Effect of Particulate Disintegration on Biomethane Potential of Particle-Rich Substrates in Batch Anaerobic Reactor
Abstract
:Featured Application
Abstract
1. Introduction
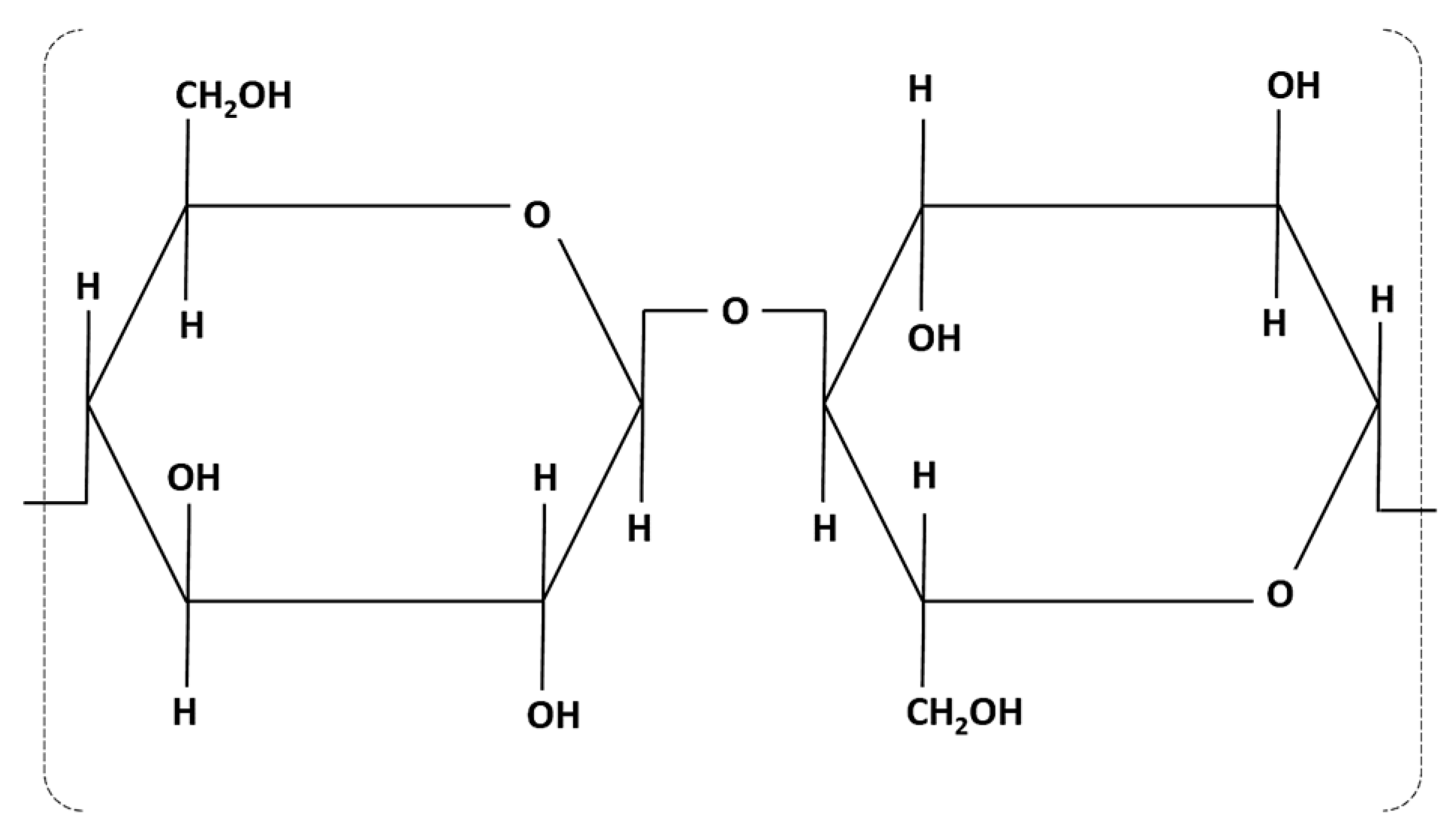
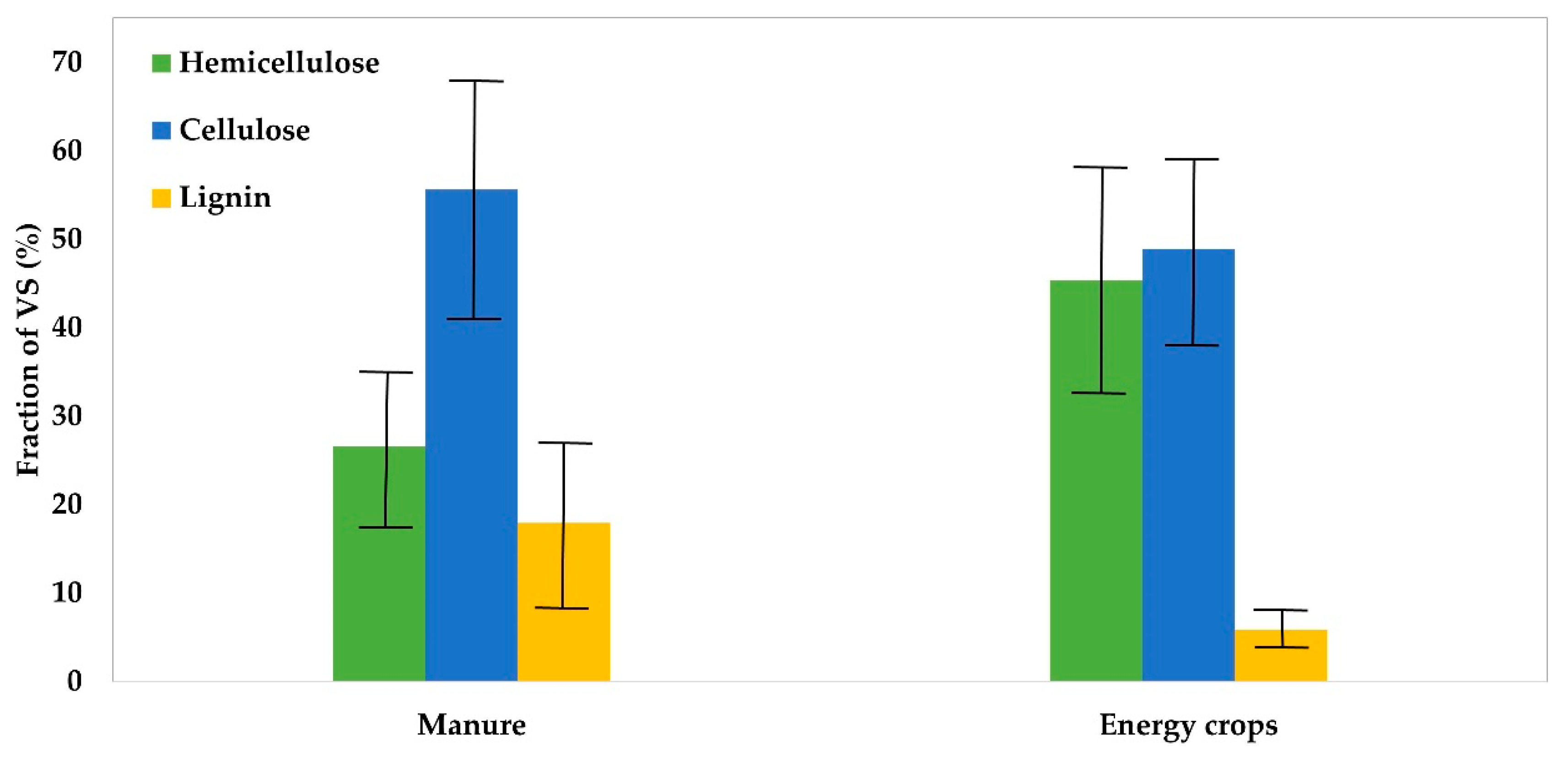
1.1. Lignocellulosic Substances
1.2. Hydrolysis Kinetics
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Analysis
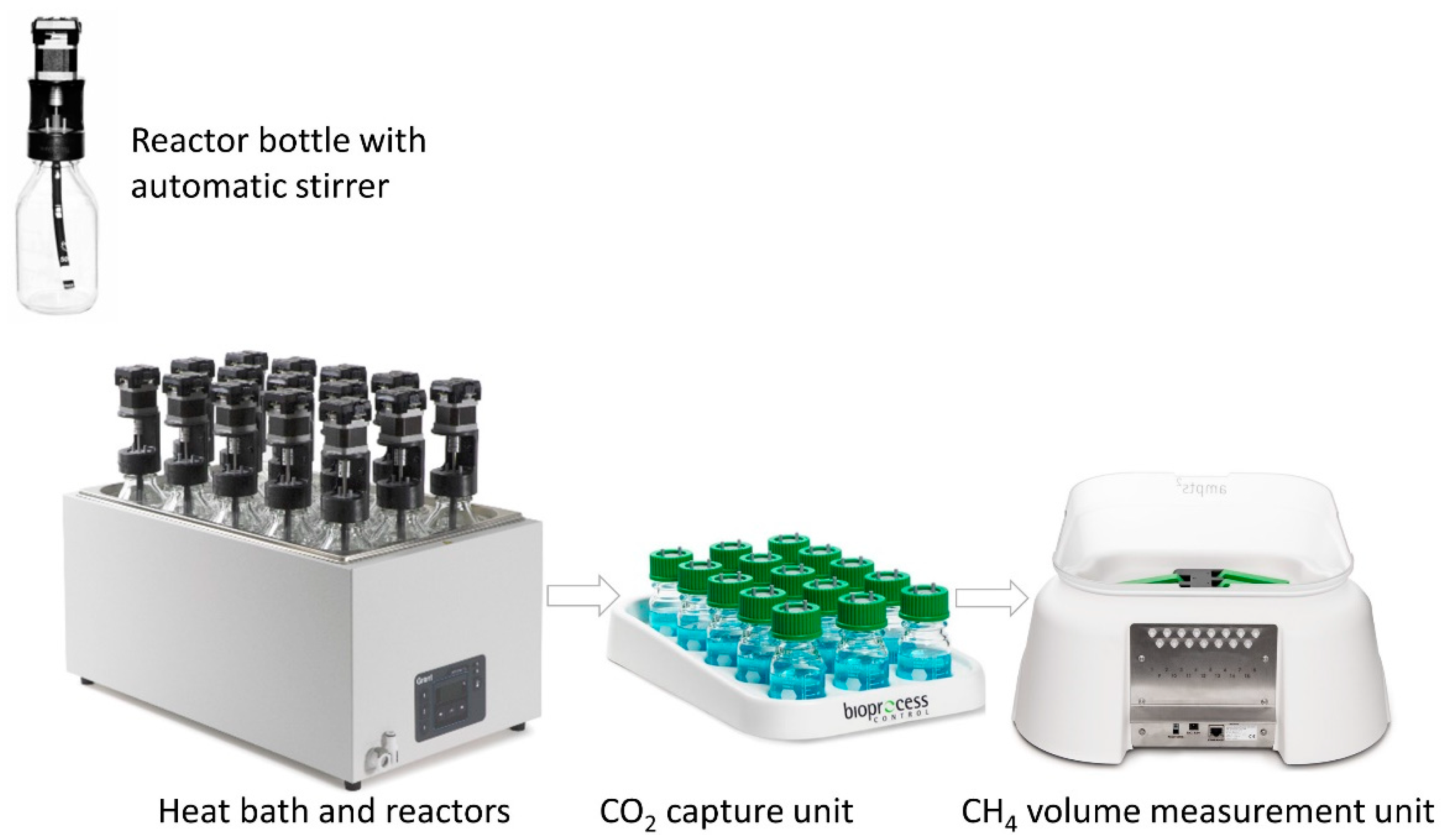
2.3. Reactor and Experimental Procedure
2.4. Granular Sludge Degassing
2.5. CO2 Removal
2.6. Theoretical Methane Yield
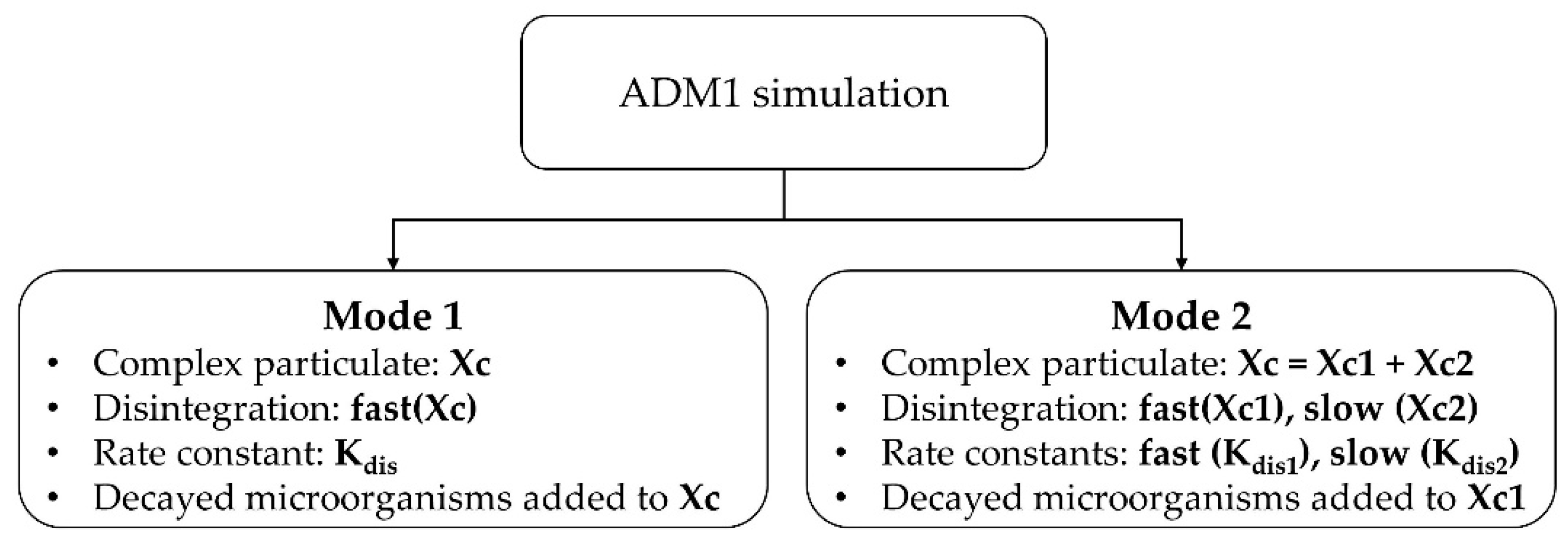
2.7. Anaerobic Digestion Model No. 1 (ADM1) Simulation
3. Result and Discussion
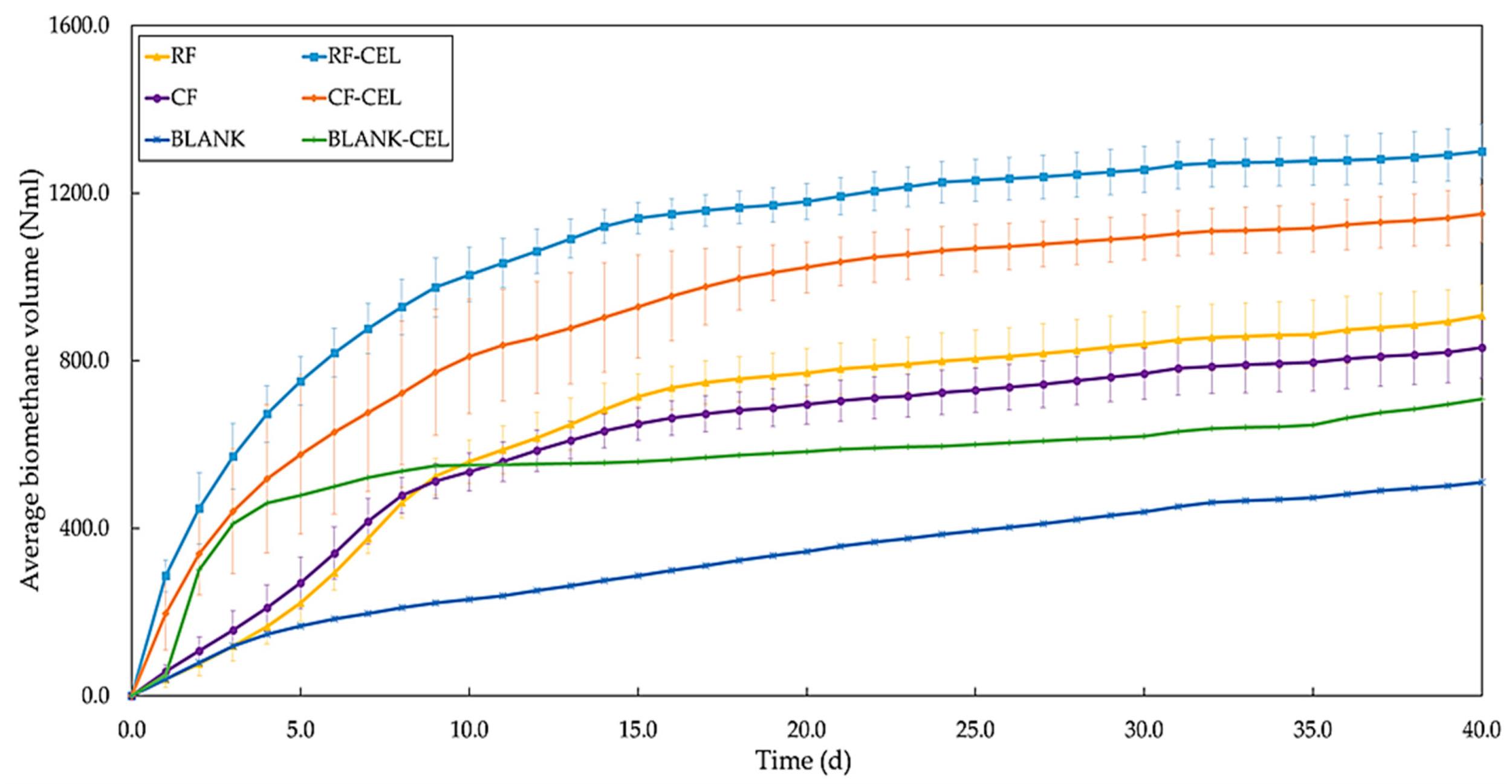
3.1. Yields
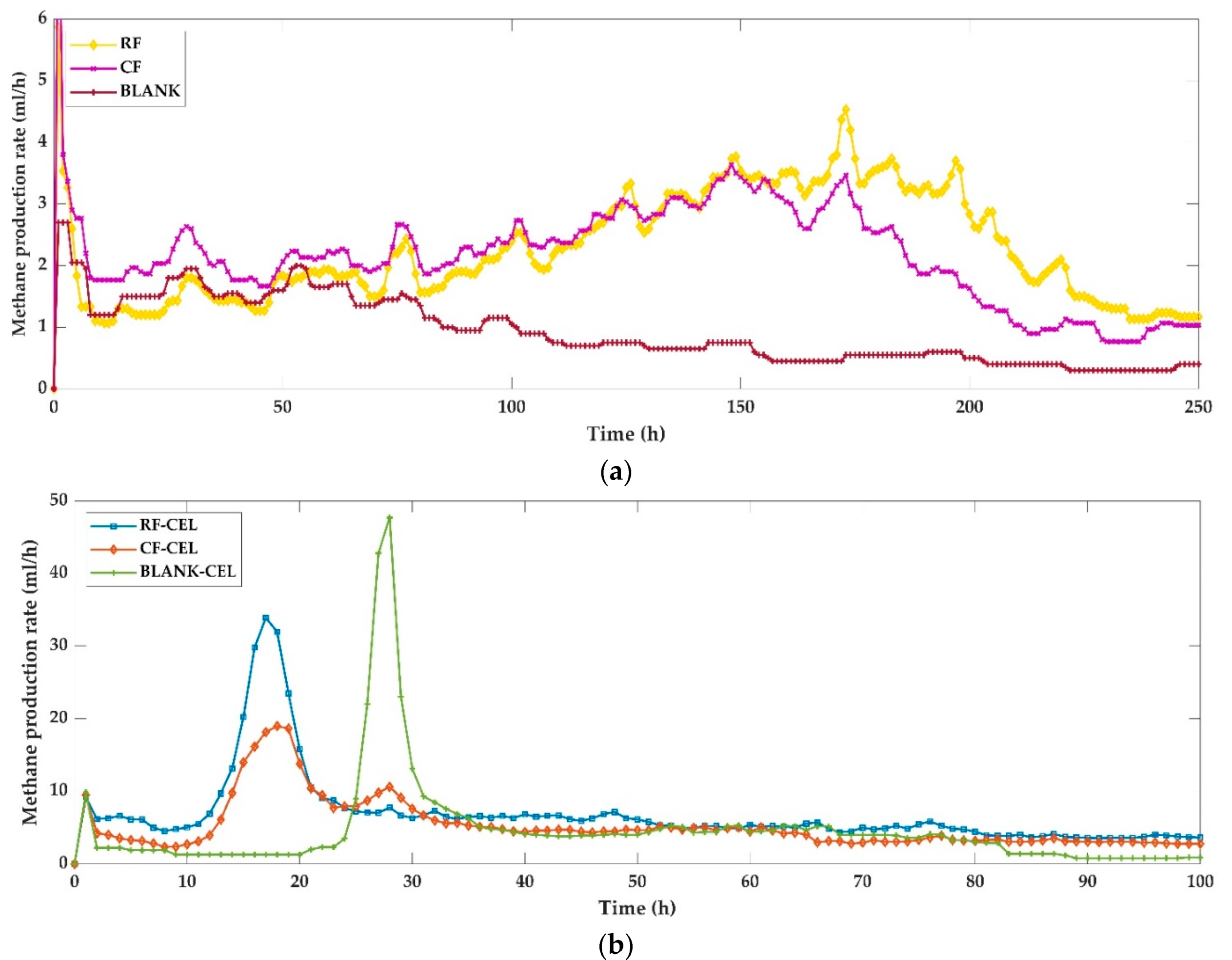
3.2. Production Rates
3.3. Effect of Cellulase Addition and Centrifugation
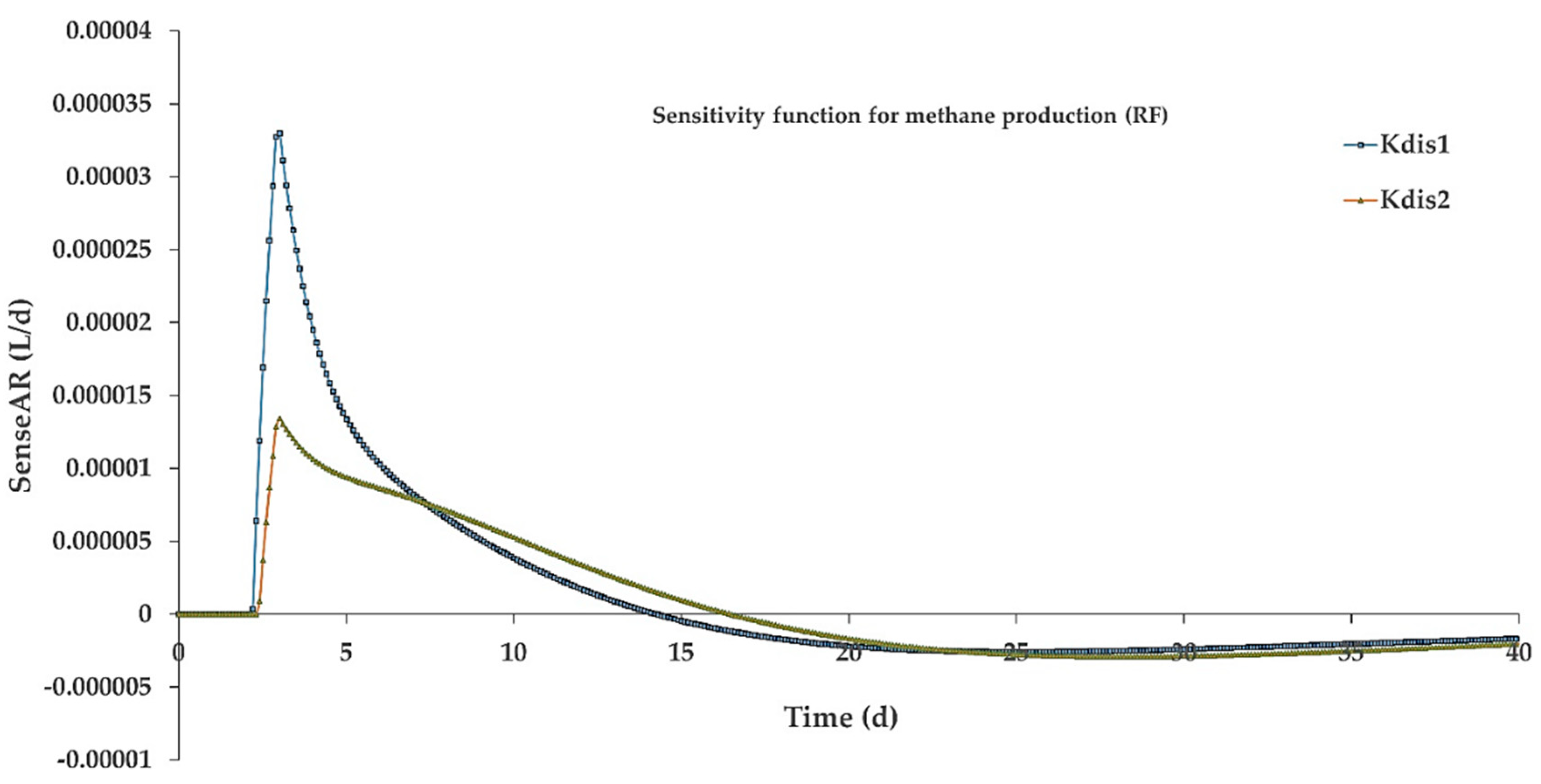
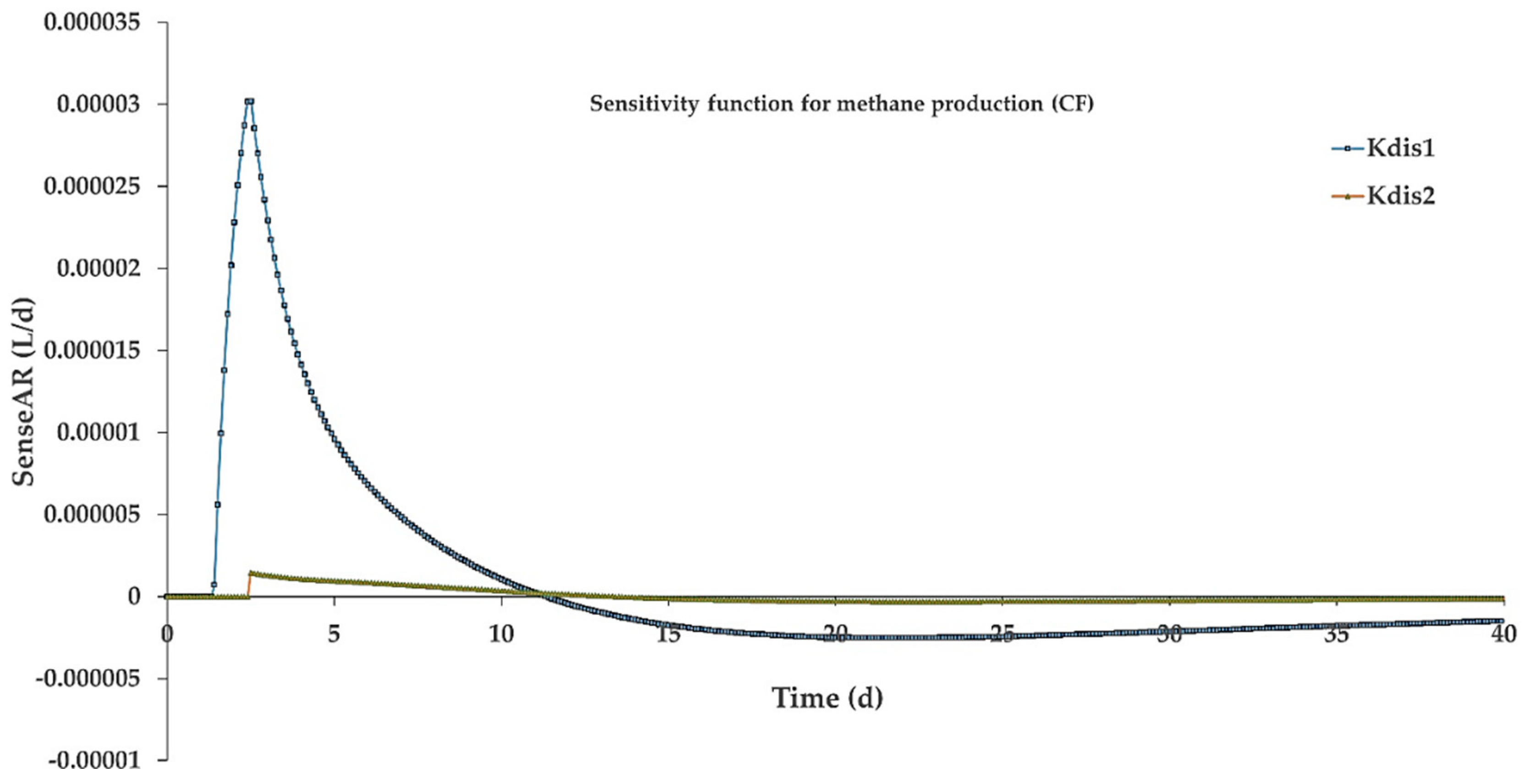
3.4. Simulation Results
4. Conclusions
- High biomethane production was observed in samples with higher particle content however, specific biomethane yield was low compared to samples with low particle contents.
- Centrifugation of samples decreased the volume of methane produced but increased the rate of methane production regardless of the addition of cellulase.
- Cellulase addition improved overall and specific methane productions both in raw and centrifuged samples but the improvement was higher in samples that contained higher suspended solids.
