Wrist Rehabilitation System Using Augmented Reality for Hemiplegic Stroke Patient Rehabilitation: A Feasibility Study
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Augmented Reality System
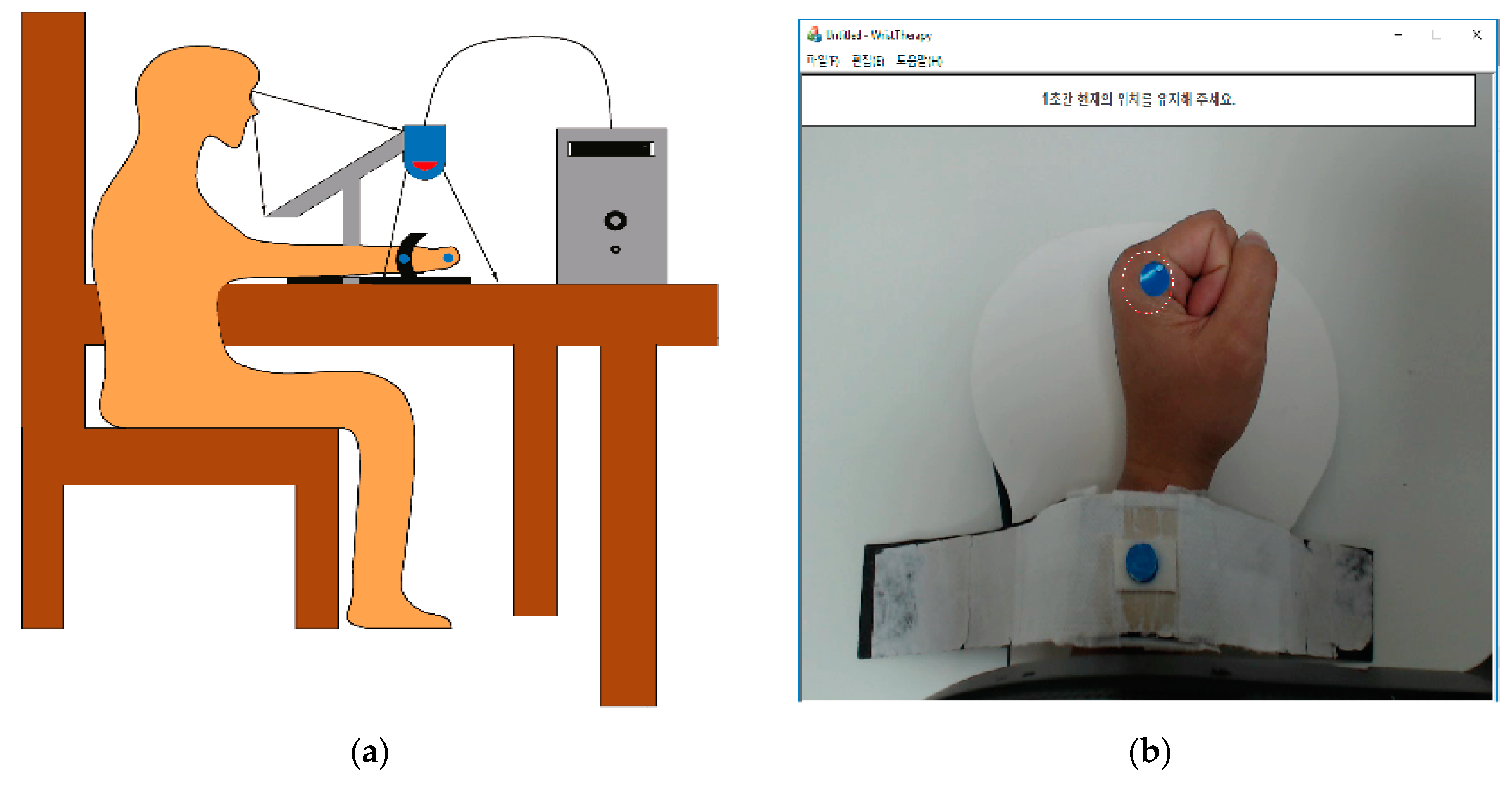
2.2.1. Hardware
2.2.2. Image Processing Program
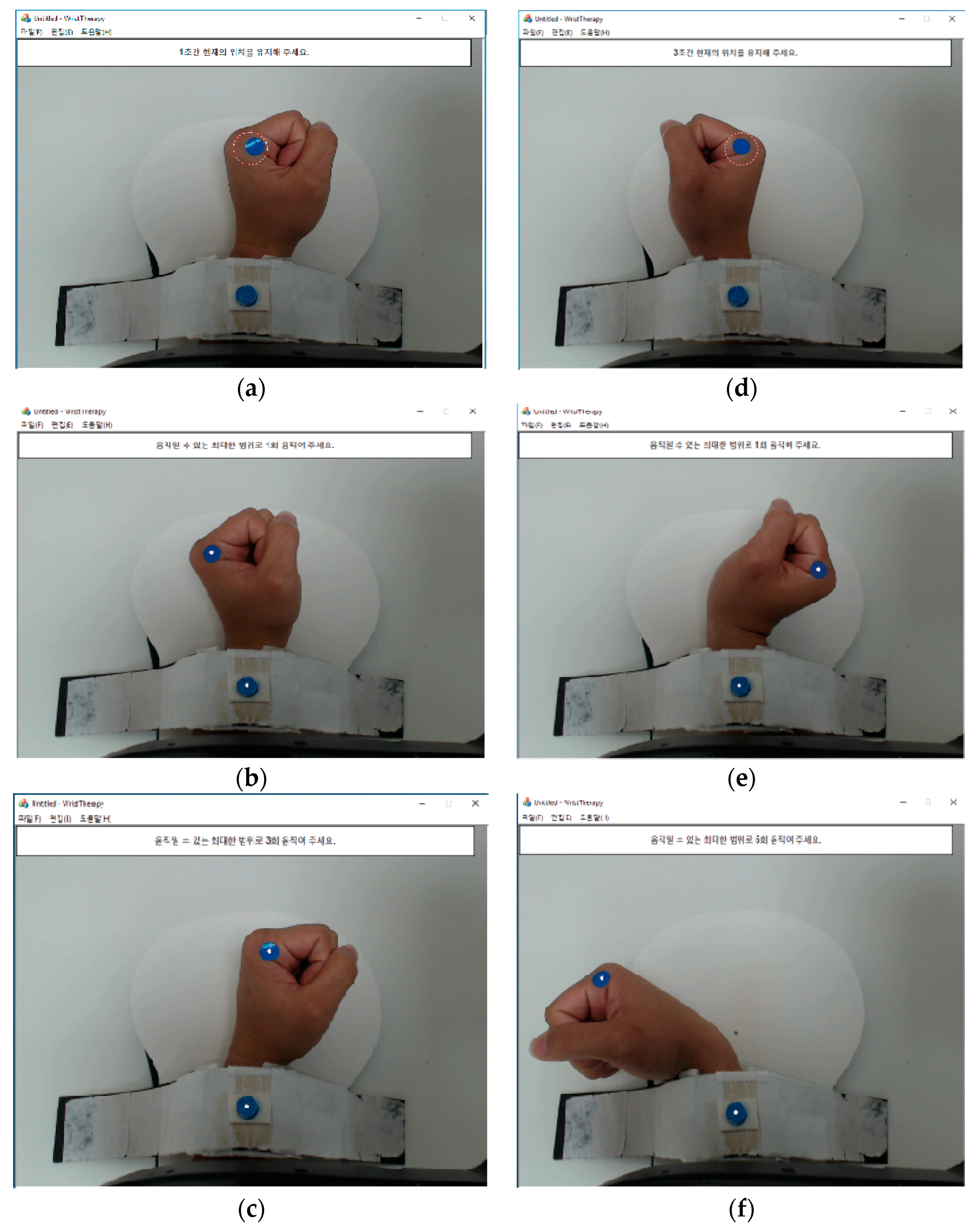
2.3. Intervention and Procedure
2.4. Outcome Measures
3. Results
3.1. Motor Function Improvement
3.2. Validation of Virtual Reality and Agmented Reality
3.3. Adverse Effects
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A. Validation of the AR System
| Validation of virtual reality | ||||||||||
| 1. Do you see the hand on the screen like yours? | ||||||||||
| −5 (Not at all.) | −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 (Perfect) |
| 2. Do you see the moving hand on the screen as your hand? | ||||||||||
| −5 (Not at all.) | −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 (Perfect) |
| 3. Do you feel your hands are getting warm? | ||||||||||
| −5 (Not at all.) | −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 (Perfect) |
| Validation of augmented reality | ||||||||||
| 1. Do you see the hand on the screen like yours? | ||||||||||
| −5 (Not at all.) | −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 (Perfect) |
| 2. Do you see the moving hand on the screen as your hand? | ||||||||||
| −5 (Not at all.) | −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 (Perfect) |
| 3. Do you feel your hands are getting warm? | ||||||||||
| −5 (Not at all.) | −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 (Perfect) |
Appendix B. Adverse Effects Assessment
| 0 [Not at all] | 2 [nervous] | 4 [a little uncomfortable] | 6 [very uncomfortable] | 8 [disturbed] | 10 [extremely disturbed] |
| Dizziness | Psychological Anxiety | Boredom | Gloomy Feeling | Muscle Twist | Motion Sickness | |
| 1 day | 8 | 0 | 0 | 0 | 4 | 8 |
| 2 day | 4 | 0 | 0 | 0 | 4 | 4 |
| 3 day | 4 | 0 | 0 | 0 | 0 | 4 |
| 4 day | 4 | 0 | 0 | 0 | 0 | 4 |
| 5 day | 4 | 0 | 0 | 0 | 0 | 0 |
| 6 day | 2 | 0 | 0 | 0 | 0 | 2 |
| 7 day | 2 | 0 | 0 | 0 | 0 | 2 |
| 8 day | 2 | 0 | 0 | 0 | 0 | 0 |
| 9 day | 2 | 0 | 0 | 0 | 0 | 2 |
| 10 day | 4 | 0 | 0 | 0 | 2 | 0 |
References
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2013, 380, 2197–2223. [Google Scholar] [CrossRef]
