Degradation of UV Filter Ethyl 4-Aminobenzoate (Et-PABA) Using a UV-Activated Persulfate Oxidation Process
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.3. Analysis Methods
3. Results and Discussion
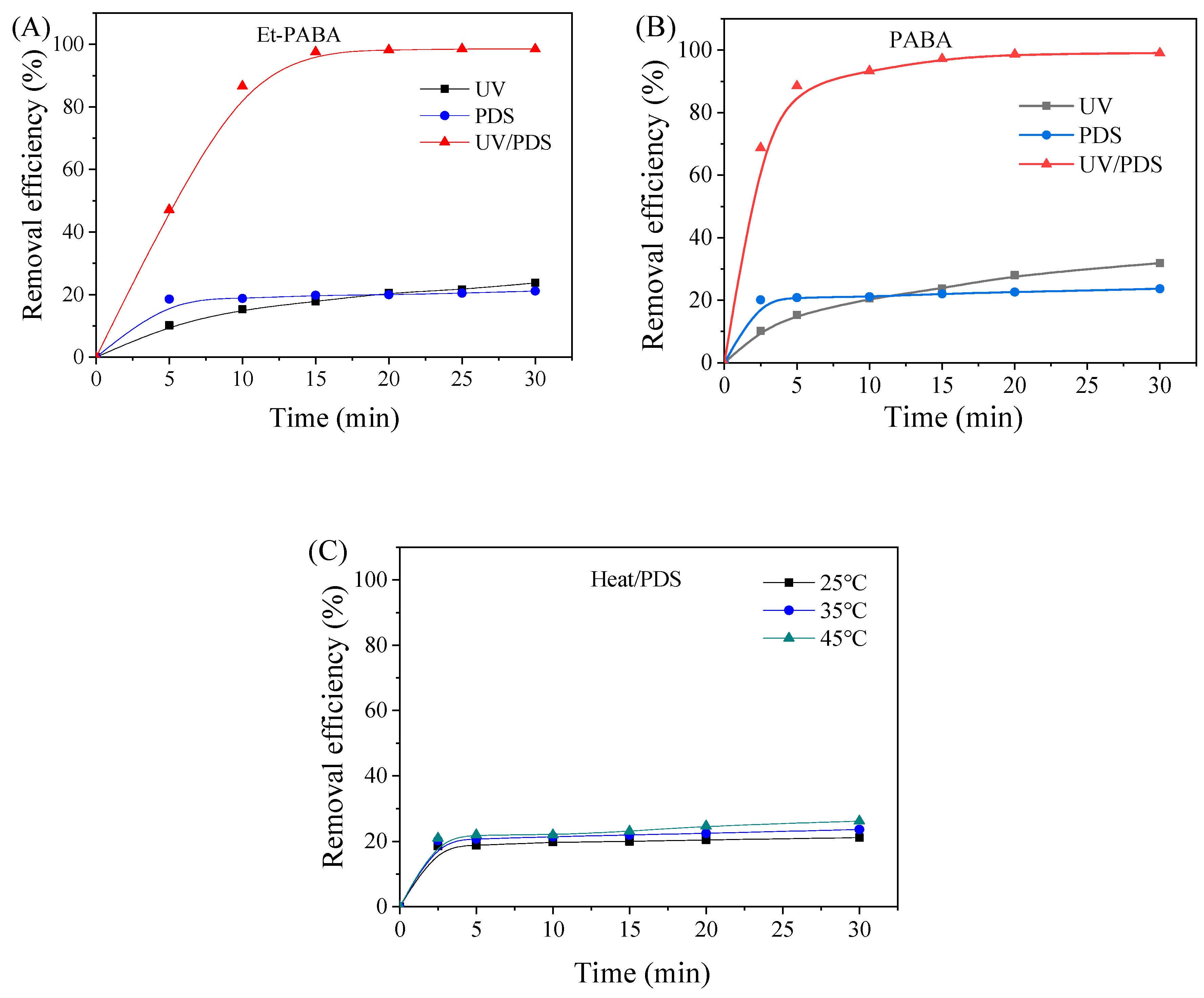
3.1. Degradation of the Et-PABA Using Different Oxidation Processes
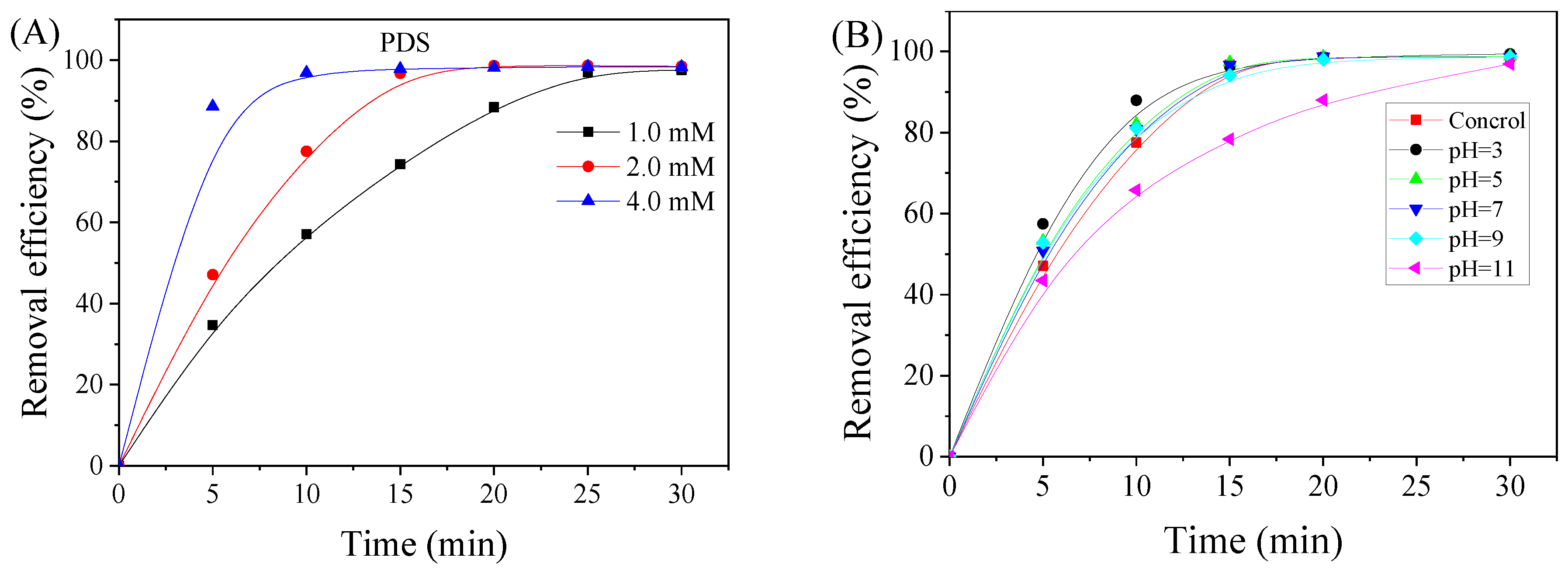
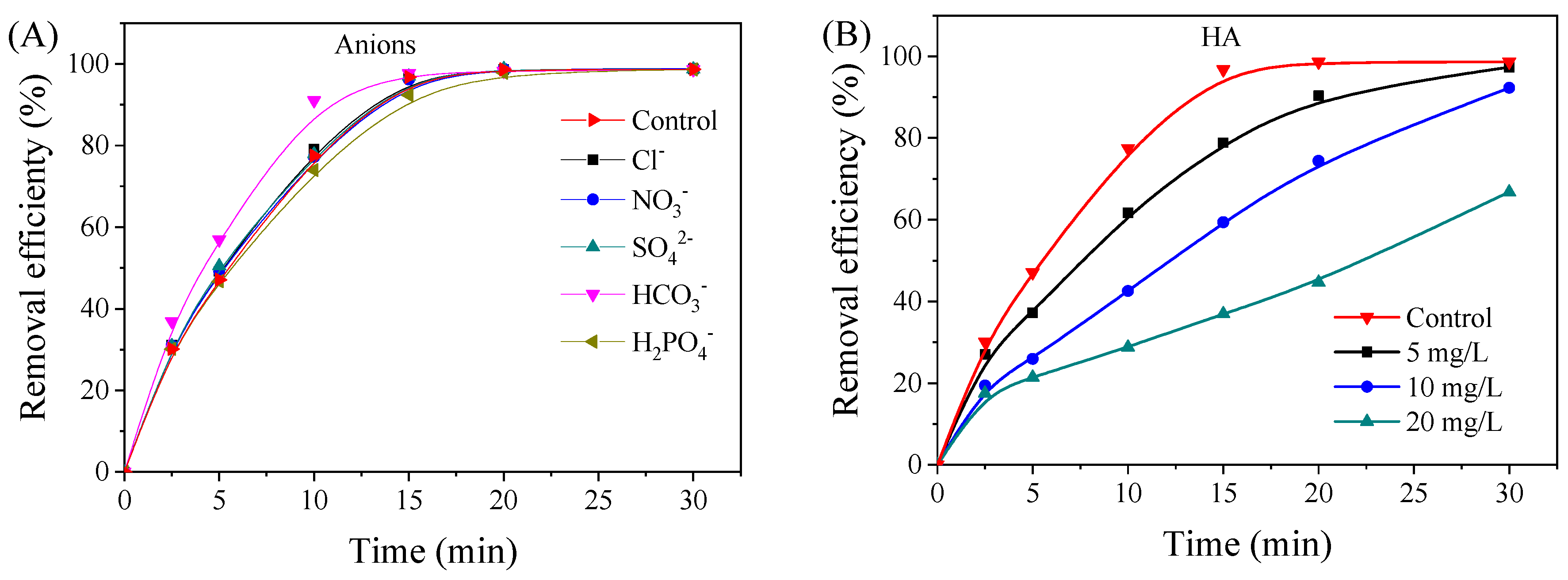
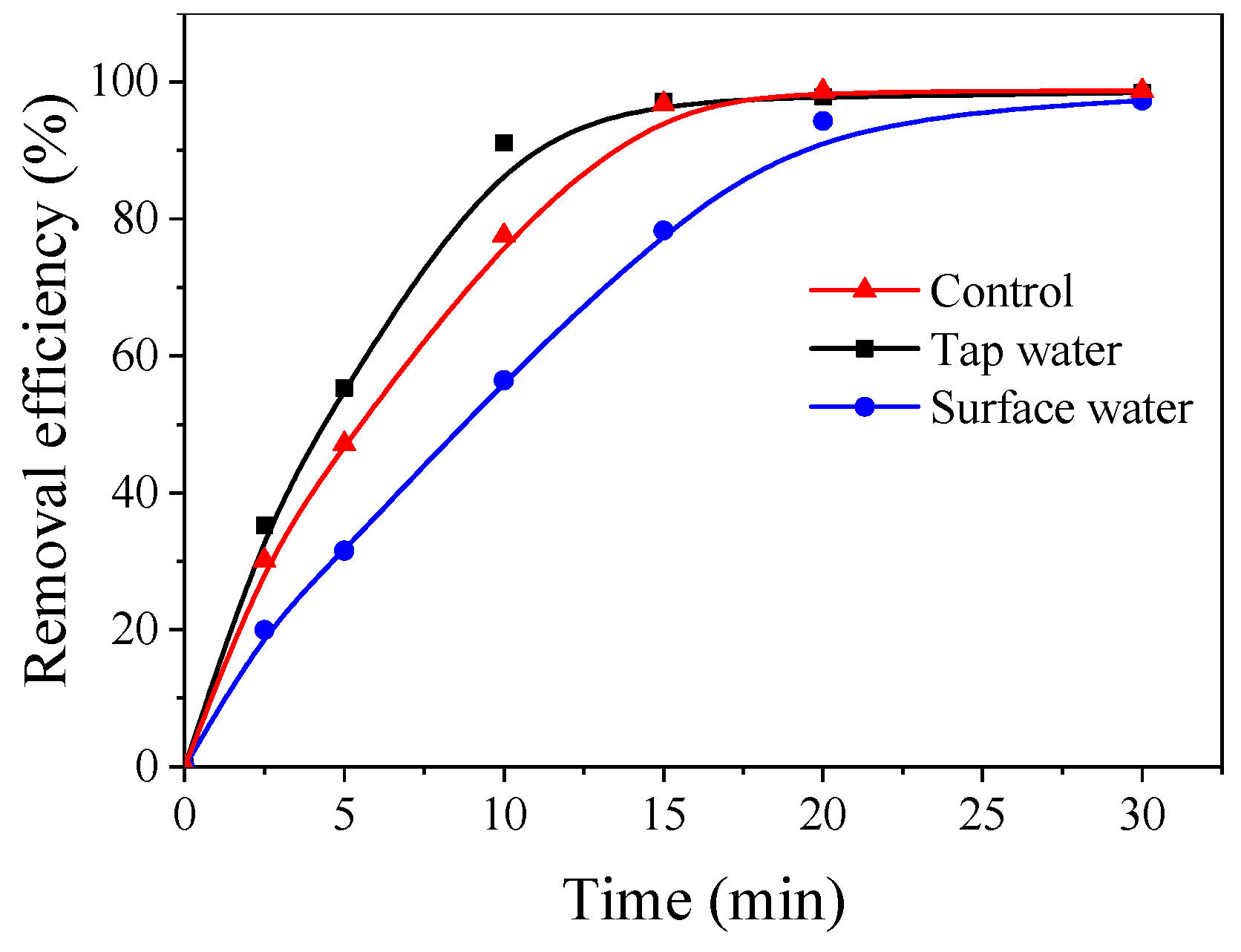
3.2. Effects of Various Factors on the Removal of Et-PABA
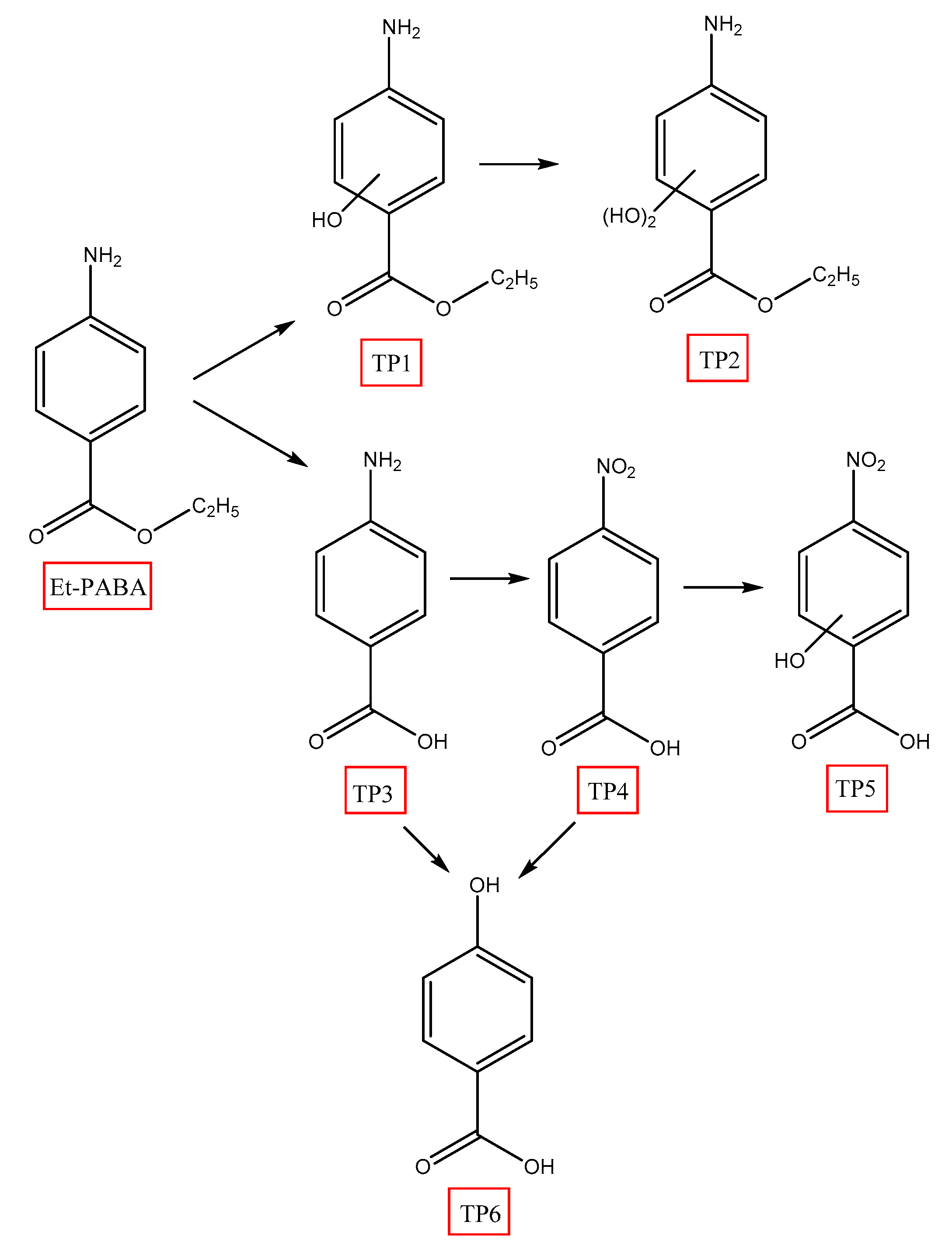
3.3. Degradation Pathways
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Vries, E.; Arnold, M.; Altsitsiadis, E.; Trakatelli, M.; Hinrichs, B.; Stockfleth, E.; Coebergh, J. Potential impact of interventions resulting in reduced exposure to ultraviolet (UV) radiation (UVA and UVB) on skin cancer incidence in four European countries, 2010–2050. Brit. J. Dermatol. 2012, 167, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Kannan, K. Widespread occurrence of benzophenone-type UV light filters in personal care products from China and the United States: An assessment of human exposure. Environ. Sci. Technol. 2014, 48, 4103–4109. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Klit, A.; Jensen, M.B.; Soeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N.E.; Drzewiecki, K.T. Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012, 35, 424–436. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Hain, E.; Timm, A.; Tarnowski, M.; Blaney, L. Occurrence of antibiotics, estrogenic hormones, and UV-filters in water, sediment, and oyster tissue from the Chesapeake Bay. Sci. Total Environ. 2019, 650, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Mitchelmore, C.L.; He, K.; Gonsior, M.