- Simulation results revealed that classifying complex particulates into fast and slow disintegrating fractions led to a more accurate modeling of particle-rich substrates.
Suggestions for Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | r(av(SensAR2)) | av(|SensAR|) | av(|ErrCont|) | |||
|---|---|---|---|---|---|---|
| Kdis1 | Kdis2 | Kdis1 | Kdis2 | Kdis1 | Kdis2 | |
| S_CH4 | 0.03 | 0.02 | 0.01 | 0 | 0.05 | 0.03 |
| S_CO2 | 0 | 0 | 0 | 0 | 0 | 0 |
| S_H2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Variable | r(av(SensAR2)) | av(|SensAR|) | av(|ErrCont|) | |||
|---|---|---|---|---|---|---|
| Kdis1 | Kdis2 | Kdis1 | Kdis2 | Kdis1 | Kdis2 | |
| S_CH4 | 0.001 | 0.001 | 0 | 0 | 0.002 | 0.001 |
| S_CO2 | 0 | 0 | 0 | 0 | 0.002 | 0.002 |
| S_H2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other parameters | ||||||
| Xc1 | 0.32 | 0.10 | 0.12 | 0.02 | 0.73 | 0.32 |
| Xc2 | 0 | 0.25 | 0 | 0.06 | 0 | 0.83 |
| Variable | r(av(SensAR2)) | av(|SensAR|) | av(|ErrCont|) | |||
|---|---|---|---|---|---|---|
| Kdis1 | Kdis2 | Kdis1 | Kdis2 | Kdis1 | Kdis2 | |
| S_CH4 | 0.09 | 0 | 0.04 | 0 | 0.24 | 0.04 |
| S_CO2 | 0 | 0 | 0 | 0 | 0.01 | 0 |
| S_H2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Variable | r(av(SensAR2)) | av(|SensAR|) | av(|ErrCont|) | |||
|---|---|---|---|---|---|---|
| Kdis1 | Kdis2 | Kdis1 | Kdis2 | Kdis1 | Kdis2 | |
| S_CH4 | 0.003 | 0 | 0.001 | 0 | 0.008 | 0.001 |
