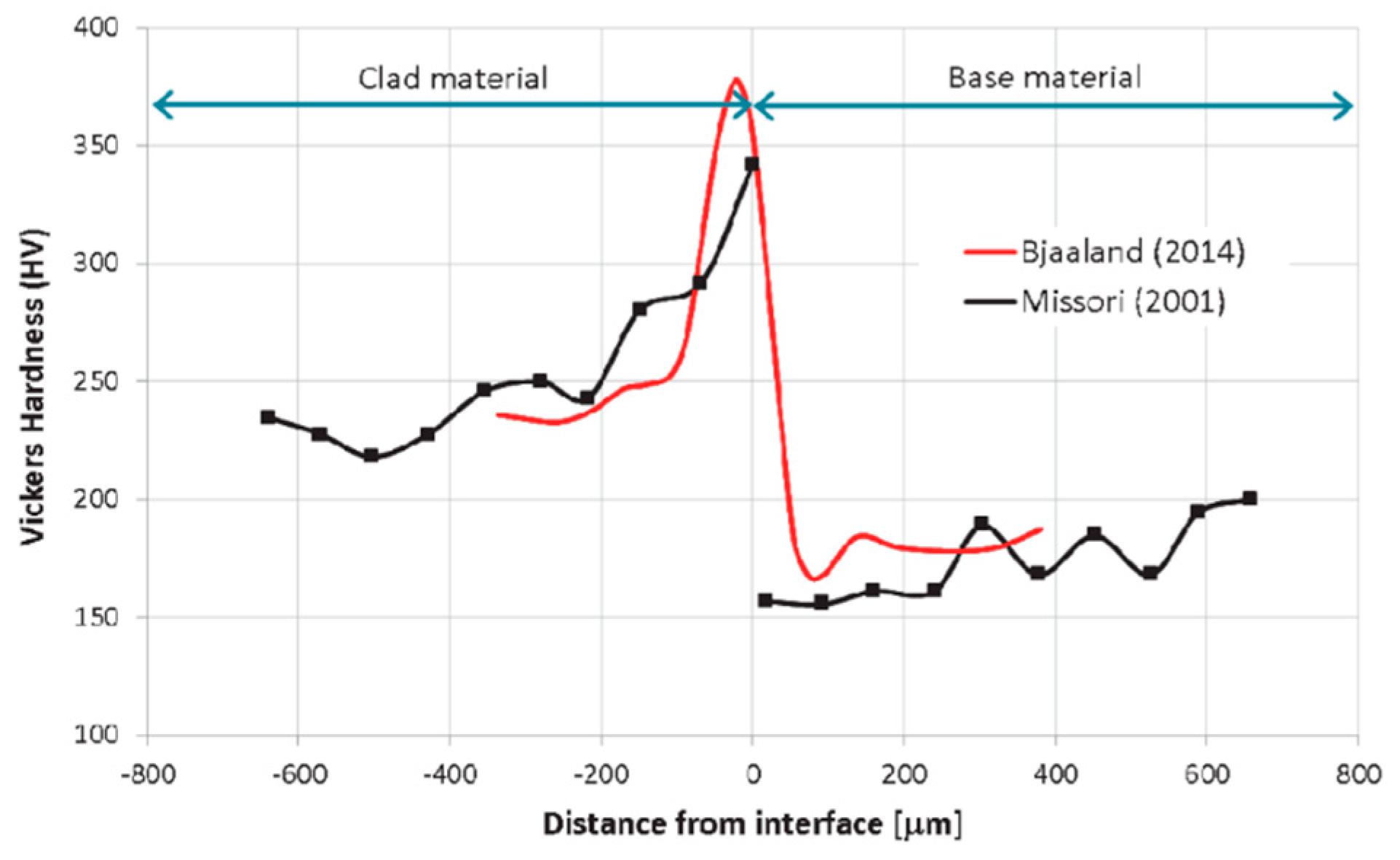
3.4.4. Bimetallic Interface After Welding
4. Potential Application of Hyperbaric Weld Repair
5. Conclusions
- Complex metallurgical phenomena present in the welding of clad pipes due to multi-material systems, especially concerning the weld metal. Clad pipes represent a solid economic alternative to pipes made of stainless steel which is vital to the oil and gas industry.
- Welding process significantly alters the base metal near the weld metal, i.e., the heat-affected zone. Therefore, in complex clad pipes, utilizing expensive materials must be considered in detail.
- Solidification parameters have very high importance on solidification behavior and resulting mechanical properties. Moreover, they can be efficiently estimated by numerical simulation at any point in welded joints.
- The subsea clad pipe network requires an emergency method for pipe repair to be developed. Therefore, the current manuscript represents an important contribution to the knowledge on microstructural changes and diffusion that may occur in welding, and how these may influence the pipeline integrity.
- During repair under hyperbaric conditions, the use of multiple filler wires is very complicated for clad pipes. Therefore, the suitability of filler wire for multi-material systems, both for carbon and nickel-based/stainless steel materials, must be carefully investigated for single filler wire applications.