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Molins, D.D.; Munoz, R.; Nogueira, S.; Alonso, M.B.; Torres, J.P.; Malm, O.; Ziolli, R.L.; Hauser-Davis, R.A.; Eljarrat, E.; Barcelo, D.; et al. Occurrence of organic UV filters and metabolites in lebranche mullet (Mugil liza) from Brazil. Sci. Total Environ. 2018, 618, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.; Joerss, H.; Ebinghaus, R. Environmental occurrence and hazard of organic UV stabilizers and UV filters in the sediment of European North and Baltic Seas. Chemosphere 2018, 212, 254–261. [Google Scholar] [CrossRef]
- Ao, J.; Yuan, T.; Gu, J.; Ma, Y.; Shen, Z.; Tian, Y.; Shi, R.; Zhou, W.; Zhang, J. Organic UV filters in indoor dust and human urine: A study of characteristics, sources, associations and human exposure. Sci. Total Environ. 2018, 640, 1157–1164. [Google Scholar] [CrossRef]
- Liu, H.; Sun, P.; Liu, H.; Yang, S.; Wang, L.; Wang, Z. Acute toxicity of benzophenone-type UV filters for Photobacterium phosphoreum and Daphnia magna: QSAR analysis, interspecies relationship and integrated assessment. Chemosphere 2015, 135, 182–188. [Google Scholar] [CrossRef]
- Liu, H.; Sun, P.; Liu, H.; Yang, S.; Wang, L.; Wang, Z. Hepatic oxidative stress biomarker responses in freshwater fish Carassius auratus exposed to four commonly benzophenone UV filters. Ecotoxicol. Environ. Saf. 2015, 119, 116–122. [Google Scholar] [CrossRef]
- Li, A.; Law, J.; Chow, C.; Huang, Y.; Li, K.; Leung, K. Joint effects of multiple UV filters on zebrafish embryo development. Environ. Sci. Technol. 2018, 52, 9460–9467. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wei, D.; Liu, W.; Geng, J.; Liu, J.; Du, Y. Formation of novel disinfection by-products chlorinated benzoquinone, phenyl benzoquinones and polycyclic aromatic hydrocarbons during chlorination treatment on UV filter 2,4-dihydroxybenzophenone in swimming pool water. J. Hazard. Mater. 