Freeze–Thaw Pre-Treatment of Cassava Tubers to Improve Efficiency of Mechanical Peeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Characterization of Cassava Tubers
2.3. Experimental Procedure
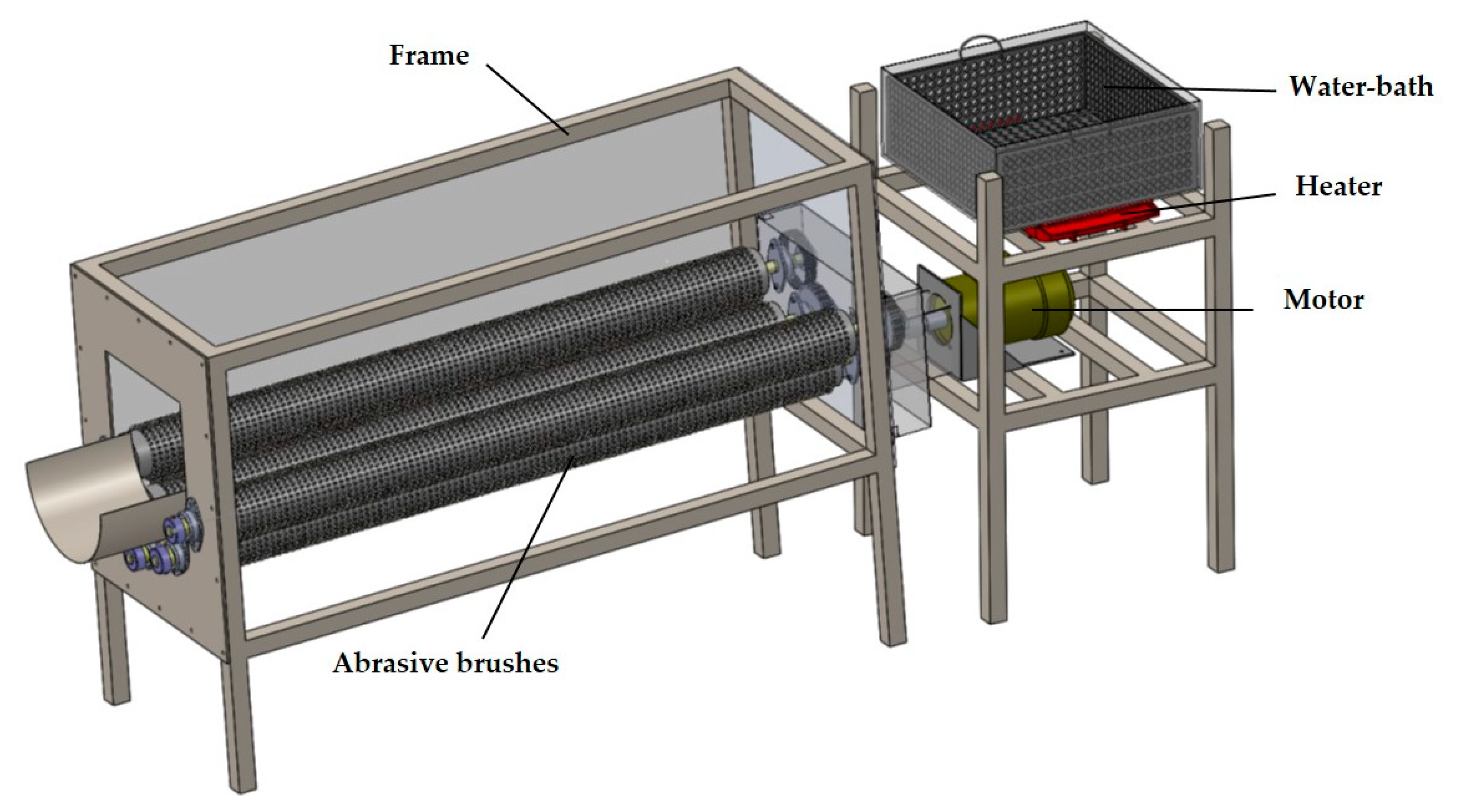
2.3.1. Description of the Prototype Cassava Peeling Machine
2.3.2. The Freeze–Thaw Pre-Treatment (FTP)
2.3.3. Peeling Process
2.4. Experimental Design
2.5. Starch Content Analysis
2.6. Scanning Electron Microscopy of Freeze–Thaw Treated Cassava Tubers
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Cassava Tubers
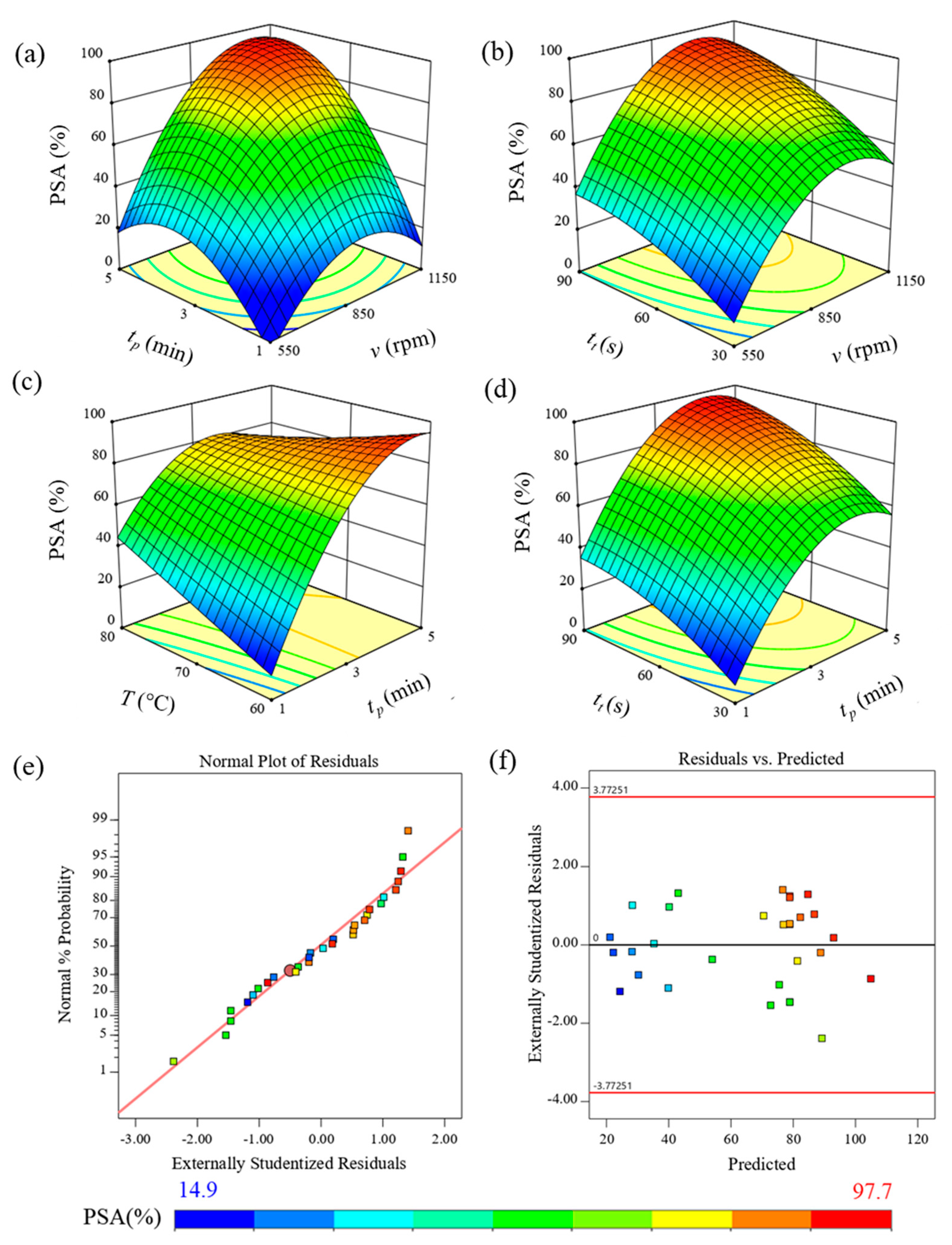
3.2. Effect of the Rotational Speed of the Brushes, the Peeling Time, and FTP on the Peeling Process for Cassava Tubers
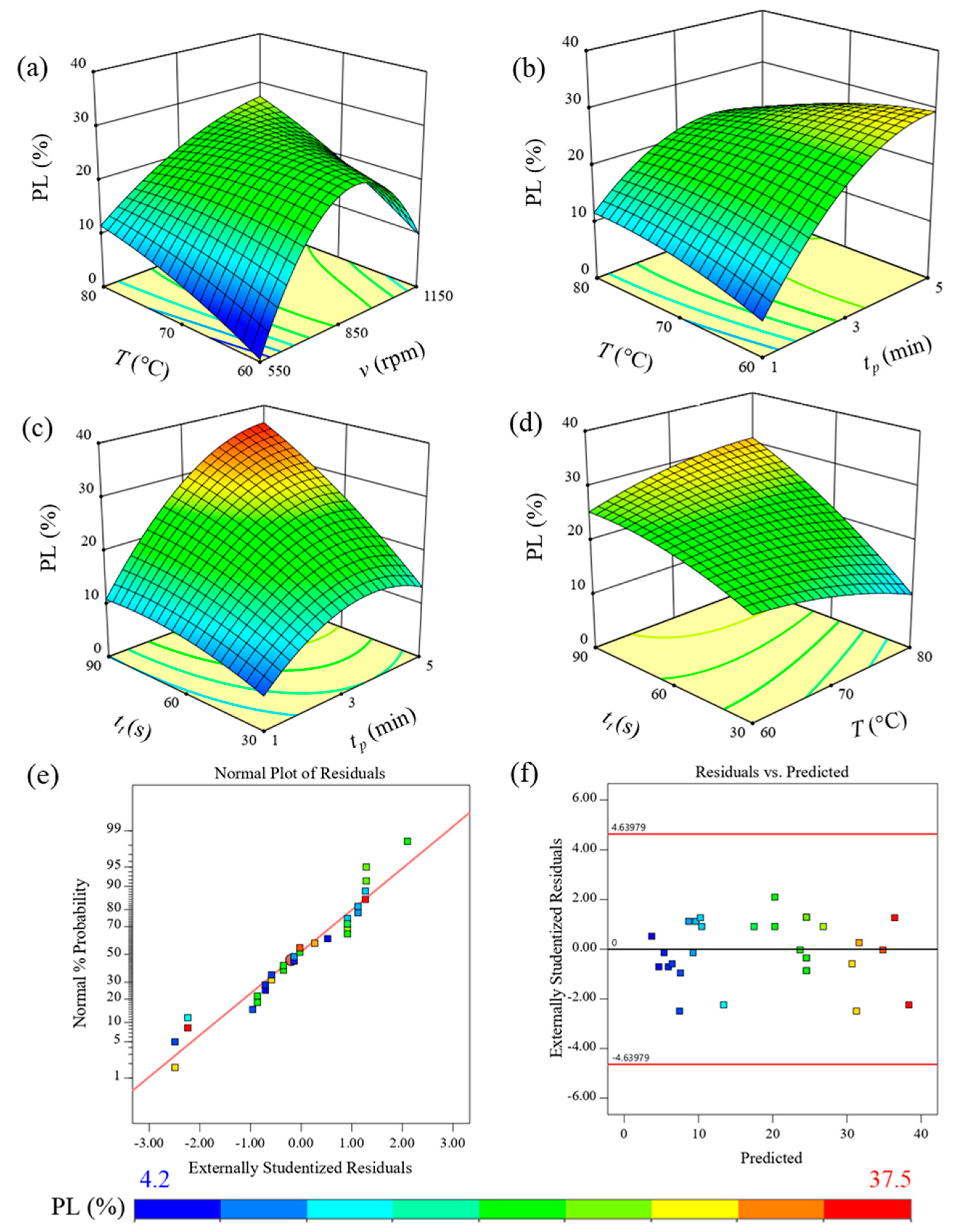
3.3. Effect of FTP on the Quality of Cassava Tubers
3.3.1. Starch Content of Peeled Cassava Tubers
3.3.2. Scanning Electron Microscopy of Peeled Cassava Tubers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Latif, S.; Müller, J. Potential of cassava leaves in human nutrition: A review. Trends Food Sci. Technol. 2015, 44, 147–158. [Google Scholar] [CrossRef]
- Alamu, E.O.; Ntawuruhunga, P.; Chibwe, T.; Mukuka, I.; Chiona, M. Evaluation of cassava processing and utilization at household level in Zambia. Food Sec. 2019, 11, 141–150. [Google Scholar] [CrossRef]
- Kolawole, P.O.; Agbetoye, L.; Ogunlowo, S.A. Sustaining World Food Security with Improved Cassava Processing Technology: The Nigeria Experience. Sustainability 2010, 2, 3681–3694. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Kumar, T.J. Roots and Tuber Crops as Functional Foods: A Review on Phytochemical Constituents and Their Potential Health Benefits. Int. J. Food Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Reilly, K.; Gomez-Vasquez, R.; Buschman, H.; Tohme, J.; Beeching, J.R. Oxidative stress responses during cassava post-harvest physiological deterioration. Plant Mol. Biol. 2003, 53, 669–685. [Google Scholar] [CrossRef]