| S_CO2 | 0.001 | 0 | 0.001 | 0 | 0.003 | 0.001 |
| S_H2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other parameters | ||||||
| Xc1 | 0.63 | 0.03 | 0.53 | 0.02 | 3.09 | 0.28 |
| Xc2 | 0 | 0.07 | 0.00 | 0.06 | 0 | 0.75 |
References
- Esposito, G. Bio-Methane Potential Tests to Measure The Biogas Production From The Digestion and Co-Digestion of Complex Organic Substrates. Open Environ. Eng. J. 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Bajpai, P. Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Bruni, E.; Jensen, A.P.; Angelidaki, I. Steam treatment of digested biofibers for increasing biogas production. Bioresour. Technol. 2010, 101, 7668–7671. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Pandey, A. Handbook of Plant-Based Biofuels; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Christy, P.M.; Gopinath, L.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Romano, R.T.; Zhang, R.; Teter, S.; McGarvey, J.A. The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass. Bioresour. Technol. 2009, 100, 4564–4571. [Google Scholar] [CrossRef] [PubMed]
- Malayil, S.; Chanakya, H. Fungal Enzyme Cocktail Treatment of Biomass for Higher Biogas Production from Leaf Litter. Procedia Environ. Sci. 2016, 35, 826–832. [Google Scholar] [CrossRef]
