A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants
Abstract
1. Introduction
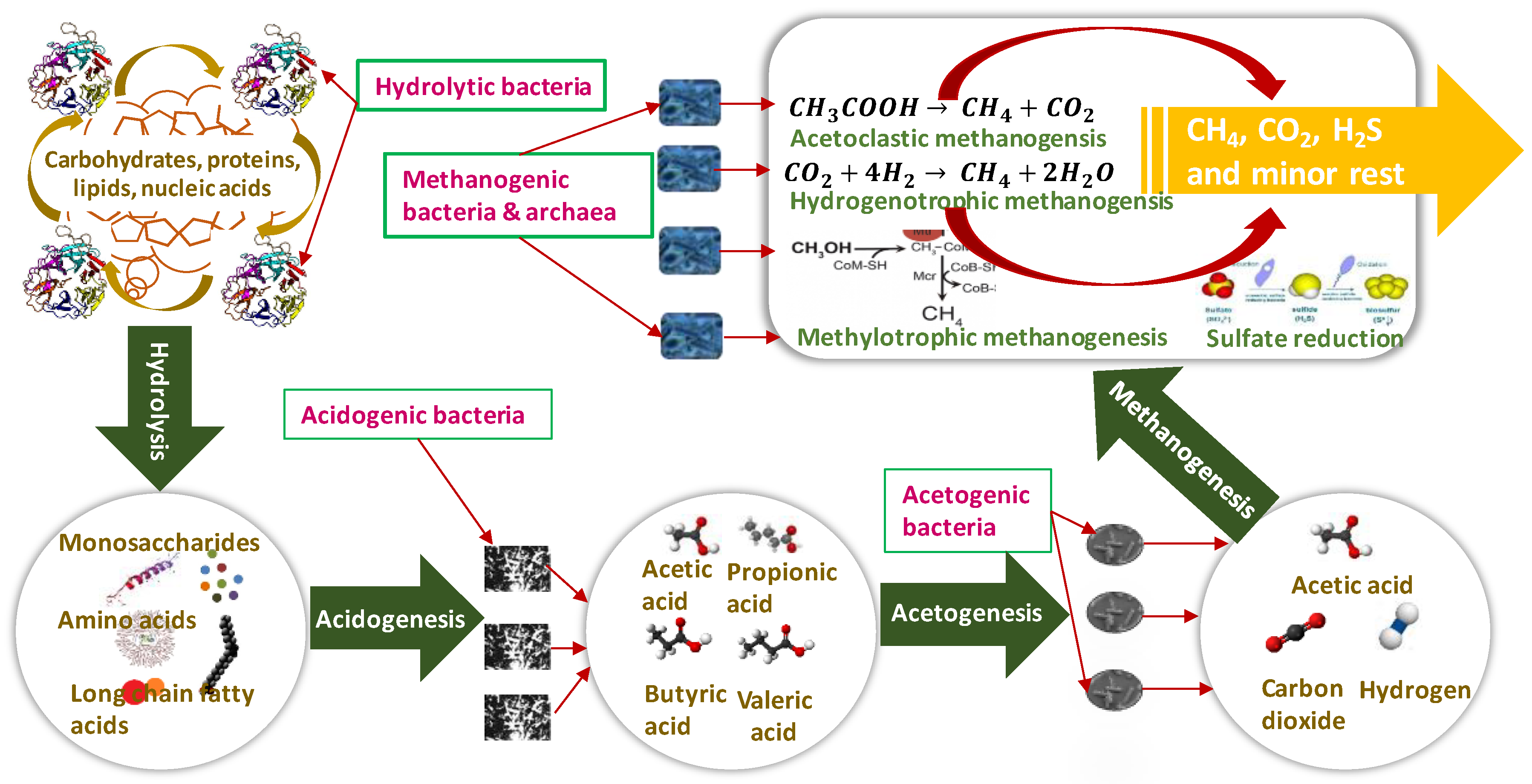
2. Anaerobic Digestion Process and Microbial Communities
3. Process Parameters Involved in a Biogas Production Plant
3.1. Feedstock
3.1.1. Substrate
3.1.2. Inoculum
3.1.3. Pretreatment
- logRo: the severity factor as a function of treatment time;
- T: the temperature in °C;
- t: is the residence time in (min); and
- 14.75: the activation energy where the process obeys first-order kinetics and the Arrhenius temperature dependence
3.1.4. Codigestion
3.2. Reactor
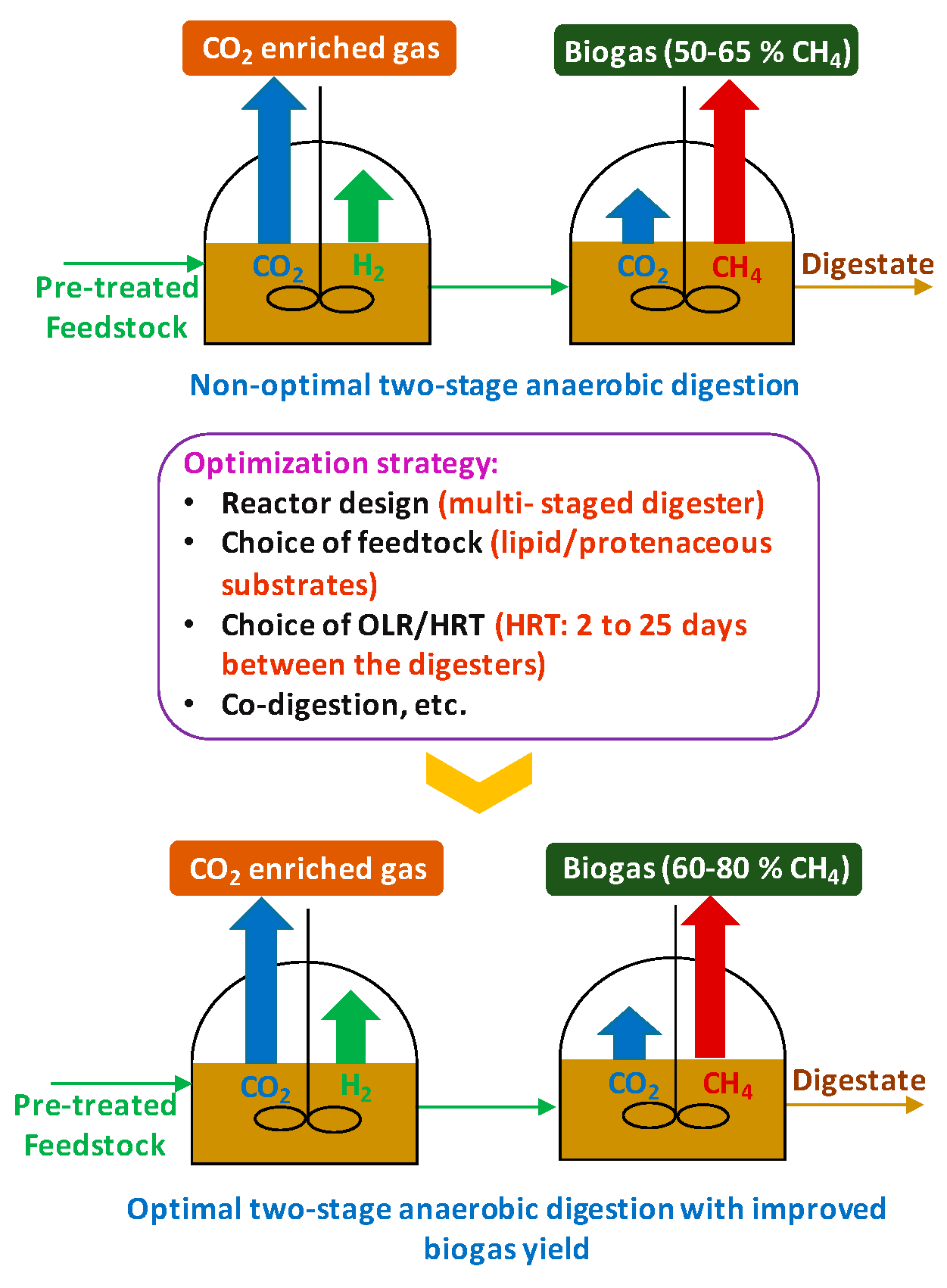
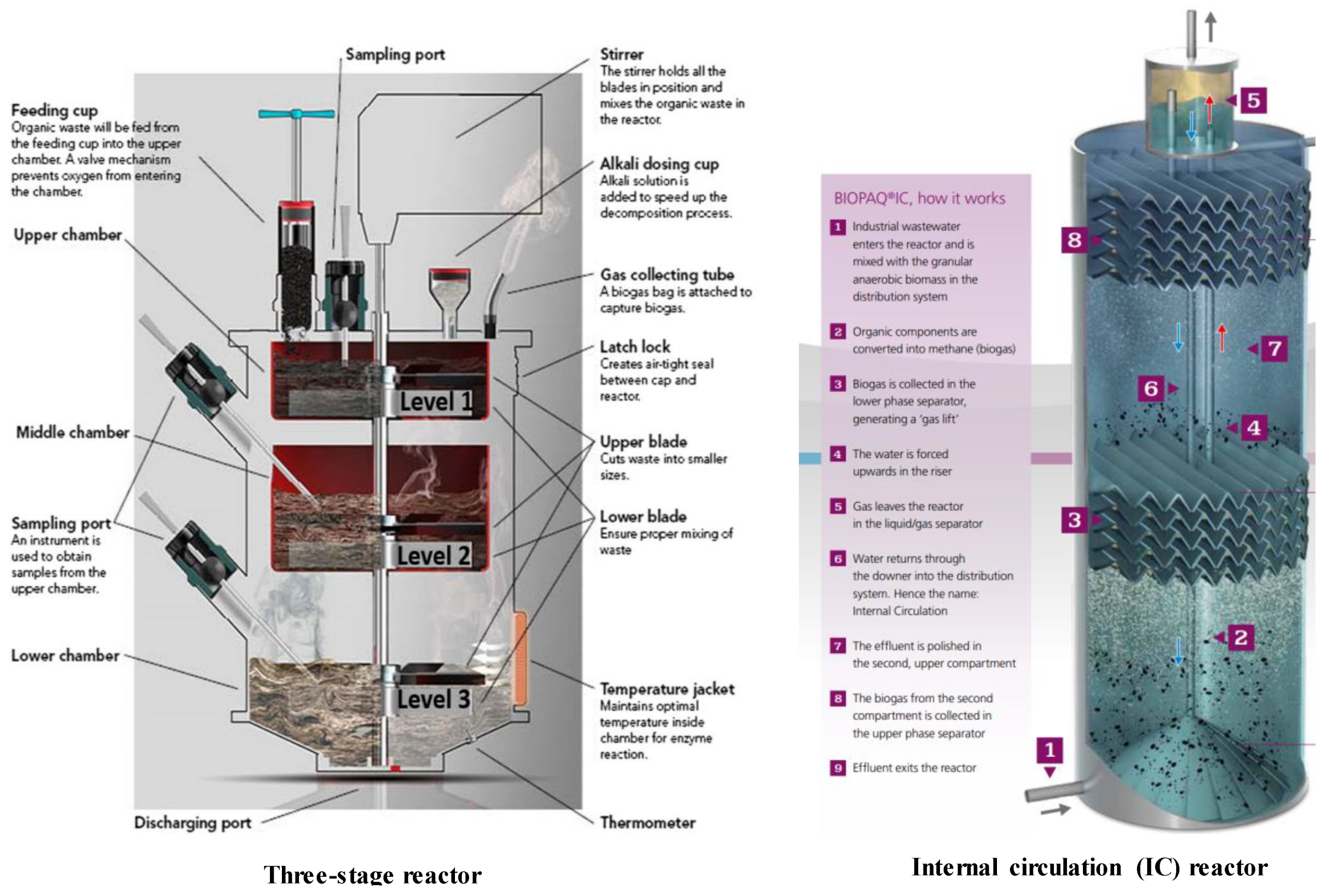
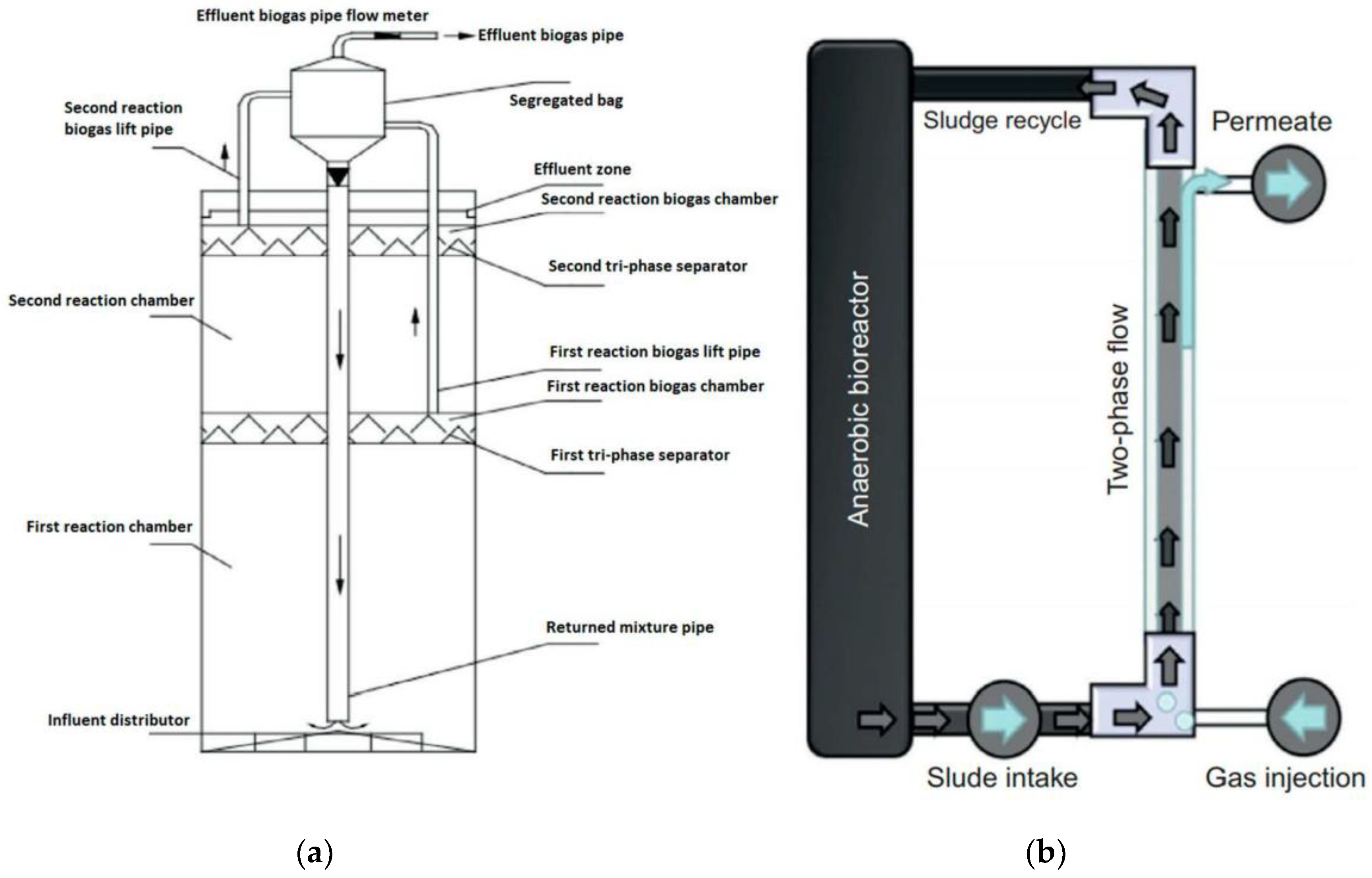
3.2.1. Configuration
3.2.2. Mixing
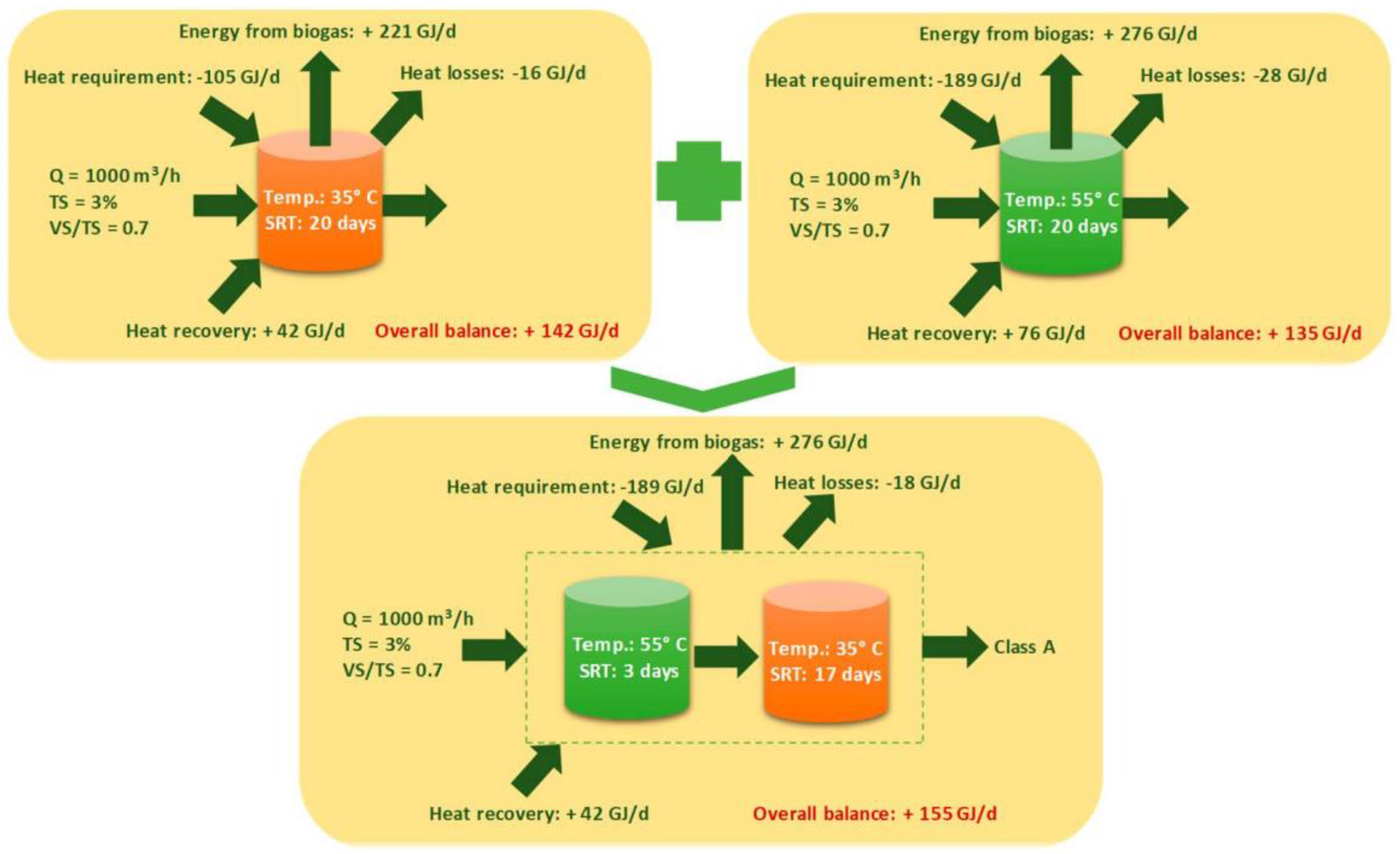
3.3. Temperature
3.4. pH
3.5. HRT
3.6. Ammonia
3.7. VFA
3.8. OLR
3.9. Pressure
4. Conclusions
- (1)
- Feedstock physical and chemical compositions substantially affect biogas production. Among the various types of feedstock materials, animal manure is still the dominant substrate or cosubstrate for biogas production because of its operational advantages of pH buffering and C:N ratio optimization. Lignin-rich substrates are found to be recalcitrant, while lipids are expected to have a high potential to boost methane. Nevertheless, LCFA inhibition from lignin remains to be of concern. Low or no lignin feedstock such as algae is an interesting biogas yield promoter. However, LCFA inhibition, increased pH level, and ammonia inhibition are highlighted as some of the barriers. For counteracting feedstock induced operational problems, codigestion, pretreatment, and use of additives are utilized in current R&D and real-life applications.
- (2)
- Enhancing feedstock accessibility allows accelerated biological degradation and consequently high AD efficiency. Pretreatment and codigestion are broadly used options promoting feedstock accessibility. However, the choice of the pretreatment method is feedstock-dependent and often is a compromise between cost and energy. For pretreatment of lignocellulosic biomass (e.g., animal manure), by overcoming the recalcitrant lignin or crystalline cellulose barrier, the biogas production can be enhanced where approaches like steam explosion, enzyme addition, and sonication at present are widespread. For substrates with high-fat content, saponification is preferably used, while for algae-like substrates, thermal pretreatment is considered an option. Despite this, almost all the conventional pretreatment methods have both success and failure, as some pretreatment options are easily amenable, while others have side-effects that counteract their positive effects. Within the novel pretreatment approaches, a combination of various pretreatment methods has been examined, and the obtained results are reported to be promising. Additionally, as for future studies, exploiting genomic sequencing as a means of understanding feedstock degradation prior to anaerobic digestion is suggested.
- (3)
- Manipulating reactor designs for achieving an optimum AD process performance has been emphasized, revealing many innovative approaches currently in practice. In terms of continuous operation, staged reactors give substantial increases in methane yields due to the establishment of appropriate microbiological conditions at different anaerobic digestion phases. Consequently, three-staged reactor configurations have been developed and reported to be an attractive option in optimizing methane production. Moreover, an anaerobic membrane reactor, internal circulation reactor, and super-high-rate reactor are some of the novel configurations shown to facilitate efficient high solid substrate treatment by increasing bacterial cells containment and separating simultaneous removal of gases, solids, and liquids. Therefore, these reactor approaches are becoming increasingly popular.