- Egbeocha, C.C.; Asoegwu, S.N.; Okereke, N.A. A review on performance of cassava peeling machines in Nigeria. FUTO J. 2016, 2, 140–168. [Google Scholar]
- Adetan, D.A.; Adekoya, L.O.; Aluko, O.B. Theory of mechanical method of peeling cassava tubers with knives. Int. Agrophys. 2006, 20, 269–276. [Google Scholar]
- Olukunle, O.J.; Ogunlowo, A.S.; Sanni, L. The search for effective cassava peeler. W. Indian J. Eng. 2010, 32, 42–47. [Google Scholar]
- Oriola, K.O.; Raji, A.O. Trends at mechanizing cassava postharvest processing operations. IJET 2013, 3, 879–887. [Google Scholar]
- Ohwovoriole, E.N.; Oboli, S.; Mgbeke, A.C. Studies and preliminary design for a cassava tuber peeling machine. Trans. ASAE 1988, 31, 380–385. [Google Scholar] [CrossRef]
- Adetan, D.A.; Adekoya, L.O.; Aluko, O.B. Characterization of some properties of cassava root tubers. J. Food Eng. 2003, 59, 349–353. [Google Scholar] [CrossRef]
- Agbetoye, L.A.S. Improving the technology of cassava harvesting and processing mechanization for food security in Nigeria. In Proceedings of the International Conference on Science and Technology, Akure, Nigeria, 14–19 August 2005; pp. 196–204. [Google Scholar]
- Ezekwe, G.O. A feature for achieving a constant depth of peel in the mechanical peeling of cassava. Nigeria J. Eng. 1976, 1, 174–181. [Google Scholar]
- Kamal, A.R.; Oyelade, O.A. Present status of cassava peeling in Nigeria. JAET 2010, 18, 7–13. [Google Scholar]
- Olukunle, O.J.; Oguntunde, P.G. Analysis of peeling pattern in an automated cassava peeling system. NJTD 2008, 6, 41–52. [Google Scholar]
- Brown, H.E.; Meredith, F.I.; Saldama, G.; Stephens, T.S. Freezing peeling improves quality of tomatoes. J. Food Sci. 1970, 35, 485–488. [Google Scholar] [CrossRef]
- Thomas, W.M.; Stanley, D.W.; Arnott, D.R. An evaluation of blanch, lye and freeze-heat methods for tomato peel removal. J. Inst. Can. Science Technol. 1976, 9, 118. [Google Scholar] [CrossRef]