Author Contributions
Funding
Conflicts of Interest
References
- Lipp, W.; Racu, A.; Moll, M. The World Market for Clad Pipe; SMI (Steel Market Intelligence) GmbH: Pflach, Austria, 2016; p. 256. [Google Scholar]
- Smith, L.M.; Celant, M. CASTI Handbook of Cladding Technology, 2nd ed.; CASTI Pub: Edmonton, AB, Canada, 2000. [Google Scholar]
- Calleja, A.; Tabernero, I.; Ealo, J.A.; Campa, F.J.; Lamikiz, A.; López de Lacalle, L.N. Feed rate calculation algorithm for the homogeneous material deposition of blisk blades by 5-axis laser cladding. Int. J. Adv. Manuf. Technol. 2014, 74, 1219–1228. [Google Scholar] [CrossRef]
- Odina, L.; Hardjanto, F.; Walker, A. Effects of impact loads on CRA-Lined pipelines. Ocean Eng. 2018, 166, 117–134. [Google Scholar] [CrossRef]
- Blondeau, R. Metallurgy and Mechanics of Welding; Wiley-ISTE: Hoboken, NJ, USA, 2008. [Google Scholar]
- Weman, K. MIG Welding Guide; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Khan, I. Welding Science and Technology; New Age International Pvt Ltd Publishers: New Delhi, India, 2009. [Google Scholar]
- Woodward, N.J.; Yapp, D.; Blackman, S.A.; Richardson, I.M.; Widgery, D.; Armstrong, M.A.P.; Verley, R.L.P.; Berge, J.O. Diverless underwater GMA welding for pipeline repair using a fillet welded sleeve. In International Pipeline Conference; American Society of Mechanical Engineers: Calgary, AB, Canada, 2004; pp. 1475–1484. [Google Scholar]
- Berge, J.O.; Armstrong, M.; Verley, R. Deep Water Remote Pipeline Repair Using Welded Sleeve Technique. In Proceedings of the International Pipeline Conference, Calgary, AB, Canada, 4–8 October 2004. [Google Scholar]
- Jemblie, L.; Olden, V.; Akselsen, O.M. A coupled diffusion and cohesive zone modelling approach for numerically assessing hydrogen embrittlement of steel structures. Int. J. Hydrog. Energy 2017, 42, 11980–11995. [Google Scholar] [CrossRef]
- Jemblie, L.; Olden, V.; Akselsen, O.M. A review of cohesive zone modelling as an approach for numerically assessing hydrogen embrittlement of steel structures. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160411. [Google Scholar] [CrossRef] [PubMed]
- Jemblie, L.; Olden, V.; Mainçon, P.; Akselsen, O.M. Cohesive zone modelling of hydrogen induced cracking on the interface of clad steel pipes. Int. J. Hydrog. Energy 2017, 42, 28622–28634. [Google Scholar] [CrossRef]
- Woodward, N.J.; Fostervoll, H.; Akselsen, O.M.; Ahlen, C.H.; Berge, J.O.; Armstrong, M. Inconel 625 Performance as Hyperbaric GMA Welding Consumable for Diverless Retrofit Tee Hot Tap Applications. In Proceedings of the International Society of Offshore and Polar Engineers (ISOPE), Lisbon, Portugal, 1–6 July 2007; pp. 3444–3451. [Google Scholar]
- Urbikain, G.; Perez, J.M.; López de Lacalle, L.N.; Andueza, A. Combination of friction drilling and form tapping processes on dissimilar materials for making nutless joints. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2018, 232, 1007–1020. [Google Scholar] [CrossRef]
- Dhib, Z.; Guermazi, N.; Ktari, A.; Gasperini, M.; Haddar, N. Mechanical bonding properties and interfacial morphologies of austenitic stainless steel clad plates. Mater. Sci. Eng. A 2017, 696, 374–386. [Google Scholar] [CrossRef]
