Numerical Modeling of the Melting Process in a Shell and Coil Tube Ice Storage System for Air-Conditioning Application
Abstract
:1. Introduction
2. Mathematical Modeling
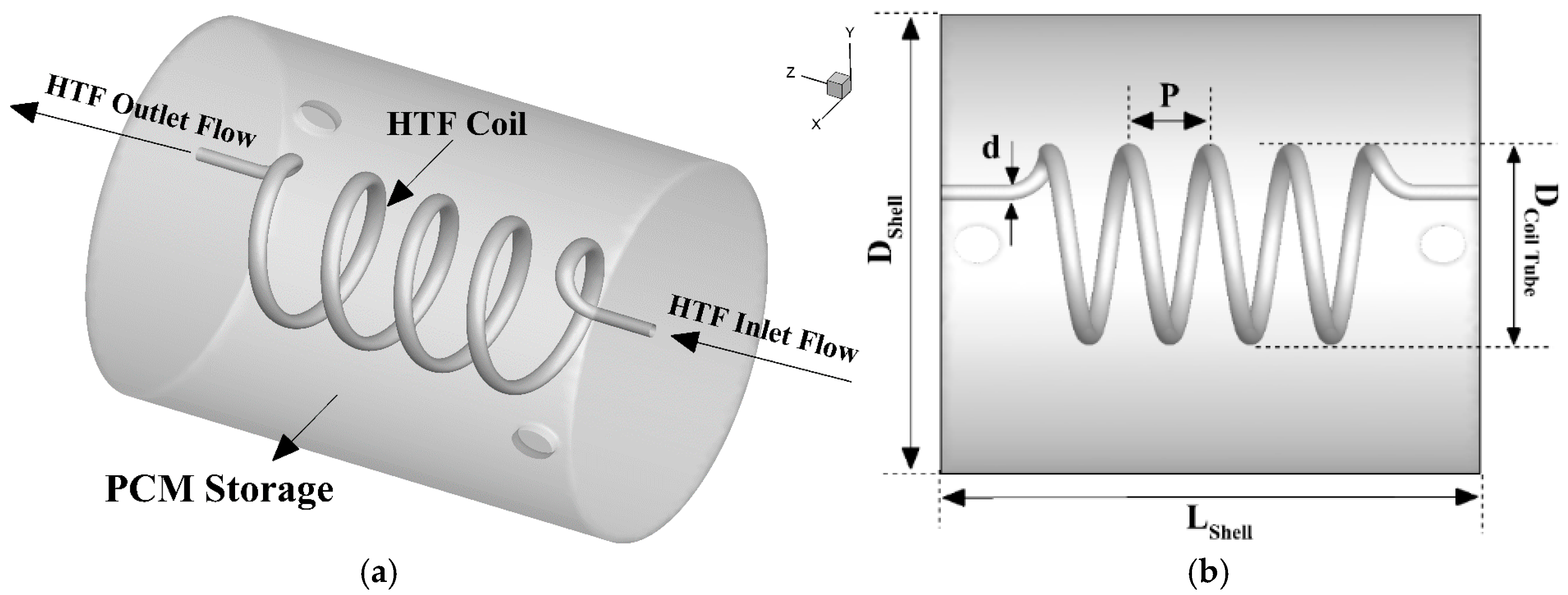
3. Numerical Model
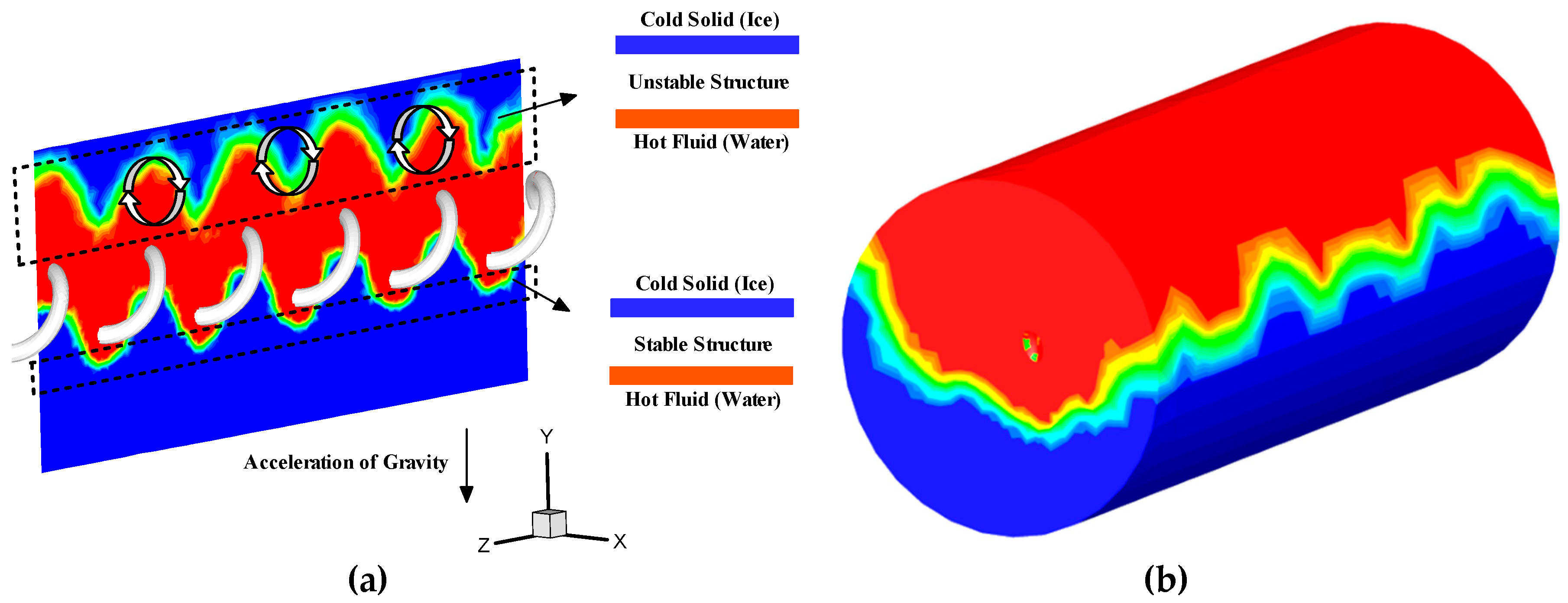
4. Results and Discussion
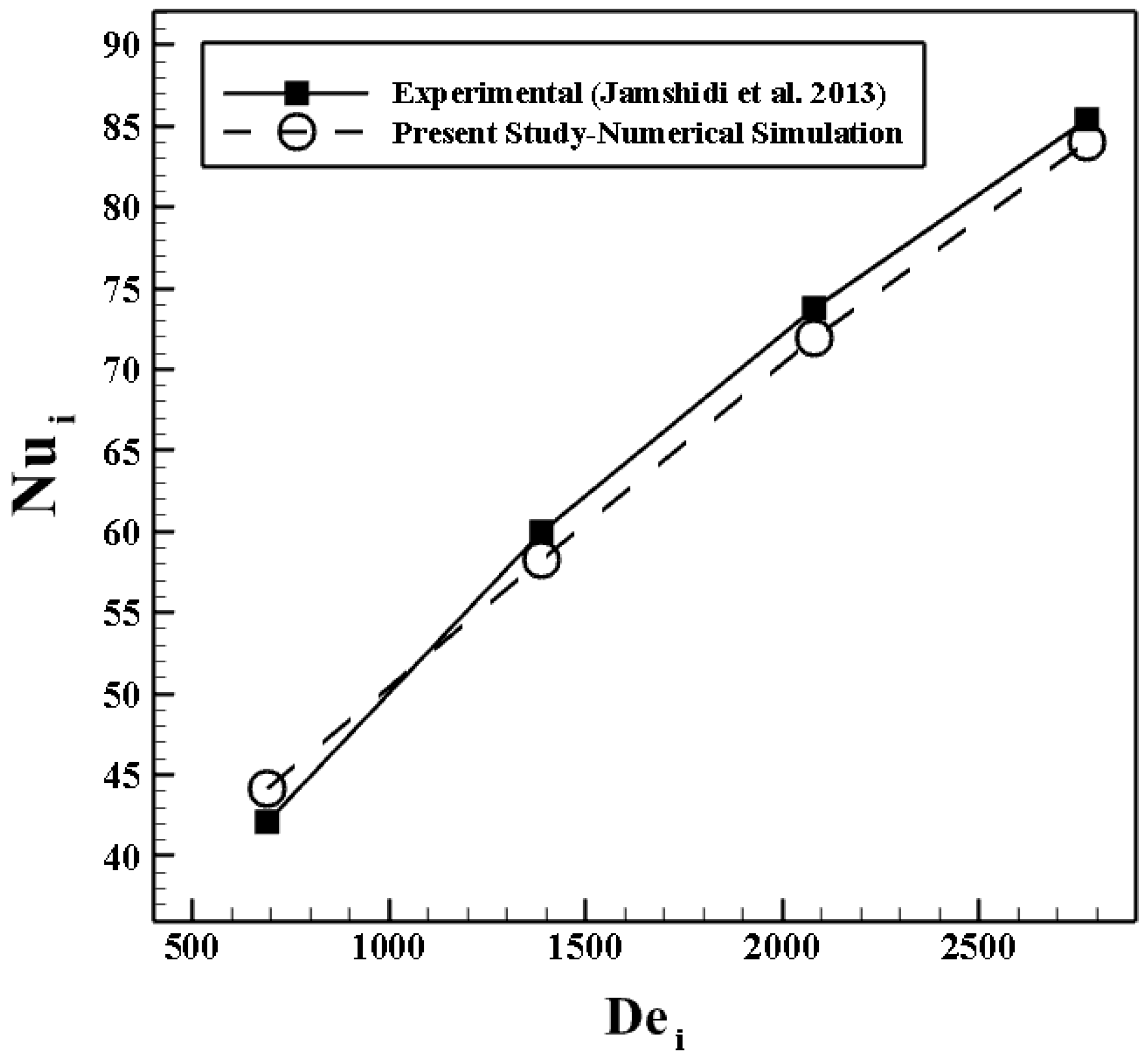
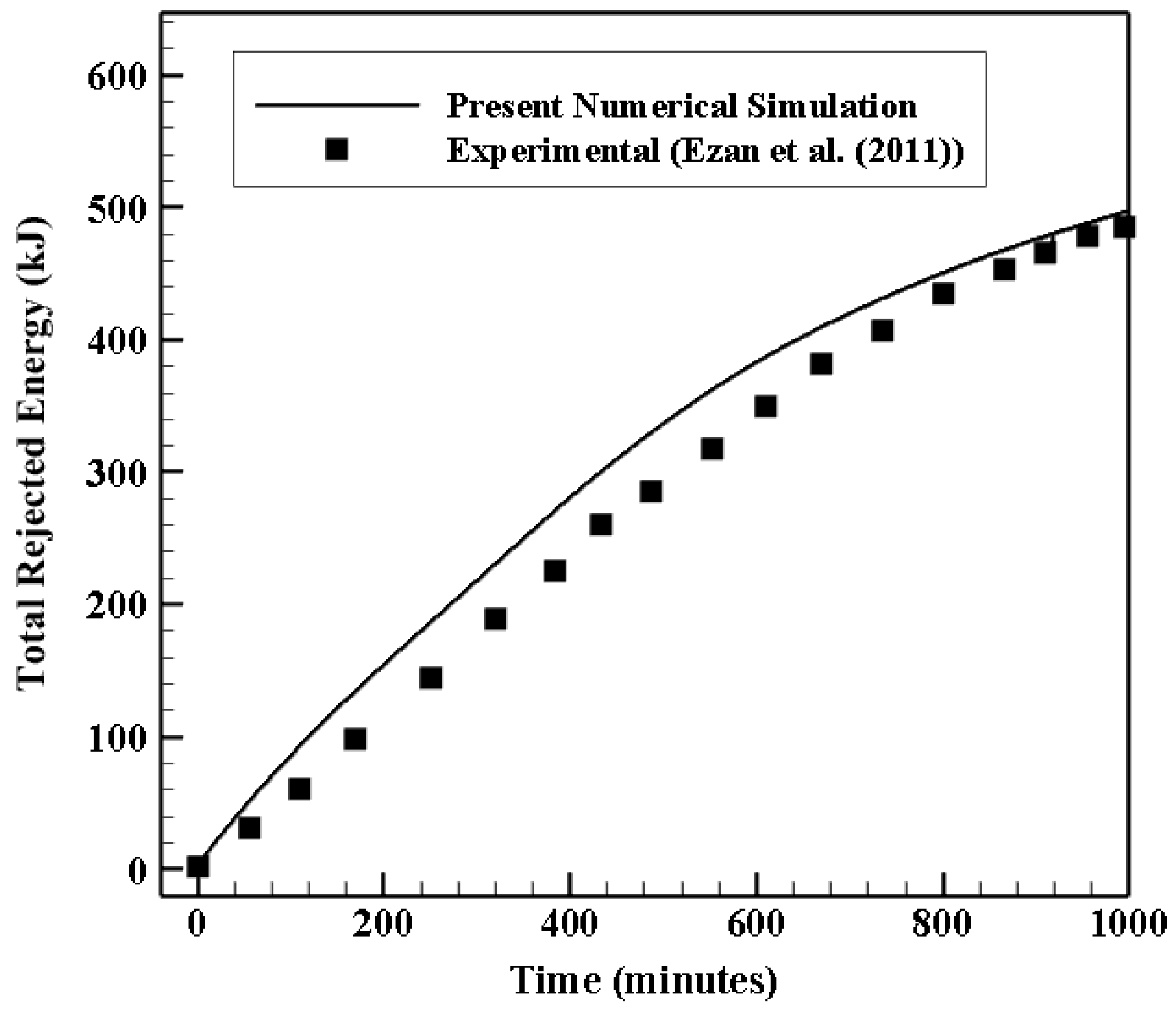
4.1. Validation Study
4.1.1. Validation of the Heat Transfer Process in a Shell and Coil Tube Heat Exchanger
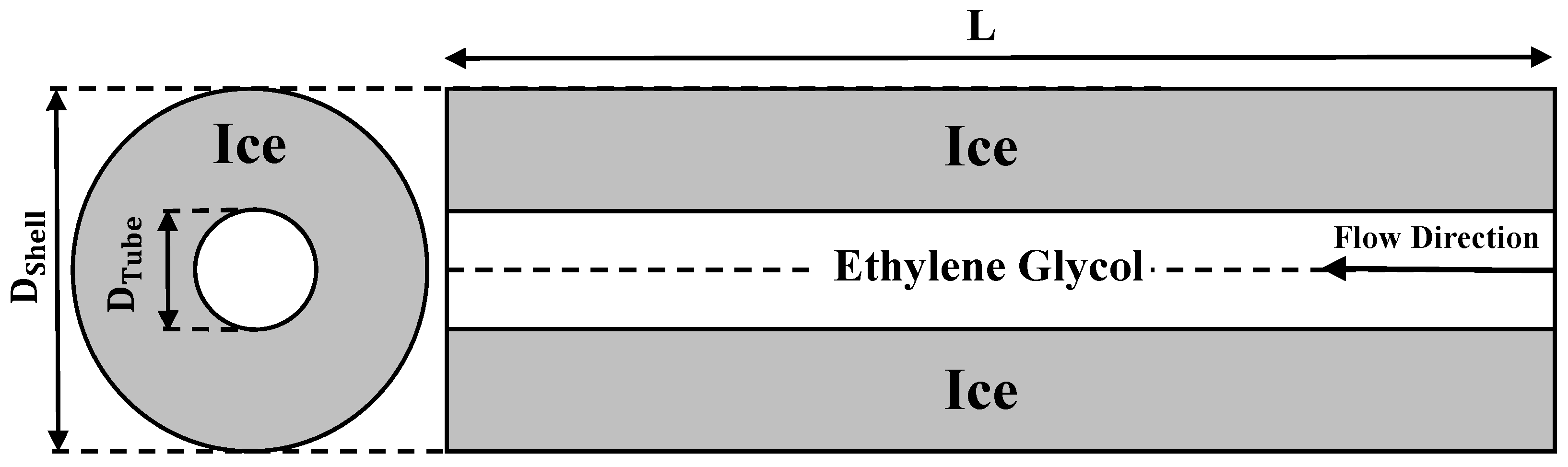
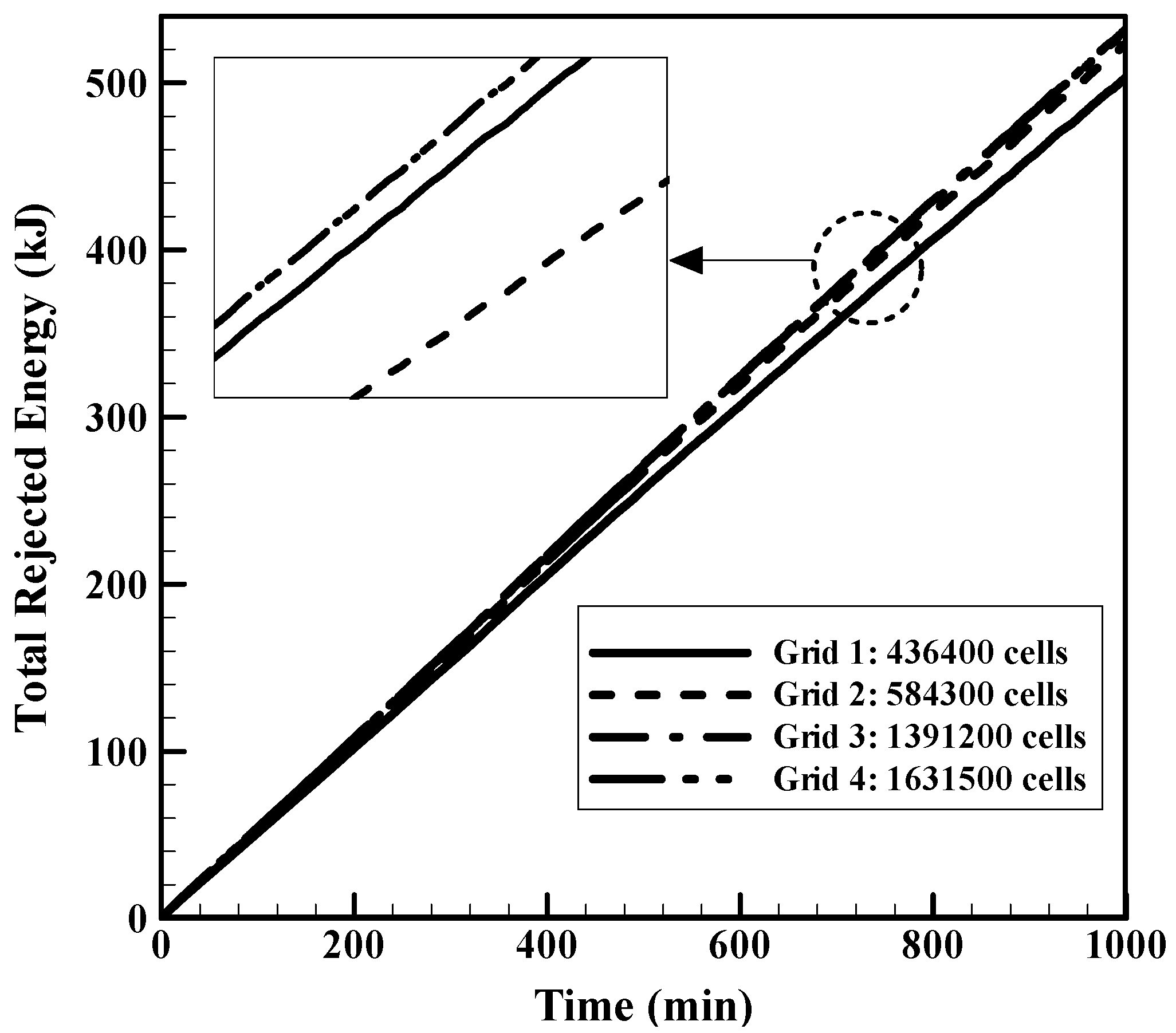
4.1.2. Validation and Mesh Independent Study of the Ice Melting Process in a Double Tube Heat Exchanger
4.2. Influence of the Operational Parameters
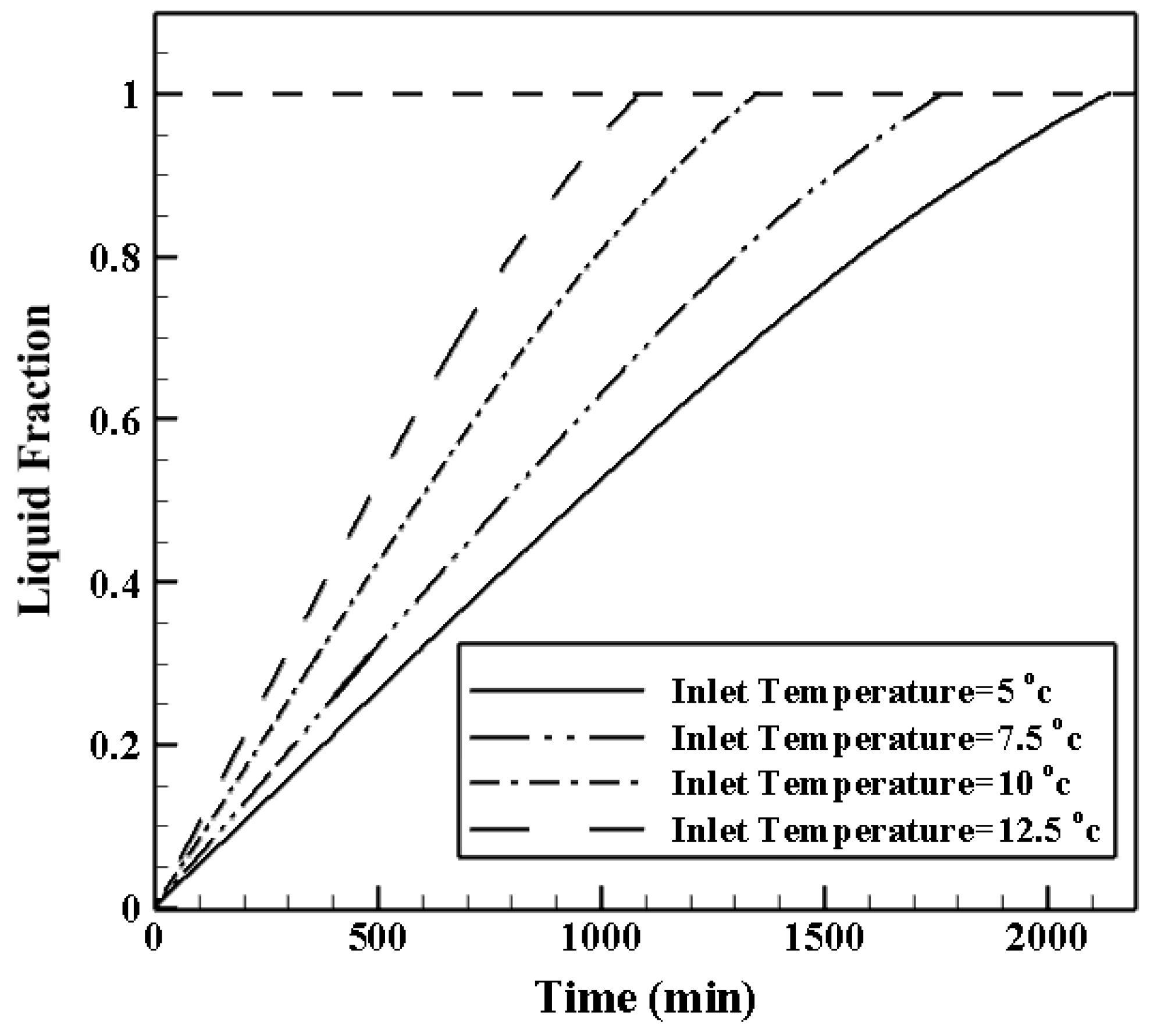
4.2.1. Effects of HTF (Heat Transfer Fluid) Inlet Temperature
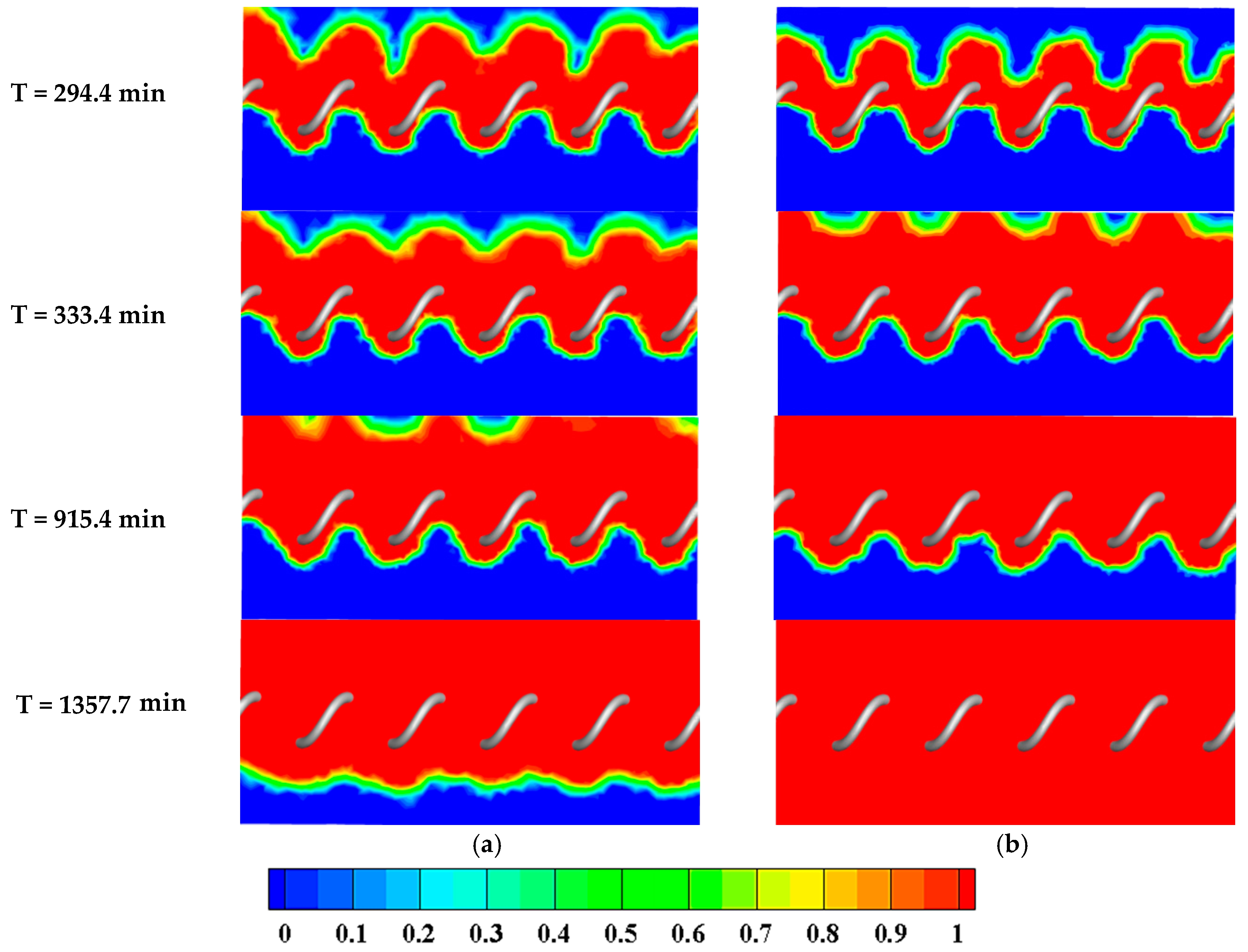
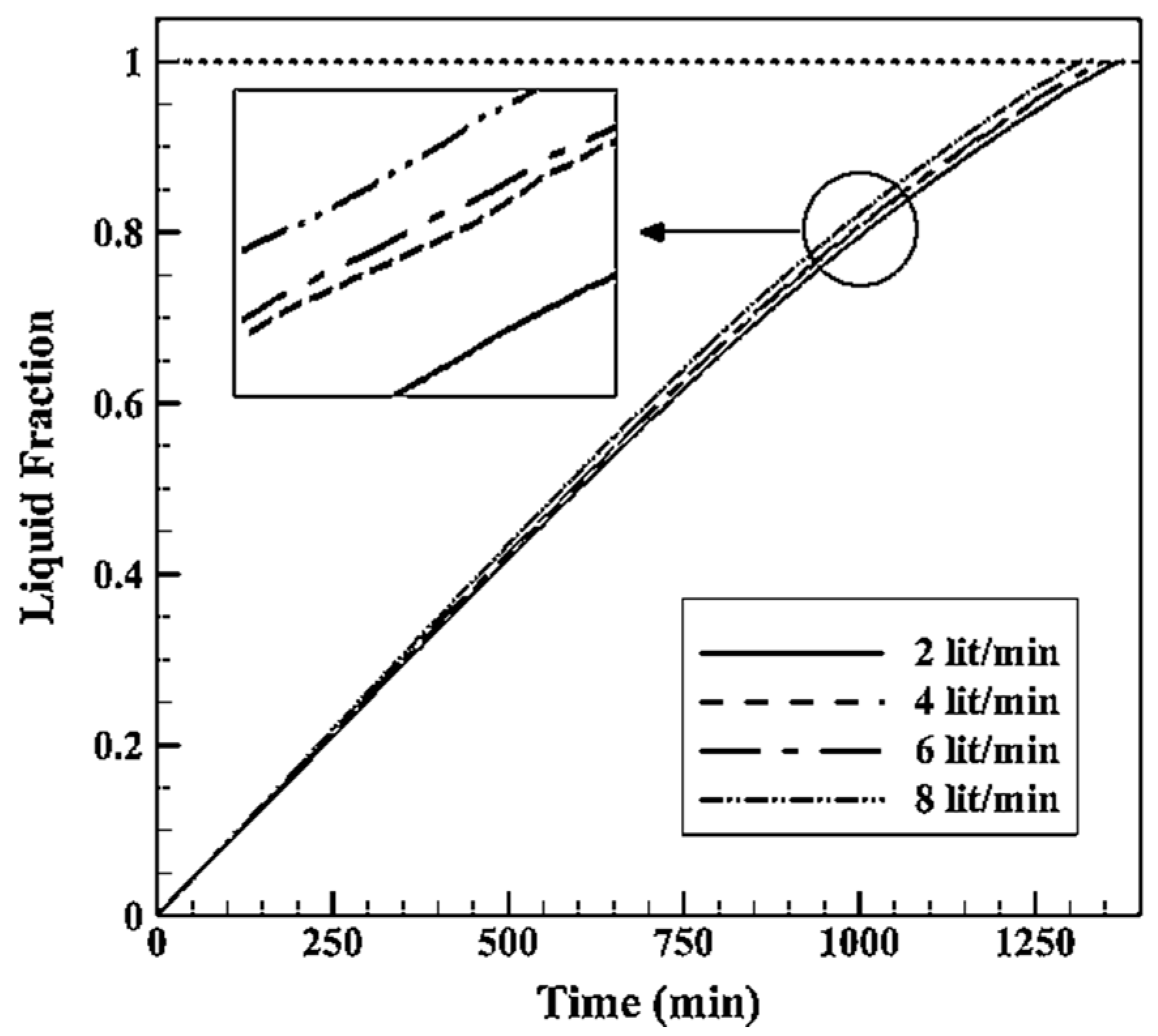
4.2.2. Effects of HTF Inlet Mass Flowrate
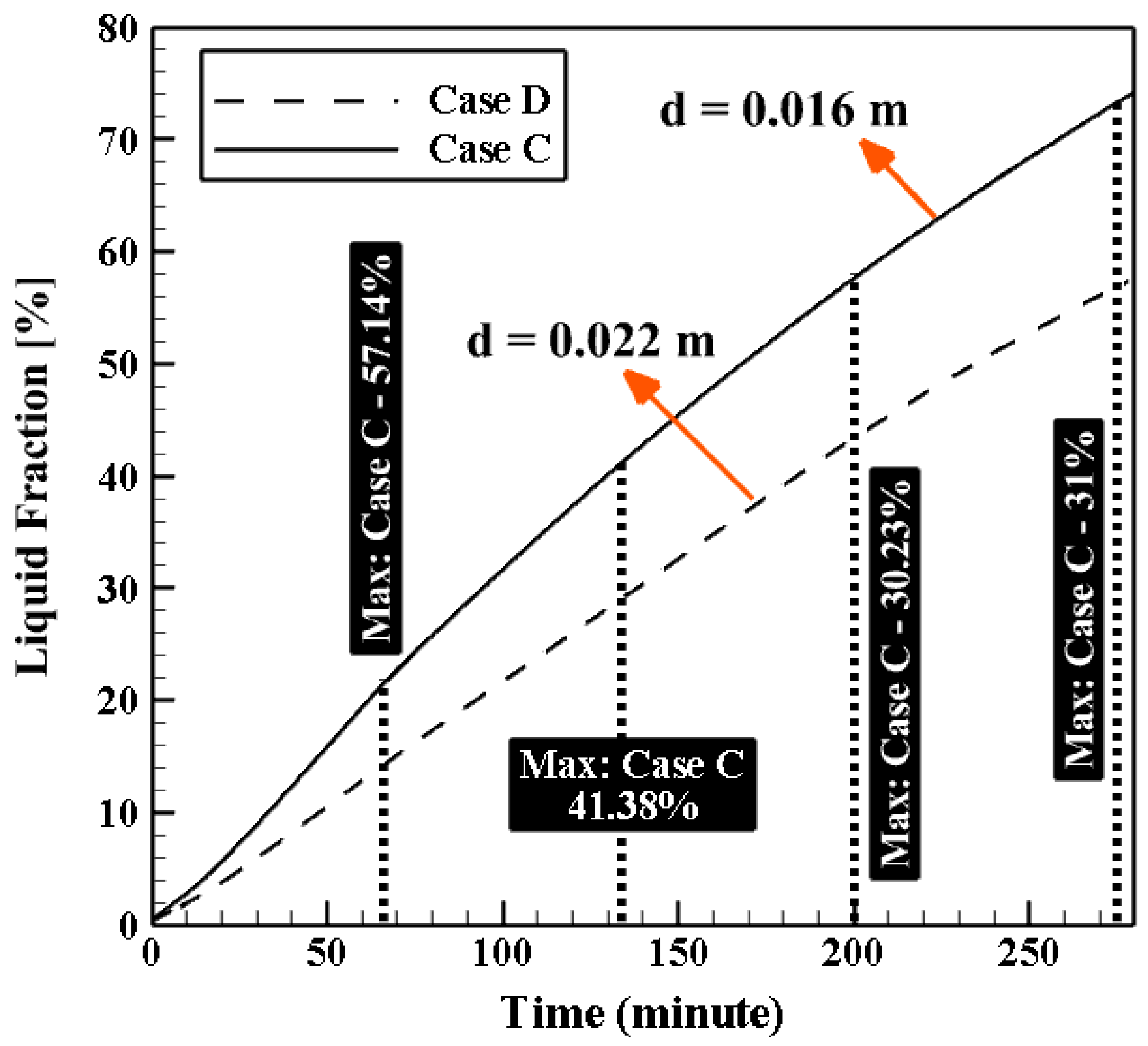
4.3. Influence of the Geometrical Parameters
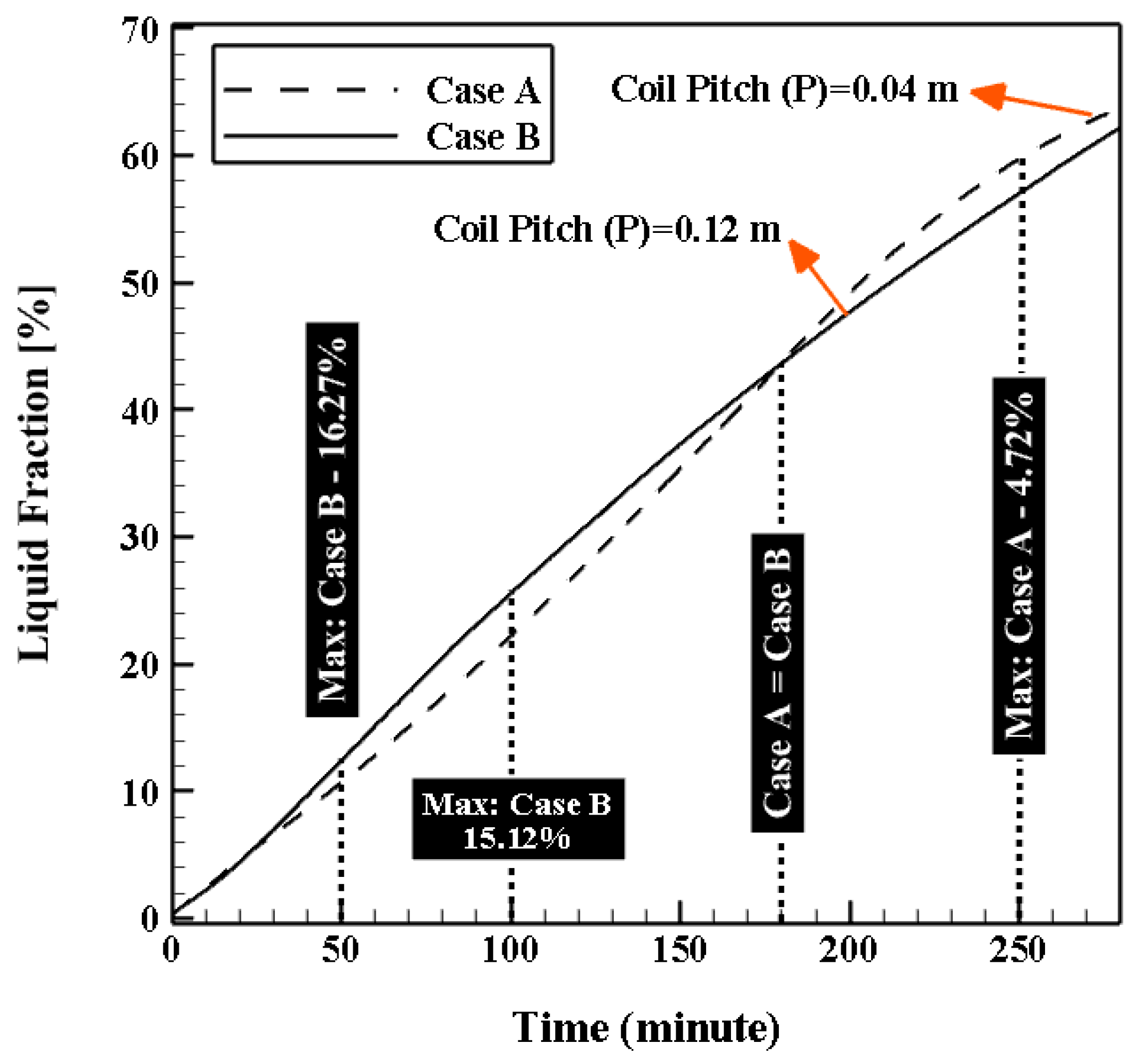
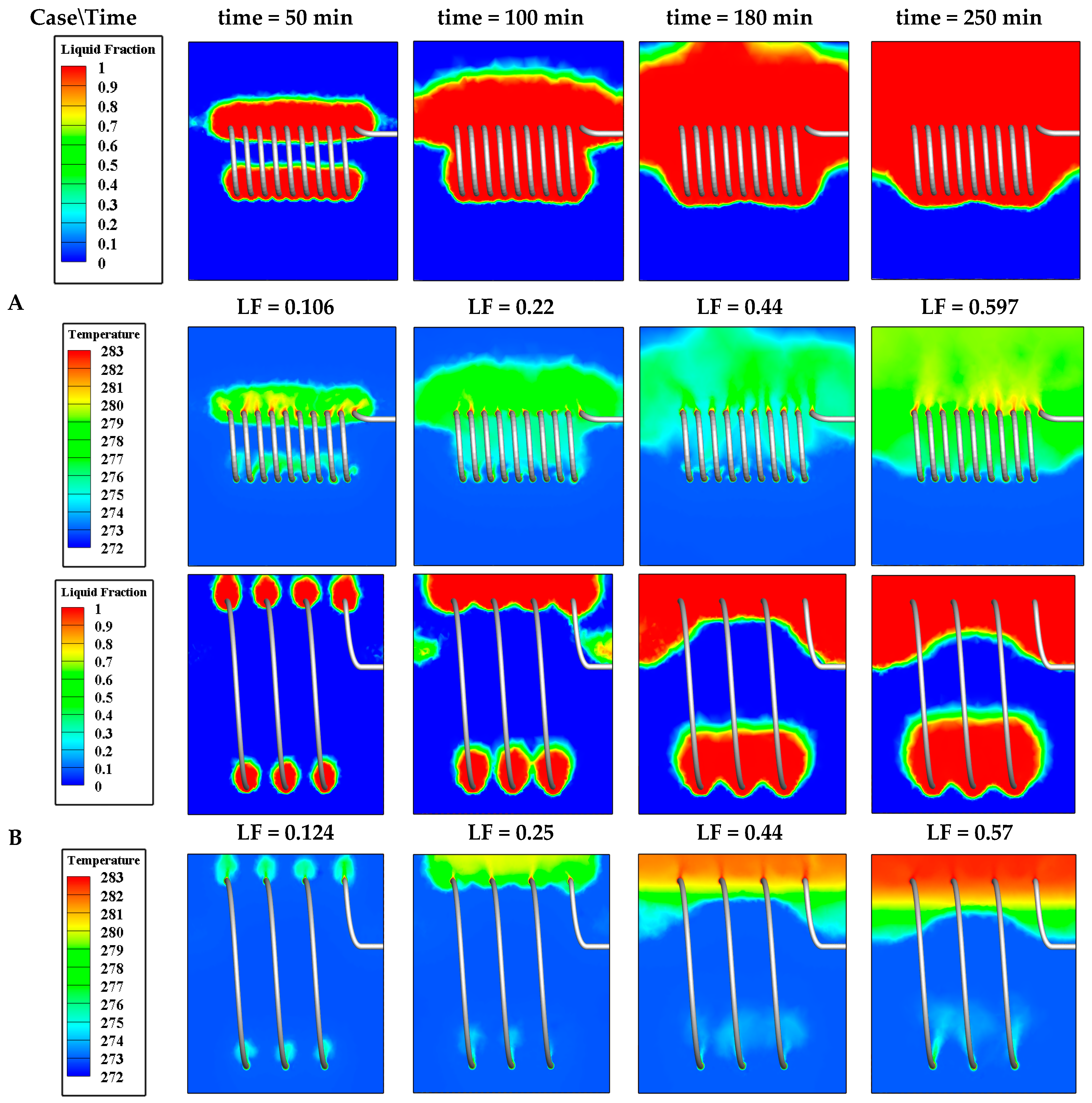
4.3.1. Effect of Coil Pitch (P)
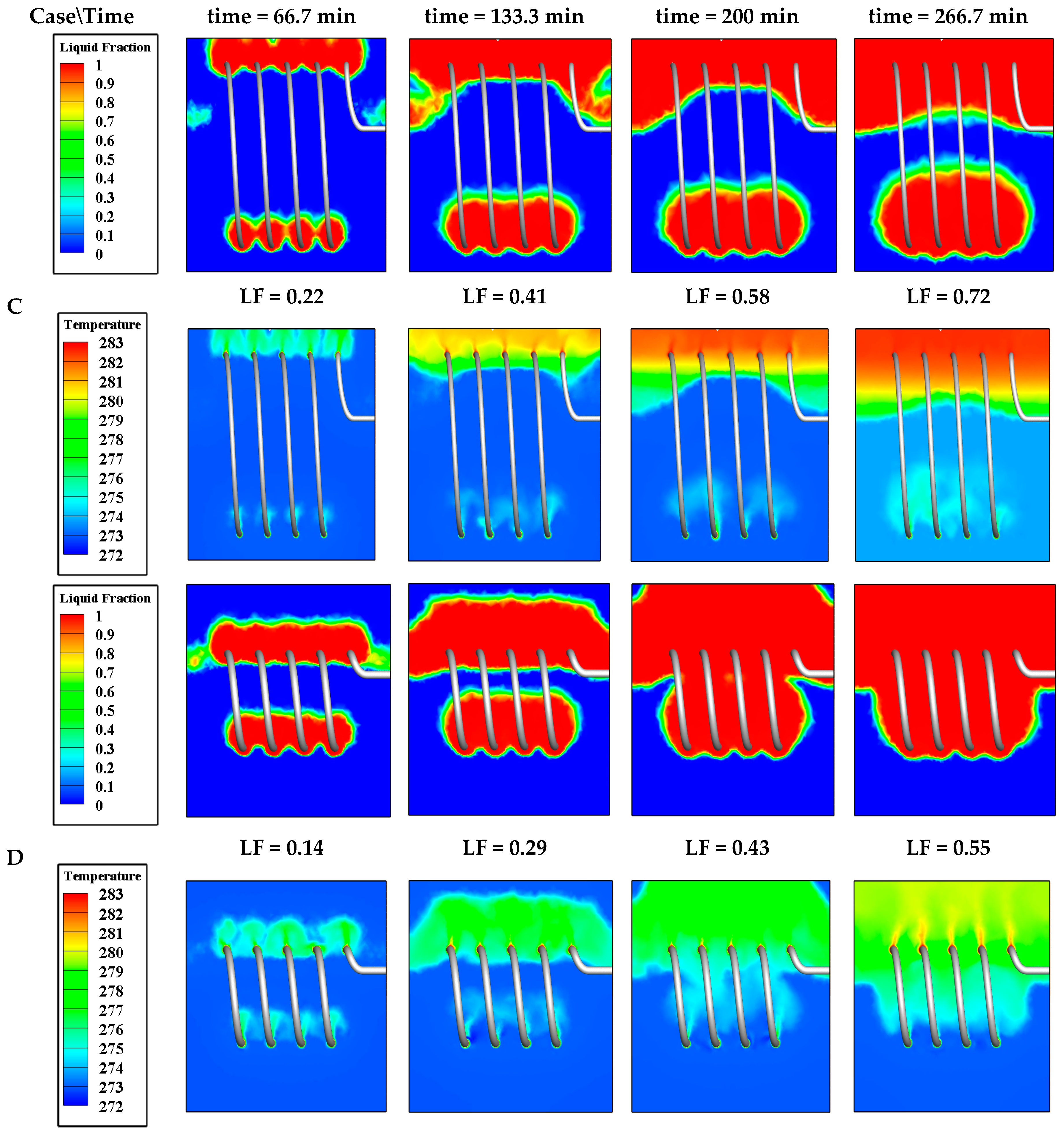
4.3.2. Effect of Coil Diameter (d)
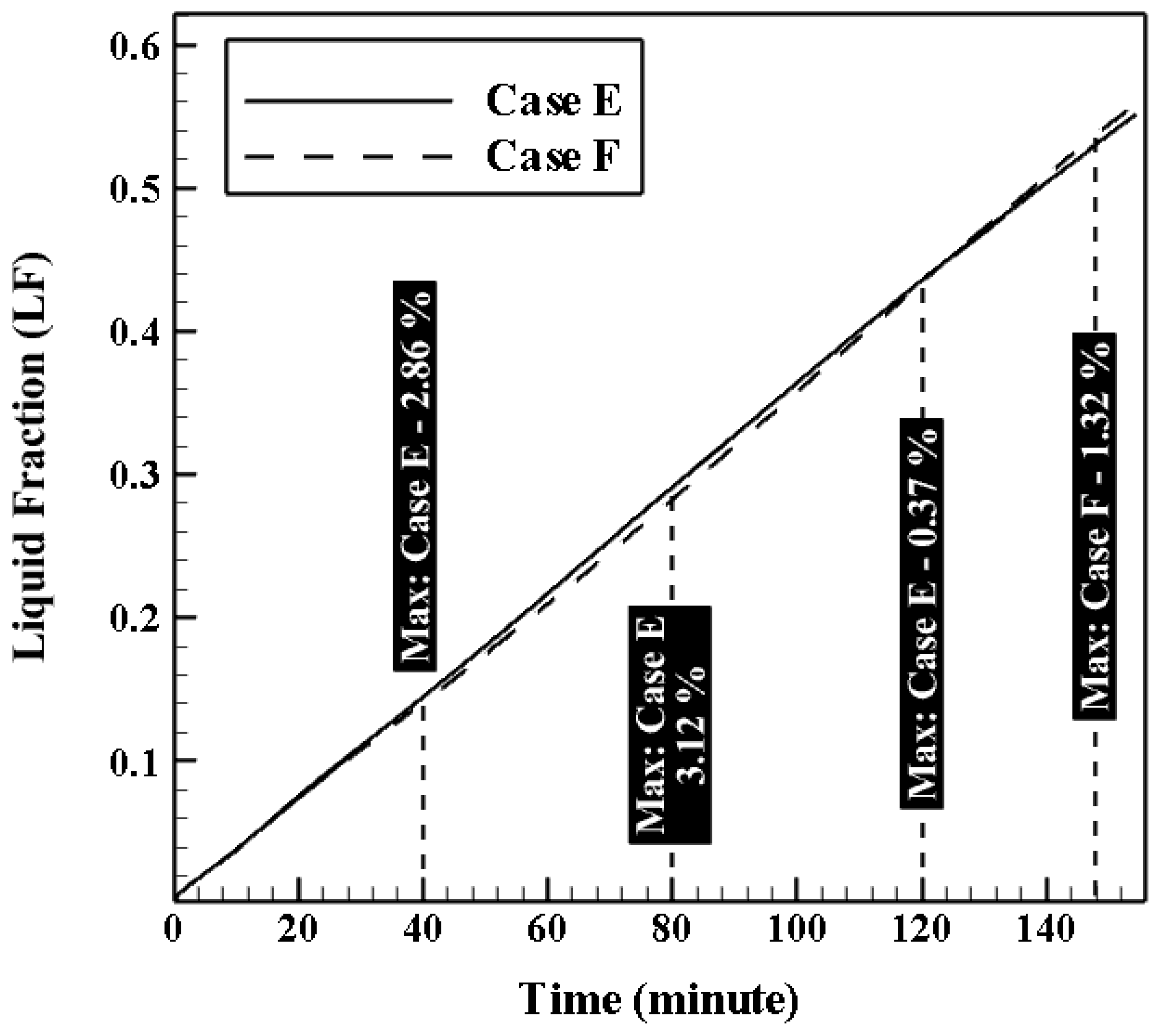
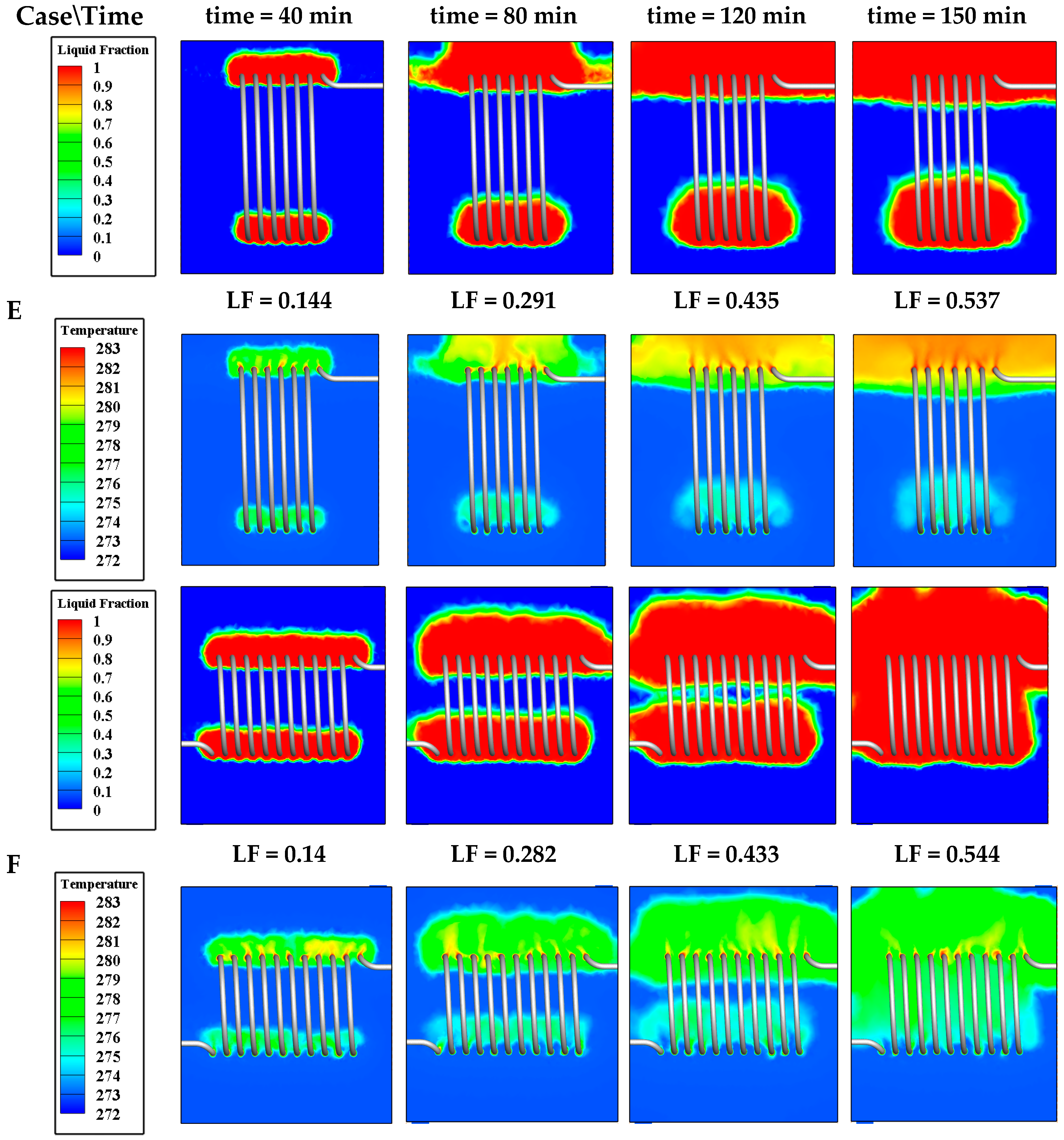