- Hochstenbach, J.; Prigatano, G.; Mulder, T. Patients’ and relatives’ reports of disturbances 9 months after stroke: Subjective changes in physical functioning, cognition, emotion, and behavior. Arch. Phys. Med. Rehab. 2005, 86, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Musampa, N.K.; Henderson, A.K.; Knaut, L.A. New Approaches to enhance motor function of the upper limb in patients with hemiparesis. Hong Kong Physiother. J. 2005, 23, 2–5. [Google Scholar] [CrossRef]
- Vaughan, N.; Dubey, V.N. Monitoring rehabilitation parameters in stroke patients. In Proceedings of the International Design Engineering Technical Conferences & Computer and Information in Engineering Conference, Cleveland, OH, USA, 6–9 August 2017; pp. 6–9. [Google Scholar]
- Gandolfi, M.; Valè, N.; Dimitrova, E.; Mazzoleni, S.; Battini, E.; Benedetti, M.; Gajofatto, A.; Ferraro, F.; Castelli, M.; Camin, M.; et al. Effects of high-intensity robot-assisted hand training on upper limb recovery and muscle activity in individuals with multiple sclerosis: A randomized, controlled, single-blinded trial. Front. Neurol. 2018, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Viglialoro, R.; Condino, S.; Turini, G.; Carbone, M.; Ferrari, V.; Gesi, M. Review of the augmented reality systems for shoulder rehabilitation. Information 2019, 10, 154. [Google Scholar] [CrossRef]
- Teasell, R.; Meyer, M.J.; McClure, A.; Pan, C.; Murie-Fernandez, M.; Foley, N.; Salter, K. Stroke rehabilitation: An international perspective. Top. Stroke Rehabilit. 2009, 16, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Cohen, L.G.; Mamdani, M.; Pooyania, S.; Ploughman, M.; Cheung, D.; Shaw, J.; Hall, J.; Nord, P.; Dukelow, S.; et al. Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation (EVREST): A randomised, multicentre, single-blind, controlled trial. Lancet Neurol. 2016, 15, 1019–1027. [Google Scholar] [CrossRef]
- Yavuzer, G.; Selles, R.; Sezer, N.; Sütbeyaz, S.; Bussmann, J.B.; Köseoğlu, F.; Atay, M.B.; Stam, H.J. Mirror therapy improves hand function in subacute stroke: A randomized controlled trial. Arch. Phys. Med. Rehab. 2008, 89, 393–398. [Google Scholar] [CrossRef]
- Yeldan, I.; Huseyınsınoglu, B.; Akıncı, B.; Tarakcı, E.; Baybas, S.; Ozdıncler, A. The effects of very early mirror therapy on functional improvement of the upper extremity in acute stroke patients. J. Phys. Ther. Sci. 2015, 27, 3519–3524. [Google Scholar] [CrossRef]
- Toh, S.; Fong, K. Systematic review on the effectiveness of mirror therapy in training upper limb hemiparesis after stroke. Hong Kong J. Occup. Ther. 2012, 22, 84–95. [Google Scholar] [CrossRef]
