Corrosion of α-Brass in Solutions Containing Chloride Ions and 3-Mercaptoalkyl-5-amino-1H-1,2,4-triazoles
Abstract
1. Introduction
2. Materials and Methods
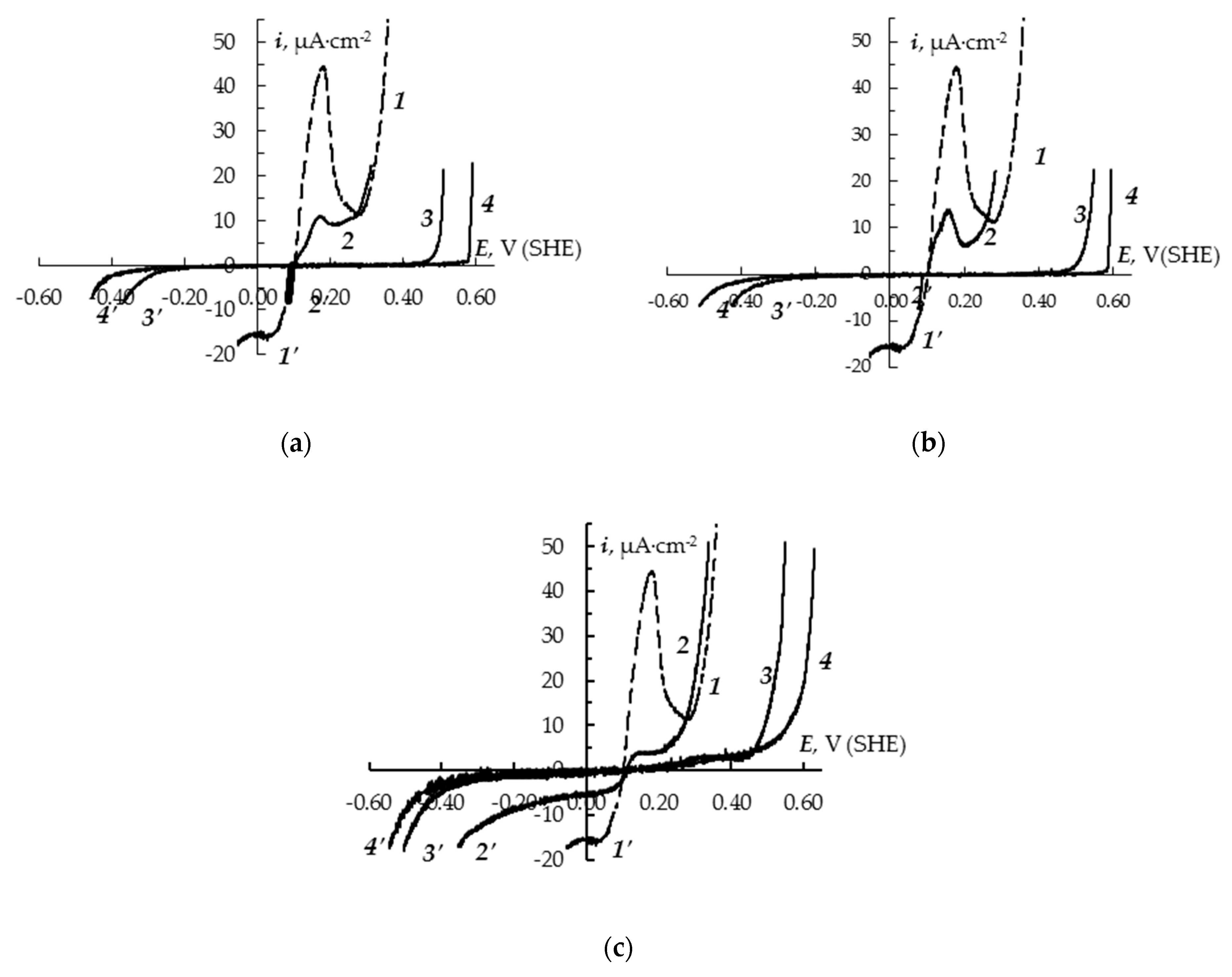
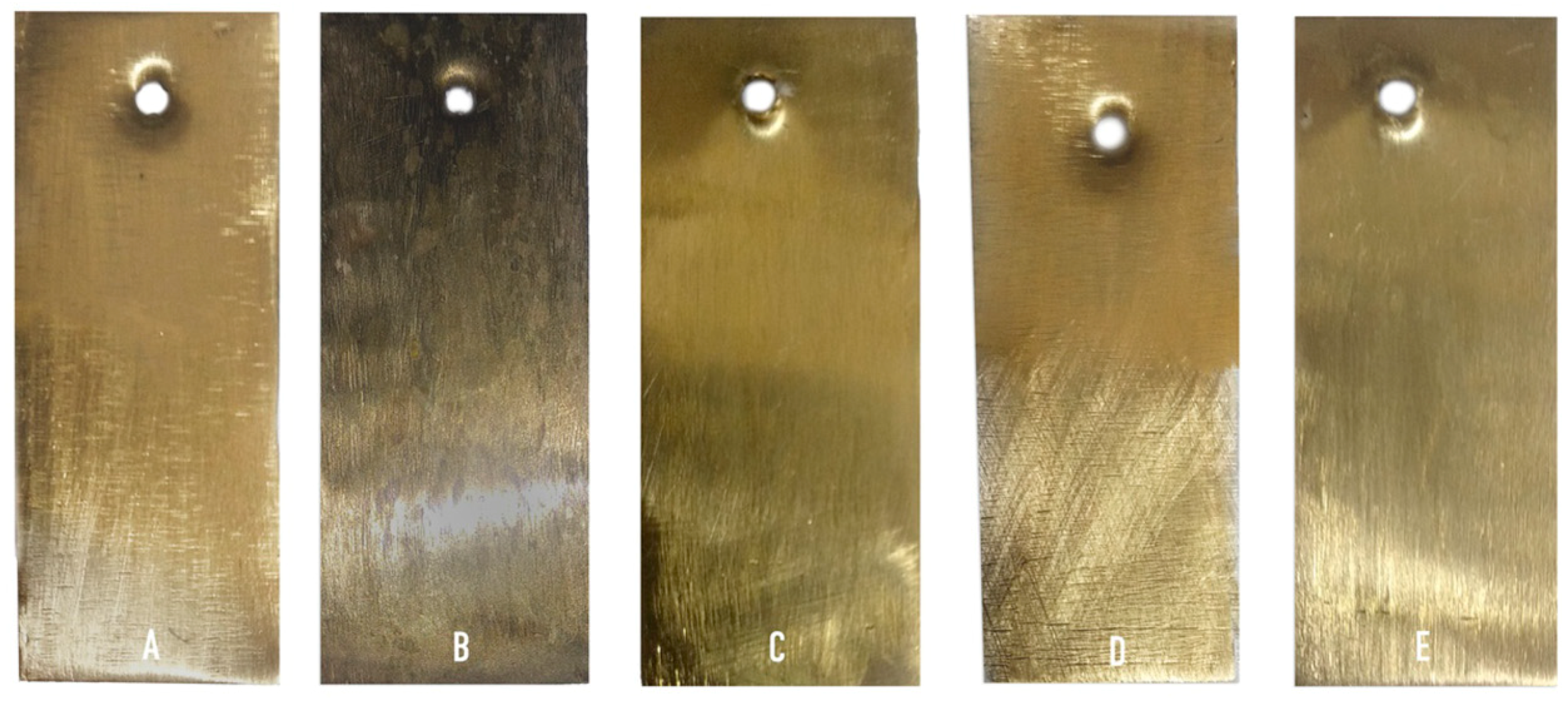
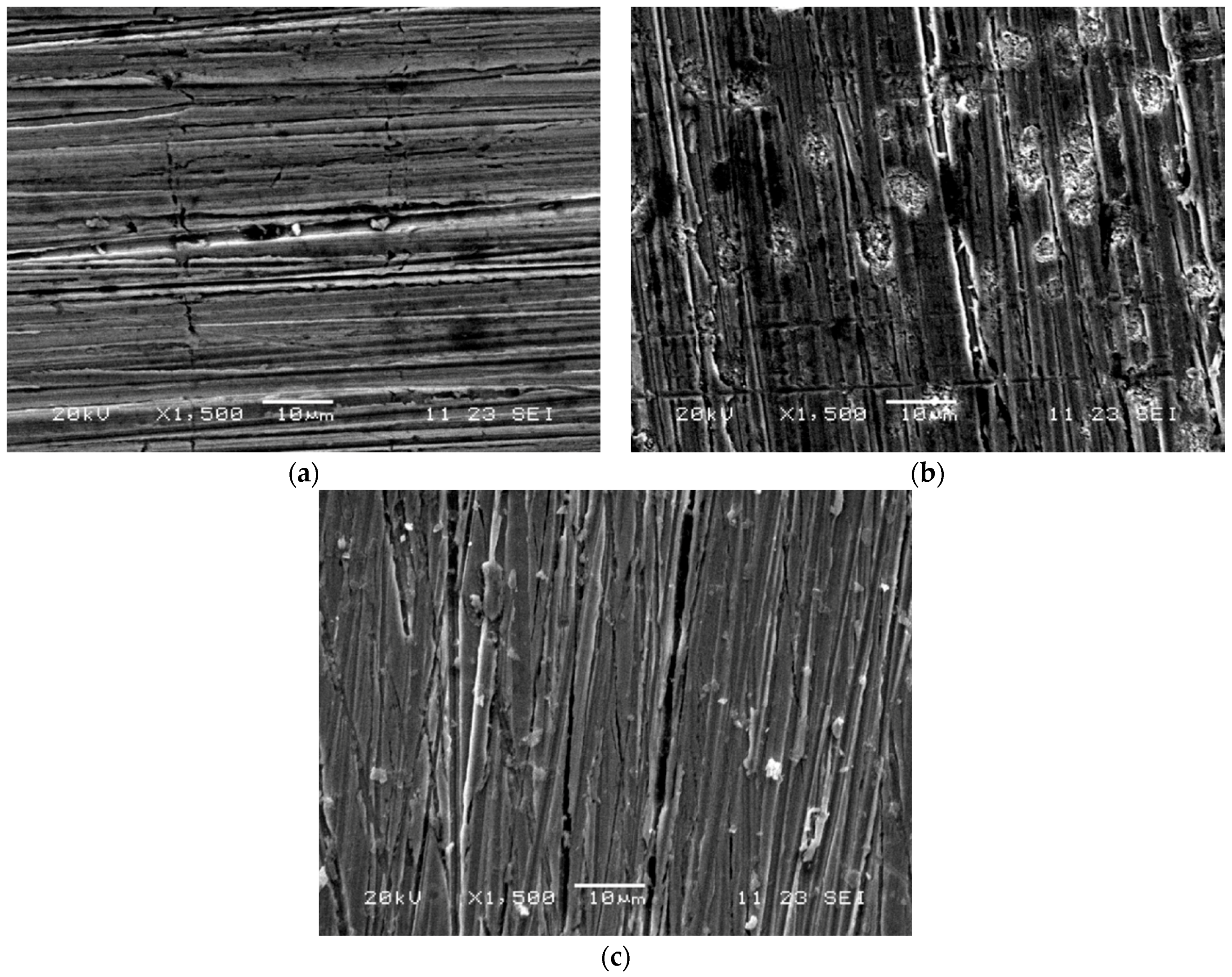
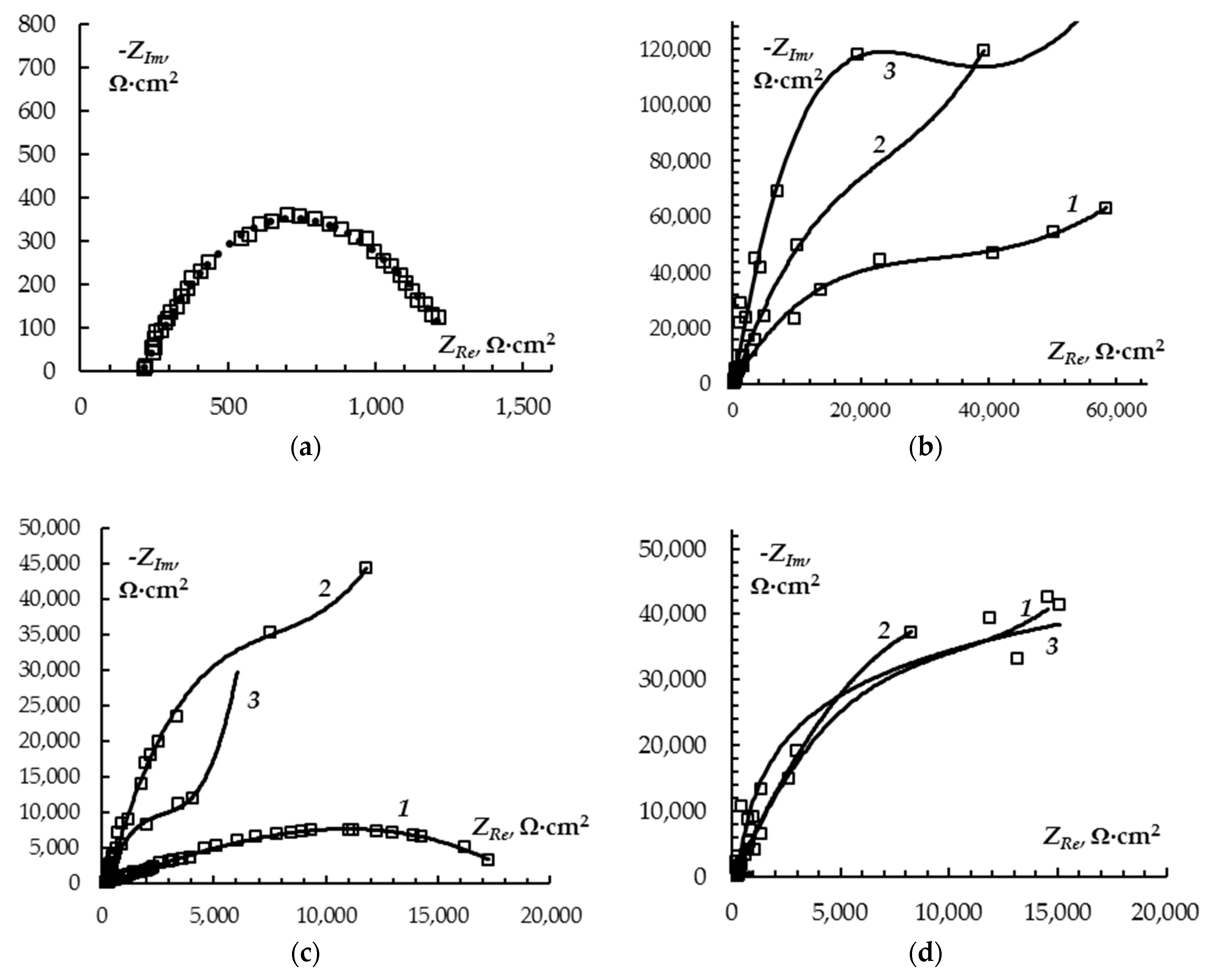
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marshakov, I.K. Corrosion resistance and dezincing of brasses. Prot. Met. 2005, 41, 205–210. [Google Scholar] [CrossRef]
- Shih, H.C.; Tzou, R.J. Effect of benzotriazole on the stress corrosion cracking and the electrochemical polarization of 70/30 brass in fluoride solutions. J. Electrochem. Soc. 1991, 138, 958–961. [Google Scholar] [CrossRef]
- Nishimura, R.; Yoshida, T. Stress corrosion cracking of Cu-30% Zn alloy in Mattsson’s solutions at pH 7.0 and 10.0 using constant load method—A proposal of SCC mechanism. Corros. Sci. 2008, 50, 1205–1213. [Google Scholar] [CrossRef]
- El-Mahdy, G.A. Electrochemical impedance study on brass corrosion in NaCl and (NH4)2SO4 solutions during cyclic wet–dry conditions. J. Appl. Electrochem. 2005, 35, 347–353. [Google Scholar] [CrossRef]
- Alfantazi, A.M.; Ahmed, T.M.; Tromans, D. Corrosion behavior of copper alloys in chloride media. Mater. Des. 2009, 30, 2425–2430. [Google Scholar] [CrossRef]
- Martin, H.; Carro, P.; Hernandez Creus, A.; Morales, J.; Fernandez, G.; Esparza, P.; Gonzales, S.; Salvarezza, R.C.; Arvia, A.J. Inerplay of surface diffusion and surface tension in the evolution of solid/liquid interfaces. Dealloying of β-brass in aqueous sodium chloride. J. Phys. Chem. B. 2000, 104, 8229–8237. [Google Scholar] [CrossRef]
- Zarnitsyn, I.D.; Vvedenskyi, A.V.; Marshakov, I.K. Nonequilibrium behavior of the surface-layer in anodic-dissolution of homogeneous alloys. Russ. J. Electrochem. 1994, 30, 492–512. [Google Scholar]
- Polunin, A.V.; Pchelnikov, A.P.; Losev, V.V.; Marshakov, I.K. Electrochemical studies of the kinetics and mechanism of brass dezincification. Electrochim. Acta 1982, 27, 467–475. [Google Scholar] [CrossRef]
- Pchelnikova, A.P.; Sitnikov, A.D.; Marshakov, I.K.; Losev, V.V. A study of the kinetics and mechanism of brass dezincification by radiotracer and electrochemical methods. Electrochim. Acta 1981, 26, 591–600. [Google Scholar] [CrossRef]
- Zhou, P.; Hutchison, M.J.; Erning, J.W.; Scully, J.R.; Ogle, K. An in situ kinetic study of brass dezincification and corrosion. Electrochim. Acta 2017, 229, 141–154. [Google Scholar] [CrossRef]