4.3.3. Effect of Coil Height (HC)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | mushy zone constant (kg × m3 × s) |
| CP | specific heat (J × kg−1 × K−1) |
| g | gravitational acceleration (m × s−2) |
| h | sensible enthalpy (J × kg−1) |
| H | enthalpy (J × kg−1) |
| k | thermal conductivity (W × m−1 × K−1) |
| L | latent heat fusion (J × kg−1) |
| T | temperature (K) |
| u | velocity (m × s−1) |
| Nu | Nusselt number, Nu = hd/K |
| De | Dean number, |
| Re | Reynolds number, Re = |
| Greek symbols | |
| µ | dynamic viscosity of the fluid (kg × m−1 × s−1) |
| ρ | density (kg × m−3) |
| ν | kinematic viscosity (m2 × s−1) |
| λ | liquid fraction (-) |
References
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Jang, M.C. Characteristics of electric power demand and promotion of the distribution of ice storage cooling system. In Proceedings of the SAREK 2000 Summer Annual Conference (I), Pyeongchang, Korea, 20 June 2000; pp. 795–804. [Google Scholar]
- Jannesari, H.; Abdollahi, N. Experimental and numerical study of thin ring and annular fin effects on improving the ice formation in ice-on-coil thermal storage systems. Appl. Energy 2017, 189, 369–384. [Google Scholar] [CrossRef]
- Torregrosa-Jaime, B.; López-Navarro, A.; Corberán, J.M.; Esteban-Matías, J.C.; Klinkner, L.; Payá, J. Experimental analysis of a paraffin-based cold storage tank. Int. J. Refrig. 2013, 36, 1632–1640. [Google Scholar] [CrossRef] [Green Version]