- Leonard, S.; Winter, F. Pilot application of freeze-heat peeling of tomatoes. J. Food Sci. 1974, 29, 162. [Google Scholar] [CrossRef]
- DIN CEN/TS 14774-3. Determination of moisture content—Oven dry method. In Part 3: Moisture in General Analysis Sample; Deutsches Institut für Normung e.V.: Berlin, Germany, 2004. [Google Scholar]
- Oluwole, O.O.; Adio, M.A. Design and construction of a batch cassava peeling machine. JMAE 2013, 3, 16–21. [Google Scholar]
- Srikaeo, K.; Khamphu, S.; Weerakul, K. Peeling of gingers as evaluated by image analysis techniques: A study for pickled ginger process. Int. Food Res. J. 2011, 18, 1387–1392. [Google Scholar]
- Association of Official Agricultural Chemists. AOAC Official Method 996.11. Starch (total) in Cereal Products, amyloglucosidase-a-amylase method. J. AOAC Int. 1997, 80, 571. [Google Scholar]
- Ayetigbo, O.; Latif, S.; Abbas, A.; Müller, J. Osmotic dehydration kinetics of biofortified yellow-flesh cassava in contrast to white-flesh cassava (Manihot esculenta). J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Ademosun, O.C.; Jimoh, M.O.; Olukunle, O.J. Effect of physical and mechanical properties of cassava tubers on the performance of an automated peeling machine. IJDS 2012, 1, 810–822. [Google Scholar]
- Jimoh, M.O.; Olukunle, O.J.; Manuwa, S.I. Modeling of cassava peeling performance using dimensional analysis. Agric. Eng. Int. CIGR J. 2016, 18, 360–367. [Google Scholar]
- Jimoh, M.O.; Olukunle, O.J. An Automated Cassava Peeling System for the Enhancement of Food Security in Nigeria. NIFOJ 2012, 30, 73–79. [Google Scholar] [CrossRef]
- Olukunle, O.J.; Akinnuli, B.O. Theory of an automated cassava peeling system. IJEIT 2013, 2, 2277–3754. [Google Scholar]