- (4)
- Among the various operational temperature regimes (psychrophilic, mesophilic, and thermophilic), the choice of an appropriate regime is largely investment and geographic specific. Thermophilic temperature enhances methane conversion rate and controls pathogens in the digestate liquids. However, the high heat requirement makes its application expensive. The recent investigations towards an optimum temperature system suggest that multitemperature, staged-digesters offer suitable conditions for diverse microbial activities, and hence give high biogas production efficiencies. By employing high-temperature post-treatment of digestate, further improvement in methane production has already been demonstrated.
- (5)
- The pH affects the degree of conversion at different AD steps and the quality of the residual digestate. Optimal methanogenesis and biogas production occurs at around pH 7. A host of factors, such as ammonium formation, bicarbonate decomposition, mineralization and reduction of multivalent ions, and struvite formation result in digestate pH fluctuations. To enable pH controlling to the desired value, adding acid or basic solutions are traditionally the major options. Moreover, online pH monitoring and alert systems are also implemented in modern day applications.
- (6)
- HRT is directly linked to the size of the anaerobic reactor, and a low HRT usually allows investment reduction. Among the all bacteria and archaea, methanogens grow slowly, and for these microorganisms, a higher HRT is required. Both feed rate and feed type influence the HRT. Consequently, the feedstock OLR regulation is a usual approach for HRT optimization. SRT, another term intertwined with HRT, represents the microbial culture and biomass retention in an operating digester. SRT is often optimized by incorporating OLR variation. A recent achievement suggests that a deliberate SRT variation can result in a shift in bacterial culture, causing a change in reaction pattern from one group to the other.
- (7)
- Ammonia is produced via the degradation of proteins and nitrogen in the feedstock. Among the two forms of ammonia (free ammonia and ionized ammonia), free ammonia is very toxic to methanogens and is a strong function of combined pH and temperature. Lowering the generation of ammonia during AD has been targeted in a variety of approaches (see Section 3.6), among which, using ammonia tolerant microorganisms (or bio-augmentation) or combined thermal stripping and absorption process are some of the novel techniques receiving constant attention.
- (8)
- VFAs are the intermediate products required for conversion to methane. Some of the VFA components are more sensitive than the others to methanogens, e.g., propionate. Unutilized VFAs accumulate, and in the worst case halt the production of biogas. To achieve increased VFA utilization and as such improved methane yield, regulation of AD process parameters such as temperature, OLR, pH, and H2 partial pressure is critical. Moreover, many additives and trace metals of various origins have been suggested to have an improved utilization of VFAs.
- (9)
- OLR refers to the amount of feedstock treated by a reactor on a daily basis. OLR variation allows the optimization of HRT, pH, VFA, ammonia, and methane production. High operational OLR enables reducing the size of the reactor and accordingly the investment cost. However, as a result of high OLR, implications such as bacteria wash out, VFA accumulation, or methane yield reduction can be experienced. In recent applications, OLR control has been used to suppress ammonia inhibition. Furthermore, a novel three-stage reactor configuration has been shown to successfully achieve high OLR treatment without compromising the production of biogas.