- Quiñones, T.S.; Plöchl, M.; Budde, J.; Heiermann, M. Results of Batch Anaerobic Digestion Test–Effect of Enzyme Addition. Agric. Eng. Int. CIGR J. 2012, 14, 38–50. [Google Scholar]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Drewes, J.E. Alternative approach to estimate the hydrolysis rate constant of particulate material from batch data. Appl. Energy 2014, 120, 11–15. [Google Scholar] [CrossRef]
- Vavilin, V.; Rytov, S.; Lokshina, L. A description of hydrolysis kinetics in anaerobic degradation of particulate organic matter. Bioresour. Technol. 1996, 56, 229–237. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- VDI—The Association of German Engineers. Vergärung organischer Stoffe. Substratcharakterisierung, Probenahme, Stoffdatenerhebung, Gärversuche; Beuth Verlag: Berlin, Germany, 2006. [Google Scholar]
- Macé, S.; Bolzonella, D.; Cecchi, F.; Mata-Álvarez, J. Comparison of the biodegradability of the grey fraction of municipal solid waste of Barcelona in mesophilic and thermophilic conditions. Water Sci. Technol. 2003, 48, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.A.; Ferguson, J.F. Solubilization of Particulate Organic Carbon during the Acid Phase of Anaerobic Digestion. J. (Water Pollut. Control Fed.) 1981, 53, 352–366. [Google Scholar]
- APHA; Water Environment Federation; American Water Works Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Bioprocess Control. AMPTS II Light Automatic Methane Potential Test System Operation and Maintenance Manual; Bioprocess Control: Lund, Sweden, 2016. [Google Scholar]
