Imaging the Vocal Folds: A Feasibility Study on Strain Imaging and Elastography of Porcine Vocal Folds
Abstract
1. Introduction
2. Materials and Methods
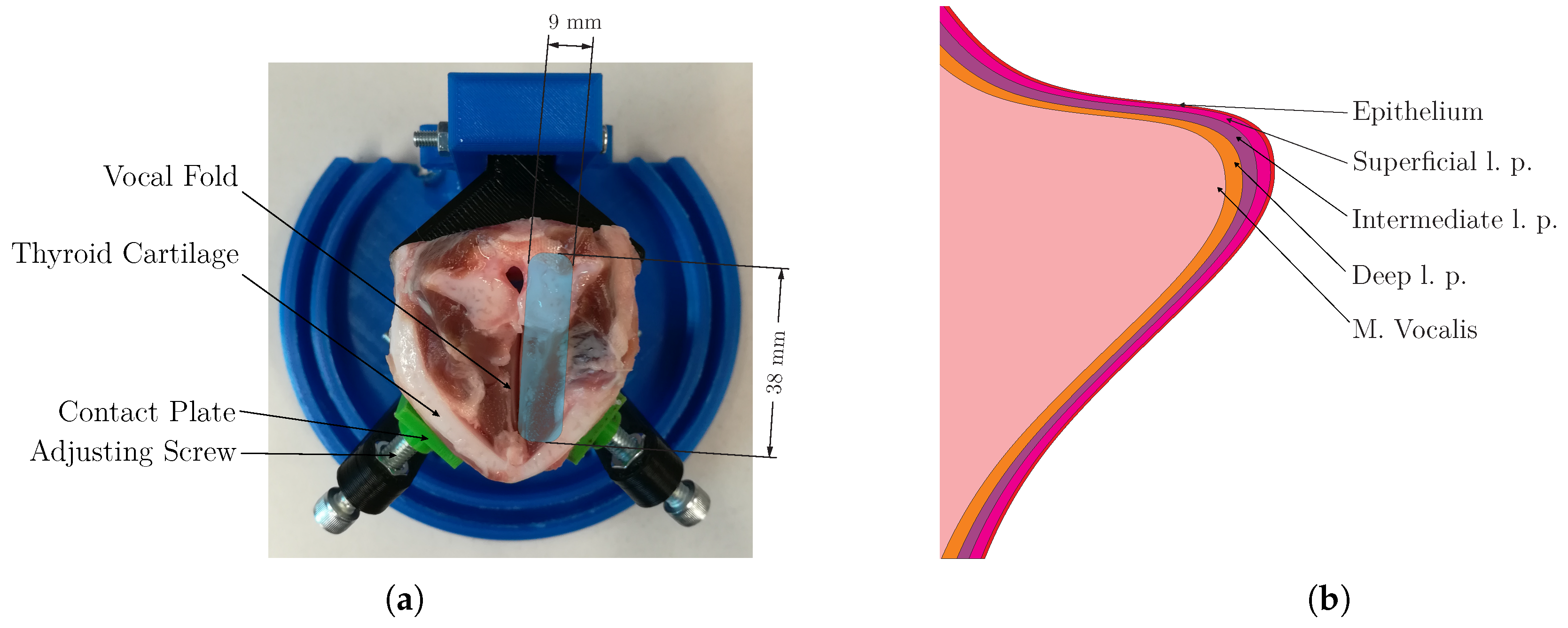
2.1. Anatomical Structure of the Vocal Folds
2.2. Preparation of Gelatin Phantoms and Porcine Larynx
2.3. Mechanical Measurements of Gelatin Phantoms
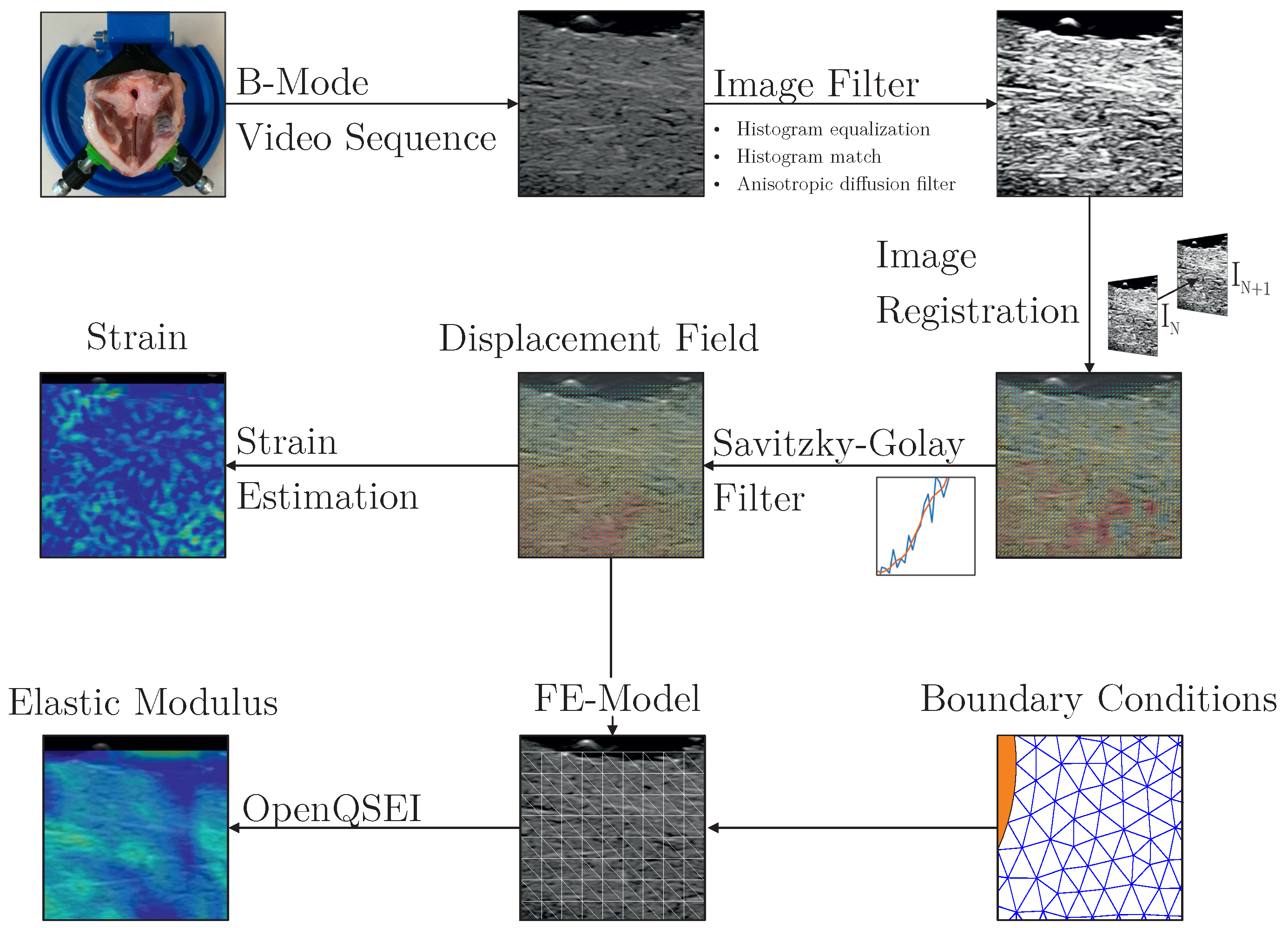
2.4. Evaluation of the Image Data
2.4.1. Image Acquisition and Preprocessing
2.4.2. Image Registration
2.4.3. Displacement Field and Strain
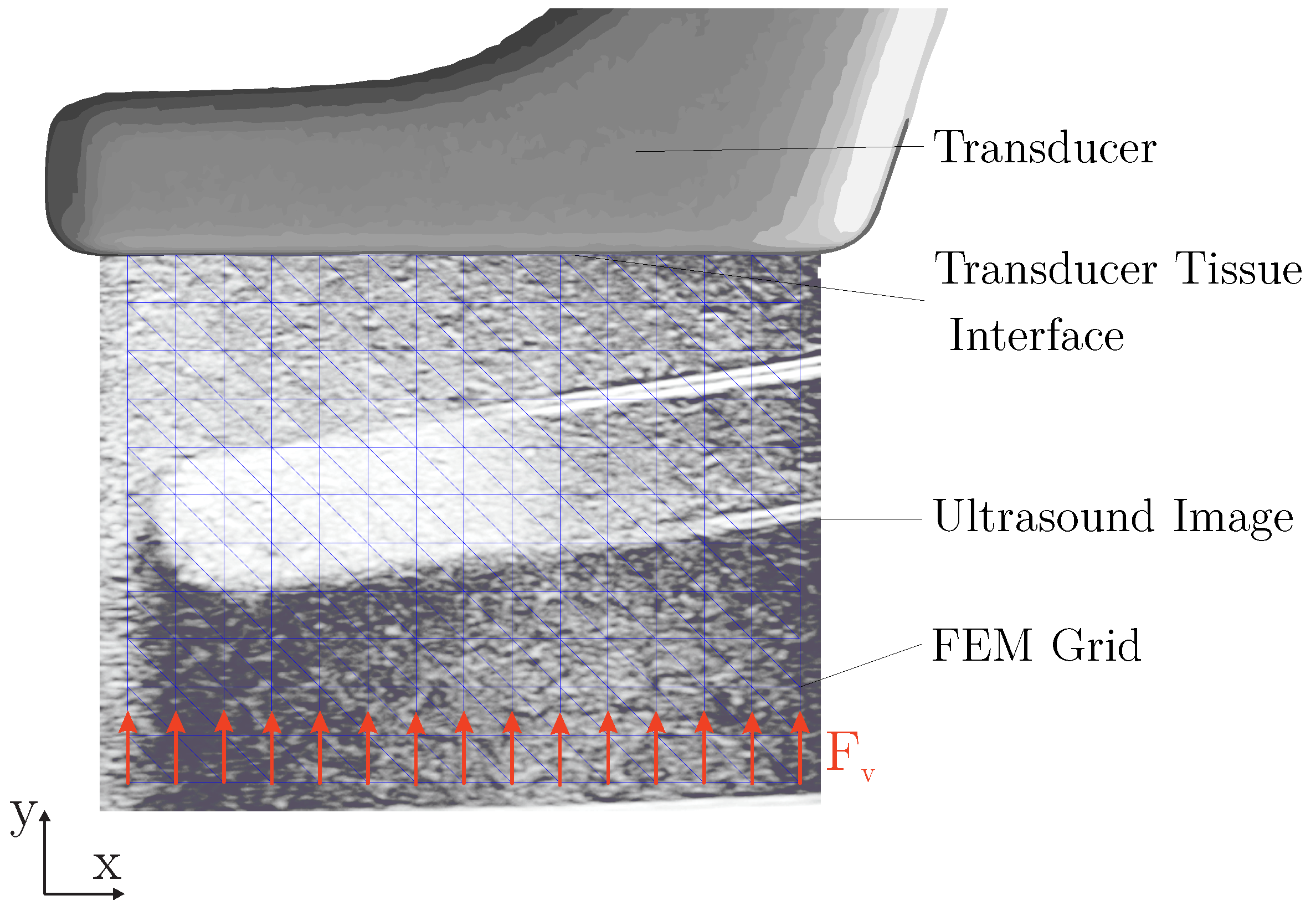
2.4.4. Elastic Properties
3. Results
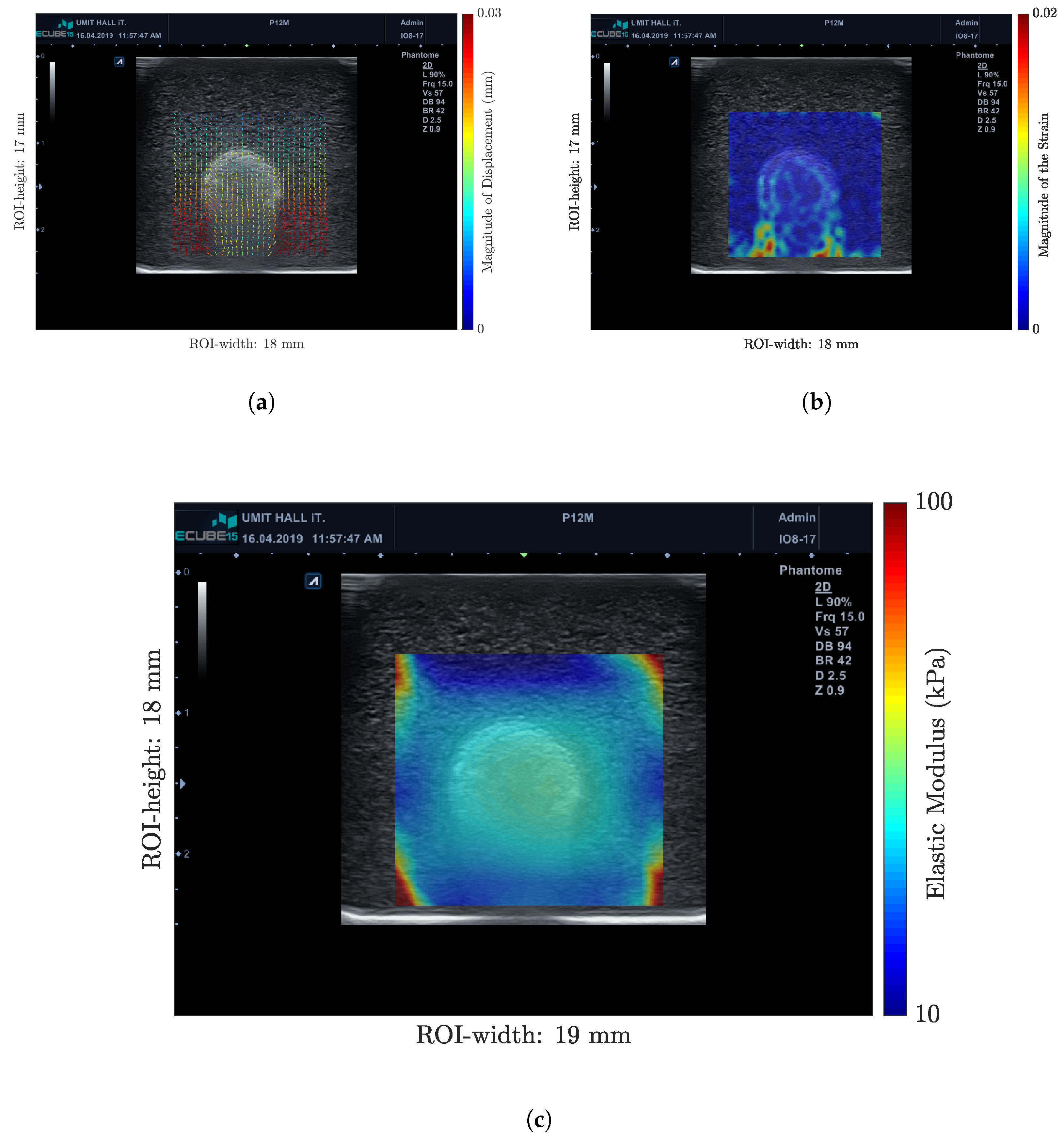
3.1. Measurements in Gelatin Phantoms
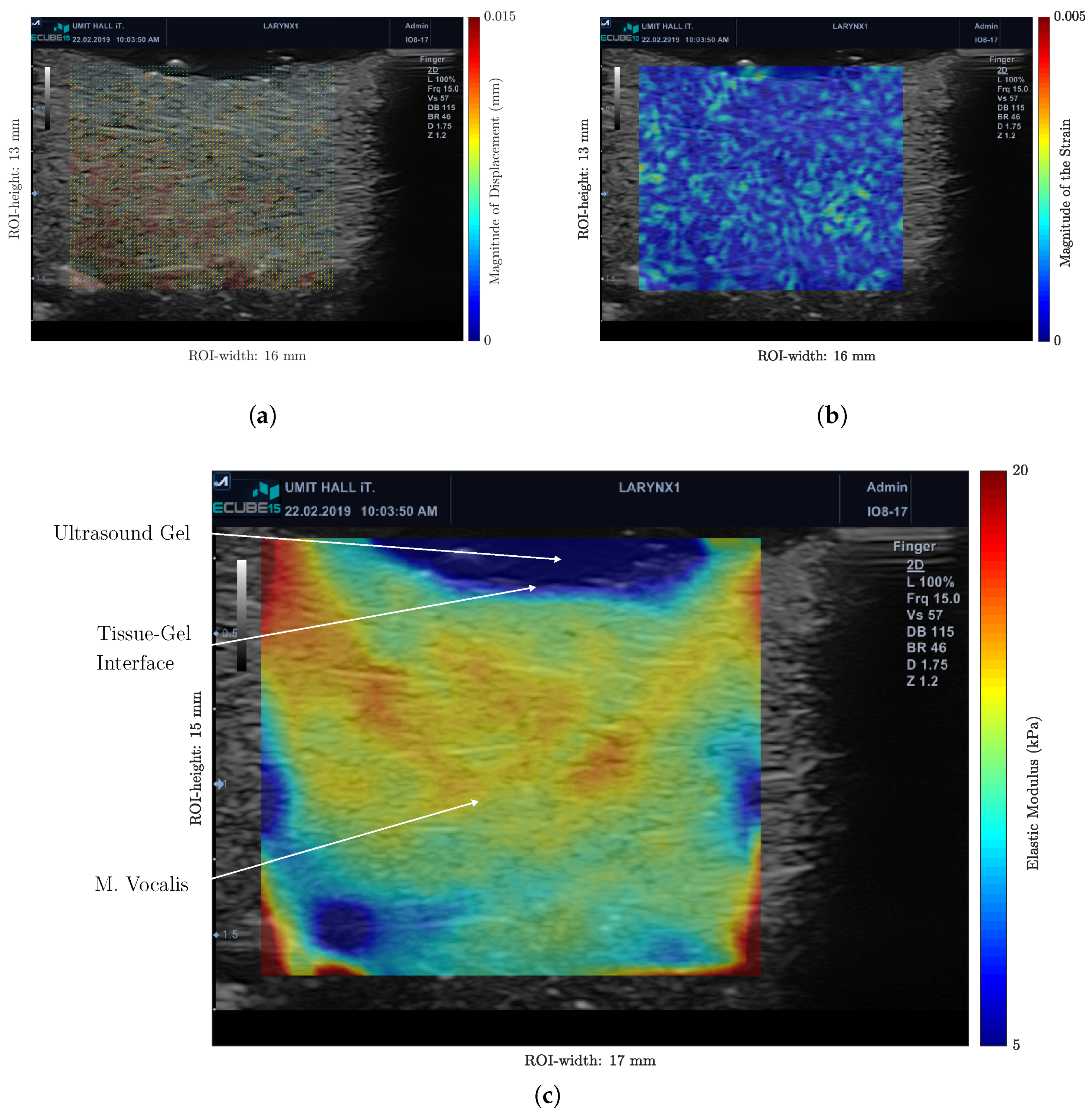
3.2. Ex Vivo Measurements on Porcine Vocal Folds
4. Discussion
4.1. Examination Method and Algorithm
4.2. Measurements on the VFs
4.3. Potential Applications and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Miri, A.K. Mechanical Characterization of Vocal Fold Tissue: A Review Study. J. Voice 2014, 28, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.R. The physics of small–amplitude oscillation of the vocal folds. J. Acoust. Soc. Am. 1988, 83, 1536–1552. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vilda, P.; Fernández-Baillo, R.; Nieto, A.; Díaz, F.; Fernández-Camacho, F.J.; Rodellar, V.; Alvarez, A.; Martínez, R. Evaluation of voice pathology based on the estimation of vocal fold biomechanical parameters. J. Voice 