- (10)
- Anaerobic digestion operates typically at atmospheric pressure, but recent investigations have identified that high-pressure systems are also possible. High pressure allows the increase of dissolved CO2 in the liquid phase and consequently increased methane composition in the biogas. The other aspects of anaerobic digester pressure, such as partial pressure of headspace gas components and variation of hydrostatic pressure levels, were mentioned as potential causes of fluctuation in the production of methane.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daioglou, V.; Doelman, J.C.; Wicke, B.; Faaij, A.; van Vuuren, D.P. Integrated assessment of biomass supply and demand in climate change mitigation scenarios. Glob. Environ. Chang. 2019, 54, 88–101. [Google Scholar] [CrossRef]
- Batstone, D.J.; Virdis, B. The role of anaerobic digestion in the emerging energy economy. Curr. Opin. Biotechnol. 2014, 27, 142–149. [Google Scholar] [CrossRef]
- Toop, T.A.; Ward, S.; Oldfield, T.; Hull, M.; Kirby, M.E.; Theodorou, M.K. AgroCycle–developing a circular economy in agriculture. Energy Procedia 2017, 123, 76–80. [Google Scholar] [CrossRef]
- Simet, A. German biogas industry adds 150 plants in 2015. Biomass Magazine, 2016. [Google Scholar]
- Zhang, T.; Yang, Y.; Xie, D. Insights into the production potential and trends of China’s rural biogas. Int. J. Energy Res. 2015, 39, 1068–1082. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Y.-X.; Wang, J.-Z.; Chen, G.; Battye, H. Where is the future of China’s biogas? Review, forecast, and policy implications. Pet. Sci. 2016, 13, 604–624. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.-C.; Lee, J.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Three-stage anaerobic co-digestion of food waste and horse manure. Sci. Rep. 2017, 7, 1269. [Google Scholar] [CrossRef] [PubMed]
- Dollhofer, V.; Podmirseg, S.M.; Callaghan, T.M.; Griffith, G.W.; Fliegerová, K. Anaerobic fungi and their potential for biogas production. In Biogas Science and Technology; Springer: Berlin, Germany, 2015; pp. 41–61. [Google Scholar]
- Sasidharan, K.; Martinez-Vernon, A.S.; Chen, J.; Fu, T.; Soyer, O. A low-cost DIY device for high resolution, continuous measurement of microbial growth dynamics. bioRxiv 2018, 407742. [Google Scholar] [CrossRef]
- Hublin, A.; Zokić, T.; Zelić, B. Optimization of biogas production from co-digestion of whey and cow manure. Biotechnol. Bioprocess Eng. 2012, 17, 1284–1293. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Mital, K. Biogas Systems: Policies, Progress and Prospects; Taylor & Francis: Abingdon, UK, 1997. [Google Scholar]
- Kim, M.D.; Song, M.; Jo, M.; Shin, S.G.; Khim, J.H.; Hwang, S. Growth condition and bacterial community for maximum hydrolysis of suspended organic materials in anaerobic digestion of food waste-recycling wastewater. Appl. Microbiol. Biotechnol. 2010, 85, 1611–1618. [Google Scholar] [CrossRef]
- Schnurer, A.; Jarvis, A. Microbiological handbook for biogas plants. Swed. Waste Manag. U 2010, 2009, 1–74. [Google Scholar]
- Angelidaki, I.; Karakashev, D.; Batstone, D.J.; Plugge, C.M.; Stams, A.J. Biomethanation and its potential. Methods Enzym. 2011, 494, 327–351. [Google Scholar]
- Nayono, S.E. Anaerobic Digestion of Organic Solid Waste for Energy Production; KIT Scientific Publishing: Karlsruhe, Germany, 2010; Volume 46. [Google Scholar]
- EInstruments. Biomass to Biogas—Anaerobic Digestion. Available online: http://www.e-inst.com/biomass-to-biogas/ (accessed on 13 March 2019).
- Felchner-Zwirello, M. Propionic Acid Degradation by Syntrophic Bacteria during Anaerobic Biowaste Digestion; KIT Scientific Publishing: Karlsruhe, Germany, 2014; Volume 49. [Google Scholar]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- André, L.; Ndiaye, M.; Pernier, M.; Lespinard, O.; Pauss, A.; Lamy, E.; Ribeiro, T. Methane production improvement by modulation of solid phase immersion in dry batch anaerobic digestion process: Dynamic of methanogen populations. Bioresour. Technol. 2016, 207, 353–360. [Google Scholar] [CrossRef]
- Steffen, R.; Szolar, O.; Braun, R. Feedstocks for Anaerobic Digestion; Institute for Agrobiotechnology Tulln, University of Agricultural Sciences: Vienna, Austria, 1998. [Google Scholar]
- Achinas, S.; Euverink, G.J.W. Theoretical analysis of biogas potential prediction from agricultural waste. Resour.-Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef]
- Martinez, E.; Redondas, V.; Fierro, J.; Gómez, X.; Morán, A. Anaerobic digestion of high lipid content wastes: FOG co-digestion and milk processing fat digestion. J. Residuals Sci. Technol. 2011, 8, 53–60. [Google Scholar]
- Pereira, M.; Sousa, D.; Mota, M.; Alves, M. Mineralization of LCFA associated with anaerobic sludge: Kinetics, enhancement of methanogenic activity, and effect of VFA. Biotechnol. Bioeng. 2004, 88, 502–511. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wang, J. An overview on biogas generation from anaerobic digestion of food waste. Int. J. Green Energy 2016, 13, 119–131. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Long, J.H.; Aziz, T.N.; Francis, L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Latha, K.; Velraj, R.; Shanmugam, P.; Sivanesan, S. Mixing strategies of high solids anaerobic co-digestion using food waste with sewage sludge for enhanced biogas production. J. Clean. Prod. 2019, 210, 388–400. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, W.; Yi, H.; Qin, Y.; Wu, J.; Liu, J.; Li, Y.-Y. Biogas production by two-stage thermophilic anaerobic co-digestion of food waste and paper waste: Effect of paper waste ratio. Renew. Energy 2019, 132, 1301–1309. [Google Scholar] [CrossRef]
- Sahoo, S.R.; Rao, P.V. Temperature-Phased Anaerobic Co-digestion of Food Waste and Rice Husk Using Response Surface Methodology. In Water Resources and Environmental Engineering II; Springer: Berlin, Germany, 2019; pp. 137–146. [Google Scholar]
- Aldin, S.; Nakhla, G.; Ray, M.B. Modeling the influence of particulate protein size on hydrolysis in anaerobic digestion. Ind. Eng. Chem. Res. 2011, 50, 10843–10849. [Google Scholar] [CrossRef]
- Moestedt, J.; Påledal, S.N.; Schnürer, A.; Nordell, E. Biogas production from thin stillage on an industrial scale—Experience and optimisation. Energies 2013, 6, 5642–5655. [Google Scholar] [CrossRef]
- Zhang, L.; Jahng, D. Enhanced anaerobic digestion of piggery wastewater by ammonia stripping: Effects of alkali types. J. Hazard. Mater. 2010, 182, 536–543. [Google Scholar] [CrossRef]
- Karlsson, A.; Ejlertsson, J. Addition of HCl as a means to improve biogas production from protein-rich food industry waste. Biochem. Eng. J. 2012, 61, 43–48. [Google Scholar] [CrossRef]
- Feedstock. The Official Innovation Portal on Anaerobic Digestion. Available online: https://www.nnfcc.co.uk/publications/report-impact-biogas-sustainability-criteria (accessed on 13 March 2019).
- Teghammar, A. Biogas Production from Lignocelluloses: Pretreatment, Substrate Characterization, Co-Digestion and Economic Evaluation; Chalmers Tekniska Högskola: Göteborg, Sweden, 2013. [Google Scholar]
- Dębowski, M.; Zieliński, M.; Grala, A.; Dudek, M. Algae biomass as an alternative substrate in biogas production technologies—Review. Renew. Sustain. Energy Rev. 2013, 27, 596–604. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res. 2013, 2, 445–454. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Q.-B.; Laurens, L.L.; Jarvis, E.E.; Nagle, N.J.; Chen, S.; Frear, C.S. Mechanism, kinetics and microbiology of inhibition caused by long-chain fatty acids in anaerobic digestion of algal biomass. Biotechnol. Biofuels 2015, 8, 141. [Google Scholar] [CrossRef]
- Peu, P.; Sassi, J.-F.; Girault, R.; Picard, S.; Saint-Cast, P.; Béline, F.; Dabert, P. Sulphur fate and anaerobic biodegradation potential during co-digestion of seaweed biomass (Ulva sp.) with pig slurry. Bioresour. Technol. 2011, 102, 10794–10802. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Heiske, S. Anaerobic digestion of macroalgae: Methane potentials, pre-treatment, inhibition and co-digestion. Water Sci. Technol. 2011, 64, 1723–1729. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J. Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour. Technol. 2012, 110, 610–616. [Google Scholar] [CrossRef]
- Tedesco, S.; Benyounis, K.; Olabi, A. Mechanical pretreatment effects on macroalgae-derived biogas production in co-digestion with sludge in Ireland. Energy 2013, 61, 27–33. [Google Scholar] [CrossRef]
- Sarker, S.; Møller, H.B.; Bruhn, A. Influence of variable feeding on mesophilic and thermophilic co-digestion of Laminaria digitata and cattle manure. Energy Convers. Manag. 2014, 87, 513–520. [Google Scholar] [CrossRef]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure. J. Environ. Qual. 2004, 33, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.S.; Tabatabaei, M.; Karimi, K.; Kumar, R. Recent updates on biogas production—A review. Biofuel Res. J. 2016, 10, 394–402. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Müller, B.; Schnürer, A. Importance of inoculum source and initial community structure for biogas production from agricultural substrates. Bioresour. Technol. 2017, 245, 768–777. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Donoso-Bravo, A.; Ruiz-Sánchez, J.C.; Valencia-Molina, K.J.; Torres-Lozada, P. Effect of inoculum on the anaerobic digestion of food waste accounting for the concentration of trace elements. Waste Manag. 2018, 71, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Nges, I.A.; Liu, J. The effects of pre-aeration and inoculation on solid-state anaerobic digestion of rice straw. Bioresour. Technol. 2017, 224, 78–86. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, R.; El-Mashad, H.M.; Dong, R. Effect of feed to inoculum ratios on biogas yields of food and green wastes. Bioresour. Technol. 2009, 100, 5103–5108. [Google Scholar] [CrossRef]
- Sunarso, S.; Johari, S.; Widiasa, I.N.; Budiyono, B. The Effect of Feed to Inoculums Ratio on Biogas Production Rate from Cattle Manure Using Rumen Fluid as Inoculums. Int. J. Sci. Eng. 2010, 1, 41–45. [Google Scholar] [CrossRef]
- González-Fernández, C.; García-Encina, P.A. Impact of substrate to inoculum ratio in anaerobic digestion of swine slurry. Biomass Bioenergy 2009, 33, 1065–1069. [Google Scholar] [CrossRef]
- Fathya, S.; Assia, K.; Hamza, M. Influence of inoculums/substrate ratios (ISRs) on the mesophilic anaerobic digestion of slaughterhouse waste in batch mode: Process stability and biogas production. Energy Procedia 2014, 50, 57–63. [Google Scholar]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Ponsá, S.; Vázquez, F.; Font, X. Increasing biogas production by thermal (70 °C) sludge pre-treatment prior to thermophilic anaerobic digestion. Biochem. Eng. J. 2008, 42, 186–192. [Google Scholar] [CrossRef]