- Holliday, J.E.; Pickering, H.W.; Holliday, J.E.; Pickering, H.W. A Soft X-Ray Study of the Near Surface Composition of Cu30Zn Alloy during Simultaneous Dissolution of Its Components. J. Electrochem. Soc. 1973, 120, 470–475. [Google Scholar] [CrossRef]
- Valcarce, M.B.; de Sanchez, S.R.; Vazquez, M. Brass dezincification in a tap water bacterial suspension. Electrochim. Acta 2006, 51, 3736–3742. [Google Scholar] [CrossRef]
- Valcarce, M.B.; de Sanchez, S.R.; Vazquez, M. A comparative analysis of copper and brass surface films in contact with tap water. J. Mater. Sci. 2006, 41, 1999–2007. [Google Scholar] [CrossRef]
- Morales, J.; Fernandez, G.T.; Esparza, P.; Gonzalez, S.; Salvarezza, R.C.; Arvia, A.J. A comparative study on the passivation and localized corrosion of α, β, and α+β brass in borate buffer solutions containing sodium chloride—I. Electrochemical data. Corros. Sci. 1995, 37, 211–229. [Google Scholar] [CrossRef]
- Morales, J.; Esparza, P.; Fernandez, G.T.; Esparza, P.; Gonzalez, S.; Salvarezza, R.C.; Arvia, A.J. A comparative study on the passivation and localized corrosion of α-and β-brass in borate buffer solutions containing sodium chloride—II. X-ray photoelectron and Auger electron spectroscopy data. Corros. Sci. 1995, 37, 231–239. [Google Scholar] [CrossRef]
- Warraky, A.E. Early stages of dezincification of α brass immersed in 4% NaCl solution. Br. Corros. J. 1997, 32, 57–62. [Google Scholar] [CrossRef]
- Milošev, I.; Mikić, T.K.; Gaberšček, M. The effect of Cu-rich sub-layer on the increased corrosion resistance of Cu–xZn alloys in chloride containing borate buffer. Electrochim. Acta 2006, 52, 415–426. [Google Scholar] [CrossRef]
- Ismail, K.M.; El-Egamy, S.S.; Abdelfatah, M. Effects of Zn and Pb as alloying elements on the electrochemical behaviour of brass in borate solutions. J. Appl. Electrochem. 2001, 31, 663–670. [Google Scholar] [CrossRef]
- El-Rahman, H.A. Passivation and pitting corrosion of α-brass (Cu/Zn: 63/37) in neutral buffer solutions containing chloride ions. Mater. Corros. 1990, 41, 635–639. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Gong, Y.L.; Jing, C.; Huang, H.J.; Li, H.R.; Zhang, S.T.; Gao, F. Synthesis of dibenzotriazole derivatives bearing alkylene linkers as corrosion inhibitors for copper in sodium chloride solution: A new thought for the design of organic inhibitors. Corros. Sci. 2016, 113, 64–77. [Google Scholar] [CrossRef]