2019, 367, 725–733. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, E.; O’Brien, J.W.; Tscharke, B.; Thomas, K.V.; Mueller, J.F. Per capita loads of organic UV filters in Australian wastewater influent. Sci. Total Environ. 2019, 662, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, P.; He, Q.; Feng, M.; Liu, H.; Yang, S.; Wang, L.; Wang, Z. Ozonation of the UV filter benzophenone-4 in aquatic environments: Intermediates and pathways. Chemosphere 2016, 149, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Binni, M.; Lu, G.; Liu, J.; Yan, Z.; Yang, H.; Pan, T. Bioconcentration and multi-biomarkers of organic UV filters (BM-DBM and OD-PABA) in crucian carp. Ecotoxicol. Environ. Saf. 2017, 141, 178–187. [Google Scholar]
- Suh, H.S.; Lim, S.K.; Kim, M.K.; Kim, M.H.; Baek, S.H. Risk assessment of ethylhexyl dimethyl PABA in sunscreen cosmetic products. Toxicol. Lett. 2017, 280, 105–106. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Liu, H.; Bruton, T.A.; Doyle, F.M.; Sedlak, D.L. In situ chemical oxidation of contaminated groundwater by persulfate: Decomposition by Fe (III)-and Mn (IV)-containing oxides and aquifer materials. Environ. Sci. Technol. 2014, 48, 10330–10336. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, P.; Chen, Y.; Ou, H.; Liu, J.; Li, C.; Li, Q. Degradation of 1H-benzotriazole using ultraviolet activating persulfate: Mechanisms, products and toxicological analysis. Chem. Eng. J. 2018, 334, 1493–1501. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef]
- Xu, H.; Wang, D.; Ma, J.; Zhang, T.; Lu, X.; Chen, Z. A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S. Appl. Catal. B Environ. 2018, 238, 557–567. [Google Scholar] [CrossRef]