- Bruni, E.; Jensen, A.P.; Angelidaki, I. Comparative study of mechanical, hydrothermal, chemical and enzymatic treatments of digested biofibers to improve biogas production. Bioresour. Technol. 2010, 101, 8713–8717. [Google Scholar] [CrossRef]
- Montingelli, M.; Benyounis, K.; Quilty, B.; Stokes, J.; Olabi, A. Influence of mechanical pretreatment and organic concentration of Irish brown seaweed for methane production. Energy 2017, 118, 1079–1089. [Google Scholar] [CrossRef]
- Tiehm, A.; Nickel, K.; Neis, U. The use of ultrasound to accelerate the anaerobic digestion of sewage sludge. Water Sci. Technol. 1997, 36, 121–128. [Google Scholar] [CrossRef]
- Park, B.; Ahn, J.-H.; Kim, J.; Hwang, S. Use of microwave pretreatment for enhanced anaerobiosis of secondary sludge. Water Sci. Technol. 2004, 50, 17–23. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Sun, S.; Qiao, W.; Xiao, M. Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour. Technol. 2011, 102, 11177–11182. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenes, J.-P.; Carrere, H. Combination of thermal treatments and anaerobic digestion to reduce sewage sludge quantity and improve biogas yield. Process Saf. Environ. Prot. 2006, 84, 280–284. [Google Scholar] [CrossRef]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge. Effect of pre-treatment at elevated temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef]
- Raju, C.S.; Sutaryo, S.; Ward, A.J.; Møller, H.B. Effects of high-temperature isochoric pre-treatment on the methane yields of cattle, pig and chicken manure. Environ. Technol. 2013, 34, 239–244. [Google Scholar] [CrossRef]
- Marañón, E.; Castrillón, L.; Quiroga, G.; Fernández-Nava, Y.; Gómez, L.; García, M. Co-digestion of cattle manure with food waste and sludge to increase biogas production. Waste Manag. 2012, 32, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Menardo, S.; Airoldi, G.; Balsari, P. The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products. Bioresour. Technol. 2012, 104, 708–714. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.Y.; Olabi, A.G. Pretreatment techniques used in biogas production from grass. Renew. Sustain. Energy Rev. 2017, 68, 1193–1204. [Google Scholar] [CrossRef]
- Schwede, S.; Rehman, Z.-U.; Gerber, M.; Theiss, C.; Span, R. Effects of thermal pretreatment on anaerobic digestion of Nannochloropsis salina biomass. Bioresour. Technol. 2013, 143, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Poulsen, T.G.; Nizami, A.-S.; Murphy, J.D.; Kiely, G. Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Carrère, H.; Sialve, B.; Bernet, N. Improving pig manure conversion into biogas by thermal and thermo-chemical pretreatments. Bioresour. Technol. 2009, 100, 3690–3694. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Estevez, M.M.; Nielsen, H.K.; Linjordet, R.; Eijsink, V.G. Biogas production and saccharification of Salix pretreated at different steam explosion conditions. Bioresour. Technol. 2011, 102, 7932–7936. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.R.; Khalid, H.; Zhang, H.; u Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. Amb Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.; Horn, S.J. Effect of different steam explosion conditions on methane potential and enzymatic saccharification of birch. Bioresour. Technol. 2013, 127, 343–349. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pretreatment of wheat straw to improve methane yields: Investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Valo, A.; Carrere, H.; Delgenes, J.P. Thermal, chemical and thermo-chemical pre-treatment of waste activated sludge for anaerobic digestion. J. Chem. Technol. Biotechnol. 2004, 79, 1197–1203. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.-K.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Mladenovska, Z.; Hartmann, H.; Kvist, T.; Sales-Cruz, M.; Gani, R.; Ahring, B.K. Thermal pretreatment of the solid fraction of manure: Impact on the biogas reactor performance and microbial community. Water Sci. Technol. 2006, 53, 59–67. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef]
- Devlin, D.; Esteves, S.; Dinsdale, R.; Guwy, A. The effect of acid pretreatment on the anaerobic digestion and dewatering of waste activated sludge. Bioresour. Technol. 2011, 102, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Vervaeren, H.; Hostyn, K.; Ghekiere, G.; Willems, B. Biological ensilage additives as pretreatment for maize to increase the biogas production. Renew. Energy 2010, 35, 2089–2093. [Google Scholar] [CrossRef]
- Bougrier, C.; Albasi, C.; Delgenès, J.-P.; Carrère, H. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem. Eng. Process. Process Intensif. 2006, 45, 711–718. [Google Scholar] [CrossRef]
- Battimelli, A.; Torrijos, M.; Moletta, R.; Delgenès, J.P. Slaughterhouse fatty waste saponification to increase biogas yield. Bioresour. Technol. 2010, 101, 3388–3393. [Google Scholar] [CrossRef]
- Ugwu, S.N.; Enweremadu, C.C. Effects of pre-treatments and co-digestion on biogas production from Okra waste. J. Renew. Sustain. Energy 2019, 11, 013101. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. The effect of aqueous ammonia soaking pretreatment on methane generation using different lignocellulosic biomasses. Waste Biomass Valorization 2015, 6, 281–291. [Google Scholar] [CrossRef]
- Romero-Güiza, M.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.; Astals, S.J.R.; Reviews, S.E. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.; De Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Cheng, J.J.; Timilsina, G.R. Status and barriers of advanced biofuel technologies: A review. Renew. Energy 2011, 36, 3541–3549. [Google Scholar] [CrossRef]
- Hjorth, M.; Gränitz, K.; Adamsen, A.P.; Møller, H.B. Extrusion as a pretreatment to increase biogas production. Bioresour. Technol. 2011, 102, 4989–4994. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef]
- Peng, H.; Chen, H.; Qu, Y.; Li, H.; Xu, J. Bioconversion of different sizes of microcrystalline cellulose pretreated by microwave irradiation with/without NaOH. Appl. Energy 2014, 117, 142–148. [Google Scholar] [CrossRef]
- Sridar, V. Microwave radiation as a catalyst for chemical reactions. Curr. Sci. 1998, 74, 446–450. [Google Scholar]
- Diaz, A.B.; de Souza Moretti, M.M.; Bezerra-Bussoli, C.; Nunes, C.D.; Blandino, A.; da Silva, R.; Gomes, E. Evaluation of microwave-assisted pretreatment of lignocellulosic biomass immersed in alkaline glycerol for fermentable sugars production. Bioresour. Technol. 2015, 185, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Biswas, A.; Cotta, M.A. Microwave pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. J. Biobased Mater. Bioenergy 2008, 2, 210–217. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Yu, Z.; Chen, Q.; Wu, G.; Yu, F.; Wang, C.; Jin, S. Microwave-assisted alkali pre-treatment of wheat straw and its enzymatic hydrolysis. Biosyst. Eng. 2006, 94, 437–442. [Google Scholar] [CrossRef]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.; Tyagi, R.; Surampalli, R. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochemistry 2011, 18, 1–18. [Google Scholar] [CrossRef]
- Ormaechea, P.; Castrillón, L.; Marañón, E.; Fernández-Nava, Y.; Negral, L.; Megido, L. Influence of the ultrasound pretreatment on anaerobic digestion of cattle manure, food waste and crude glycerine. Environ. Technol. 2017, 38, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.; Delgenès, J.; Steyer, J.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Cesaro, A.; Naddeo, V.; Amodio, V.; Belgiorno, V. Enhanced biogas production from anaerobic codigestion of solid waste by sonolysis. Ultrason. Sonochem. 2012, 19, 596–600. [Google Scholar] [CrossRef]
- Pérez-Elvira, S.; Fdz-Polanco, M.; Plaza, F.; Garralón, G.; Fdz-Polanco, F. Ultrasound pre-treatment for anaerobic digestion improvement. Water Sci. Technol. 2009, 60, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Tanjore, D.; Richard, T.L. A systems view of lignocellulose hydrolysis. In Advances in Bioprocess Technology; Springer: Berlin, Germany, 2015; pp. 387–419. [Google Scholar]
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.; Raes, K. Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour. Technol. 2015, 192, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Schimpf, U.; Hanreich, A.; Mähnert, P.; Unmack, T.; Junne, S.; Renpenning, J.; Lopez-Ulibarri, R. Improving the efficiency of large-scale biogas processes: Pectinolytic enzymes accelerate the lignocellulose degradation. J. Sustain. Energy Environ. 2013, 4, 53–60. [Google Scholar]
- Jagadabhi, P.S.; Kaparaju, P.; Väisänen, A.; Rintala, J. Effect of macro-and micro-nutrients addition during anaerobic mono-digestion of grass silage in leach-bed reactors. Environ. Technol. 2019, 40, 418–429. [Google Scholar] [CrossRef]
- Facchin, V.; Cavinato, C.; Fatone, F.; Pavan, P.; Cecchi, F.; Bolzonella, D. Effect of trace element supplementation on the mesophilic anaerobic digestion of foodwaste in batch trials: The influence of inoculum origin. Biochem. Eng. J. 2013, 70, 71–77. [Google Scholar] [CrossRef]
- Jiang, Y.; Heaven, S.; Banks, C.J. Strategies for stable anaerobic digestion of vegetable waste. Renew. Energy 2012, 44, 206–214. [Google Scholar] [CrossRef]
- Funk, T.; Gu, W.; Friedrich, S.; Wang, H.; Gencic, S.; Grahame, D.A.; Cramer, S.P. Chemically Distinct Ni Sites in the A-Cluster in Subunit β of the Acetyl-CoA Decarbonylase/Synthase Complex from Methanosarcina t hermophila: Ni L-Edge Absorption and X-ray Magnetic Circular Dichroism Analyses. J. Am. Chem. Soc. 2004, 126, 88–95. [Google Scholar] [CrossRef]
- Chen, S.; Yakunin, A.F.; Kuznetsova, E.; Busso, D.; Pufan, R.; Proudfoot, M.; Kim, R.; Kim, S.-H. Structural and functional characterization of a novel phosphodiesterase from Methanococcus jannaschii. J. Biol. Chem. 2004, 279, 31854–31862. [Google Scholar] [CrossRef]
- Kumar, A.; Miglani, P.; Gupta, R.; Bhattacharya, T. Impact of Ni (II), Zn (II) and Cd (II) on biogassification of potato waste. J. Environ. Biol. 2006, 27, 61–66. [Google Scholar] [PubMed]
- Climenhaga, M.; Banks, C. Anaerobic digestion of catering wastes: Effect of micronutrients and retention time. Water Sci. Technol. 2008, 57, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, G.; Seghezzo, L.; Lettinga, G.; Kleerebezem, R. Kinetics and mass-transfer phenomena in anaerobic granular sludge. Biotechnol. Bioeng. 2001, 73, 125–134. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Huttunen, S.; Rintala, J. Laboratory investigations on co-digestion of energy crops and crop residues with cow manure for methane production: Effect of crop to manure ratio. Resour. Conserv. Recycl. 2007, 51, 591–609. [Google Scholar] [CrossRef]