- Stevens, J.; Stoykov, M. Using motor imagery in the rehabilitation of hemiparesis. Arch. Phys. Med. Rehab. 2003, 84, 1090–1092. [Google Scholar] [CrossRef]
- Koo, K.; Park, D.; Youm, Y.; Cho, S.; Hwang, C. Enhanced reality showing long-lasting analgesia after total knee arthroplasty: Prospective, randomized clinical trial. Sci. Rep. 2018, 8, 2343. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Shin, W.S.; Bang, D.H. Mirror therapy using gesture recognition for upper limb function, neck discomfort, and quality of life after chronic stroke: A single-blind randomized controlled trial. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3271–3278. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.; Szczudlik, A.; Agostini, M.; Opara, J.; Nowobilski, R.; Ventura, L.; Tonin, P.; Turolla, A. Virtual reality for upper limb rehabilitation in sub-acute and chronic stroke: A randomized controlled trial. Arch. Phys. Med. Rehab. 2018, 99, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, N.; Gabrys, B.; Dubey, V.N. An overview of self-adaptive technologies within virtual reality training. Comput. Sci. Rev. 2016, 22, 65–87. [Google Scholar] [CrossRef]
- Turolla, A.; Dam, M.; Ventura, L.; Tonin, P.; Agostini, M.; Zucconi, C.; Kiper, P.; Cagnin, A.; Piron, L. Virtual reality for the rehabilitation of the upper limb motor function after stroke: A prospective controlled trial. J. Neuroeng. Rehabil. 2013, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.; Lee, B. Effect of virtual reality-based bilateral upper extremity training on upper extremity function after stroke: A randomized controlled clinical trial. Occup. Ther. Int. 2016, 23, 357–368. [Google Scholar] [CrossRef]
- Soufi, A.H.; Hassani, M.A.; Amini, M.; Sheikhi, M. The effects of virtual reality on upper limb function in chronic stroke patients: A clinical trial. Iranian Rehabilit. J. 2019, 17, 81–89. [Google Scholar] [CrossRef]
- Azuma, R.; Baillot, Y.; Behringer, R.; Feiner, S.; Julier, S.; MacIntyre, B. Recent advances in augmented reality. IEEE Comput. Graph. 2001, 21, 34–47. [Google Scholar] [CrossRef]
- Tang, A.; Biocca, F.; Lim, L. Comparing differences in presence during social interaction in augmented reality versus virtual reality environments: An exploratory study. Proc. Presence 2004, 204–208. [Google Scholar]