- Dhib, Z.; Guermazi, N.; Gaspérini, M.; Haddar, N. Cladding of low-carbon steel to austenitic stainless steel by hot-roll bonding: Microstructure and mechanical properties before and after welding. Mater. Sci. Eng. A 2016, 656, 130–141. [Google Scholar] [CrossRef]
- Jemblie, L.; Bjaaland, H.; Nyhus, B.; Olden, V.; Akselsen, O.M. Fracture toughness and hydrogen embrittlement susceptibility on the interface of clad steel pipes with and without a Ni-interlayer. Mater. Sci. Eng. A 2017, 685, 87–94. [Google Scholar] [CrossRef]
- Bjaaland, H. Evaluation of Welded Clad Pipe—Microstructures and Properties. Master’s Thesis, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2015. [Google Scholar]
- Bjaaland, H. Metallurgical Reactions in Welding of Clad X60/X65 Pipelines; Norwegian University of Science and Technology (NTNU): Gjøvik, Norway, 2014. [Google Scholar]
- Koshy, P. Alloy 625 Weld Cladding of Wellheads and Valves: Review of Dilution-Control Techniques and Weld Process Development. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 1985; p. 10. [Google Scholar]
- Bjaaland, H.; Akselsen, O.M.; Olden, V.; Nyhus, B.; Karlsen, M.; Hjelen, J. Metallurgical reactions in welding of clad X60/X65 pipelines. In Proceedings of the Twenty-fifth International Ocean and Polar Engineering Conference—In International Offshore and Polar Engineering Conference, Kona, HI, USA, 21–26 June 2015; pp. 61–66. [Google Scholar]
- Specification, A.P.I. 5LD, Specification for CRA Clad or Lined Steel Pipe, 3rd ed.; American Petroleum Institute: Washington, DC, USA, 2009. [Google Scholar]
- Veritas, D.N. Offshore Standard DNV-OS-F101, Submarine Pipeline Systems; Det, DNV; Norske Veritas: Høvik, Norway, 2012. [Google Scholar]
- Wu, C.; Li, S.; Zhang, C.; Wang, X. Microstructural evolution in 316LN austenitic stainless steel during solidification process under different cooling rates. J. Mater. Sci. 2016, 51, 2529–2539. [Google Scholar] [CrossRef]
- Suutala, N. Effect of solidification conditions on the solidification mode in austenitic stainless steels. Metall. Trans. A 1983, 14, 191–197. [Google Scholar] [CrossRef]
- Olson, D.L. Prediction of austenitic weld metal microstructure and properties. Weld. J. 1985, 64, 281–295. [Google Scholar]
- Akselsen, O.M.; Rørvik, G.; van der Eijk, C.; Kvaale, P.E. Mechanical properties of experimental 13% Cr stainless steel weld deposits. In Proceedings of the Nordic welding conference, Reykjavik, Iceland, 20–22 September 2000. [Google Scholar]
- Hammar, O.; Svensson, U. Solidification and Casting of Metals: Proceedings of an International Conference on Solidification; The Metals Societ: London, UK, 1979. [Google Scholar]
- Cieslak, M.J.; Headley, T.J.; Romig, A.D.; Kollie, T. A melting and solidification study of alloy 625. Metall. Trans. A 1988, 19, 2319–2331. [Google Scholar] [CrossRef]
- Knorovsky, G.A.; Cieslak, M.J.; Headley, T.J.; Romig, A.D.; Hammetter, W.F. INCONEL 718: A solidification diagram. Metall. Trans. A 1989, 20, 2149–2158. [Google Scholar] [CrossRef]
- DuPont, J.N.; Marder, A.R.; Notis, M.R.; Robino, C.V. Solidification of Nb-bearing superalloys: Part II. Pseudoternary solidification surfaces. Metall. Mater. Trans. A 1998, 29, 2797–2806. [Google Scholar] [CrossRef]
- DuPont, J.N.; Robino, C.V. The influence of Nb and C on the solidification microstructures of Fe-Ni-Cr alloys. Scr. Mater. 1999, 41, 449–454. [Google Scholar] [CrossRef]