- López-Navarro, A.; Biosca-Taronger, J.; Torregrosa-Jaime, B.; Martínez-Galván, I.; Corberán, J.M.; Esteban-Matías, J.C.; Payá, J. Experimental investigation of the temperatures and performance of a commercial ice-storage tank. Int. J. Refrig. 2013, 36, 1310–1318. [Google Scholar] [CrossRef] [Green Version]
- López-Navarro, A.; Biosca-Taronger, J.; Torregrosa-Jaime, B.; Corberán, J.M.; Bote-García, J.L.; Payá, J. Experimental investigations on the influence of ice floating in an internal melt ice-on-coil tank. Energy Build. 2013, 57, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Ezan, M.A.; Ozdogan, M.; Erek, A. Experimental study on charging and discharging periods of water in a latent heat storage unit. Int. J. Therm. Sci. 2011, 50, 2205–2219. [Google Scholar] [CrossRef]
- Fertelli, A.; Büyükalaca, O.; Yilmaz, A. Ice formation around a horizontal tube in a rectangular vessel. J. Therm. Sci. Technol. 2009, 29, 75–87. [Google Scholar]
- Habeebullah, B.A. An experimental study on ice formation around horizontal long tubes. Int. J. Refrig. 2007, 30, 789–797. [Google Scholar] [CrossRef]