2007, 21, 450–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Characteristics of phonation onset in a two-layer vocal fold model. J. Acoust. Soc. Am. 2009, 125, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Dion, G.R.; Jeswani, S.; Roof, S.; Fritz, M.; Coelho, P.G.; Sobieraj, M.; Amin, M.R.; Branski, R.C. Functional assessment of the ex vivo vocal folds through biomechanical testing: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Goodyer, E.; Jiang, J.J.; Devine, E.; Sutor, A.; Rupitsch, S.; Zorner, S.; Stingl, M.; Schmidt, B. Devices and Methods on Analysis of Biomechanical Properties of Laryngeal Tissue and Substitute Materials. Curr. Bioinform. 2011, 6, 344–361. [Google Scholar] [CrossRef]
- Ruffing, S.; Struffert, T.; Grgic, A.; Reith, W. Bildgebende Diagnostik von Pharynx und Larynx. Der Radiol. 2005, 45, 828–837. [Google Scholar] [CrossRef]
- Bailly, L.; Cochereau, T.; Orgéas, L.; Bernardoni, N.H.; Du Roscoat, S.R.; McLeer-Florin, A.; Robert, Y.; Laval, X.; Laurencin, T.; Chaffanjon, P.; et al. 3D multiscale imaging of human vocal folds using synchrotron X-ray microtomography in phase retrieval mode. Sci. Rep. 2018, 8, 14003:1–14003:20. [Google Scholar] [CrossRef]
- Hawkshaw, M.J.; Sataloff, J.B.; Sataloff, R.T. New concepts in vocal fold imaging: A review. J. Voice 2013, 27, 738–743. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, S.Y.; Luo, F.; Gao, Y.; Yang, X.Y. High-Frequency Sonographic Measurements of True and False Vocal Cords. J. Ultrasound Med. 2010, 29, 1023–1030. [Google Scholar] [CrossRef]