- DuPont, J.N.; Banovic, S.W.; Marder, A.R. Microstructural Evolution and Weldability of Dissimilar Welds between a Super Austenitic Stainless Steel and Nickel Base Alloys. Weld. J. 2003, 82, 125–135. [Google Scholar]
- DuPont, J.N. Fundamentals of Weld Solidification. In ASM Handbook—Welding Fundamentals and Processes; Lienert, T., Siewert, T., Babu, S., Acoff, V., Eds.; ASM International: Almere, The Netherlands, 2011; Volume 6, pp. 96–114. [Google Scholar]
- David, S.A.; Babu, S.S.; Vitek, J.M. Welding: Solidification and microstructure. JOM 2003, 55, 14–20. [Google Scholar] [CrossRef]
- Lienert, T.; Siewert, T.; Babu, S.; Acoff, V. ASM Handbook; Welding Fundamentals and Processes (ASM): Almere, The Netherlands, 2011; Volume 6. [Google Scholar]
- Blecher, J.J.; Palmer, T.A.; DebRoy, T. Solidification Map of a Nickel-Base Alloy. Metall. Mater. Trans. A 2014, 45, 2142–2151. [Google Scholar] [CrossRef]
- Aarbogh, H.M.; Hamide, M.; Fjær, H.G.; Mo, A.; Bellet, M. Experimental validation of finite element codes for welding deformations. J. Mater. Process. Technol. 2010, 210, 1681–1689. [Google Scholar] [CrossRef]
- Yang, Z.; Debroy, T. Modeling macro-and microstructures of Gas-Metal-Arc Welded HSLA-100 steel. Metall. Mater. Trans. B 1999, 30, 483–493. [Google Scholar] [CrossRef]
- Bunaziv, I.; Akselsen, O.M.; Frostevarg, J.; Kaplan, A.F.H. Laser-arc hybrid welding of thick HSLA steel. J. Mater. Process. Technol. 2018, 259, 75–87. [Google Scholar] [CrossRef]
- Azar, A.S.; Ås, S.K.; Akselsen, O.M. Analytical Modeling of Weld Bead Shape in Dry Hyperbaric GMAW Using Ar-He Chamber Gas Mixtures. J. Mater. Eng. Perform. 2013, 22, 673–680. [Google Scholar] [CrossRef]
- Heigel, J.C.; Gouge, M.F.; Michaleris, P.; Palmer, T.A. Selection of powder or wire feedstock material for the laser cladding of Inconel® 625. J. Mater. Process. Technol. 2016, 231, 357–365. [Google Scholar] [CrossRef]
- Tan, W.; Shin, Y.C. Multi-scale modeling of solidification and microstructure development in laser keyhole welding process for austenitic stainless steel. Comput. Mater. Sci. 2015, 98, 446–458. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhang, J.X.; Gumenyuk, A.; Rethmeier, M.; Na, S.J. Numerical simulation of full penetration laser welding of thick steel plate with high power high brightness laser. J. Mater. Process. Technol. 2014, 214, 1710–1720. [Google Scholar] [CrossRef]
- Easterling, K. Introduction to the Physical Metallurgy of Welding; Butterworth-Heinemann: Oxford, UK, 1983. [Google Scholar]
- Wei, H.L.; Elmer, J.W.; DebRoy, T. Crystal growth during keyhole mode laser welding. Acta Mater. 2017, 133, 10–20. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Dendrite growth at the limit of stability: Tip radius and spacing. Acta Metall. 1981, 29, 11–20. [Google Scholar] [CrossRef]
- Franke, M.M.; Singer, R.F.; Steinbach, I. Tertiary dendritic instability in late stage solidification of Ni-based superalloys. Model. Simul. Mater. Sci. Eng. 2014, 22, 025026. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 3rd ed.; Trans Tech Publ: Aedermannsdorf, Switzerland, 1989; p. 305. [Google Scholar]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; Chapman & Hall (CRC Press): Boca Raton, FL, USA, 1992. [Google Scholar]