- Darmana, D.; Henket, R.; Deen, N.G.; Kuipers, J. Detailed modelling of hydrodynamics, mass transfer and chemical reactions in a bubble column using a discrete bubble model: Chemisorption of CO2 into NaOH solution, numerical and experimental study. Chem. Eng. Sci. 2007, 62, 2556–2575. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering Treatment and Reuse; McGraw-Hill Education: Boston, MA, USA, 2003. [Google Scholar]
- Gali, A.; Benabdallah, T.; Astals, S.; Mata-Alvarez, J. Modified version of ADM1 model for agro-waste application. Bioresour. Technol. 2009, 100, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Batstone, D.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.; Pavlostathis, S.; Rozzi, A.; Sanders, W.; Siegrist, H.; Vavilin, V. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Reichert, P. Aquasim 2.0-User Manual; Swiss Federal Institute for Environmental Science and Technology: Dubendorf, Switzerland, 1998. [Google Scholar]


| Property | Raw Feed (RF) | Centrifuged Feed (RF) |
|---|---|---|
| TS (g/L) | 21.5 | 12.2 |
| VS (g/L) | 13.8 | 5.9 |
| TSS (g/L) | 14.2 | 2.5 |
| VSS (g/L) | 12.0 | 2.3 |
| TDS (g/L) | 7.3 | 9.7 |
| VDS (g/L) | 1.7 | 3.6 |
| CODtotal (g/L) | 33.2 | 19.7 |
| CODsoluble (g/L) | 16.6 | 11.4 |
| NH4+ (g/L) | 1.8 | 1.3 |
| pH | 7.0 | 7.0 |
| Sample Name | Sample Description | Feed (mL) | Granule (mL) | Total (mL) | Headspace (mL) |
|---|---|---|---|---|---|
| RF1 | Raw feed parallel 1 | 75 (sample) | 200 | 275 | 200 |