- Sajeev, M.S.; Sreekumar, J.; Unnikrishnan, J.; Moorthy, M.S.N.; Shanavas, S. Kinetics of thermal softening of cassava tubers and rheological modeling of the starch. J. Food Sci. Technol. 2009, 47, 507–518. [Google Scholar] [CrossRef]
- Rahman, M.S. Food preservation by freezing. In Handbook of Food Preservation, 2nd ed.; Rahman, M.S., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 259–284. [Google Scholar]
- Lee, M.H.; Baek, M.H.; Cha, D.S.; Park, H.J.; Lim, S.T. Freeze‚ thaw stabilization of sweet potato starch gel by polysaccharide gums. Food Hydrocoll. 2002, 16, 345–352. [Google Scholar] [CrossRef]
- Muadklay, J.; Charoenrein, S. Effects of hydrocolloids and freezing rates on freeze-thaw stability of tapioca starch gels. Food Hydrocoll. 2008, 22, 1268–1272. [Google Scholar] [CrossRef]
- Guraya, H.S.; James, C.; Champagne, E.T. Effect of cooling, and freezing on the digestibility of debranched rice starch and physical properties of the resulting material. Starch 2001, 53, 64–74. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Ajayi, O.I.; Gbadebo, C.T.; Kayode, R.M.O.; Karim, O.R.; Adeloye, A.A. Physicochemical properties of gari prepared from frozen cassava roots. LWT Food Sci. Technol. 2019, 99, 594–599. [Google Scholar] [CrossRef]
- Christianson, D.D. Hydrocolloid interactions with starches. In Food Carbohydrates; Lineback, D.R., Inglett, G.E., Eds.; AVI Publishing Company, Inc.: Westport, CT, USA, 1982; pp. 399–419. [Google Scholar]
- Ferrero, C.; Martino, M.N.; Zaritzky, N.E. Stability of frozen starch pastes: Effect of freezing, storage and xanthan gum addition. J. Food Process. Preserv. 1993, 17, 191–211. [Google Scholar] [CrossRef]

| Variables | Unit | Coded Factors | Levels | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |||
| Rotational speed of brushes | rpm | X1 | 550 | 700 | 850 | 1000 | 1150 |
| Peeling time | min | X2 | 1 | 2 | 3 | 4 | 5 |
| Thawing temperature | °C | X3 | 50 | 60 | 70 | 80 | 90 |
| Incubation time | s | X4 | 0 | 30 | 60 | 90 | 120 |
| Parameters | Mean ± SD |