- Ma, F.B.; Li, W.H.; Tian, H.W.; Hou, B.R. The use of a new thiadiazole derivative as a highly efficient and durable copper inhibitor in 3.5% NaCl solution. Corros. Sci. 2015, 10, 5862–5879. [Google Scholar]
- Gong, Y.L.; Wang, Z.Q.; Gao, F.; Zhang, S.T.; Li, H.R. Synthesis of new benzotriazole derivatives containing carbon chains as the corrosion inhibitors for copper in sodium chloride solution. Ind. Eng. Chem. Res. 2015, 54, 12242–12253. [Google Scholar] [CrossRef]
- Finšgar, M. EQCM and XPS analysis of 1,2,4-triazole and 3-amino-1,2,4-triazole as copper corrosion inhibitors in chloride solution. Corros. Sci. 2013, 77, 350–359. [Google Scholar] [CrossRef]
- Chirkunov, A.A.; Kuznetsov, Y.I.; Shikhaliev, K.S.; Agafonkina, M.O.; Andreeva, N.P.; Kazansky, L.P.; Potapov, A.Y. Adsorption of 5-alkyl-3-amino-1, 2, 4-triazoles from aqueous solutions and protection of copper from atmospheric corrosion. Corros. Sci. 2018, 144, 230–236. [Google Scholar] [CrossRef]
- Bi, H.; Burstein, G.T.; Rodriguez, B.B.; Kawaley, G. Some aspects of the role of inhibitors in the corrosion of copper in tap water as observed by cyclic voltammetry. Corros. Sci. 2016, 102, 510–516. [Google Scholar] [CrossRef]
- Qin, T.T.; Li, J.; Luo, H.Q.; Li, M.; Li, N.B. Corrosion inhibition of copper by 2,5-dimercapto-1,3,4-thiadiazole monolayer in acidic solution. Corros. Sci. 2011, 53, 1072–1078. [Google Scholar] [CrossRef]
- Sherif, E.M.; El Shamy, A.M.; Ramla, M.M.; El Nazhawy, A.O.H. 5-(Phenyl)-4H-1,2,4-triazole-3-thiol as a corrosion inhibitor for copper in 3.5% NaCl solutions. Mater. Chem. Phys. 2007, 102, 231–239. [Google Scholar] [CrossRef]
- Otmaĉić, H.; Stupnišek-Lisac, E. Copper corrosion inhibitors in near neutral media. Electrochim. Acta 2003, 48, 985–991. [Google Scholar] [CrossRef]
- Fan, H.; Li, S.; Zhao, Z.; Wang, H.; Shi, Z.; Zhang, L. Inhibition of brass corrosion in sodium chloride solutions by self-assembled silane films. Corros. Sci. 2011, 53, 4273–4281. [Google Scholar] [CrossRef]
- Fouda, A.S.; Ismael, M.A.; Shahba, R.A.; Kamel, L.A.; El-Nagggar, A.A. Corrosion Inhibition of Copper and α-Brass in 1 M HNO3 Solution using New arylpyrimido [5, 4-c] quinoline-2, 4-dione derivative. Int. J. Electrochem. Sci. 2017, 12, 3361–3384. [Google Scholar] [CrossRef]