- Alexopoulou, C.; Petala, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Kondarides, D.I.; Mantzavinos, D. Copper phosphide and persulfate salt: A novel catalytic system for the degradation of aqueous phase micro-contaminants. Appl. Catal. B Environ. 2019, 244, 178–187. [Google Scholar] [CrossRef]
- Deng, J.; Ya, C.; Ge, Y.; Cheng, Y.; Chen, Y.; Xu, M.; Wang, H. Activation of peroxymonosulfate by metal (Fe, Mn, Cu and Ni) doping ordered mesoporous Co3O4 for the degradation of enrofloxacin. RSC Adv. 2018, 8, 2338–2349. [Google Scholar] [CrossRef]
- Li, R.; Cai, M.; Xie, Z.; Zhang, Q.; Zeng, Y.; Liu, H.; Liu, G.; Lv, W. Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B Environ. 2019, 244, 974–982. [Google Scholar] [CrossRef]
- Duan, X.; Su, C.; Miao, J.; Zhong, Y.; Shao, Z.; Wang, S.; Sun, H. Insights into perovskite-catalyzed peroxymonosulfate activation: Maneuverable cobalt sites for promoted evolution of sulfate radicals. Appl. Catal. B Environ. 2018, 220, 626–634. [Google Scholar] [CrossRef]
- Ahn, Y.Y.; Bae, H.; Kim, H.I.; Kim, S.H.; Kim, J.H.; Lee, S.G.; Lee, J. Surface-loaded metal nanoparticles for peroxymonosulfate activation: Efficiency and mechanism reconnaissance. Appl. Catal. B Environ. 2019, 241, 561–569. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Zhu, C.; Dionysiou, D.D.; Zhao, G.; Fang, G.; Zhou, D. New insight into the mechanism of peroxymonosulfate activation by sulfur-containing minerals: Role of sulfur conversion in sulfate radical generation. Water Res. 2018, 142, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Shi, D.; Wu, Z.; Zhang, L.; Zhai, Z.; Fang, Y.; Sun, P.; Han, R.; Wu, J.; Liu, H. CoMn2O4 