- Hoermann, S.; Santos, F.L.; Morkisch, N.; Jettkowski, K.; Sillis, M.; Cutfield, N.J.; Schmidt, H.; Hale, L.; Kruger, J.; Regenbrecht, H.; et al. Computerized mirror therapy with augmented reflection technology for stroke rehabilitation. In Proceedings of the International Conference on Virtual Rehabilitation, Valencia, Spain, 9–12 June 2015; pp. 2331–9569. [Google Scholar]
- Mouraux, D.; Brassinne, E.; Sobczak, S.; Nonclercq, A.; Warzée, N.; Sizer, P.S.; Tuna, T.; Penelle, B. 3D augmented reality mirror visual feedback therapy applied to the treatment of persistent, unilateral upper extremity neuropathic pain: A preliminary study. J. Man. Manip. Ther. 2016, 25, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient a method for evaluation of physical performance. Scand. J. Rehab. Med. 1975, 7, 13–31. [Google Scholar]
- Mathiowetz, V.; Weber, K.; Kashman, N.; Volland, G. Adult norms for the nine hold peg test of finger dexterity. Occup. Ther. J. Res. 1985, 5, 24–38. [Google Scholar] [CrossRef]
- Alexander, M.P.; Baker, E.; Naeser, M.; Kaplan, E.; Palumbo, C. Neuropsychological and neuroanatomical dimensions of ideomotor apraxia. Brain 1992, 115, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.; Marler, J.; Barsan, W.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, N.; Jackson, J.; Adams, S. Reliability and revision of the nottingham sensory assessment for stroke patients. Physiotherapy 1998, 84, 358–365. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of beck depression inventories-IA and-II in psychiatric outpatients. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Romano, D.; Bottini, G.; Maravita, A. Perceptual effects of the mirror box training in normal subjects. Restor. Neurol. Neurosci. 2013, 31, 373–386. [Google Scholar]
- Jebsen, R.; Taylor, N.; Trieschmann, R.; Trotter, M.; Howard, L. An objective and standardized test of hand function. Arch. Phys. Med. Rehab. 1969, 50, 311–319. [Google Scholar]
- Page, S.J.; Levine, P.; Hade, E. Psychometric properties and administration of the wrist/hand subscales of the fugl-meyer assessment in minimally impaired upper extremity hemiparesis in stroke. Arch. Phys. Med. Rehab. 2012, 93, 2373–2376.e5. [Google Scholar] [CrossRef]
- Kellor, M.; Frost, J.; Silberberg, N.; Iversen, I.; Cummings, R. Hand strength and dexterity. Am. J. Occup. Ther. 1971, 25, 77–83. [Google Scholar] [PubMed]