- Gao, G.; Liang, C.H. 1, 3-Bis-diethylamino-propan-2-ol as volatile corrosion inhibitor for brass. Corros. Sci. 2007, 49, 3479–3493. [Google Scholar] [CrossRef]
- Keleş, H.; Akça, S. The effect of Variamine Blue B on brass corrosion in NaCl solution. Arabian J. Chem. 2019, 12, 236–248. [Google Scholar] [CrossRef]
- Asan, A.; Kabasakaloglu, M.; Işiklan, M.; Kiliç, Z. Corrosion inhibition of brass in presence of terdentate ligands in chloride solution. Corros. Sci. 2005, 47, 1534–1544. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Petrović, M.B. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Antonijević, M.M.; Milić, S.M.; Petrović, M.B. Films formed on copper surface in chloride media in the presence of azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Kazansky, L.P. Physicochemical aspects of metal protection by azoles as corrosion inhibitors. Russ. Chem. Rev. 2008, 77, 219–232. [Google Scholar] [CrossRef]
- Mihajlović, M.B.P.; Antonijević, M.M. Copper Corrosion Inhibitors. Period 2008–2014. A Review. Int. J. Electrochem. Sci. 2015, 10, 1027–1053. [Google Scholar]
- Milošev, I.; Kovačević, N.; Kovač, J.; Kokalj, A. The roles of mercapto, benzene and methyl groups in the corrosion inhibition of imidazoles on copper: I. Experimental characterization. Corros. Sci. 2015, 98, 107–118. [Google Scholar] [CrossRef]
- Finšgar, M.; Petovar, B.; Xhanari, K.; Maver, U. The corrosion inhibition of certain azoles on steel in chloride media: Electrochemistry and surface analysis. Corros. Sci. 2016, 111, 370–381. [Google Scholar] [CrossRef]
- Kovačević, N.; Milošev, I.; Kokalj, A. How relevant is the adsorption bonding of imidazoles and triazoles for their corrosion inhibition of copper? Corros. Sci. 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Allah, A.G.; Badawy, M.W.; Rehan, H.H.; Abou-Romia, M.M. Inhibition of corrosion of α-brass (Cu-Zn, 67/33) in acid chloride solutions by some amino pyrazole derivatives. J. Appl. Electrochem. 1989, 19, 928–932. [Google Scholar] [CrossRef]
- Walker, R. Triazole, benzotriazole, and naphthotriazole as corrosion inhibitors for brass. Corrosion 1976, 32, 414–417. [Google Scholar] [CrossRef]
- Kosec, T.; Milošev, I.; Pihlar, B. Benzotriazole as an inhibitor of brass corrosion in chloride solution. Appl. Surf. Sci. 2007, 253, 8863–8873. [Google Scholar] [CrossRef]
- Kosec, T.; Merl, D.K.; Milošev, I. Impedance and XPS study of benzotriazole films formed on copper, copper–zinc alloys and zinc in chloride solution. Corros. Sci. 2008, 50, 1987–1997. [Google Scholar] [CrossRef]