- Attia, A.A.; Altohamy, A.A.; Rabbo, M.A.; Sakr, R.Y. Comparative study on Al2O3 nanoparticle addition on cool storage system performance. Appl. Therm. Eng. 2016, 94, 449–457. [Google Scholar] [CrossRef]
- Yan, C.; Shi, W.; Li, X.; Zhao, Y. Optimal design and application of a compound cold storage system combining seasonal ice storage and chilled water storage. Appl. Energy 2016, 171, 1–11. [Google Scholar] [CrossRef]
- Erek, A.; Akif Ezan, M. Experimental and numerical study on charging processes of an ice-on-coil thermal energy storage system. Int. J. Energy Res. 2007, 31, 158–176. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Ji, C.; Wang, W.X. Numerical simulation of internal melt ice-on-coil thermal storage system. Energy Procedia 2011, 12, 1042–1048. [Google Scholar] [CrossRef]
- Xie, J.; Yuan, C. Numerical study of thin layer ring on improving the ice formation of building thermal storage system. Appl. Therm. Eng. 2014, 69, 46–54. [Google Scholar] [CrossRef]
- Xie, J.; Yuan, C. Parametric study of ice thermal storage system with thin layer ring by Taguchi method. Appl. Therm. Eng. 2016, 98, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Buyruk, E.; Fertelli, A.; Sonmez, N. Numerical investigation for solidification around various cylinder geometries. CSIR 2009, 68, 122–129. [Google Scholar]