| RF2 | Raw feed parallel 2 | 75 (sample) | 200 | 275 | 200 |
| RF3 | Raw feed parallel 3 | 75 (sample) | 200 | 275 | 200 |
| RF-CEL1 | Raw feed and cellulase parallel 1 | 1.2 g cellulase + 75 (sample) | 200 | 275 | 200 |
| RF-CEL2 | Raw feed and cellulase parallel 2 | 1.2 g cellulase + 75 (sample) | 200 | 275 | 200 |
| RF-CEL3 | Raw feed and cellulase parallel 3 | 1.2 g cellulase + 75 (sample) | 200 | 275 | 200 |
| CF1 | Centrifuged feed parallel 1 | 75 (sample) | 200 | 275 | 200 |
| CF2 | Centrifuged feed parallel 2 | 75 (sample) | 200 | 275 | 200 |
| CF3 | Centrifuged feed parallel 3 | 75 (sample) | 200 | 275 | 200 |
| CF-CEL1 | Centrifuged feed and cellulase parallel 1 | 1.2 g cellulase + 75 (sample) | 200 | 275 | 200 |
| CF-CEL2 | Centrifuged feed and cellulase parallel 2 | 1.2 g cellulase + 75 (sample) | 200 | 275 | 200 |
| CF-CEL3 | Centrifuged feed and cellulase parallel 3 | 1.2 g cellulase + 75 (sample) | 200 | 275 | 200 |
| BLANK1 | Blank parallel 1 | 75 (distilled water) | 200 | 275 | 200 |
| BLANK2 | Blank parallel 2 | 75 (distilled water) | 200 | 275 | 200 |
| BLANK-CEL | Blank and cellulase parallel 1 | 1.2 g cellulase + 75 (distilled water) | 200 | 275 | 200 |
| VFA | Concentration (g/L) |
|---|---|
| Acetic acid | 3.9 |
| Propionic acid | 0.2 |
| Isobutyric acid | 0.0 |
| Butyric acid | 0.6 |
| Isovaleric acid | 0.2 |
| Valeric acid | 0.1 |
| Isocaproic acid | 0.0 |