- Silva, C.C.; Miranda, H.C.D.; Motta, M.F.; Farias, J.P.; Afonso, C.R.M.; Ramirez, A.J. New insight on the solidification path of an alloy 625 weld overlay. J. Mater. Res. Technol. 2013, 2, 228–237. [Google Scholar] [CrossRef]
- Epishin, A.; Link, T.; Brückner, U.; Fedelich, B.; Portella, P. Effects of segregation in nickel-base superalloys: Dendritic stresses. Superalloys 2004, 2004, 537–543. [Google Scholar]
- Andrews, K. Empirical formulae for the calculation of some transformation temperatures. J. Iron Steel Inst. 1965, 203, 721–727. [Google Scholar]
- Shushan, S.M.; Charles, E.A.; Congleton, J. The environment assisted cracking of diffusion bonded stainless to carbon steel joints in an aqueous chloride solution. Corros. Sci. 1996, 38, 673–686. [Google Scholar] [CrossRef]
- McGuire, M. Austenitic Stainless Steels. In Stainless Steels for Design Engineers; ASM International: Almere, The Netherlands, 2008. [Google Scholar]
- Guiraldenq, P.; Duparc, O.H. The genesis of the Schaeffler diagram in the history of stainless steel. Metall. Res. Technol. 2017, 114, 613. [Google Scholar] [CrossRef]
- Lippold, J.C.; Kotecki, D.J. Welding Metallurgy and Weldability of Stainless Steels; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Kotecki, D.J.; Siewert, T.A. WRC-1992 Constitution Diagram for Stainless Steel Weld Metals: A Modification of the WRC-1988 Diagram. Weld. J. 1992, 71, 171–178. [Google Scholar]
- Feng, J.; Zhang, H.; He, P. The CMT short-circuiting metal transfer process and its use in thin aluminium sheets welding. Mater. Des. 2009, 30, 1850–1852. [Google Scholar] [CrossRef]
- Azar, A.S.; Akselsen, O.M.; Fostervoll, H. Prediction of the Thermal Cycles in Dry Hyperbaric GMA Welding Using Partial Differential Heat Transfer Equations. In Proceedings of the 9th International Conference, Trends in Welding Research, Chicago, IL, USA, 4–8 June 2012; pp. 738–745. [Google Scholar]
- Shakil, M.; Ahmad, M.; Tariq, N.H.; Hasan, B.A.; Akhter, J.I.; Ahmed, E.; Mehmood, M.; Choudhry, M.A.; Iqbal, M. Microstructure and hardness studies of electron beam welded Inconel 625 and stainless steel 304L. Vacuum 2014, 110, 121–126. [Google Scholar] [CrossRef]
- Shah Hosseini, H.; Shamanian, M.; Kermanpur, A. Microstructural and weldability analysis of Inconel617/AISI 310 stainless steel dissimilar welds. Int. J. Press. Vessel. Pip. 2016, 144, 18–24. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, D.; Yan, Y.T.; Guo, N.; Feng, J.C. Microstructural characteristics and mechanical properties of underwater wet flux-cored wire welded 316L stainless steel joints. J. Mater. Process. Technol. 2016, 238, 423–430. [Google Scholar] [CrossRef]
- Suutala, N.; Takalo, T.; Moisio, T. Ferritic-austenitic solidification mode in austenitic stainless steel welds. Metall. Trans. A 1980, 11, 717–725. [Google Scholar] [CrossRef]
- DuPont, J.N.; Lippold, J.C.; Kiser, S.D. Welding Metallurgy and Weldability of Nickel-Base Alloys; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ren, W.; Lu, F.; Yang, R.; Liu, X.; Li, Z. Liquation cracking in fiber laser welded joints of inconel 617. J. Mater. Process. Technol. 2015, 226, 214–220. [Google Scholar] [CrossRef]
- Hua, C.; Lu, H.; Yu, C.; Chen, J.-M.; Zhang, M.-L.; Li, D.-Y. Reduction of Laves phase in nickel-alloy welding process under ultrasonic Ampère’s force. J. Mater. Process. Technol. 2018, 252, 389–397. [Google Scholar] [CrossRef]