|---|---|
| Mass of tuber (g) | 733.4 ± 254.7 |
| Length of tuber (mm) | 240 ± 40.0 |
| Diameter of tuber (mm) | 66.7 ± 9.9 |
| Peel thickness (mm) | 2.9 ± 0.2 |
| Penetration force, flesh (N/mm2) | 3.3 ± 0.7 |
| Penetration force, peel (N/mm2) | 5.0 ± 0.4 |
| Dry matter, flesh (%) | 32.9 ± 4.1 |
| Dry matter, peel (%) | 43.3 ± 19.4 |
| Proportion of peel by mass (%) | 14.7 ± 0.9 |
| Factor Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Rotational Speed of Brushes (rpm) | Peeling Time (min) | Thawing Temperature (°C) | Incubation Time (s) | Peeled Surface Area (%) | Peel Loss (%) | ||
| X1 | X2 | X3 | X4 | Exp a | Pred b | Exp | Pred | |
| 1 | 1000 | 4 | 60 | 30 | 94.9 | 84.7 | 30.4 | 31.3 |
| 2 | 700 | 4 | 60 | 30 | 48.0 | 40.2 | 9.2 | 8.7 |
| 3 | 1000 | 2 | 60 | 90 | 76.6 | 70.5 | 12.5 | 13.4 |
| 4 | 850 | 5 | 70 | 60 | 78.2 | 81.3 | 27.2 | 26.8 |
| 5 | 1000 | 2 | 80 | 90 | 72.2 | 89.2 | 34.8 | 34.8 |
| 6 | 700 | 4 | 80 | 30 | 26.8 | 28.2 | 5.6 | 6.0 |
| 7 | 550 | 3 | 70 | 60 | 24.4 | 30.3 | 6.8 | 7.6 |
| 8 | 700 | 2 | 80 | 90 | 50.9 | 54.0 | 6.2 | 6.5 |
| 9 | 850 | 3 | 70 | 60 | 63.5 | 78.9 | 26.0 | 24.5 |
| 10 | 700 | 4 | 80 | 90 | 81.1 | 76.8 | 37.0 | 36.4 |
| 11 | 700 | 2 | 60 | 30 | 14.9 | 24.3 | 4.3 | 4.7 |
| 12 | 1000 | 4 | 60 | 90 | 97.9 | 104.9 c | 23.7 | 23.7 |
| 13 | 1150 | 3 | 70 | 60 | 87.1 | 76.6 | 21.8 | 20.3 |
| 14 | 850 | 3 | 70 | 60 | 84.9 | 78.9 | 23.5 | 24.5 |
| 15 | 1000 | 2 | 60 | 30 | 22.8 | 21.2 | 5.3 | 5.4 |
| 16 | 700 | 4 | 60 | 90 | 87.2 | 88.8 | 30.4 | 30.7 |
| 17 | 850 | 3 | 70 | 60 | 92.3 | 78.9 | 24.1 | 24.5 |
| 18 | 850 | 3 | 90 | 60 | 89.3 | 82.3 | 17.8 | 17.5 |
| 19 | 1000 | 2 | 80 | 30 | 31.1 | 39.9 | 6.6 | 7.5 |
| 20 | 1000 | 4 | 80 | 30 | 60.8 | 72.8 | 9.2 | 9.3 |
| 21 | 850 | 3 | 70 | 120 | 92.8 | 86.8 | 31.9 | 31.6 |
| 22 | 850 | 3 | 70 | 0 | 20.7 | 22.2 | 4.2 | 3.7 |
| 23 | 700 | 2 | 60 | 90 | 35.5 | 35.3 | 10.8 | 10.3 |