- Hsiao, T.Y.; Wang, C.L.; Chen, C.N.; Hsieh, F.J.; Shau, Y.W. Elasticity of human vocal folds measured in vivo using color Doppler imaging. Ultrasound Med. Biol. 2002, 28, 1145–1152. [Google Scholar] [CrossRef]
- Tsui, P.H.; Wan, Y.L.; Chen, C.K. Ultrasound imaging of the larynx and vocal folds: Recent applications and developments. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, Q.; Xia, C.X.; Shin, J.; Shih, G.; Min, R. Shear Wave Elastography to Assess False Vocal Folds in Healthy Adults: A Feasibility Study. J. Ultrasound Med. 2018, 37, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xia, C.X.; Zhu, Q.; Hamilton, J.; Min, R. Ultrasound strain imaging in assessment of false vocal folds in adults: A feasibility study. Clin. Imaging 2018, 51, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tridandapani, S.; Yoshida, E.; Moore, C.; Beitler, J.; Jani, A.; Liu, T. TU–E–201C–01: Ultrasound Elasticity Evaluation of Vocal Cord Function. Med. Phys. 2010, 37, 3404. [Google Scholar] [CrossRef]
- Sigrist, R.M.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- DeWall, R.J. Ultrasound Elastography: Principles, Techniques, and Clinical Applications. Crit. Rev. Biomed. Eng. 2013, 41, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.G.; Chen, J.H.; Shau, Y.W.; Hsiao, T.Y. Dynamic B-mode ultrasound imaging of vocal