- Ismail, K.A.; Sousa, L.M.; Lino, F.A. Solidification of PCM around curved tubes including natural convection effects. Int. J. Energy Eng. 2015, 5, 57–74. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Q.; Wennersten, R. The impact of refrigerant inlet temperature on the ice storage process in an ice-on-coil storage plate. Energy Procedia 2018, 145, 82–87. [Google Scholar] [CrossRef]
- Kim, M.H.; Duong, X.Q.; Chung, J.D. Performance enhancement of fin attached ice-on-coil type thermal storage tank for different fin orientations using constrained and unconstrained simulations. Heat Mass Transf. 2017, 53, 1005–1015. [Google Scholar] [CrossRef]
- Seddegh, S.; Wang, X.; Henderson, A.D. A comparative study of thermal behaviour of a horizontal and vertical shell-and-tube energy storage using phase change materials. Appl. Therm. Eng. 2016, 93, 348–358. [Google Scholar] [CrossRef]
- Brent, A.D.; Voller, V.R.; Reid, K.T.J. Enthalpy-Porosity technique for modeling convection-diffusion phase change: Application to the melting of a pure metal. Numer. Heat Transf. A Appl. 1988, 13, 297–318. [Google Scholar]
- Faghri, A.; Zhang, Y. Transport Phenomena in Multiphase Systems; Elsevier Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Mousavi Ajarostaghi, S.S.; Aghajani Delavar, M.; Dolati, A. Numerical investigation of melting process in phase change material (PCM) cylindrical storage considering different geometries. Heat Transf. Res. 2017, 48, 1515–1529. [Google Scholar] [CrossRef]