- Sarker, S.; Møller, H.B. Boosting biogas yield of anaerobic digesters by utilizing concentrated molasses from 2nd generation bioethanol plant. Int. J. Energy Environ. 2013, 4, 199–210. [Google Scholar]
- Sarker, S.; Møller, H.B. Regulating feeding and increasing methane yield from co-digestion of C 5 molasses and cattle manure. Energy Convers. Manag. 2014, 84, 7–12. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R. Biogas production from co-digestion of dairy manure and food waste. Bioresour. Technol. 2010, 101, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Fatone, F.; Bolzonella, D.; Pavan, P. Thermophilic anaerobic co-digestion of cattle manure with agro-wastes and energy crops: Comparison of pilot and full scale experiences. Bioresour. Technol. 2010, 101, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, F.; Wase, D.; Thayanithy, K.; Forster, C. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass Bioenergy 2002, 22, 71–77. [Google Scholar] [CrossRef]
- Poulsen, T.G.; Adelard, L. Improving biogas quality and methane yield via co-digestion of agricultural and urban biomass wastes. Waste Manag. 2016, 54, 118–125. [Google Scholar] [CrossRef]
- Mata-Alvarez, J. Biomethanization of the Organic Fraction of Municipal Solid Wastes; IWA Publishing: London, UK, 2003. [Google Scholar]
- Bardiya, N.; Gaur, A. Effects of carbon and nitrogen ratio on rice straw biomethanation. J. Rural Energy 1997, 4, 1–16. [Google Scholar]
- Muzenda, E. Bio-Methane Generation from Organic Waste: A Review. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 22–24 October 2014. [Google Scholar]
- Vintiloiu, A.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Mineral substances and macronutrients in the anaerobic conversion of biomass: An impact evaluation. Eng. Life Sci. 2012, 12, 287–294. [Google Scholar] [CrossRef]
- Gil, A.; Siles, A.J.; Serrano, A.; Chica, A.; Martín, M. Effect of variation in the C/[N+P] ratio on anaerobic digestion. Environ. Prog. Sustain. Energy 2018, 38, 228–236. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.; Kumar, S.; Tyagi, V.; Tyagi, S. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Lay, C.-H.; Huang, C.-Y.; Chen, C.-C.; Lin, C.-Y. Biohydrogen production in an anaerobic baffled stacking reactor: Recirculation strategy and substrate concentration effects. Biochem. Eng. J. 2016, 109, 59–64. [Google Scholar] [CrossRef]
- Hamdi, M.; Garcia, J.-L. Comparison between anaerobic filter and anaerobic contact process for fermented olive mill wastewaters. Bioresour. Technol. 1991, 38, 23–29. [Google Scholar] [CrossRef]
- Elmitwalli, T.A.; Sklyar, V.; Zeeman, G.; Lettinga, G. Low temperature pre-treatment of domestic sewage in an anaerobic hybrid or an anaerobic filter reactor. Bioresour. Technol. 2002, 82, 233–239. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Tay, J.-H.; Show, K.-Y.; Yan, R.; Liang, D.T.; Lee, D.-J.; Jiang, W.-J. Biohydrogen production in a granular activated carbon anaerobic fluidized bed reactor. Int. J. Hydrogen Energy 2007, 32, 185–191. [Google Scholar] [CrossRef]
- Hung, C.-H.; Lee, K.-S.; Cheng, L.-H.; Huang, Y.-H.; Lin, P.-J.; Chang, J.-S. Quantitative analysis of a high-rate hydrogen-producing microbial community in anaerobic agitated granular sludge bed bioreactors using glucose as substrate. Appl. Microbiol. Biotechnol. 2007, 75, 693–701. [Google Scholar] [CrossRef]
- Saddoud, A.; Hassaïri, I.; Sayadi, S. Anaerobic membrane reactor with phase separation for the treatment of cheese whey. Bioresour. Technol. 2007, 98, 2102–2108. [Google Scholar] [CrossRef]
- Zhang, R.; Yin, Y.; Sung, S.; Dague, R. Anaerobic treatment of swine waste by the anaerobic sequencing batch reactor. Trans. ASAE 1997, 40, 767. [Google Scholar] [CrossRef]
- Angenent, L.T.; Sung, S. Development of anaerobic migrating blanket reactor (AMBR), a novel anaerobic treatment system. Water Res. 2001, 35, 1739–1747. [Google Scholar] [CrossRef]
- Xie, S.; Lawlor, P.G.; Frost, J.P.; Hu, Z.; Zhan, X. Effect of pig manure to grass silage ratio on methane production in batch anaerobic co-digestion of concentrated pig manure and grass silage. Bioresour. Technol. 2011, 102, 5728–5733. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Wu, J.F.; Lo, Y.S.; Lo, Y.C.; Lin, P.J.; Chang, J.S. Anaerobic hydrogen production with an efficient carrier-induced granular sludge bed bioreactor. Biotechnol. Bioeng. 2004, 87, 648–657. [Google Scholar] [CrossRef]
- Wu, S.Y.; Hung, C.H.; Lin, C.N.; Chen, H.W.; Lee, A.S.; Chang, J.S. Fermentative hydrogen production and bacterial community structure in high-rate anaerobic bioreactors containing silicone-immobilized and self-flocculated sludge. Biotechnol. Bioeng. 2006, 93, 934–946. [Google Scholar] [CrossRef]
- Fang, C.; Boe, K.; Angelidaki, I. Biogas production from potato-juice, a by-product from potato-starch processing, in upflow anaerobic sludge blanket (UASB) and expanded granular sludge bed (EGSB) reactors. Bioresour. Technol. 2011, 102, 5734–5741. [Google Scholar] [CrossRef]
- Alves, M.; Vieira, J.M.; Pereira, R.A.; Pereira, M.; Mota, M. Effects of lipids and oleic acid on biomass development in anaerobic fixed-bed reactors. Part II: Oleic acid toxicity and biodegradability. Water Res. 2001, 35, 264–270. [Google Scholar] [CrossRef]
- Jagadish, K.; Chanakya, H.; Rajabapaiah, P.; Anand, V. Plug flow digestors for biogas generation from leaf biomass. Biomass Bioenergy 1998, 14, 415–423. [Google Scholar] [CrossRef]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Submerged anaerobic membrane bioreactor for low-strength wastewater treatment: Effect of HRT and SRT on treatment performance and membrane fouling. Water Res. 2011, 45, 705–713. [Google Scholar] [CrossRef]
- Chen, J.; Tang, C.; Zheng, P.; Zhang, L. Performance of lab-scale SPAC anaerobic bioreactor with high loading rate. Chin. J. Biotechnol. 2008, 24, 1413–1419. [Google Scholar] [CrossRef]
- Sung, S.; Santha, H. Performance of temperature-phased anaerobic digestion (TPAD) system treating dairy cattle wastes. Water Res. 2003, 37, 1628–1636. [Google Scholar] [CrossRef]
- Bouallagui, H.; Cheikh, R.B.; Marouani, L.; Hamdi, M. Mesophilic biogas production from fruit and vegetable waste in a tubular digester. Bioresour. Technol. 2003, 86, 85–89. [Google Scholar] [CrossRef]
- Chiang, C.-F. Effects of Reactor Configuration on the Performance Of Static-Bed Submerged Media Anaerobic Reactors. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1988. [Google Scholar]
- Intanoo, P.; Chaimongkol, P.; Chavadej, S. Hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactors (UASB) with an emphasis on maximum hydrogen production. Int. J. Hydrogen Energy 2016, 41, 6107–6114. [Google Scholar] [CrossRef]
- Chiumenti, A.; da Borso, F.; Limina, S. Dry anaerobic digestion of cow manure and agricultural products in a full-scale plant: Efficiency and comparison with wet fermentation. Waste Manag. 2018, 71, 704–710. [Google Scholar] [CrossRef]
- Lissens, G.; Vandevivere, P.; De Baere, L.; Biey, E.; Verstraete, W. Solid waste digestors: Process performance and practice for municipal solid waste digestion. Water Sci. Technol. 2001, 44, 91–102. [Google Scholar] [CrossRef]
- Heo, N.H.; Park, S.C.; Kang, H. Effects of mixture ratio and hydraulic retention time on single-stage anaerobic co-digestion of food waste and waste activated sludge. J. Environ. Sci. Health Part A 2004, 39, 1739–1756. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Cecchi, F.; Llabrés, P.; Pavan, P. Anaerobic digestion of the Barcelona central food market organic wastes. Plant design and feasibility study. Bioresour. Technol. 1992, 42, 33–42. [Google Scholar] [CrossRef]
- Nasir, I.M.; Mohd Ghazi, T.I.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Estevez, M.M.; Sapci, Z.; Linjordet, R.; Schnürer, A.; Morken, J. Semi-continuous anaerobic co-digestion of cow manure and steam-exploded Salix with recirculation of liquid digestate. J. Environ. Manag. 2014, 136, 9–15. [Google Scholar] [CrossRef]
- Nordberg, Å.; Jarvis, Å.; Stenberg, B.; Mathisen, B.; Svensson, B.H. Anaerobic digestion of alfalfa silage with recirculation of process liquid. Bioresour. Technol. 2007, 98, 104–111. [Google Scholar] [CrossRef]
- Verrier, D.; Roy, F.; Albagnac, G. Two-phase methanization of solid vegetable wastes. Biol. Wastes 1987, 22, 163–177. [Google Scholar] [CrossRef]
- Bouallagui, H.; Touhami, Y.; Cheikh, R.B.; Hamdi, M. Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Kübler, H.; Schertler, C. Three-phase anaerobic digestion of organic wastes. Water Sci. Technol. 1994, 30, 367–374. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, J.Y.; Kim, J.K.; Cho, J.H.; Chun, Y.N.; Lee, I.H.; Lee, J.S.; Park, J.S.; Park, D.-H. Development of a modified three-stage methane production process using food wastes. Appl. Biochem. Biotechnol. 2000, 84, 731–741. [Google Scholar] [CrossRef]
- Rodriguez, R. Upflow Anaerobic Sludge Blanket Reactor: Modelling; The Royal Institute of Technology: Stockholm, Sweden, 2011. [Google Scholar]
- Ding, J.N.; Wang, D.Z. Influence of external circulation on sludge characteristics during start-up of internal circulation reactor. J. Cent. South Univ. Technol. 2005, 12, 425–429. [Google Scholar] [CrossRef]
- Visvanathan, C.; Abeynayaka, A. Developments and future potentials of anaerobic membrane bioreactors (AnMBRs). Membr. Water Treat 2012, 3, 1–23. [Google Scholar] [CrossRef]
- Gaida, D.; Wolf, C.; Bongards, M. Feed control of anaerobic digestion processes for renewable energy production: A review. Renew. Sustain. Energy Rev. 2017, 68, 869–875. [Google Scholar] [CrossRef]
- Barjenbruch, M.; Hoffmann, H.; Kopplow, O.; Tränckner, J. Minimizing of foaming in digesters by pre-treatment of the surplus-sludge. Water Sci. Technol. 2000, 42, 235–241. [Google Scholar] [CrossRef]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M. Anaerobic digestion of animal waste: Effect of mode of mixing. Water Res. 2005, 39, 3597–3606. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chynoweth, P.; Biljetina, R. Anaerobic digestion of municipal solid waste in a nonmixed solids concentrating digestor. Appl. Biochem. Biotechnol. 1990, 24, 533–544. [Google Scholar] [CrossRef]
- Özdemir, G. Optimering av Biogasproduktion Från Gödsel; Linnaeus University: Växjö, Sweden, 2009. [Google Scholar]
- Wang, B.; Wu, D.; Ekama, G.A.; Huang, H.; Lu, H.; Chen, G.-H. Optimizing mixing mode and intensity to prevent sludge flotation in sulfidogenic anaerobic sludge bed reactors. Water Res. 2017, 122, 481–491. [Google Scholar] [CrossRef]
- Ong, H.; Greenfield, P.; Pullammanappallil, P. Effect of mixing on biomethanation of cattle-manure slurry. Environ. Technol. 2002, 23, 1081–1090. [Google Scholar] [CrossRef]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidakia, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef]