fold vibration during phonation. Ultrasound Med. Biol. 2009, 35, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Semmler, M.; Döllinger, M.; Patel, R.R.; Ziethe, A.; Schützenberger, A. Clinical relevance of endoscopic three-dimensional imaging for quantitative assessment of phonation. Laryngoscope 2018, 128, 2367–2374. [Google Scholar] [CrossRef]
- Noordzij, J.P.; Ossoff, R.H. Anatomy and Physiology of the Larynx. Otolaryngol. Clin. N. Am. 2006, 39, 1–10. [Google Scholar] [CrossRef]
- Garrett, C.G.; Coleman, J.R.; Reinisch, L. Comparative Histology and Vibration of the Vocal Folds: Implications for Experimental Studies in Microlaryngeal Surgery. Laryngoscope 2000, 110, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Kakita, Y.; Ohmaru, K.; Kurita, S. Structure and Mechanical Properties of the Vocal Fold. Speech Lang. 1982, 7, 271–297. [Google Scholar] [CrossRef]
- Cook, D.D.; Nauman, E.; Mongeau, L. Ranking vocal fold model parameters by their influence on modal frequencies. J. Acoust. Soc. Am. 2009, 126, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Kazuto, N. A comparative Study of the Layer Structure of the Vocal Fold: A morphological investigation of 11 mammalian species. Otol. Fukuoka 1982, 28, 699–738. [Google Scholar]
- Culjat, M.O.; Goldenberg, D.; Tewari, P.; Singh, R.S. A Review of Tissue Substitutes for Ultrasound Imaging. Ultrasound Med. Biol. 2010, 36, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Bude, R.O.; Adler, R.S. An easily made, low-cost, tissue-like ultrasound phantom material. J. Clin. Ultrasound 1995, 23, 271–273. [Google Scholar] [CrossRef]