| Caproic acid | 0.0 |
| Heptanoic acid | 0.0 |
| Total | 4.9 |
| Parameter | RF | RF | CF | CF |
|---|---|---|---|---|
| Kdis | Kdis1 and Kdis2 | Kdis | Kdis1 and Kdis2 | |
| Disintegration constant (d−1) | 0.17 | 0.17, 0.075 | 0.17 | 0.17, 0.075 |
| Amino acid degrading organisms (kg COD/m3) | 1.70 | 1.70 | 1.70 | 1.70 |
| Acetate degrading organisms (kg COD/m3) | 2.23 | 2.23 | 2.23 | 2.23 |
| Butyrate/valerate degrading organisms (kg COD/m3) | 0.69 | 0.69 | 0.69 | 0.69 |
| Fatty acid degrading organisms (kg COD/m3) | 1.85 | 1.85 | 1.85 | 1.85 |
| Hydrogen degrading organisms (kg COD/m3) | 1.05 | 1.05 | 1.05 | 1.05 |
| Propionate degrading organisms (kg COD/m3) | 0.29 | 0.29 | 0.29 | 0.29 |
| Sugar degrading organisms (kg COD/m3) | 1.68 | 1.68 | 1.68 | 1.68 |
| Soluble amino acids (kg COD/m3) | 0.2 | 0.2 | 0.2 | 0.2 |
| Soluble fatty acids (kg COD/m3) | 0.25 | 0.25 | 0.25 | 0.25 |
| Soluble acetates (kg COD/m3) | 0.66 | 0.66 | 0.66 | 0.66 |
| Soluble butyrates (kg COD/m3) | 0.15 | 0.15 | 0.15 | 0.15 |
| Soluble propionates (kg COD/m3) | 0.05 | 0.05 | 0.05 | 0.05 |
| Soluble valerates (kg COD/m3) | 0.08 | 0.08 | 0.08 | 0.08 |
| Sample | Experimental (mL) | Theoretical (mL) | Efficiency (%) |
|---|---|---|---|
| RF | 403 ± 73 | 898 | 45 |
| RF-CEL | 621 ± 54 | 898 | 69 |
| CF | 331 ± 61 | 533 | 62 |
| CF-CEL | 462 ± 57 | 533 | 87 |
| Specific Methane Yield | RF | RF-CEL | CF | CF-CEL |
|---|---|---|---|---|