- Duncan, P.W.; Wallace, D.; Lai, S.; Johnson, D.; Embretson, S.; Laster, L. The stroke impact scale version 2.0. Stroke 1999, 30, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.; Lim, Y.; Han, H.; Hay, C.; Woo, H.S. Application of the Korean version of the modified barthel index: Development of a keyform for use in clinical practice. Hong Kong J. Occup. Ther. 2017, 29, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Culicchia, G.; Nobilia, M.; Asturi, M.; Santilli, V.; Paoloni, M.; Santis, R.; Galeoto, G. Cross-cultural adaptation and validation of the Jebsen-Taylor hand function test in an Italian population. Rehabilit. Res. Pract. 2016, 2016, 8970917. [Google Scholar] [CrossRef] [PubMed]
- Merians, A.S.; Jack, D.; Boian, R.; Tremaine, M.; Burdea, G.C.; Adamovich, S.V.; Recce, M.; Poizner, H. Virtual reality–augmented rehabilitation for patients following stroke. Phys. Ther. 2002, 82, 898–915. [Google Scholar] [PubMed]
- Santisteban, L.; Térémetz, M.; Bleton, J.P.; Baron, J.C.; Maier, M.A.; Lindberg, P.G. Upper limb outcome measures used in stroke rehabilitation studies: A systematic literature review. PloS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef]
- Faria, A.L.; Cameirão, M.S.; Couras, J.F.; Aguiar, J.R.; Costa, G.M.; i Badia, S.B. Combined cognitive-motor rehabilitation in virtual reality improves motor outcomes in chronic stroke—A pilot study. Front. Psychol. 2018, 9, 854. [Google Scholar] [CrossRef]
- Hwang, C.; Seong, J.; Son, D.S. Individual finger synchronized robot-assisted hand rehabilitation in subacute to chronic stroke: A prospective randomized clinical trial of efficacy. Clin. Rehabil. 2011, 26, 696–704. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Patterson, D.R.; Carrougher, G.J.; Sharar, S.R. Effectiveness of virtual reality–based pain control with multiple treatments. Clin. J. Pain 2001, 17, 229–235. [Google Scholar] [CrossRef]
- Sharar, S.R.; Carrougher, G.J.; Nakamura, D.; Hoffman, H.G.; Blough, D.K.; Patterson, D.R. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: Preliminary results from 3 ongoing studies. Arch. Phys. Med. Rehab. 2007, 88, S43–S49. [Google Scholar] [CrossRef]
- Casale, R.; Damiani, C.; Rosati, V. Mirror therapy in the rehabilitation of lower-limb amputation. Am. J. Phys. Med. Rehab. 2009, 88, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Darnall, B.; Li, H. Home-based self-delivered mirror therapy for phantom pain: A pilot study. J. Rehabil. Med. 2012, 44, 254–260. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | |
|---|---|
| Age (years old) | 56 |
| Sex | Male |
| Weight (kg) | 77 |
| Height (cm) | 174 |
| Paralysis | Yes (۷)/No ( ) |