- Cardarelli, F. Properties of Materials. In Materials Handbook; Cardarelli, F., Ed.; Springer: Cham, Switzerland, 2018; pp. 1–99. [Google Scholar]
- Cornbleet, P.J.; Gochman, N. Incorrect least-squares regression coefficients in method-comparison analysis. Clin. Chem. 1979, 25, 432–438. [Google Scholar]
- Hall, T.J.; Bilgen, M.; Insana, M.F.; Krouskop, T.A. Phantom materials for elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997, 44, 1355–1365. [Google Scholar] [CrossRef]
- Markidou, A.; Shih, W.Y.; Shih, W.H. Soft-materials elastic and shear moduli measurement using piezoelectric cantilevers. Rev. Sci. Instrum. 2005, 76, 064302. [Google Scholar] [CrossRef]
- Parker, J.R. Algorithms for Image Processing and Computer Vision, 2nd ed.; Wiley: Indianapolis, IN, USA, 2011. [Google Scholar]
- Ugural, A.C.; Fenster, S.K. Advanced Mechanics of Materials and Applied Elasticity, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Kavanagh, J.L.; Menand, T.; Daniels, K.A. Gelatine as a crustal analogue: Determining elastic properties for modeling magmatic intrusions. Tectonophysics 2013, 582, 101–111. [Google Scholar] [CrossRef]