catalyst prepared using the sol-gel method for the activation of peroxymonosulfate and degradation of UV filter 2-phenylbenzimidazole-5-sulfonic acid (PBSA). Nanomaterials 2019, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, P.; Feng, M.; Liu, H.; Yang, S.; Wang, L.; Wang, Z. Nitrogen and sulfur co-doped CNT-COOH as an efficient metal-free catalyst for the degradation of UV filter BP-4 based on sulfate radicals. Appl. Catal. B Environ. 2016, 187, 1–10. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Zhai, Z.; Zhang, X.; Fang, Y.; Tan, J.; Wu, J. Degradation of UV filter BP-1 with nitrogen-doped industrial graphene as a metal-free catalyst of peroxymonosulfate activation. Chem. Eng. J. 2019, 356, 262–271. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.; Guo, L.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Khan, M.A.; Paeng, K.-J.; Kurade, M.B.; Kim, S.-J.; Jeon, B.-H. Aqueous phase degradation of methyl paraben using UV-activated persulfate method. Chem. Eng. J. 2017, 321, 11–19. [Google Scholar] [CrossRef]
- Verma, S.; Nakamura, S.; Sillanpȁȁ, M. Application of UV-C LED activated PMS for the degradation of anatoxin-a. Chem. Eng. J. 2016, 284, 122–129. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Rios Quintero, R.; Acevedo-Merino, A.; Nebot, E. Disinfection performance using a UV/persulfate system: Effects derived from different aqueous matrices. Photochem. Photobiol. Sci. 2019, 18, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Dong, W.; Wang, X.; Bi, W.; Zhai, P.; Li, H.; Nie, M. Degradation of sunscreen agent p-aminobenzoic acid using a combination system of UV irradiation, persulphate and iron (II). Environ. Sci. Pollut. Res. 2016, 23, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- Tsoumachidou, S.; Velegraki, T.; Poulios, I. TiO2 photocatalytic degradation of UV filter para-aminobenzoic acid under artificial and solar illumination. J. Chem. Technol. Biotechnol. 2016, 91, 1773–1781. [Google Scholar] [CrossRef]