| L CH4/g TS | 0.25 | 0.38 | 0.36 | 0.51 |
| L CH4/g VS | 0.4 | 0.6 | 0.7 | 1.0 |
| L CH4/g CODtotal | 0.16 | 0.25 | 0.22 | 0.31 |
| L CH4/g CODsoluble | 0.32 | 0.5 | 0.39 | 0.54 |
| g CODCH4/g CODtotal | 0.44 | 0.69 | 0.61 | 0.86 |
| Sample | Kh (d−1) |
|---|---|
| RF | 0.088 |
| RF-CEL | 0.154 |
| CF | 0.094 |
| CF-CEL | 0.120 |
| BLANK | 0.062 |
| BLANK-CEL | 0.148 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassew, F.A.; Bergland, W.H.; Dinamarca, C.; Bakke, R. Effect of Particulate Disintegration on Biomethane Potential of Particle-Rich Substrates in Batch Anaerobic Reactor. Appl. Sci. 2019, 9, 2880. https://doi.org/10.3390/app9142880
Tassew FA, Bergland WH, Dinamarca C, Bakke R. Effect of Particulate Disintegration on Biomethane Potential of Particle-Rich Substrates in Batch Anaerobic Reactor. Applied Sciences. 2019; 9(14):2880. https://doi.org/10.3390/app9142880
Chicago/Turabian StyleTassew, Fasil Ayelegn, Wenche Hennie Bergland, Carlos Dinamarca, and Rune Bakke. 2019. "Effect of Particulate Disintegration on Biomethane Potential of Particle-Rich Substrates in Batch Anaerobic Reactor" Applied Sciences 9, no. 14: 2880. https://doi.org/10.3390/app9142880
APA StyleTassew, F. A., Bergland, W. H., Dinamarca, C., & Bakke, R. (2019). Effect of Particulate Disintegration on Biomethane Potential of Particle-Rich Substrates in Batch Anaerobic Reactor. Applied Sciences, 9(14), 2880. https://doi.org/10.3390/app9142880