- Hochhauser, F.; Ernst, W.; Rauch, R.; Vallant, R.; Enzinger, N. Influence of the Soft Zone on The Strength of Welded Modern Hsla Steels. Weld. World 2012, 56, 77–85. [Google Scholar] [CrossRef]
- Onsøien, M.; M’Hamdi, M.; Mo, A. A CCT Diagram for an Offshore Pipeline Steel of X70 Type. Weld. J. 2009, 88, 1–6. [Google Scholar]
- Akselsen, O.M.; Solberg, J.K.; Grong, Ø. Effects of martensite-austenite (M-A) islands on intercritical heat-affected zone toughness of low carbon microalloyed steels. Scand. J. Metall. 1988, 17, 194–200. [Google Scholar]
- Akselsen, O.M.; Grong, Ø.; Solberg, J.K. Structure–property relationships in intercritical heat affected zone of low-carbon microalloyed steels. Mater. Sci. Technol. 1987, 3, 649–655. [Google Scholar] [CrossRef]
- Mohseni, P.; Solberg, J.K.; Østby, E.; Akselsen, O.M. Application of Electron Backscatter Diffraction (EBSD) On Facet Crystallographic Orientation Studies In Arctic Steels. In Proceedings of the Twenty-first International Offshore and Polar Engineering Conference, Maui, HI, USA, 16–21 June 2011; p. 5. [Google Scholar]
- Mohseni, P.; Solberg, J.K.; Karlsen, M.; Akselsen, O.M.; Østby, E. Application of combined EBSD and 3D-SEM technique on crystallographic facet analysis of steel at low temperature. J. Microsc. 2013, 251, 45–56. [Google Scholar] [CrossRef]
- Mohseni, P.; Solberg, J.K.; Karlsen, M.; Akselsen, O.M.; Østby, E. Cleavage Fracture Initiation at M–A Constituents in Intercritically Coarse-Grained Heat-Affected Zone of a HSLA Steel. Metall. Mater. Trans. A 2014, 45, 384–394. [Google Scholar] [CrossRef]
- Mohseni, P.; Solberg, J.K.; Karlsen, M.; Akselsen, O.M.; Østby, E. Investigation of mechanism of cleavage fracture initiation in intercritically coarse grained heat affected zone of HSLA steel. Mater. Sci. Technol. 2012, 28, 1261–1268. [Google Scholar] [CrossRef]
- Haugen, V.G.; Rogne, B.R.S.; Akselsen, O.M.; Thaulow, C.; Østby, E. Local mechanical properties of intercritically reheated coarse grained heat affected zone in low alloy steel. Mater. Des. 2014, 59, 135–140. [Google Scholar] [CrossRef]
- Davis, C.L.; King, J.E. Cleavage initiation in the intercritically reheated coarse-grained heat-affected zone: Part I. Fractographic evidence. Metall. Mater. Trans. A 1994, 25, 563–573. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels: Microstructure and Properties, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar]
- Kou, S. Welding Metallurgy, 2nd ed.; Wiley: Hoboken, NJ, USA, 2003; p. 480. [Google Scholar]
- Lippold, J.C. Welding Metallurgy and Weldability; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Baeslack III, W.A.; Lippold, J.C.; Savage, W.F. Unmixed zone formation in austenitic stainless steel weldments. Weld. J. 1979, 58, 168. [Google Scholar]
- Feng, Y.; Luo, Z.; Liu, Z.; Li, Y.; Luo, Y.; Huang, Y. Keyhole gas tungsten arc welding of AISI 316L stainless steel. Mater. Des. 2015, 85, 24–31. [Google Scholar] [CrossRef]
- Sabzi, M.; Dezfuli, S.M. Drastic improvement in mechanical properties and weldability of 316L stainless steel weld joints by using electromagnetic vibration during GTAW process. J. Manuf. Process. 2018, 33, 74–85. [Google Scholar] [CrossRef]