- Soto-Vazquez, L.; Cotto, M.; Duconge, J.; Morant, C.; Marquez, F. Synthesis and photocatalytic activity of TiO2 nanowires in the degradation of p-aminobenzoic acid: A comparative study with a commercial catalyst. J. Environ. Manag. 2016, 167, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ji, Y.; Zeng, C.; Zhang, Y.; Wang, Z.; Yang, X. Aquatic photodegradation of sunscreen agent p-aminobenzoic acid in the presence of dissolved organic matter. Water Res. 2013, 47, 153–162. [Google Scholar] [CrossRef]
- Mao, L.; Meng, C.; Zeng, C.; Ji, Y.; Yang, X.; Gao, S. The effect of nitrate, bicarbonate and natural organic matter on the degradation of sunscreen agent p-aminobenzoic acid by simulated solar irradiation. Sci. Total Environ. 2011, 409, 5376–5381. [Google Scholar] [CrossRef]
- Li, A.J.; Sang, Z.Y.; Chow, C.H.; Law, J.C.F.; Guo, Y.; Leung, K.S.Y. Environmental behavior of 12 UV filters and photocatalytic profile of ethyl-4-aminobenzoate. J. Hazard. Mater. 2017, 337, 115–125. [Google Scholar] [CrossRef]
- Mc Kay, G.; Dong, M.; Kleinman, J.L.; Mezyk, S.P.; Rosario-Ortiz, F.L. Temperature dependence of the reaction between the hydroxyl radical and organic matter. Environ. Sci. Technol. 2011, 45, 6932–6937. [Google Scholar] [CrossRef] [PubMed]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for advanced oxidation processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, J.M.; Duran, A.; Gonzalez, R.; Exposito, A.J. In situ chemicaloxidation of carbamazepine solutions using persulfate simultaneously activated by heat energy, UV light, Fe2+ions, and H2O2. Appl. Catal. B Environ. 