- Dachs, G.; Rehm, W. Der Eigenstromverbrauch von Biogasanlagen und Potenziale zu Dessen Reduzierung; Solarenergieförderverein Bayern: München, Germany, 2006. [Google Scholar]
- Kowalczyk, A.; Harnisch, E.; Schwede, S.; Gerber, M.; Span, R. Different mixing modes for biogas plants using energy crops. Appl. Energy 2013, 112, 465–472. [Google Scholar] [CrossRef]
- Kowalczyk, A.A. Untersuchung der Übertragbarkeit und Reproduzierbarkeit von Versuchen zur Biogasbildung in kontinuierlichen Prozessen sowie erste Studien zu deren Optimierung. Ph.D. Thesis, Ruhr-Universität Bochum, Bochum, Germany, 2012. [Google Scholar]
- Teng, Z.; Hua, J.; Wang, C.; Lu, X. Chapter 4—Design and optimization principles of biogas reactors in large scale applications A2—Shi, Fan. In Reactor and Process Design in Sustainable Energy Technology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 99–134. [Google Scholar]
- Prieto, A.L.; Futselaar, H.; Lens, P.N.; Bair, R.; Yeh, D.H. Development and start up of a gas-lift anaerobic membrane bioreactor (Gl-AnMBR) for conversion of sewage to energy, water and nutrients. J. Membr. Sci. 2013, 441, 158–167. [Google Scholar] [CrossRef]
- Xu, F.; Huang, Z.; Miao, H.; Ren, H.; Zhao, M.; Ruan, W. Identical full-scale biogas-lift reactors (BLRs) with anaerobic granular sludge and residual activated sludge for brewery wastewater treatment and kinetic modeling. J. Environ. Sci. 2013, 25, 2031–2040. [Google Scholar] [CrossRef]
- Schnürer, A.; Jarvis, Å. Microbiological Handbook for Biogas Plants-Swedish Waste Management U2009: 03, Swedish Gas Centre Report 207; Avfall Sverige, Svenskt Gastekniskt Center AB: Malmo, Sweden, 2009. [Google Scholar]
- Bramstedt, S. Temperature Optimization of Anaerobic Digestion at the Käppala Waste Water Treatment Plant; The Royal Institute of Technology: Stockholm, Sweden, 2016. [Google Scholar]
- Bouallagui, H.; Torrijos, M.; Godon, J.; Moletta, R.; Cheikh, R.B.; Touhami, Y.; Delgenes, J.; Hamdi, M. Two-phases anaerobic digestion of fruit and vegetable wastes: Bioreactors performance. Biochem. Eng. J. 2004, 21, 193–197. [Google Scholar] [CrossRef]
- Cysneiros, D.; Thuillier, A.; Villemont, R.; Littlestone, A.; Mahony, T.; O’Flaherty, V. Temperature effects on the trophic stages of perennial rye grass anaerobic digestion. Water Sci. Technol. 2011, 64, 70–76. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Nelson, R. Methane Generation from Anaerobic Digesters: Considering Different Substrates; Environmental Biotechnology, Lowa State University: Ames, IA, USA, 2010. [Google Scholar]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. Applications of the anaerobic digestion process. In Biomethanation II; Springer: Berlin, Germany, 2003; pp. 1–33. [Google Scholar]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef]
- Nielsen, M.; Holst-Fischer, C.; Malmgren-Hansen, B.; Bjerg-Nielsen, M.; Kragelund, C.; Møller, H.B.; Ottosen, L.D. Small temperature differences can improve the performance of mesophilic sludge-based digesters. Biotechnol. Lett. 2017, 39, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- David, B.; Francesco, F.; Paolo, P.; Franco, C. Mesophilic, thermophilic and temperature phased anaerobic digestion of waste activated sludge. Chem. Eng. Trans. 2008, 17, 385–390. [Google Scholar]
- Voelklein, M.A.; O’Shea, R.; Jacob, A.; Murphy, J.D. Role of trace elements in single and two-stage digestion of food waste at high organic loading rates. Energy 2017, 121, 185–192. [Google Scholar] [CrossRef]
- Puchajda, B.; Oleszkiewicz, J. Impact of sludge thickening on energy recovery from anaerobic digestion. Water Sci. Technol. 2008, 57, 395–401. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. A novel process configuration for anaerobic digestion of source-sorted household waste using hyper-thermophilic post-treatment. Biotechnol. Bioeng. 2005, 90, 830–837. [Google Scholar] [CrossRef]
- Alepu, O.; Li, Z.; Ikhumhen, H.; Kalakodio, L.; Wang, K. Effect of Hydraulic Retention Time on Anaerobic Digestion of Xiao Jiahe Municipal Sludge. Int. J. Waste Resour. 2016, 6, 2. [Google Scholar]
- Passos, F.; García, J.; Ferrer, I. Impact of low temperature pretreatment on the anaerobic digestion of microalgal biomass. Bioresour. Technol. 2013, 138, 79–86. [Google Scholar] [CrossRef]
- Lohani, S.P.; Wang, S.; Bergland, W.H.; Khanal, S.N.; Bakke, R. Modeling temperature effects in anaerobic digestion of domestic wastewater. Water-Energy Nexus 2018, 1, 56–60. [Google Scholar] [CrossRef]
- Verma, S. Anaerobic Digestion of Biodegradable Organics in Municipal Solid Wastes. Master’s Thesis, Columbia University, New York, NY, USA, 2002. [Google Scholar]
- Liu, D.; Zeng, R.J.; Angelidaki, I. Effects of pH and hydraulic retention time on hydrogen production versus methanogenesis during anaerobic fermentation of organic household solid waste under extreme-thermophilic temperature (70 °C). Biotechnol. Bioeng. 2008, 100, 1108–1114. [Google Scholar] [CrossRef]
- Sibiya, N.T.; Muzenda, E.; Tesfagiorgis, H.B. Effect of Temperature and pH on the Anaerobic Digestion of Grass Silage. In Proceedings of the 6th International Conference on Green Technology, Renewable Energy and Environmental Engineering, Cape Town, South Africa, 27–28 November 2014. [Google Scholar]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe 0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Georgacakis, D.; Sievers, D.; Iannotti, E.J. Buffer stability in manure digesters. Agric. Wastes 1982, 4, 427–441. [Google Scholar] [CrossRef]
- Sommer, S.G.; Husted, S. A simple model of pH in slurry. J. Agric. Sci. 1995, 124, 447–453. [Google Scholar] [CrossRef]
- Kovács, E.; Wirth, R.; Maróti, G.; Bagi, Z.; Nagy, K.; Minárovits, J.; Rákhely, G.; Kovács, K.L. Augmented biogas production from protein-rich substrates and associated metagenomic changes. Bioresour. Technol. 2015, 178, 254–261. [Google Scholar] [CrossRef]
- Yu, D.; Liu, J.; Sui, Q.; Wei, Y. Biogas-pH automation control strategy for optimizing organic loading rate of anaerobic membrane bioreactor treating high COD wastewater. Bioresour. Technol. 2016, 203, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis. Bioresour. Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O. Anaerobic acidogenesis of dairy wastewater: The effects of variations in hydraulic retention time with no pH control. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2004, 79, 755–760. [Google Scholar] [CrossRef]
- Nayono, S.E.; Gallert, C.; Winter, J. Co-digestion of press water and food waste in a biowaste digester for improvement of biogas production. Bioresour. Technol. 2010, 101, 6987–6993. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Kim, H.-S.; Yeom, I.-T. The optimum operational condition of membrane bioreactor (MBR): Cost estimation of aeration and sludge treatment. Water Res. 2004, 38, 37–46. [Google Scholar] [CrossRef]
- Buysman, E. Anaerobic Digestion for Developing Countries with Cold Climates. Master’s Thesis, Wageningen University, Wageningen, The Netherlands, 2009. [Google Scholar]
- Ueno, Y.; Tatara, M.; Fukui, H.; Makiuchi, T.; Goto, M.; Sode, K. Production of hydrogen and methane from organic solid wastes by phase-separation of anaerobic process. Bioresour. Technol. 2007, 98, 1861–1865. [Google Scholar] [CrossRef]
- Yang, F.; Chen, R.; Yue, Z.; Liao, W.; Marsh, T.L. Phylogenetic analysis of anaerobic co-digestion of animal manure and corn stover reveals linkages between bacterial communities and digestion performance. AiM 2016, 6, 879. [Google Scholar] [CrossRef][Green Version]
- Nges, I.A.; Liu, J. Effects of solid retention time on anaerobic digestion of dewatered-sewage sludge in mesophilic and thermophilic conditions. Renew. Energy 2010, 35, 2200–2206. [Google Scholar] [CrossRef]
- Akram, A.; Stuckey, D. Biomass acclimatisation and adaptation during start-up of a submerged anaerobic membrane bioreactor (SAMBR). Environ. Technol. 2008, 29, 1053–1065. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Jensen, P.D.; Rabaey, K.; Tyson, G.W. Temperature and solids retention time control microbial population dynamics and volatile fatty acid production in replicated anaerobic digesters. Sci. Rep. 2015, 5, 8496. [Google Scholar] [CrossRef]
- Gagliano, M.; Braguglia, C.; Gallipoli, A.; Gianico, A.; Rossetti, S. Microbial diversity in innovative mesophilic/thermophilic temperature-phased anaerobic digestion of sludge. Environ. Sci. Pollut. Res. 2015, 22, 7339–7348. [Google Scholar] [CrossRef] [PubMed]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Yabu, H.; Sakai, C.; Fujiwara, T.; Nishio, N.; Nakashimada, Y. Thermophilic two-stage dry anaerobic digestion of model garbage with ammonia stripping. J. Biosci. Bioeng. 2011, 111, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Gallert, C.; Winter, J. Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: Effect of ammonia on glucose degradation and methane production. Appl. Microbiol. Biotechnol. 1997, 48, 405–410. [Google Scholar] [CrossRef]
- Bujoczek, G.; Oleszkiewicz, J.; Sparling, R.; Cenkowski, S. High solid anaerobic digestion of chicken manure. J. Agric. Eng. Res. 2000, 76, 51–60. [Google Scholar] [CrossRef]
- Sahu, N.; Deshmukh, S.; Chandrashekhar, B.; Sharma, G.; Kapley, A.; Pandey, R. Optimization of hydrolysis conditions for minimizing ammonia accumulation in two-stage biogas production process using kitchen waste for sustainable process development. J. Environ. Chem. Eng. 2017, 5, 2378–2387. [Google Scholar] [CrossRef]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous ammonia equilibrium calculations: Effect of pH and temperature. J. Fish. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- De Baere, L.; Devocht, M.; Van Assche, P.; Verstraete, W. Influence of high NaCl and NH4Cl salt levels on methanogenic associations. Water Res. 1984, 18, 543–548. [Google Scholar] [CrossRef]
- Farrow, C. Anaerobic Digestion of Poultry Manure: Implementation of Ammonia Control to Optimize Biogas Yield; University of Guelph: Guelph, ON, Canada, 2016. [Google Scholar]
- Kroeker, E.; Schulte, D.; Sparling, A.; Lapp, H. Anaerobic treatment process stability. J. Water Pollut. Control Fed. 1979, 51, 718–727. [Google Scholar]
- Niu, Q.; Qiao, W.; Qiang, H.; Li, Y.-Y. Microbial community shifts and biogas conversion computation during steady, inhibited and recovered stages of thermophilic methane fermentation on chicken manure with a wide variation of ammonia. Bioresour. Technol. 2013, 146, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Shin, S.G.; Hwang, S. Methanogenic population dynamics assessed by real-time quantitative PCR in sludge granule in upflow anaerobic sludge blanket treating swine wastewater. Bioresour. Technol. 2010, 101, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, K.F.; Saulnier, M.; Ley, A. Inhibition of methanogenesis in pure cultures by ammonia, fatty acids, and heavy metals, and protection against heavy metal toxicity by sewage sludge. Can. J. Microbiol. 1987, 33, 551–554. [Google Scholar] [CrossRef]