| Lesion location | Cerebrum (۷) Brainstem ( ) Cerebellum ( ) |
| Treatment duration after paralysis | 17 days |
| Patient Characteristics | Pre-Intervention | Post-Intervention (10 Days) | ||||
|---|---|---|---|---|---|---|
| Jebsen Taylor test (s) | 19; 8; 10; 11; 6; 5; 6 | 17; 10; 11; 11; 5; 5; 6 | ||||
| Arm Motor Fugl-Meyer scale; wrist/hand | 19 | 23 | ||||
| Arm Motor Fugl-Meyer scale; proximal arm | 31 | 34 | ||||
| Ashworth scale; wrist | 1 | 0 | ||||
| Ashworth scale; elbow | 1 | 0 | ||||
| Nine-hole pegboard (s) | 30 | 25 | ||||
| Stroke impact scale, hand function | 46 | 54 | ||||
| Grasp force in Newtons (kg) | 12.7 | 17.7 | ||||
| Active range of motion (ROM) of wrist joint | Flexion: 60°; extension: 30° | Flexion: 70°; extension: 33° | ||||
| Korean version-Modified Barthel Index (K-MBI) | 92 | 95 | ||||
| Peak to peak Motor Evoked Potential | Mean amplitude | Maximal amplitude (mV) | Amplitude (M-wave−1) | Mean amplitude | Maximal amplitude (mV) | Amplitude (M-wave−1) |
| - | 3.5/4.9 | 0.3043/0.7903 | - | - | - | |
| Post-Intervention (10 Days) | |
|---|---|
| Validation of virtual reality (−5 to 5) | 0.8 ± 1.79 |
| Validation of augmented reality (−5 to 5) | −4.16 ± 1.89 |
| Side Effect Assessment | Post-Intervention (10 Days) |
|---|---|
| Dizziness | 3.6 ± 1.83 |
| Psychological anxiety | 0 |
| Boredom | 0 |
| Gloomy feeling | 0 |
| Muscle twist | 1 ± 1.69 |
| Motion sickness | 2.6 ± 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, H.L.; Kim, J.P.; Kim, K.; Hwang, C.H.; Koo, K.-i. Wrist Rehabilitation System Using Augmented Reality for Hemiplegic Stroke Patient Rehabilitation: A Feasibility Study. Appl. Sci. 2019, 9, 2892. https://doi.org/10.3390/app9142892
Phan HL, Kim JP, Kim K, Hwang CH, Koo K-i. Wrist Rehabilitation System Using Augmented Reality for Hemiplegic Stroke Patient Rehabilitation: A Feasibility Study. Applied Sciences. 2019; 9(14):2892. https://doi.org/10.3390/app9142892
Chicago/Turabian StylePhan, Huu Lam, Jong Pal Kim, Kwangsoo Kim, Chang Ho Hwang, and Kyo-in Koo. 2019. "Wrist Rehabilitation System Using Augmented Reality for Hemiplegic Stroke Patient Rehabilitation: A Feasibility Study" Applied Sciences 9, no. 14: 2892. https://doi.org/10.3390/app9142892
APA StylePhan, H. L., Kim, J. P., Kim, K., Hwang, C. H., & Koo, K.-i. (2019). Wrist Rehabilitation System Using Augmented Reality for Hemiplegic Stroke Patient Rehabilitation: A Feasibility Study. Applied Sciences, 9(14), 2892. https://doi.org/10.3390/app9142892