- Chaudhry, A. Estimation of effective Poissons ratio in non-homogeneous porous media using two ultrasound transducers: A feasibility study. Imaging Med. 2016, 8, 105–111. [Google Scholar] [CrossRef]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J.P.W. elastix: A toolbox for intensity-based medical image registration. IEEE Trans. Med Imaging 2010, 29, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Perona, P.; Malik, J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans. Pattern Anal. Mach. Intell. 1990, 12, 629–639. [Google Scholar] [CrossRef]
- Shamonin, D.P.; Bron, E.E.; Lelieveldt, B.P.F.; Smits, M.; Klein, S.; Staring, M. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front. Neuroinform. 2013, 7, 50:1–50:15. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elmoniem, K.Z.; Stuber, M.; Prince, J.L. Direct three-dimensional myocardial strain tensor quantification and tracking using zHARP. Med. Image Anal. 2008, 12, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Pluim, J.P.W.; Staring, M.; Viergever, M.A. Adaptive Stochastic Gradient Descent Optimisation for Image Registration. Int. J. Comput. Vis. 2008, 81, 227. [Google Scholar] [CrossRef]
- Metz, C.T.; Klein, S.; Schaap, M.; van Walsum, T.; Niessen, W.J. Nonrigid registration of dynamic medical imaging data using nD + t B-splines and a groupwise optimization approach. Med. Image Anal. 2011, 15, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Staring, M.; Klein, S. Itk: Transforms Supporting Spatial Derivatives. Insight J. 2010. Available online: http://hdl.handle.net/10380/3215 (accessed on 2 July 2019).