- Mamaş, S.; Kiyak, T.; Kabasakaloğlu, M.; Koc, A. The effect of benzotriazole on brass corrosion. Mater. Chem. Phys. 2005, 93, 41–47. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Milić, S.M.; Šerbula, S.M.; Bogdanović, G.D. The influence of chloride ions and benzotriazole on the corrosion behavior of Cu37Zn brass in alkaline medium. Electrochim. Acta 2005, 50, 3693–3701. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, N. Influence of benzotriazole derivatives on the dezincification of 65–35 brass in sodium chloride. Appl. Surf. Sci. 2005, 239, 182–192. [Google Scholar] [CrossRef]
- Obot, I.B.; Edouk, U.M. Benzimidazole: Small planar molecule with diverse anti-corrosion potentials. J. Mol. Liq. 2017, 246, 66–90. [Google Scholar] [CrossRef]
- Ravichandran, R.; Nanjundan, S.; Rajendran, N. Effect of benzotriazole derivatives on the corrosion and dezincification of brass in neutral chloride solution. J. Appl. Electrochem. 2004, 34, 1171–1176. [Google Scholar] [CrossRef]
- Ravichandran, R.; Nanjundan, S.; Rajendran, N. Effect of benzotriazole derivatives on the corrosion of brass in NaCl solutions. Appl. Surf. Sci. 2004, 236, 241–250. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, N. Electrochemical behaviour of brass in artificial seawater: Effect of organic inhibitors. Appl. Surf. Sci. 2005, 241, 449–458. [Google Scholar] [CrossRef]
- Stupnišek-Lisac, E.; Gazivoda, A.; Madžarac, M. Evaluation of non-toxic corrosion inhibitors for copper in sulphuric acid. Electrochim. Acta 2002, 47, 4189–4194. [Google Scholar] [CrossRef]
- Okafor, P.C.; Ikpi, M.E.; Uwah, I.E.; Ebenso, E.E.; Ekpe, U.J.; Umoren, S.A. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 2008, 50, 2310–2317. [Google Scholar] [CrossRef]
- Fouda, A.S.; Shalabi, K.; Idress, A.A. Ceratonia siliqua extract as a green corrosion inhibitor for copper and brass in nitric acid solutions. Green Chem. Lett. Rev. 2015, 8, 17–29. [Google Scholar] [CrossRef]
- Nagiub, A.; Mansfeld, F. Evaluation of corrosion inhibition of brass in chloride media using EIS and ENA. Corros. Sci. 2001, 43, 2147–2171. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Antón, J.G.; Guiñón, J.L.; Herranz, V.P. Comparison of inorganic inhibitors of copper, nickel and copper–nickels in aqueous lithium bromide solution. Electrochim. Acta 2004, 50, 957–966. [Google Scholar] [CrossRef]