| 24 | 850 | 3 | 70 | 60 | 63.5 | 78.9 | 26.0 | 24.5 |
| 25 | 1000 | 4 | 80 | 90 | 94.5 | 93.0 | 37.5 | 38.3 |
| 26 | 850 | 1 | 70 | 60 | 36.0 | 28.3 | 10.8 | 10.4 |
| 27 | 700 | 2 | 80 | 30 | 53.5 | 43.1 | 10.2 | 9.7 |
| 28 | 850 | 3 | 50 | 60 | 65.5 | 75.5 | 20.6 | 20.3 |
| 29 | 850 | 3 | 70 | 60 | 84.7 | 78.9 | 23.5 | 24.5 |
| 30 | 850 | 3 | 70 | 60 | 91.9 | 78.9 | 24.1 | 24.5 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Intercept | 18615.46 | 12.00 | 1551.29 | 11.51 | <0.0001 |
| X1-Rotational speed of brushes | 3225.20 | 1.00 | 3225.20 | 23.93 | 0.0001 |
| X2-Peeling time | 4214.43 | 1.00 | 4214.43 | 31.27 | <0.0001 |
| X3-Thawing temperature | 68.81 | 1.00 | 68.81 | 0.51 | 0.4846 |
| X4-Incubation time | 6252.99 | 1.00 | 6252.99 | 46.39 | <0.0001 |
| X1X2 | 205.05 | 1.00 | 205.05 | 1.52 | 0.2342 |
| X1X4 | 25.05 | 1.00 | 25.05 | 0.19 | 0.6718 |
| X2X3 | 940.43 | 1.00 | 940.43 | 6.98 | 0.0171 |
| X2X4 | 18.39 | 1.00 | 18.39 | 0.14 | 0.7164 |
| X12 | 1131.89 | 1.00 | 1131.89 | 8.40 | 0.01 |
| X22 | 1012.01 | 1.00 | 1012.01 | 7.51 | 0.014 |
| X42 | 1041.20 | 1.00 | 1041.20 | 7.72 | 0.0129 |
| X1X2X4 | 1116.43 | 1.00 | 1116.43 | 8.28 | 0.0104 |
| Residual | 2291.34 | 17.00 | 134.78 | – | – |
| Lack-of-fit | 1407.20 | 12.00 | 117.27 | 0.66 | 0.7412 |
| Pure error | 884.14 | 5.00 | 176.83 | – | – |
| Correction total | 20906.81 | 29.00 | – | – | – |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Intercept | 3333.06 | 20.00 | 166.65 | 100.03 | <0.0001 |
| X1-Rotational speed of brushes | 242.44 | 1.00 | 242.44 | 145.52 | <0.0001 |
| X2-Peeling time | 134.14 | 1.00 | 134.14 | 80.51 | <0.0001 |
| X3-Thawing temperature | 3.91 | 1.00 | 3.91 | 2.35 | 0.1597 |
| X4-Incubation time | 1170.61 | 1.00 | 1170.61 | 702.65 | <0.0001 |
| X1X2 | 5.27 | 1.00 | 5.27 | 3.16 | 0.1090 |
| X1X3 | 8.98 | 1.00 | 8.98 | 5.39 | 0.0453 |
| X1X4 | 0.25 | 1.00 | 0.25 | 0.15 | 0.7062 |
| X2X3 | 52.94 | 1.00 | 52.94 | 31.78 | 0.0003 |