- Rueckert, D.; Sonoda, L.I.; Hayes, C.; Hill, D.L.; Leach, M.O.; Hawkes, D.J. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Trans. Med. Imaging 1999, 18, 712–721. [Google Scholar] [CrossRef]
- Pan, B. Full-field strain measurement using a two-dimensional Savitzky-Golay digital differentiator in digital image correlation. Opt. Eng. 2007, 46, 033601:1–033601:10. [Google Scholar] [CrossRef]
- Lai, W.M.; Rubin, D.; Krempl, E. Introduction to Continuum Mechanics, 3rd ed.; Pergamon Press: Oxford, NY, USA, 1993. [Google Scholar]
- Barbone, P.E.; Bamber, J.C. Quantitative elasticity imaging: What can and cannot be inferred from strain images. Phys. Med. Biol. 2002, 47, 2147–2164. [Google Scholar] [CrossRef] [PubMed]
- Burks, G.; de Vita, R.; Leonessa, A. Characterization of the Continuous Elastic Parameters of Porcine Vocal Folds. J. Voice Off. J. Voice Found. 2018. [Google Scholar] [CrossRef] [PubMed]
- Smyl, D.; Antin, K.N.; Liu, D.; Bossuyt, S. Coupled digital image correlation and quasi-static elasticity imaging of inhomogeneous orthotropic composite structures. Inverse Probl. 2018, 34, 124005. [Google Scholar] [CrossRef]
- Smyl, D.; Bossuyt, S.; Liu, D. OpenQSEI: A MATLAB package for quasi static elasticity imaging. SoftwareX 2019, 9, 73–76. [Google Scholar] [CrossRef]
- Richards, M.S.; Barbone, P.E.; Oberai, A.A. Quantitative three-dimensional elasticity imaging from quasi-static deformation: A phantom study. Phys. Med. Biol. 2009, 54, 757–779. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.L.; Siltanen, S. Linear and Nonlinear Inverse Problems with Practical Applications, 1st ed.; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2012. [Google Scholar]
- Aster, R.C.; Borchers, B.; Thurber, C.H. Parameter Estimation and Inverse Problems, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Cosgrove, D.O.; Berg, W.A.; Doré, C.J.; Skyba, D.M.; Henry, J.P.; Gay, J.; Cohen-Bacrie, C. Shear wave elastography for breast masses is highly reproducible. Eur. Radiol. 2012, 22, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Ren, R.; Dong, W.; Ma, J.; Xu, Z.; Mao, Y.; Jiang, L. Assessment of vocal cord movement by ultrasound in the ICU. Intensive Care Med. 2018, 44, 2145–2152. [Google Scholar] [CrossRef]
- Fukuhara, T.; Donishi, R.; Matsuda, E.; Koyama, S.; Fujiwara, K.; Takeuchi, H. A Novel Lateral Approach to the Assessment of Vocal Cord Movement by Ultrasonography. World J. Surg. 2018, 42, 130–136. [Google Scholar] [CrossRef]
- Deliyski, D.D.; Hillman, R.E. State of the Art Laryngeal Imaging: Research and Clinical Implications. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 147–152. [Google Scholar] [CrossRef]
- Bakhshaee, H.; Young, J.; Yang, J.C.W.; Mongeau, L.; Miri, A.K. Determination of strain field on the superior surface of excised larynx vocal folds using DIC. J. Voice 2013, 27, 659–667. [Google Scholar] [CrossRef][Green Version]
- Wong, B.J.F.; Jackson, R.P.; Guo, S.; Ridgway, J.M.; Mahmood, U.; Su, J.; Shibuya, T.Y.; Crumley, R.L.; Gu, M.; Armstrong, W.B.; et al. In Vivo Optical Coherence Tomography of the Human Larynx: Normative and Benign Pathology in 82 Patients. Laryngoscope 2005, 115, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.A.; Zeitels, S.M.; Anderson, R.R.; Kobler, J.B.; Pierce, M.C.; de Boer, J.F. Imaging the mucosa of the human vocal fold with optical coherence tomography. Ann. Otol. Rhinol. Laryngol. 2005, 114, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, G.; Rubinstein, M.; Saidi, A.; Wong, B.J.; Chen, Z. Office-based dynamic imaging of vocal cords in awake patients with swept-source optical coherence tomography. J. Biomed. Opt. 2009, 14, 064020:1–064020:4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burns, J.A.; Kim, K.H.; Kobler, J.B.; deBoer, J.F.; Lopez-Guerra, G.; Zeitels, S.M. Real-time tracking of vocal fold injections with optical coherence tomography. Laryngoscope 2009, 119, 2182–2186. [Google Scholar] [CrossRef] [PubMed]