- Xu, Q.J.; Zhou, G.D.; Wang, H.F.; Cai, W.B. Electrochemical studies of polyaspartic acid and sodium tungstate as corrosion inhibitors for brass and Cu30Ni alloy in simulated cooled water solutions. Anti-Corros. Met. Mater. 2006, 53, 207–211. [Google Scholar] [CrossRef]
- Nihorimbere, M.; Kerroum, Y.; Guenbour, A.; Kacimi, M.; Bellaouchou, A.; Touir, R.; Zarrouk, A. Corrosion inhibition of brass in artificial drinking water by mineral compound. J. Mater. Environ. Sci. 2016, 7, 4121–4128. [Google Scholar]
- Abd El-Rahman, H.A. Evaluation of AHT as corrosion inhibitor for α-brass in acid chloride solutions. Corrosion 1991, 47, 424–428. [Google Scholar] [CrossRef]
- Mountassir, Z.; Srhiri, A. Electrochemical behaviour of Cu–40Zn in 3% NaCl solution polluted by sulphides: Effect of aminotriazole. Corros. Sci. 2007, 49, 1350–1361. [Google Scholar] [CrossRef]
- Elbakri, M.; Touir, R.; Touhami, M.E.; Srhiri, A.; Benmessaoud, M. Electrosynthesis of adherent poly (3-amino-1,2,4-triazole) films on brass prepared in nonaqueous solvents. Corros. Sci. 2008, 50, 1538–1545. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part I. Long-term immersion, 3D-profilometry, and electrochemistry. Corros. Sci. 2013, 72, 82–89. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part II. Surface analysis using X-ray photoelectron spectroscopy. Corros. Sci. 2013, 72, 90–98. [Google Scholar] [CrossRef]
- Finšgar, M.; Kek Merl, D. 2-Mercaptobenzoxazole as a copper corrosion inhibitor in chloride solution: Electrochemistry, 3D-profilometry, and XPS surface analysis. Corros. Sci. 2013, 80, 82–95. [Google Scholar] [CrossRef]
- Finšgar, M.; Merl, D.K. An electrochemical, long-term immersion, and XPS study of 2-mercaptobenzothiazole as a copper corrosion inhibitor in chloride solution. Corros. Sci. 2014, 83, 164–175. [Google Scholar] [CrossRef]
- Agafonkina, M.O.; Kuznetsov, Y.I.; Andreeva, N.P.; Shikhaliev, K.S.; Potapov, A.Y. Adsorption and passivation of copper by S-containing heterocyclic compounds in neutral aqueous solutions. Corros. Mater. Prot. 2016, 1, 29–38. [Google Scholar]
- Arkhipushkin, I.A.; Vagramyan, T.A.; Shikhaliev, K.S.; Kazansky, L.P. The study of adsorption of 5-mercaptomethyl-3-amino-1,2,4-triazole on copper in neutral solutions. Corros. Mater. Prot. 2016, 7, 18–24. [Google Scholar]