- Schnürer, A.; Nordberg, Å. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740. [Google Scholar] [CrossRef]
- Gallert, C.; Bauer, S.; Winter, J. Effect of ammonia on the anaerobic degradation of protein by a mesophilic and thermophilic biowaste population. Appl. Microbiol. Biotechnol. 1998, 50, 495–501. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B. Anaerobic thermophilic digestion of manure at different ammonia loads: Effect of temperature. Water Res. 1994, 28, 727–731. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Bonmatı, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre-or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Submersible microbial desalination cell for simultaneous ammonia recovery and electricity production from anaerobic reactors containing high levels of ammonia. Bioresour. Technol. 2015, 177, 233–239. [Google Scholar] [CrossRef]
- Bodalo, A.; Gomez, J.-L.; Gomez, E.; Leon, G.; Tejera, M. Ammonium removal from aqueous solutions by reverse osmosis using cellulose acetate membranes. Desalination 2005, 184, 149–155. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C. Biogas stripping of ammonia from fresh digestate from a food waste digester. Bioresour. Technol. 2015, 190, 66–75. [Google Scholar] [CrossRef]
- Nzila, A. Mini review: Update on bioaugmentation in anaerobic processes for biogas production. Anaerobe 2016, 46, 3–12. [Google Scholar] [CrossRef]
- Kimura, Y.; Isaka, K.; Kazama, F.; Sumino, T. Effects of nitrite inhibition on anaerobic ammonium oxidation. Appl. Microbiol. Biotechnol. 2010, 86, 359–365. [Google Scholar] [CrossRef]
- Lin, L.; Chen, J.; Xu, Z.; Yuan, S.; Cao, M.; Liu, H.; Lu, X. Removal of ammonia nitrogen in wastewater by microwave radiation: A pilot-scale study. J. Hazard. Mater. 2009, 168, 862–867. [Google Scholar] [CrossRef]
- Çelen, I.; Türker, M. Recovery of ammonia as struvite from anaerobic digester effluents. Environ. Technol. 2001, 22, 1263–1272. [Google Scholar] [CrossRef]
- Zeshan; Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef]
- Ukwuani, A.T.; Tao, W. Developing a vacuum thermal stripping–acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res. 2016, 106, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Labatut, R.A.; Gooch, C.A. Monitoring of Anaerobic Digestion Process to Optimize Performance and Prevent System Failure. In Proceedings of the Got Manure? Enhancing Environmental and Economic Sustainability Conference, Liverpool, NY, USA, 28–29 March 2012. [Google Scholar]
- Inanc, B.; Matsui, S.; Ide, S. Propionic acid accumulation in anaerobic digestion of carbohydrates: An investigation on the role of hydrogen gas. Water Sci. Technol. 1999, 40, 93–100. [Google Scholar] [CrossRef]
- Khan, M.; Ngo, H.H.; Guo, W.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.; Wang, J.; Wu, Y. Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef]
- Hao, T.-W.; Luo, J.-H.; Wei, L.; Mackey, H.R.; Chi, K.; Chen, G.-H. Example study for granular bioreactor stratification: Three-dimensional evaluation of a sulfate-reducing granular bioreactor. Sci. Rep. 2016, 6, 31718. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of organic loading rate on VFA production, organic matter removal and microbial activity of a two-stage thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef]
- Yuan, Q.; Sparling, R.; Oleszkiewicz, J.A. VFA generation from waste activated sludge: Effect of temperature and mixing. Chemosphere 2011, 82, 603–607. [Google Scholar] [CrossRef]
- Öztürk, M. Degradation of acetate, propionate, and butyrate under shock temperature. J. Environ. Eng. 1993, 119, 321–331. [Google Scholar] [CrossRef]
- Yu, L.; Bule, M.; Ma, J.; Zhao, Q.; Frear, C.; Chen, S. Enhancing volatile fatty acid (VFA) and bio-methane production from lawn grass with pretreatment. Bioresour. Technol. 2014, 162, 243–249. [Google Scholar] [CrossRef]
- Wu, H.; Gao, J.; Yang, D.; Zhou, Q.; Liu, W. Alkaline fermentation of primary sludge for short-chain fatty acids accumulation and mechanism. Chem. Eng. J. 2010, 160, 1–7. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. Overview of recent progress towards in-situ biogas upgradation techniques. Fuel 2018, 226, 686–697. [Google Scholar] [CrossRef]
- Speece, R.; Boonyakitsombut, S.; Kim, M.; Azbar, N.; Ursillo, P. Overview of Anaerobic Treatment: Thermophilic and Propionate Implications-Keynote Address—Association of Environmental Engineering and Science Professors—78th Annual Water Environment Federation Technical Exposition and Conference, Washington, DC, Oct. 29–Nov. 2, 2005. Water Environ. Res. 2006, 78, 460–473. [Google Scholar]
- Fukuzaki, S.; Nishio, N.; Shobayashi, M.; Nagai, S. Inhibition of the fermentation of propionate to methane by hydrogen, acetate, and propionate. Appl. Environ. Microbiol. 1990, 56, 719–723. [Google Scholar]
- Liu, X.; Wang, W.; Shi, Y.; Zheng, L.; Gao, X.; Qiao, W.; Zhou, Y. Pilot-scale anaerobic co-digestion of municipal biomass waste and waste activated sludge in China: Effect of organic loading rate. Waste Manag. 2012, 32, 2056–2060. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Anwar, N.; Ma, Z.; Liu, G.; Zhang, R. Effect of organic loading rate on anaerobic digestion of food waste under mesophilic and thermophilic conditions. Energy Fuels 2017, 31, 2976–2984. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, Q.; Pan, Z.; Jiang, Y.; Liebetrau, J.; Nelles, M.; Dong, H.; Dong, R. Performance evaluation of a novel anaerobic digestion operation process for treating high-solids content chicken manure: Effect of reduction of the hydraulic retention time at a constant organic loading rate. Waste Manag. 2017, 64, 340–347. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Wang, Z.; Tan, T.; Qin, P. Biogas by semi-continuous anaerobic digestion of food waste. Appl. Biochem. Biotechnol. 2015, 175, 3901–3914. [Google Scholar] [CrossRef]
- Ferguson, R.M.; Coulon, F.; Villa, R. Organic loading rate: A promising microbial management tool in anaerobic digestion. Water Res. 2016, 100, 348–356. [Google Scholar] [CrossRef]
- Shen, F.; Li, H.; Wu, X.; Wang, Y.; Zhang, Q. Effect of organic loading rate on anaerobic co-digestion of rice straw and pig manure with or without biological pretreatment. Bioresour. Technol. 2018, 250, 155–162. [Google Scholar] [CrossRef]
- Ding, L.; Gutierrez, E.C.; Cheng, J.; Xia, A.; O’Shea, R.; Guneratnam, A.J.; Murphy, J.D. Assessment of continuous fermentative hydrogen and methane co-production using macro-and micro-algae with increasing organic loading rate. Energy 2018, 151, 760–770. [Google Scholar] [CrossRef]
- Zhang, E.; Li, J.; Zhang, K.; Wang, F.; Yang, H.; Zhi, S.; Liu, G. Anaerobic digestion performance of sweet potato vine and animal manure under wet, semi-dry, and dry conditions. AMB Express 2018, 8, 45. [Google Scholar] [CrossRef]
- Polizzi, C.; Alatriste-Mondragón, F.; Munz, G. The role of organic load and ammonia inhibition in anaerobic digestion of tannery fleshing. Water Resour. Ind. 2018, 19, 25–34. [Google Scholar] [CrossRef]
- Wang, S.; Hawkins, G.L.; Kiepper, B.H.; Das, K.C. Treatment of slaughterhouse blood waste using pilot scale two-stage anaerobic digesters for biogas production. Renew. Energy 2018, 126, 552–562. [Google Scholar] [CrossRef]
- Solli, L.; Schnürer, A.; Horn, S.J. Process performance and population dynamics of ammonium tolerant microorganisms during co-digestion of fish waste and manure. Renew. Energy 2018, 125, 529–536. [Google Scholar] [CrossRef]
- Muñoz, P.J.W.; Valorization, B. Assessment of Batch and Semi-continuous Anaerobic Digestion of Food Waste at Psychrophilic Range at Different Food Waste to Inoculum Ratios and Organic Loading Rates. Waste Biomass Valoriz. 2018, 1–10. [Google Scholar] [CrossRef]
- Micolucci, F.; Gottardo, M.; Pavan, P.; Cavinato, C.; Bolzonella, D. Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste. J. Clean. Prod. 2018, 171, 1376–1385. [Google Scholar] [CrossRef]
- Fontana, A.; Campanaro, S.; Treu, L.; Kougias, P.G.; Cappa, F.; Morelli, L.; Angelidaki, I. Performance and genome-centric metagenomics of thermophilic single and two-stage anaerobic digesters treating cheese wastes. Water Res. 2018, 134, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kumar, G.; Yun, Y.-M.; Kwon, J.-C.; Kim, S.-H. Effect of feeding mode and dilution on the performance and microbial community population in anaerobic digestion of food waste. Bioresour. Technol. 2018, 248, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Loh, K.-C.; Zhang, J.; Tong, Y.W.; Dai, Y. Two-stage anaerobic digestion of food waste and horticultural waste in high-solid system. Appl. Energy 2018, 209, 400–408. [Google Scholar] [CrossRef]
- Morken, J.; Gjetmundsen, M.; Fjørtoft, K. Determination of kinetic constants from the co-digestion of dairy cow slurry and municipal food waste at increasing organic loading rates. Renew. Energy 2018, 117, 46–51. [Google Scholar] [CrossRef]
- Eslami, H.; Hashemi, H.; Fallahzadeh, R.A.; Khosravi, R.; Fard, R.F.; Ebrahimi, A.A. Effect of organic loading rates on biogas production and anaerobic biodegradation of composting leachate in the anaerobic series bioreactors. Ecol. Eng. 2018, 110, 165–171. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Andersen, M.; Olesen, C.B.; Feilberg, A.; Kofoed, M.V. Parameters affecting acetate concentrations during in-situ biological hydrogen methanation. Bioresour. Technol. 2018, 258, 33–40. [Google Scholar] [CrossRef]
- Wickham, R.; Xie, S.; Galway, B.; Bustamante, H.; Nghiem, L.D. Anaerobic digestion of soft drink beverage waste and sewage sludge. Bioresour. Technol. 2018, 262, 141–147. [Google Scholar] [CrossRef]
- Petersson, A.; Wellinger, A. Biogas upgrading technologies—Developments and innovations. IEA Bioenergy 2009, 20, 1–19. [Google Scholar]
- Vavilin, V.; Vasiliev, V.; Rytov, S. Modelling of gas pressure effects on anaerobic digestion. Bioresour. Technol. 1995, 52, 25–32. [Google Scholar] [CrossRef]
- Singh, G.; Jain, V.K.; Singh, A. Effect of Temperature and other factors on Anaerobic Digestion Process, responsible for Bio Gas Production. Int. J. Theor. Appl. Mech. 2017, 12, 637–657. [Google Scholar]
- Lindeboom, R.; Fermoso, F.; Weijma, J.; Zagt, K.; Van Lier, J. Autogenerative high pressure digestion: Anaerobic digestion and biogas upgrading in a single step reactor system. Water Sci. Technol. 2011, 64, 647–653. [Google Scholar] [CrossRef] [PubMed]