2015, 176, 120–129. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J.; Zhou, Q. Heat-activated persulfate oxidation of atrazine: Implications for remediation of groundwater contaminated by herbicides. Chem. Eng. J. 2015, 263, 45–54. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, P.; Boyer, T.H.; Zhao, L.; Huang, C. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS. Environ. Sci. Technol. 2015, 49, 3056–3066. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Kinetics and mechanism investigation on the destruction of oxytetracycline by UV-254 nm activation of persulfate. J. Hazard. Mater. 2016, 305, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Santos, A.; Vicente, F.; Gonzalez, C. Diuron abatement using activated persulphate: Effect of pH, Fe (II) and oxidant dosage. Chem. Eng. J. 2010, 162, 257–265. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.; Bruell, C.J. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 2007, 66, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.C.; Gmurek, M.; Rossi, A.F.; Corceiro, V.; Costa, R.; Quinta-Ferreira, M.E.; Ledakowicz, S.; Quinta-Ferreira, R.M. Application of Fenton oxidation to reduce the toxicity of mixed parabens. Water Sci. Technol. 2016, 74, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Gao, N.; Deng, Y.; Zhang, Y.; Sui, M.; Deng, J.; Zhou, S. Degradation of antipyrine by UV, UV/H2O2 and UV/PS. J. Hazard. Mater. 2013, 260, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; He, X.; Khan, H.M.; Shah, N.S.; Dionysiou, D.D. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82−/Fe2+ and UV/HSO5/Fe2+ processes: A comparative study. Chem. Eng. J. 2013, 218, 376–383. [Google Scholar] [CrossRef]