- Arkhipushkin, I.A.; Shikhaliev, K.S.; Potapov, A.Y.; Sapronova, L.V.; Kazansky, L.P. Inhibition of Brass (80/20) by 5-Mercaptopentyl-3-Amino-1,2,4-Triazole in Neutral Solutions. Metals 2017, 7, 488. [Google Scholar] [CrossRef]
- Kazansky, L.P.; Pronin, Y.E.; Arkhipushkin, I.A. XPS study of adsorption of 2-mercaptobenzothiazole on a brass surface. Corros. Sci. 2014, 89, 21–29. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel slopes and corrosion rates obtained in the pre-Tafel region of polarization curves. Corros. Sci. 2005, 47, 3178–3186. [Google Scholar] [CrossRef]
- Shih, H.; Mansfeld, F. Software for quantitative analysis of polarization curves. Comput. Model. Corros. ASTM Int. 1992, 174–183. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford CT, UK, 2016. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Özcan, M.; Dehri, I.; Erbil, M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: Correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004, 236, 155–164. [Google Scholar] [CrossRef]
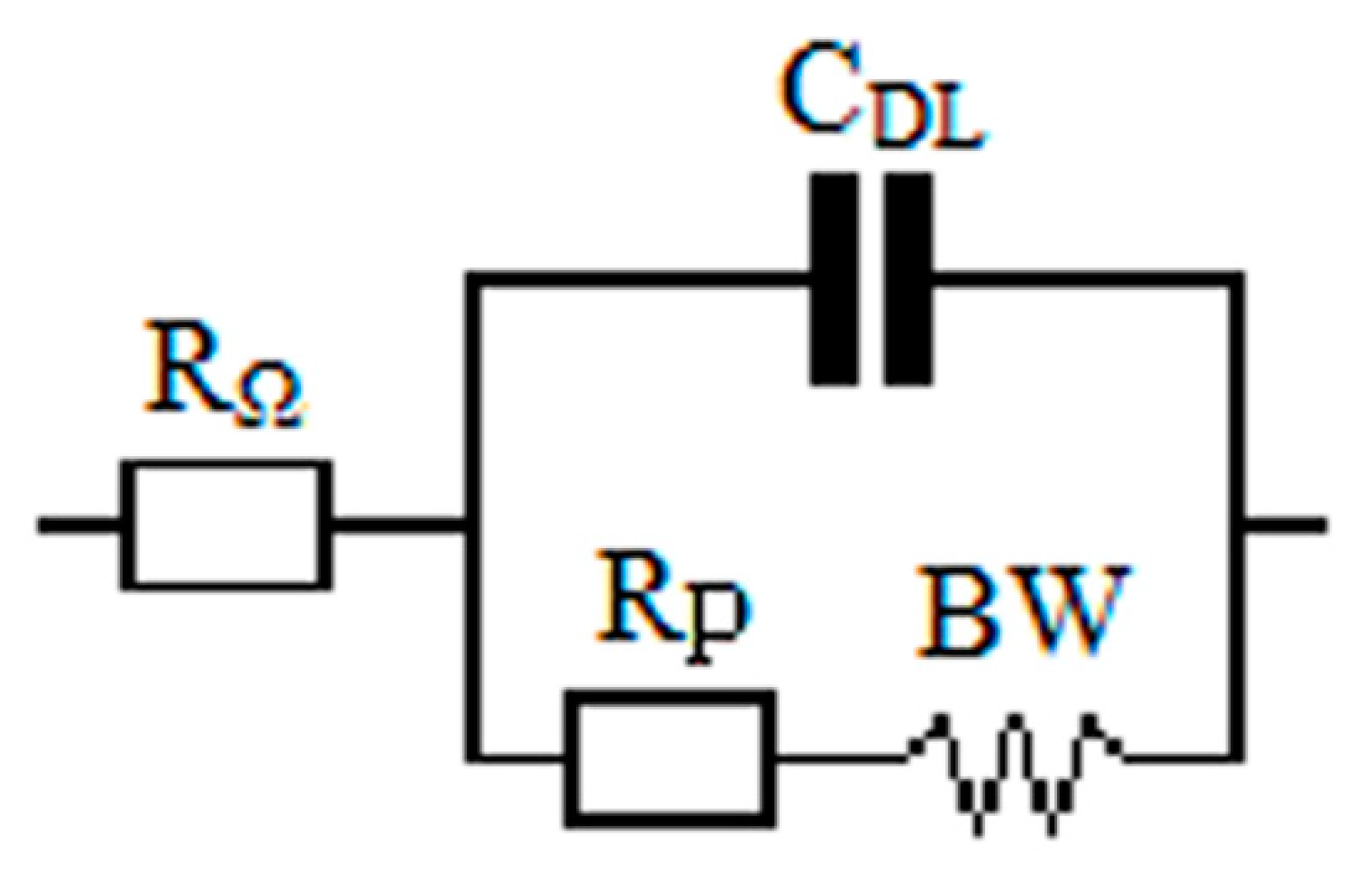
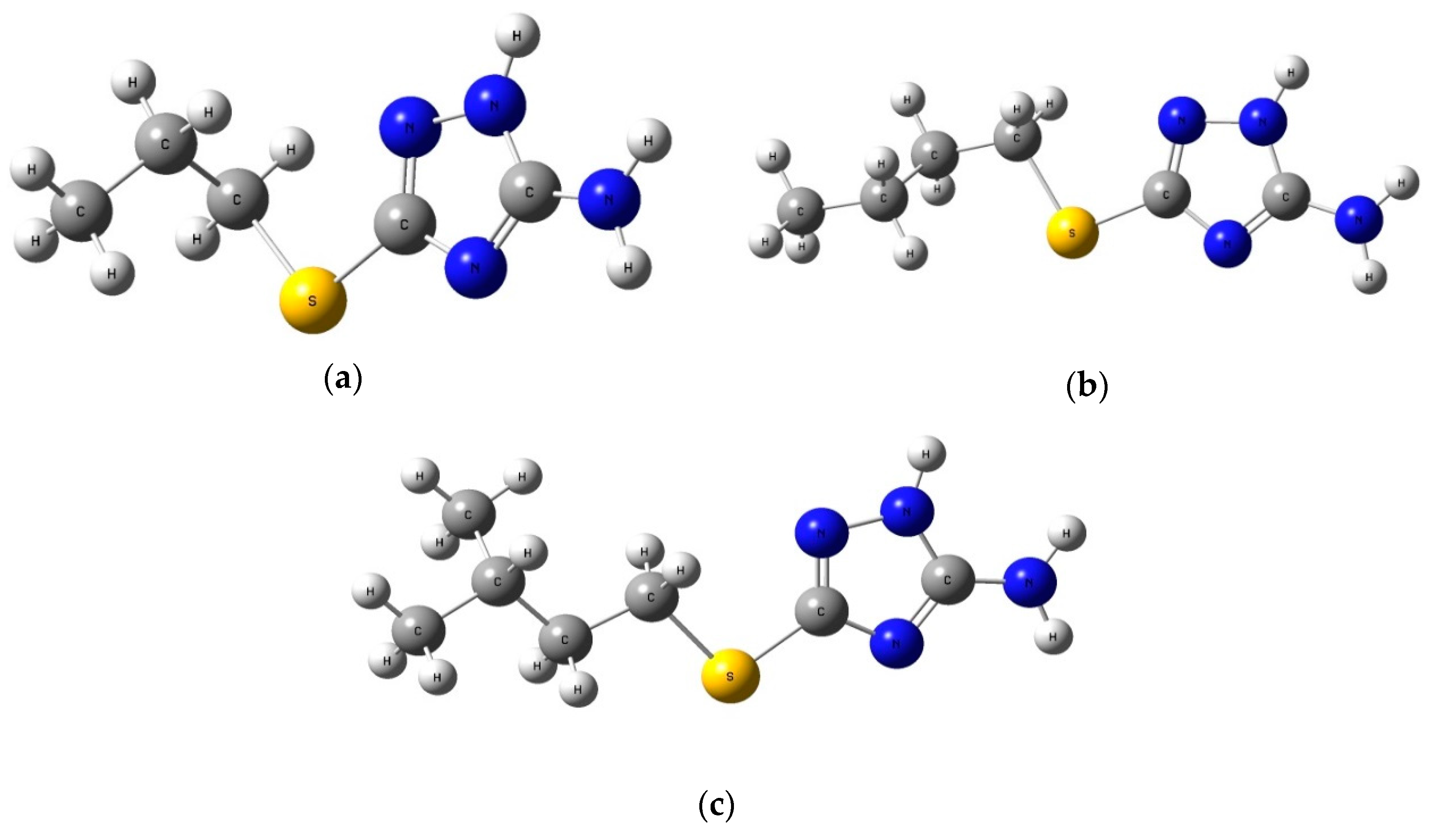
| Symbol | Name | Formula | Solubility |
|---|---|---|---|
| A | 3-mercaptopropyl-5-amino-1H-1,2,4-triazole | 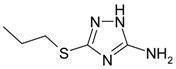 | >10 mM |
| B | 3-mercaptobutyl-5-amino-1H-1,2,4-triazole | 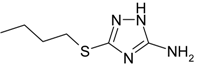 | |
| C | 3-mercapto(3-methylbutyl)-5-amino-1H-1,2,4-triazole | 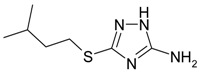 |
| Inhibitor | Cinh, mM | Ecorr, V | Rp, kΩ∙cm2 | icorr μA∙cm−2 | Zi, % |
|---|---|---|---|---|---|
| - | - | 0.111 | 1.51 ± 0.11 | 26 ± 4 | - |
| A | 0.01 | 0.100 | 1.73 ± 0.10 | 38 ± 5 | −46.2 |
| 0.10 | 0.200 | 382 ± 24 | 0.06 ± 0.02 | 99.8 | |
| 1.00 | 0.200 | 244 ± 18 | 0.11 ± 0.03 | 99.6 | |
| B | 0.01 | 0.110 | 0.76 ± 0.09 | 13 ± 3 | 50.0 |
| 0.10 | 0.200 | 195 ± 13 | 0.12 ± 0.04 | 99.5 | |
| 1.00 | 0.250 | 170 ± 15 | 0.19 ± 0.06 | 99.3 | |
| C | 0.01 | 0.120 | 20.9 ± 2.3 | 4.0 ± 0.7 | 84.6 |
| 0.10 | 0.150 | 143 ± 18 | 0.22 ± 0.05 | 99.2 | |
| 1.00 | 0.180 | 281 ± 24 | 0.12 ± 0.03 | 99.5 |
| Inhibitor | Cinh, mM | |||
|---|---|---|---|---|
| 0.0 | 1.0 | 5.0 | 10.0 | |
| A | 3 | 68 | 98 | 152 |
| B | 173 | 194 | 212 | |
| C | 94 | 117 | 283 | |
| Polarization Mode | Element | |||
|---|---|---|---|---|
| Cu | Zn | O | Cu/Zn | |
| No polarization | 61.16 | 38.84 | 0.00 | 61/39 |
| After polarization in borate buffer + 10 mM NaCl | 59.04 | 37.51 | 3.45 | 61/39 |
| After polarization in borate buffer + 10 mM NaCl + 1.00 mM of 3-mercapto(3-methylbutyl)-5-amino-1H-1,2,4-triazole | 57.13 | 35.47 | 7.39 | 62/38 |