- Kikuchi, H.; Takahashi, H.; Yanagiwara, H.; Murakami, T. Relationship between ferromagnetic properties and grain size of Inconel alloy 600. J. Magn. Magn. Mater. 2015, 381, 56–64. [Google Scholar] [CrossRef]
- Duvall, D.S.; Owczarski, W.A. Further heat affected zone studies in heat-resistant nickel alloys. Weld. J. 1967, 46, 423–432. [Google Scholar]
- Lippold, J.C.; Beaslack III, W.A.; Varol, I. Heat-Affected Zone Liquation Cracking in Austenitic and Duplex Stainless Steels. Weld. J. 1992, 71, 1–14. [Google Scholar]
- Li, S.; Li, K.; Cai, Z.; Pan, J. Behavior of M23C6 phase in Inconel 617B superalloy during welding. J. Mater. Process. Technol. 2018, 258, 38–46. [Google Scholar] [CrossRef]
- Korrapati, P.K.; Avasarala, V.K.; Bhushan, M.; Ramkumar, K.D.; Arivazhagan, N.N.; Narayanan, S. Assessment of Mechanical Properties of PCGTA Weldments of Inconel 625. Procedia Eng. 2014, 75, 9–13. [Google Scholar] [CrossRef]
- Vigraman, T.; Ravindran, D.; Narayanasamy, R. Diffusion bonding of AISI 304L steel to low-carbon steel with AISI 304L steel interlayer. Mater. Des. 2012, 34, 594–602. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.X.; Chen, C.X.; Feng, J.H.; Yin, F.X. Microstructure, mechanical properties and interface bonding mechanism of hot-rolled stainless steel clad plates at different rolling reduction ratios. J. Alloy Compd. 2018, 766, 517–526. [Google Scholar] [CrossRef]
- Lindholm, D. Carbon Diffusion across the Bimetallic Interface of Welded Clad Pipes. In Proceedings of the 28th International Journal of Offshore and Polar Engineering, Sapporo, Japan, 10–15 June 2018; pp. 165–172. [Google Scholar]
- Olden, V.; Jemblie, L.; Akselsen, O.M.; Utvær, A. Hydrogen embrittlement susceptibility on the interface of clad steel pipes, before and after welding. In Proceedings of the International Conference on Metals & Hydrogen, Ghent, Belgium, 28–30 May 2018. [Google Scholar]
- Wang, Y.; Tsai, H.L. Impingement of filler droplets and weld pool dynamics during gas metal arc welding process. Int. J. Heat Mass Transf. 2001, 44, 2067–2080. [Google Scholar] [CrossRef]
- Hu, J.; Tsai, H.L. Effects of current on droplet generation and arc plasma in gas metal arc welding. J. Appl. Phys. 2006, 100, 053304. [Google Scholar] [CrossRef]
- Hu, J.; Tsai, H.L. Heat and mass transfer in gas metal arc welding. Part I: The arc. Int. J. Heat Mass Transf. 2007, 50, 833–846. [Google Scholar] [CrossRef]
- Rao, Z.H.; Hu, J.; Liao, S.M.; Tsai, H.L. Modeling of the transport phenomena in GMAW using argon–helium mixtures. Part I—The arc. Int. J. Heat Mass Transf. 2010, 53, 5707–5721. [Google Scholar] [CrossRef]
- Rao, Z.H.; Hu, J.; Liao, S.M.; Tsai, H.L. Modeling of the transport phenomena in GMAW using argon–helium mixtures. Part II—The metal. Int. J. Heat Mass Transf. 2010, 53, 5722–5732. [Google Scholar] [CrossRef]
- Rao, Z.H.; Zhou, J.; Tsai, H.L. Determination of equilibrium wire-feed-speeds for stable gas metal arc welding. Int. J. Heat Mass Transf. 2012, 55, 6651–6664. [Google Scholar] [CrossRef]
- Zhao, Y.; Chung, H. Numerical simulation of droplet transfer behavior in variable polarity gas metal arc welding. Int. J. Heat Mass Transf. 2017, 111, 1129–1141. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, Z.; Chen, X.L. Three-Dimensional Simulation of Transient GMA Weld Pool with Free Surface. Weld. J. 2004, 83, 169–176. [Google Scholar]