- Nie, M.; Yang, Y.; Zhang, Z.; Yan, C.; Wang, X.; Li, H.; Dong, W. Degradation of chloramphenicol by thermally activated persulfate in aqueous solution. Chem. Eng. J. 2014, 246, 373–382. [Google Scholar] [CrossRef]
- Luo, C.; Ma, J.; Jiang, J.; Liu, Y.; Song, Y.; Yang, Y.; Guan, Y.; Wu, D. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2, UV/HSO5− and UV/S2O82−. Water Res. 2015, 80, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, N.; Yang, J.; Wang, C.; Gu, Z.; Jiang, C. Study on the characteristics of 2,4-dichlorophenol in water degraded by UV/PS. Chin. Environ. Sci. 2017, 37, 2145–2149. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, R.; Wu, Q.; Lin, C.; Zhang, L.; Zhai, Z.; Sun, P.; Fang, Y.; Wu, J.; Liu, H. Degradation of UV Filter Ethyl 4-Aminobenzoate (Et-PABA) Using a UV-Activated Persulfate Oxidation Process. Appl. Sci. 2019, 9, 2873. https://doi.org/10.3390/app9142873
Han R, Wu Q, Lin C, Zhang L, Zhai Z, Sun P, Fang Y, Wu J, Liu H. Degradation of UV Filter Ethyl 4-Aminobenzoate (Et-PABA) Using a UV-Activated Persulfate Oxidation Process. Applied Sciences. 2019; 9(14):2873. https://doi.org/10.3390/app9142873
Chicago/Turabian StyleHan, Ruirui, Qiang Wu, Chihao Lin, Lingfeng Zhang, Zhicai Zhai, Ping Sun, Yingsen Fang, Jiaqiang Wu, and Hui Liu. 2019. "Degradation of UV Filter Ethyl 4-Aminobenzoate (Et-PABA) Using a UV-Activated Persulfate Oxidation Process" Applied Sciences 9, no. 14: 2873. https://doi.org/10.3390/app9142873
APA StyleHan, R., Wu, Q., Lin, C., Zhang, L., Zhai, Z., Sun, P., Fang, Y., Wu, J., & Liu, H. (2019). Degradation of UV Filter Ethyl 4-Aminobenzoate (Et-PABA) Using a UV-Activated Persulfate Oxidation Process. Applied Sciences, 9(14), 2873. https://doi.org/10.3390/app9142873