- Shih, Y.C.; Chou, H. Numerical study of solidification around staggered cylinders in a fixed space. Numer. Heat Transf. A Appl. 2005, 48, 239–260. [Google Scholar] [CrossRef]
- Sugawara, M.; Beer, H. Numerical analysis for freezing/melting around vertically arranged four cylinders. Heat Mass Transf. 2009, 45, 1223–1231. [Google Scholar] [CrossRef]
- Ashby, M.F.; Shercliff, H.; Cebon, D. Materials: Engineering, Science, Processing and Design; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar]
- Jamshidi, N.; Farhadi, M.; Ganji, D.D.; Sedighi, K. Experimental analysis of heat transfer enhancement in shell and helical tube heat exchangers. Appl. Therm. Eng. 2013, 51, 644–652. [Google Scholar] [CrossRef]
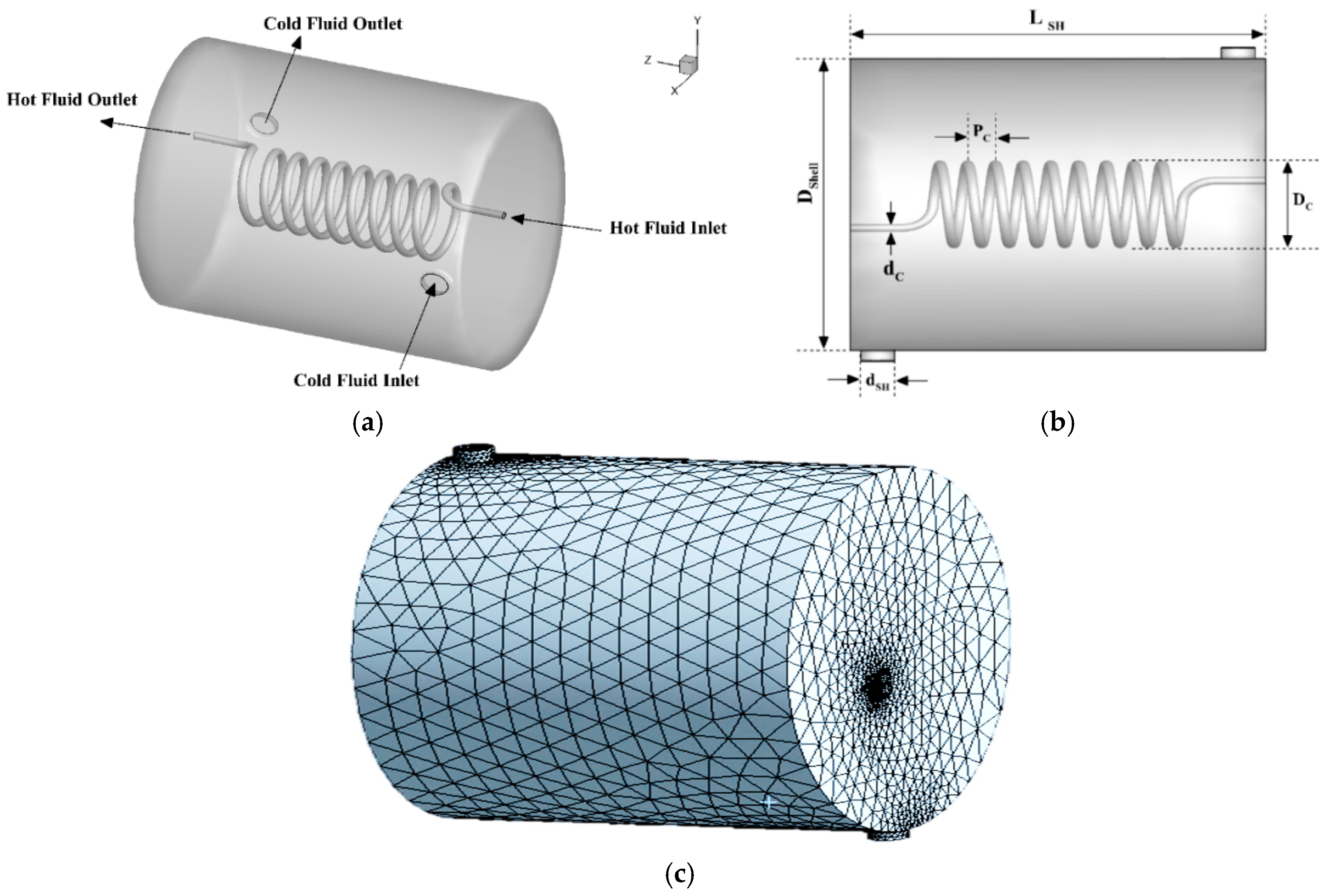

| Parameter | Notation | Value |
|---|---|---|
| Helix Diameter | DC | 81.3 mm |
| Coil Diameter | dC | 9 mm |
| Coil Pitch | PC | 15 mm |
| Shell Length | LSH | 250 mm |
| Shell Diameter | DSH | 140 mm |
| Hot Flow Inlet Temperature | THot-Inlet | 50 °C |
| Cold Flow Inlet Temperature | TCold-Inlet | 20 °C |
| Cold Flow Inlet Velocity (Shell) | VCold-Inlet | 0.008 m/s |
| Hot Flow Inlet Velocity (Tube) | VHot-Inlet | 0.08, 0.165, 0.248 and 0.331 m/s |