- Chen, J.; Schwenk, C.; Wu, C.S.; Rethmeier, M. Predicting the influence of groove angle on heat transfer and fluid flow for new gas metal arc welding processes. Int. J. Heat Mass Transf. 2012, 55, 102–111. [Google Scholar] [CrossRef]
- Hertel, M.; Spille-Kohoff, A.; Füssel, U.; Schnick, M. Numerical simulation of droplet detachment in pulsed gas–metal arc welding including the influence of metal vapour. J. Phys. D Appl. Phys. 2013, 46, 224003. [Google Scholar] [CrossRef]
- Cheon, J.; Kiran, D.V.; Na, S.-J. CFD based visualization of the finger shaped evolution in the gas metal arc welding process. Int. J. Heat Mass Transf. 2016, 97, 1–14. [Google Scholar] [CrossRef]
- Ogino, Y.; Hirata, Y.; Murphy, A.B. Numerical simulation of GMAW process using Ar and an Ar–CO2 gas mixture. Weld. World 2016, 60, 345–353. [Google Scholar] [CrossRef]



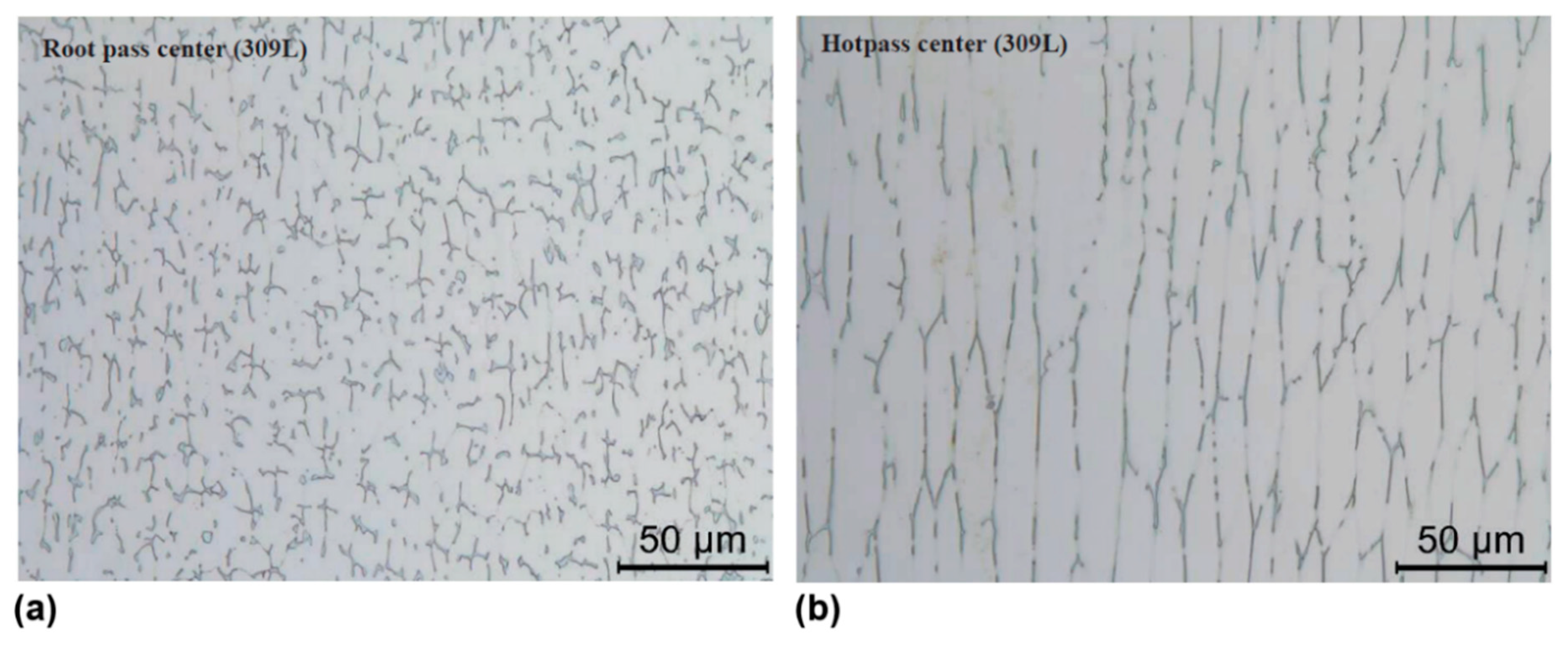
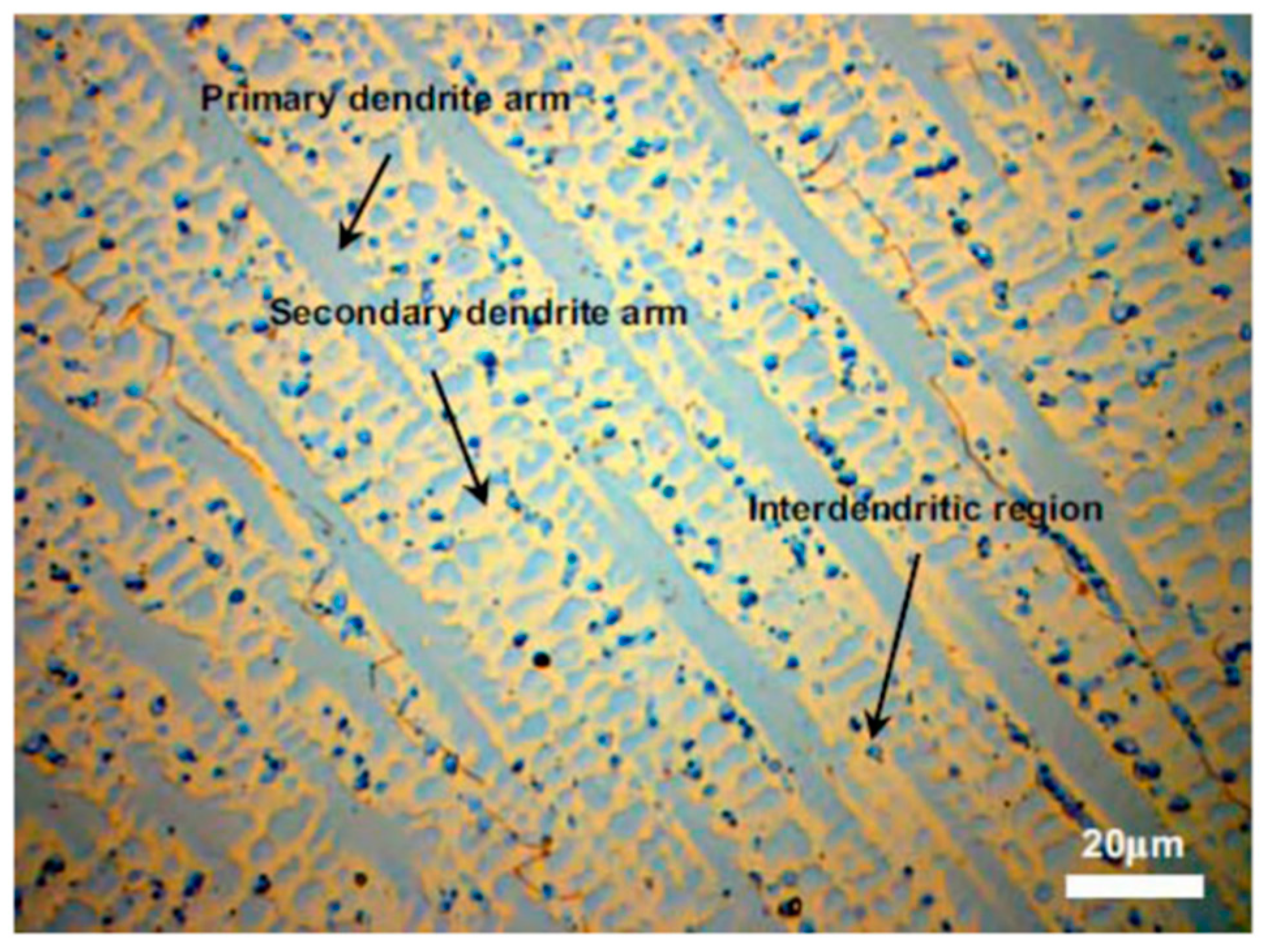
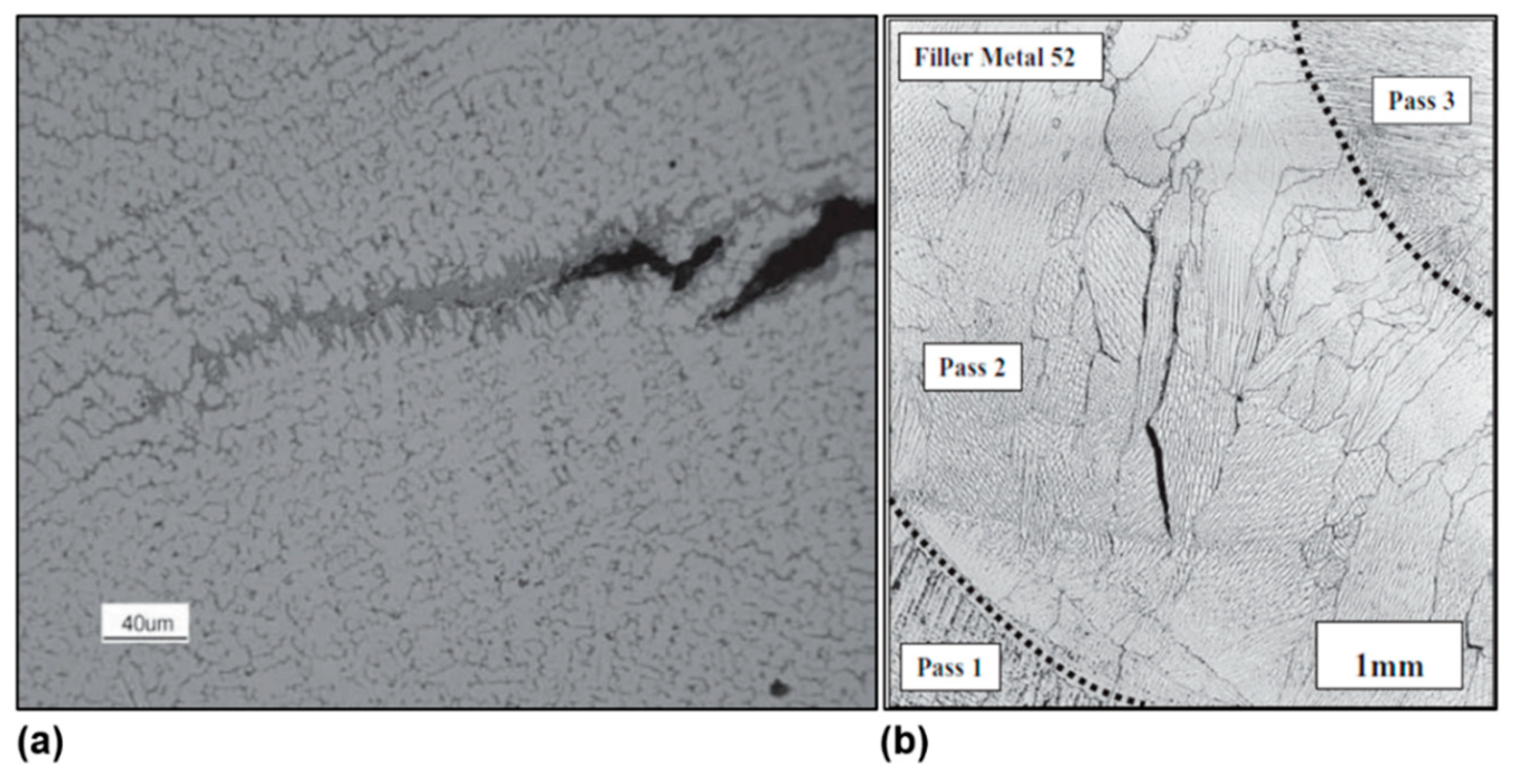
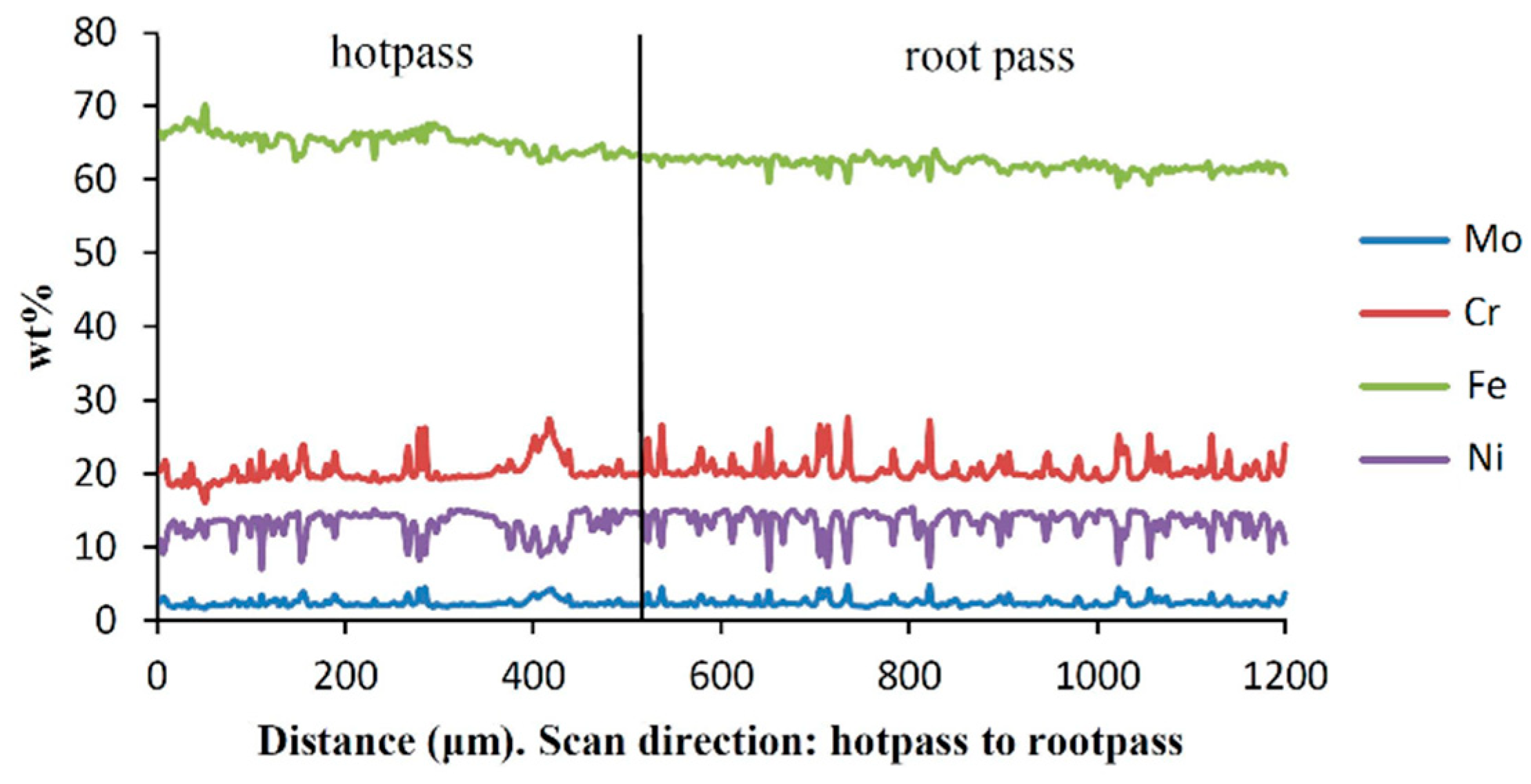

| Alloy | Ni | Cr | Mo | Fe | Nb | Co | Mn | Ti | Si | C |
|---|---|---|---|---|---|---|---|---|---|---|
| Alloy 59 | 59.0 | 22.0–24.0 | 15.0–16.0 | ≤1.5 | - | ≤0.3 | ≤0.5 | - | ≤0.1 | ≤0.01 |
| Inconel 625 | 58.0 | 20.0–23.0 | 8.0–10.0 | 5.0 | 3.15–4.15 | 1.0 | ≤0.5 | 0.4 | 0.5 | 0.1 |
| X65 | 0.5 | 0.02 | - | 98.5 | 0.05 | - | ≤1.4 | 0.04 | 0.25 | 0.07 |
| 316L | 10.0–14.0 | 16.0–18.0 | 3.0 | 63.0–64.0 | - | - | ≤1.3 | - | 0.75 | ≤0.03 |
| Material | Density (kg m−3) | Thermal Conductivity (W m−1 °C−1) | Specific Heat (J kg−1 °C−1) | Solidus Point (°C) | Latent Heat of Fusion (J kg−1 °C−1) |
|---|---|---|---|---|---|
| Inconel 625/Alloy 59 [42] | 8440 | 10 | 410 | 1290 | 227000 |
| 316L SS [43] | 7200 | 19 | 712 | 1424 | 274000 |
| X65 carbon steel [44] | 7800 | 32 | 726 | 1494 | 277000 |
| Material | Cooling Rate, ε (°C/s) | Temperature Gradient, G (°C/mm) | Solidification Growth Rate, R (mm/s) | F-Factor = G/R |
|---|---|---|---|---|
| Inconel 625/Alloy 59 | 1122 | 160 | 7.0 | 23 |
| 316L SS | 925 | 132 | 7.0 | 19 |
| X65 carbon steel | 874 | 125 | 7.0 | 18 |
| Material | Cooling Rate, ε (°C/s) | Temperature Gradient, G (°C/mm) | α Angle (Degree) | Solidification Growth Rate, R (mm/s) | F-Factor = G/R |
|---|---|---|---|---|---|
| Inconel 625/Alloy 59 | 323 | 242 | 79 | 1.336 | 181 |
| 316L SS | 241 | 166 | 78 | 1.455 | 114 |
| X65 carbon steel | 373 | 149 | 69 | 2.509 | 59 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunaziv, I.; Olden, V.; Akselsen, O.M. Metallurgical Aspects in the Welding of Clad Pipelines—A Global Outlook. Appl. Sci. 2019, 9, 3118. https://doi.org/10.3390/app9153118
Bunaziv I, Olden V, Akselsen OM. Metallurgical Aspects in the Welding of Clad Pipelines—A Global Outlook. Applied Sciences. 2019; 9(15):3118. https://doi.org/10.3390/app9153118
Chicago/Turabian StyleBunaziv, Ivan, Vigdis Olden, and Odd M. Akselsen. 2019. "Metallurgical Aspects in the Welding of Clad Pipelines—A Global Outlook" Applied Sciences 9, no. 15: 3118. https://doi.org/10.3390/app9153118
APA StyleBunaziv, I., Olden, V., & Akselsen, O. M. (2019). Metallurgical Aspects in the Welding of Clad Pipelines—A Global Outlook. Applied Sciences, 9(15), 3118. https://doi.org/10.3390/app9153118