| X2X4 | 81.73 | 1.00 | 81.73 | 49.06 | <0.0001 |
| X3X4 | 193.85 | 1.00 | 193.85 | 116.35 | <0.0001 |
| X12 | 193.16 | 1.00 | 193.16 | 115.94 | <0.0001 |
| X22 | 60.03 | 1.00 | 60.03 | 36.03 | 0.0002 |
| X32 | 54.87 | 1.00 | 54.87 | 32.94 | 0.0003 |
| X42 | 80.95 | 1.00 | 80.95 | 48.59 | <0.0001 |
| X1X2X3 | 66.69 | 1.00 | 66.69 | 40.03 | 0.0001 |
| X1X2X4 | 256.22 | 1.00 | 256.22 | 153.80 | <0.0001 |
| X1X3X4 | 198.61 | 1.00 | 198.61 | 119.21 | <0.0001 |
| X2X3X4 | 74.87 | 1.00 | 74.87 | 44.94 | <0.0001 |
| X12X2 | 14.87 | 1.00 | 14.87 | 8.92 | 0.0153 |
| X12X3 | 20.77 | 1.00 | 20.77 | 12.47 | 0.0064 |
| Residual | 14.99 | 9.00 | 1.67 | – | – |
| Lack-of-fit | 8.20 | 4.00 | 2.05 | 1.51 | 0.3268 |
| Pure error | 6.79 | 5.00 | 1.36 | – | – |
| Correction total | 3348.05 | 29.00 | – | – | – |
| Treatment | Total Starch Content (%, d.b.) |
|---|---|
| Manually peeled cassava tubers | 70.92 a ± 3.13 |
| Peeled cassava tubers after FTP using the peeling machine | 70.08 a ± 2.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barati, Z.; Latif, S.; Romuli, S.; Müller, J. Freeze–Thaw Pre-Treatment of Cassava Tubers to Improve Efficiency of Mechanical Peeling. Appl. Sci. 2019, 9, 2856. https://doi.org/10.3390/app9142856
Barati Z, Latif S, Romuli S, Müller J. Freeze–Thaw Pre-Treatment of Cassava Tubers to Improve Efficiency of Mechanical Peeling. Applied Sciences. 2019; 9(14):2856. https://doi.org/10.3390/app9142856
Chicago/Turabian StyleBarati, Ziba, Sajid Latif, Sebastian Romuli, and Joachim Müller. 2019. "Freeze–Thaw Pre-Treatment of Cassava Tubers to Improve Efficiency of Mechanical Peeling" Applied Sciences 9, no. 14: 2856. https://doi.org/10.3390/app9142856
APA StyleBarati, Z., Latif, S., Romuli, S., & Müller, J. (2019). Freeze–Thaw Pre-Treatment of Cassava Tubers to Improve Efficiency of Mechanical Peeling. Applied Sciences, 9(14), 2856. https://doi.org/10.3390/app9142856