| Parameter | Symbol | Value |
|---|---|---|
| Density of Fluid (kg/m3) | ρf | 999.833 |
| Specific Heat of the Fluid (J/(kg·K)) | Cl | 4180 |
| Specific Heat of the Solid (J/(kg·K)) | CS | 2217 |
| Thermal conductivity of liquid water (W/(m·K)) | KS | 1.918 |
| Thermal conductivity of ice (W/(m·K)) | Kl | 0.578 |
| Viscosity of the fluid (kg/(m·s)) | µf | 0.00162 |
| Latent Heat of Phase Change (J/kg) | L | 334,000 |
| Melting Temperature (K) | Tf | 273.15 |
| Density of Ethylene Glycol (kg/m3) | ρEG | 1111.4 |
| Specific Heat of Ethylene Glycol (J/(kg·K)) | CEG | 2415 |
| Thermal conductivity of Ethylene Glycol (W/(m·K)) | KEG | 0.252 |
| Viscosity of Ethylene Glycol (kg/(m·s)) | µEG | 0.0157 |
| Models | Height of Spiral Coil | Coil Pitch | Helix Diameter | Spiral Coil Length | Coil Diameter | Heat Transfer Area | |
|---|---|---|---|---|---|---|---|
| HC (m) | P (m) | DC (m) | L (m) | d (m) | A (m2) | ||
| Effect of P | Case A | 0.36 | 0.04 | 0.17 | 4.83 | 0.016 | 9.7 × 10−4 |
| Case B | 0.36 | 0.12 | 0.48 | 4.55 | 0.016 | 9.7 × 10−4 | |
| Effect of d | Case C | 0.36 | 0.09 | 0.48 | 6.05 | 0.016 | 1.2 × 10−3 |
| Case D | 0.36 | 0.09 | 0.25 | 3.17 | 0.022 | 1.2 × 10−3 | |
| Effect of HC | Case E | 0.24 | 0.04 | 0.43 | 8.13 | 0.016 | 1.6 × 10−3 |
| Case F | 0.42 | 0.04 | 0.25 | 8.27 | 0.016 | 1.6 × 10−3 | |