| Inhibitor | Cinh, mM | RΩ, Ω∙cm2 | CDL, μF∙cm−2 | RpEIS, kΩ∙cm2 | BW, kΩ∙cm2∙s−0,5 | Degree of protection, ηinh, % | |
|---|---|---|---|---|---|---|---|
| BWa | BWb | ||||||
| - | 0.00 | 240 | 26.2 | 0.176 | 1.28 | 0.20 | - |
| A | 0.01 | 256 | 1.57 | 72 | 73 | 1.77 | 99.76 |
| 0.10 | 250 | 1.00 | 141 | 298 | 6.00 | 99.88 | |
| 1.00 | 258 | 1.54 | 473 | 135 | 0.26 | 99.96 | |
| B | 0.01 | 200 | 2.56 | 0.9 | 13 | 0.81 | 79.86 |
| 0.10 | 255 | 1.58 | 149 | 151 | 23.27 | 99.88 | |
| 1.00 | 244 | 1.02 | 35 | 9336 | 10−6 | 99.50 | |
| C | 0.01 | 275 | 0.86 | 52 | 239 | 2.87 | 99.66 |
| 0.10 | 275 | 1.42 | 158 | 20605 | 10−6 | 99.89 | |
| 1.00 | 275 | 0.79 | 111 | 23526 | 10−6 | 99.84 | |
| Molecule | HOMO | LUMO | HLG | IP | EA | χ | η | σ |
|---|---|---|---|---|---|---|---|---|
| A | −6.36 | −0.61 | 5.75 | 6.36 | 0.61 | 3.49 | 2.88 | 0.35 |
| B | −6.38 | −0.61 | 5.77 | 6.38 | 0.61 | 3.50 | 2.89 | 0.34 |
| C | −6.35 | −0.62 | 5.73 | 6.35 | 0.62 | 3.48 | 2.87 | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozaderov, O.; Shikhaliev, K.; Prabhakar, C.; Tripathi, A.; Shevtsov, D.; Kruzhilin, A.; Komarova, E.; Potapov, A.; Zartsyn, I.; Kuznetsov, Y. Corrosion of α-Brass in Solutions Containing Chloride Ions and 3-Mercaptoalkyl-5-amino-1H-1,2,4-triazoles. Appl. Sci. 2019, 9, 2821. https://doi.org/10.3390/app9142821
Kozaderov O, Shikhaliev K, Prabhakar C, Tripathi A, Shevtsov D, Kruzhilin A, Komarova E, Potapov A, Zartsyn I, Kuznetsov Y. Corrosion of α-Brass in Solutions Containing Chloride Ions and 3-Mercaptoalkyl-5-amino-1H-1,2,4-triazoles. Applied Sciences. 2019; 9(14):2821. https://doi.org/10.3390/app9142821
Chicago/Turabian StyleKozaderov, Oleg, Khidmet Shikhaliev, Chetti Prabhakar, Anuj Tripathi, Dmitry Shevtsov, Alexei Kruzhilin, Ekaterina Komarova, Andrei Potapov, Ilya Zartsyn, and Yuri Kuznetsov. 2019. "Corrosion of α-Brass in Solutions Containing Chloride Ions and 3-Mercaptoalkyl-5-amino-1H-1,2,4-triazoles" Applied Sciences 9, no. 14: 2821. https://doi.org/10.3390/app9142821
APA StyleKozaderov, O., Shikhaliev, K., Prabhakar, C., Tripathi, A., Shevtsov, D., Kruzhilin, A., Komarova, E., Potapov, A., Zartsyn, I., & Kuznetsov, Y. (2019). Corrosion of α-Brass in Solutions Containing Chloride Ions and 3-Mercaptoalkyl-5-amino-1H-1,2,4-triazoles. Applied Sciences, 9(14), 2821. https://doi.org/10.3390/app9142821