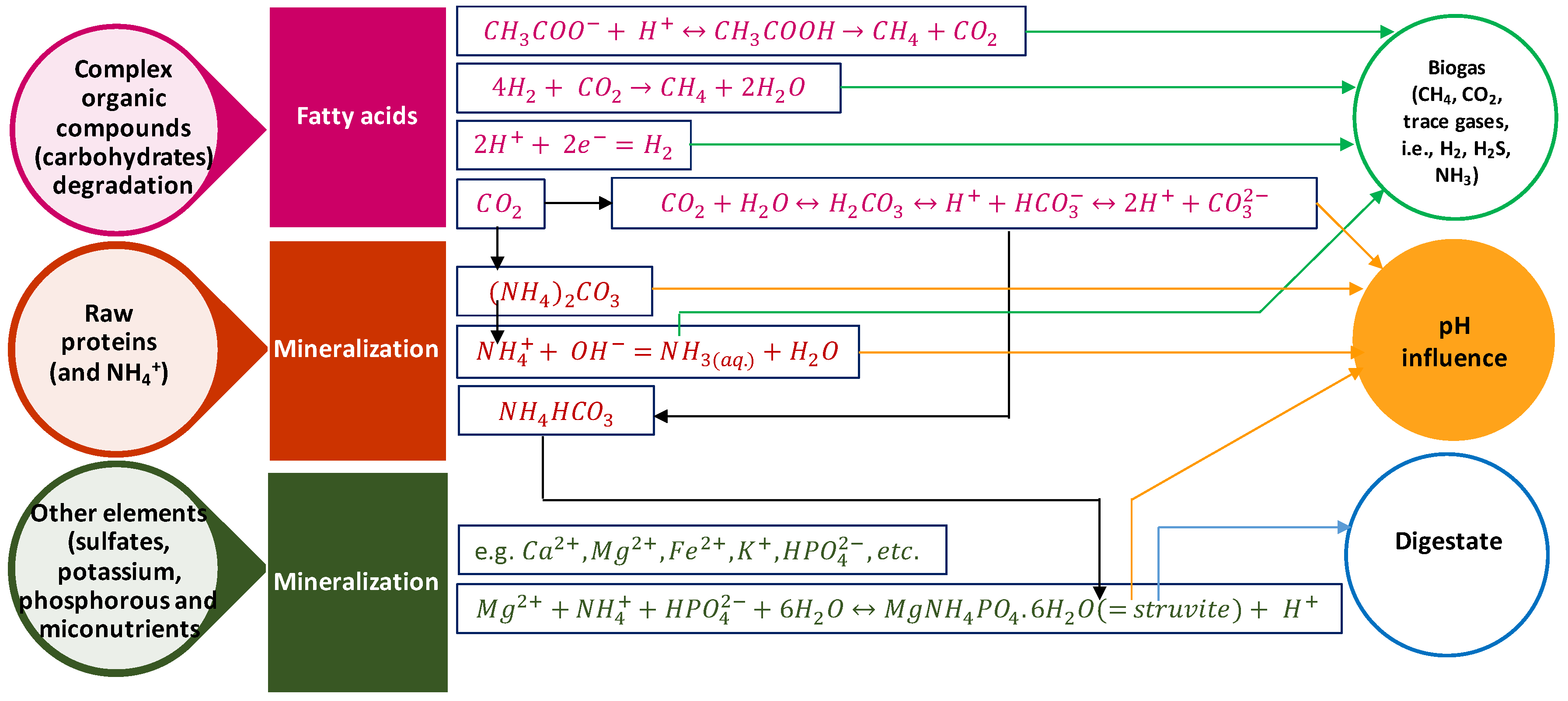
| Primary Substrate Components | Hydrolyzed Products | Bacterial Group |
|---|---|---|
| Carbohydrates | Soluble sugars | Clostridium, Acitovibrio celluliticus, Staphylococcus, Bacteriodes |
| Lipids | Higher fatty acids or alcohols and glycerol | Clostridium, Staphylococcus, Micrococcus |
| Proteins | Soluble peptides and amino acids | Clostridium, Proteus vulgaris, Peptococcus, Bacteriods, Bacillus, Vibrio |
| Feedstock | Methane Formation Stoichiometry | Methane Concentration, % |
|---|---|---|
| Carbohydrate | 50 | |
| Lipid | 69.5 | |
| Protein | 68.8 |
| Additive | Element or Compound | Benefit | Adverse Effect |
|---|---|---|---|
| Macro-nutrients | P, N and S | Methane production improvement and enhanced process stability | Methane or biomass inhibition by overdosing |
| Micro-nutrients, heavy metals | Cu2+, Zn2+, Cr3+, Cd, Ni, Pb4+ and Hg2+ | Promoting various enzymatic reactions | Inhibition to the acetogens |
| Micro-nutrients, light metals | Na+, K+, Mg2+, Ca2+ and Al3+ | Enhancing microbial growth | Restricting production of double cells (Mg2+), Inhibition of acetoclastic methanogens (Na+), Destabilizing buffering system (Ca2+), etc. |
| Iron | Zero valent iron, Clean scrap, rusty scrap and iron additives | Sulfide fixation, biomass stimulation, etc. | Precipitation and clogging risk |
| Ash | Bottom ash and fly ash | ||
| Inorganic absorbent materials | MgCl2, MgCl2.6H2O, MgPO4.3H2O | Ammonia sequestration through struvite formation | |
| Inorganic nitrogen | Availability of nitrogen as nutrient | Inhibition of methane producing enzymes, VFA accumulation | |
| Nano particles | Ag, Au, Fe, Al2O3, SiO2, TiO, ZnO | Methane production improvement | Inhibition of hydrolytic and methanogenic biomass |
| Biological additives (bioaugmentation and enzyme) | Compost, C. proteolyticus, SAO co-culture and Methanoculleus bourgensis MS2 | Increased methanogenic activity, increased hydrolytic activity | Cost, process control |
| Others | Biochar, activated carbon, sand, zeolite, Ni-Zeo, Co-Zeo, Mg-Zeo, rockwool, membrane, molecular sieve, polyurethane foam and loofah | Biomass immobilization, buffering agents, enhanced VFA degradation |
| Reactor Configuration | Application | Operational Parameters (Reactor Size, Feedstock Type, Reactor Temperature, pH, OLR and HRT) | Results | Reference |
|---|---|---|---|---|
| ABSR | Biohydrogen production | 3 L, Sucrose, 37 °C, 5.5 ± 0.2 (adjusted), 10–30 gCOD/L, 8 h | HPR: 10.9 ± 1.5 L/L-d, HY: 1.7 ± 0.2 mol/mol-sucrose | [129] |
| ACP | Methane production | 10 L, Olive mill waste water and urea, 35 °C, 7.5 (adjusted), 2 gCOD/L/d, 15 days | MY: 0.15 L-CH4/gCOD removed | [130] |
| AF | Pre-treatment & process performance | 60 L, Domestic sewage, 13 °C, N/G, 234 mgCOD/L, 4 h | MC: 70.7 ± 2.9%; MC in AF was found higher than that of AH | [131] |
| AFBR | Biohydrogen production and waste water treatment | ca 4 L, Synthetic waste water, 37 °C, 4 (adjusted), 10 g/L, 0.5 to 4 h | Max. HPR: 2.36 L/L-h, Max. HY: 1.16 mol/mol-glucose | [132] |
| AGSB | Biohydrogen production | ca 0.9 L, Glucose, 40 °C, 6.5, 20 gCOD/L, 4, 2, 1 and 0.5 h | HC: 36–41%, HY: 1.4 to 31.5 mol/mol-glucose | [133] |
| AH | Pre-treatment & process performance of sewage treatment plant | 88 L, Domestic sewage, 13 °C, N/G, 340 mgCOD/L, 4 h | MC: 58.9 ± 3.2% (see also AF, given above) | [131] |
| AnMBR | Biogas generation | 2-phase (7 L & 20 L), Cheese whey, 37 ± 2 °C (both phases), 6.5 at start (acidogenic), Max. 19.78 gCOD/L-d (methanogenic), 1 d & 4 d | MC: Max. 70% (methanogenic); biogas production exceeded 10 times reactor volume increased with OLR | [134] |
| AMBR | Methane production & waste water treatment | 12 L, Sucrose base synthetic wastewater, 35 ± 1 °C, 6.5 (adjusted), 30 g/L/d, 12 h | MPR: 6.5 L/L/d with 62.2% average methane based COD removal efficiency | [136] |
| ASBR | Biogas generation | N/A, Swine waste, 25 °C, 6.8 to 7.4, 0.9 to 5.5 g/L/d, 2 to 6 days | Biogas production rate: 0.9 to 1.8 L/L/d | [135] |
| BSAR | Biogas generation | 1 L (5 units, equal volume), Pig manure (PM) and grass silage (GS), 35 °C, 6.5 to 8.0, 5 PM:GS (1:1, 1:3, 3:1, 1:0, 0:1), 90 days | Max. MY: 304.2 mL/gVS (at OLR 3:1 for PM:GS) Max. cumulative MY: 8517 L (at OLR 3:1 for PM:GS) | [137] |
| CIGBR | Biohydrogen production & waste water treatment | ca 1 L, Sucrose base waste water, 35 °C, 3 (adjusted), 2.5 to 5 gCOD/L/h, 4 to 8 h | Max. HPR: ~7.3 L/L-h, Max. HY: 3.03 mol/mol-sucrose | [138] |
| CSAB | Biohydrogen production | ca 1 L, Sucrose, inoculum heat shock, 40 °C, 6.6 ± 0.2, 30 to 40 gCOD/L/h, 0.5 to 6 h | Max. HPR: 15 L/L-h, Optimal HY: 3.5 mol/mol-sucrose | [139] |
| CSTR | Methane production | 5 L, CM and Laminaria digitata, 35 ± 2 °C & 50 ± 1 °C, 8.0 ± 0.3, 2.5 to 2.9 gVS/L/d, 22 days | MY avg.: ca 225 L/kg VS (meso), ca 170 L/kg VS (thermo) | [118] |
| EGSB | Biogas generation | 1 L, Potato-juice, 37 °C, 8 (adjusted to 4, 5, 6 & 7), 2.5 to 4.2 gCOD/L-d, 6 to 10 days | MY avg.: 385 mLCH4/gVS; MPR avg.: 1496 mLCH4/L-d | [140] |
| FBR | Biogas generation and biomass development | 86 L, Skim milk, whole milk and oleate (variable feeding in 3 periods), 35 °C, 7 to 7.2 (adjusted), 12 g/L (2.4 - 4.15 g oleate/L skim. milk), 426 days | Max. MPR: 33 and 46 mLCH4/gVS-d | [141] |
| PFR | Biogas generation | ca 5 m3 (field scale plant), Terrestrial weeds and leafy biomass, 25 to 35 °C, N/D, 50 to 100 kg leafy biomass/day, 35 to 70 days | Average biogas yield: 50 L/kg fresh biomass (at OLR: 50 kg/day); 30 to 45 L/kg fresh biomass (at OLR: 100 kg/day) | [142] |
| SAnMBRs | Biogas generation & waste water treatment | 6 L (3 units), Synthetic low strength waste water, 25 to 30 °C, 7.0 ± 0.5 (adjusted), 1.1 to 1.65 kg COD/m3/day, 8 to 12 h | Max. MPR avg.: ca 2.9 L/d (HRT: 8, SRT: infinitive), Max. MY average: 0.29 L/gCOD (HRT: 8, SRT: infinitive), Max. specific MY: 0.068 L/MLVSS/d (HRT: 12, SRT: infinitive) | [143] |
| TPAD | Biogas generation and performance analysis | 30 L (meso) & 20 L (thermo), CM, 38 °C (meso) and 58 °C (thermo), 7.00 to 7.75, 2 to 8 gVS/L/d, 14 days | MY: 0.21 to 0.22 L/gVS fed (thermo); 0.15 L/gVS (meso) | [145] |
| TR | Biogas generation | 18 L (4 units), Fruit and vegetable waste, 35 ± 1 °C, 6.8 to 7.6, 2 to 8 gVS/L/d, 12 to 20 days | Max. biogas production rate: 2.62 L/L/d, Max. biogas yield: 707 L/kgVS fed, Max. MC: 65% | [146] |
| USMAR | Methane production | 85 L (3 equal cylinders), Synthetic waste & dry milk, 35 °C, 4.5 to 7.2, 1 to 12 gCOD/L/d, 0.5 to 2 days | MY: 0.1 to 0.2 L/gVS fed with 5 to 13% increase in MC as a result of OLR at 10 gCOD/L/d | [147] |
| UASB | Hydrogen and methane production | 24 L, Cassava waste water, 37 °C, 5.5 (with control), 10 to 30 kg/m3-d (biohydrogen reactor) & 2 to 10 kg/m3-d (methane reactor, Short (N/G) | Max. HPR: 0.39 L/L-d (at OLR: 25 kg/m3-d), Max. HY: 39.83 L/kgCOD fed (at OLR: 25 kg/m3-d), Max. MPR: 0.91 L/L-d (at OLR: 8 kg/m3-d), Max. MY: 115.23 L/kgCOD (at OLR: 8 kg/m3-d) | [148] |
| OLR | CH4 Yield | Aim | Feedstock | Reference |
|---|---|---|---|---|
| 0.4 to 3.1 kg COD/m3 | Maximum (0.46 LCH4/gCODremoved) at OLR of 2.5 kg COD/m3 | Co-digestion | Rice straw and pig manure | [257] |
| 1 to 4 gVS/L/d for methane reactor; 3 to 12 gVS/L/d for H2 reactor | Methane production maximized at OLR of 2 gVS/L/d and thereafter decreased. H2 production maximized at OLR of 6 gVS/L/d | Co-production of H2 and CH4 | Macro-algae Laminaria digitata and micro-algae Arthrospira plantensis | [258] |
| 30, 60 and 90 gVS/L | Methane yield for all the co-digestion types maximized at OLR of 30 gvs/l and 60 gVS/L | Co-digestion performance | Sweet potato vine and animal manure | [259] |
| 2.5 to 27.7 gVS/L | Ammonia inhibition at OLR > 20 gVS/L | Ammonia inhibition | Tannery fleshing, municipal solid waste, chrome shaving and others | [260] |
| 0.4 to 0.7 gCOD/L/d | Methane yield decreased with increased OLR | Pilot scale two stage AD | Slaughter house waste | [261] |
| 1.5 to 4.3 g/L/d | Maximum methane yield at OLR of 3.5 g/L/d | Methane production by ammonium tolerant microorganisms | Protein rich fish silage | [262] |
| 1, 2 & 3 gVS/L/d | 70% and 73% reduction of SMY and SCOD for OLR increment from 1 to 3 gVS/L/d | Semi-continuous AD at different psychrophilic range | Food waste | [263] |
| Various | Specific gas production (0.88 m3biogas/kgvs)at two stage reactor was found higher than that of (0.75 m3 biogas/kgvs)single stage reactor for an optimum OLR of 3.5 kgvs/m3/d | Comparison between single and two stage reactor performance | Food waste | [264] |
| 2.4 and 3.6 gCOD/d | Higher OLR led to reactor’s acidification problem and hence affected methane yield | Performance and metagenomics analyses of single and two stage thermophilic anaerobic digestion | Cheese wastes | [265] |
| 4.6 and 8.6 kgCOD/m3/d | The maximum methane productivity peaked to 2.78 L/L/d at OLR of 8.6 kgCOD/m3/d, but the system was unstable | Effect of feeding with or without dilution | Food waste | [266] |
| 2.0 to 6.0 gVS/L/d | Methane yield decreased as OLR increased for both two-stage and co-digestion reactors | Comparison between two-stage and co.digestion AD | Food waste and horticulture waste | [267] |
| 1.53 to 5.04 gVS/L | 0.44 LCH4/gVS at OLR of 5.04 gVS/L | Determination of kinetics constant | Co-digestion of cattle manure and municipal food waste | [268] |
| Reactor ASBR: 0.93–25.0 gCOD/L.d Reactor AMBR: 1.04–19.65 gCOD/L.d | Maximum biogas yield at OLR of 10.08 gCOD/L.d, Biogas production decreased for OLR > 18.52 gCOD/L.d | Effect of OLR and series reactor AD | Composting leachate | [269] |
| 0.5, 1.5 and 2.0 VS/Lsludge/d | H2 uptake by homoacetogens increased at higher OLR resulting acetate accumulation | Acetate concentration during in situ methane upgradation | Sludge and H2; fluromethane as inhibitor | [270] |
| 1.12 to 3.88 kgCOD/m3/d | Methane yield continued to increase up to OLR of 2 kgCOD/m3/d. Methane production inhibited at OLR > 3.8 kgCOD/m3/d | Co-digestion | Beverage waste and sewage sludge | [271] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. https://doi.org/10.3390/app9091915
Sarker S, Lamb JJ, Hjelme DR, Lien KM. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Applied Sciences. 2019; 9(9):1915. https://doi.org/10.3390/app9091915
Chicago/Turabian StyleSarker, Shiplu, Jacob J. Lamb, Dag R. Hjelme, and Kristian M. Lien. 2019. "A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants" Applied Sciences 9, no. 9: 1915. https://doi.org/10.3390/app9091915
APA StyleSarker, S., Lamb, J. J., Hjelme, D. R., & Lien, K. M. (2019). A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Applied Sciences, 9(9), 1915. https://doi.org/10.3390/app9091915