| Parameters | Symbol | Value (mm) |
|---|---|---|
| Height of the shell | L | 700 |
| Shell diameter | DSh | 620 |
| Shell inlet and outlet diameter | dSh | 25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi Ajarostaghi, S.S.; Poncet, S.; Sedighi, K.; Aghajani Delavar, M. Numerical Modeling of the Melting Process in a Shell and Coil Tube Ice Storage System for Air-Conditioning Application. Appl. Sci. 2019, 9, 2726. https://doi.org/10.3390/app9132726
Mousavi Ajarostaghi SS, Poncet S, Sedighi K, Aghajani Delavar M. Numerical Modeling of the Melting Process in a Shell and Coil Tube Ice Storage System for Air-Conditioning Application. Applied Sciences. 2019; 9(13):2726. https://doi.org/10.3390/app9132726
Chicago/Turabian StyleMousavi Ajarostaghi, Seyed Soheil, Sébastien Poncet, Kurosh Sedighi, and Mojtaba Aghajani Delavar. 2019. "Numerical Modeling of the Melting Process in a Shell and Coil Tube Ice Storage System for Air-Conditioning Application" Applied Sciences 9, no. 13: 2726. https://doi.org/10.3390/app9132726
APA StyleMousavi Ajarostaghi, S. S., Poncet, S., Sedighi, K., & Aghajani Delavar, M. (2019). Numerical Modeling of the Melting Process in a Shell and Coil Tube Ice Storage System for Air-Conditioning Application. Applied Sciences, 9(13), 2726. https://doi.org/10.3390/app9132726