- Benboujja, F.; Garcia, J.A.; Beaudette, K.; Strupler, M.; Hartnick, C.J.; Boudoux, C. Intraoperative imaging of pediatric vocal fold lesions using optical coherence tomography. J. Biomed. Opt. 2016, 21, 016007:1–016007:10. [Google Scholar] [CrossRef] [PubMed]
- Englhard, A.S.; Betz, T.; Volgger, V.; Lankenau, E.; Ledderose, G.J.; Stepp, H.; Homann, C.; Betz, C.S. Intraoperative assessment of laryngeal pathologies with optical coherence tomography integrated into a surgical microscope. Lasers Surg. Med. 2017, 49, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Sun, L.; Dailey, S.H.; Wang, S.H.; Shung, K.K. High frequency ultrasonic characterization of human vocal fold tissue. J. Acoust. Soc. Am. 2007, 122, 1827–1832. [Google Scholar] [CrossRef]
- Mariappan, Y.K.; Glaser, K.J.; Ehman, R.L. Magnetic resonance elastography: A review. Clin. Anat. 2010, 23, 497–511. [Google Scholar] [CrossRef]
- Zourmand, A.; Mirhassani, S.M.; Ting, H.N.; Bux, S.I.; Ng, K.H.; Bilgen, M.; Jalaludin, M.A. A magnetic resonance imaging study on the articulatory and acoustic speech parameters of Malay vowels. Biomed. Eng. Online 2014, 13, 103:1–103:20. [Google Scholar] [CrossRef]
- Echternach, M.; Birkholz, P.; Traser, L.; Flügge, T.V.; Kamberger, R.; Burk, F.; Burdumy, M.; Richter, B. Articulation and vocal tract acoustics at soprano subject’s high fundamental frequencies. J. Acoust. Soc. Am. 2015, 137, 2586–2595. [Google Scholar] [CrossRef]
- Taylor, C.J.; Tarbox, G.J.; Bolster, B.D., Jr.; Bangerter, N.K.; Thomson, S.L. Magnetic resonance imaging-based measurement of internal deformation of vibrating vocal fold models. J. Acoust. Soc. Am. 2019, 145, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Shaath, G.A.; Jijeh, A.; Alkurdi, A.; Ismail, S.; Elbarbary, M.; Kabbani, M.S. Ultrasonography assessment of vocal cords mobility in children after cardiac surgery. J. Saudi Heart Assoc. 2012, 24, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.P.A. Use of ultrasound in the evaluation of the vocal folds following thyroidectomy. Colomb. J. Anesthesiol. 2014, 42, 238–242. [Google Scholar] [CrossRef]
- Kandil, E.; Deniwar, A.; Noureldine, S.I.; Hammad, A.Y.; Mohamed, H.; Al-Qurayshi, Z.; Tufano, R.P. Assessment of Vocal Fold Function Using Transcutaneous Laryngeal Ultrasonography and Flexible Laryngoscopy. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.P.; Woo, J.W.; Youn, Y.K.; Chow, F.C.L.; Lee, K.E.; Lang, B.H.H. The importance of sonographic landmarks by transcutaneous laryngeal ultrasonography in post-thyroidectomy vocal cord assessment. Surgery 2014, 156, 1590–1596. [Google Scholar] [CrossRef]
- Alipour, F.; Jaiswal, S.; Vigmostad, S. Vocal Fold Elasticity in the Pig, Sheep, and Cow Larynges. J. Voice 2011, 25, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Barthelat, F.; Mongeau, L. Effects of Dehydration on the Viscoelastic Properties of Vocal Folds in Large Deformations. J. Voice 2012, 26, 688–697. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamprecht, R.; Maghzinajafabadi, M.; Semmler, M.; Sutor, A. Imaging the Vocal Folds: A Feasibility Study on Strain Imaging and Elastography of Porcine Vocal Folds. Appl. Sci. 2019, 9, 2729. https://doi.org/10.3390/app9132729
Lamprecht R, Maghzinajafabadi M, Semmler M, Sutor A. Imaging the Vocal Folds: A Feasibility Study on Strain Imaging and Elastography of Porcine Vocal Folds. Applied Sciences. 2019; 9(13):2729. https://doi.org/10.3390/app9132729
Chicago/Turabian StyleLamprecht, Raphael, Mohammadali Maghzinajafabadi, Marion Semmler, and Alexander Sutor. 2019. "Imaging the Vocal Folds: A Feasibility Study on Strain Imaging and Elastography of Porcine Vocal Folds" Applied Sciences 9, no. 13: 2729. https://doi.org/10.3390/app9132729
APA StyleLamprecht, R., Maghzinajafabadi, M., Semmler, M., & Sutor, A. (2019). Imaging the Vocal Folds: A Feasibility Study on Strain Imaging and Elastography of Porcine Vocal Folds. Applied Sciences, 9(13), 2729. https://doi.org/10.3390/app9132729




