Erosion Resistance Properties of Iron–Carbon Composite Plating to Molten Lead-Free Solder
Abstract
1. Introduction
2. Experimental Procedure
2.1. Preparation of Specimens
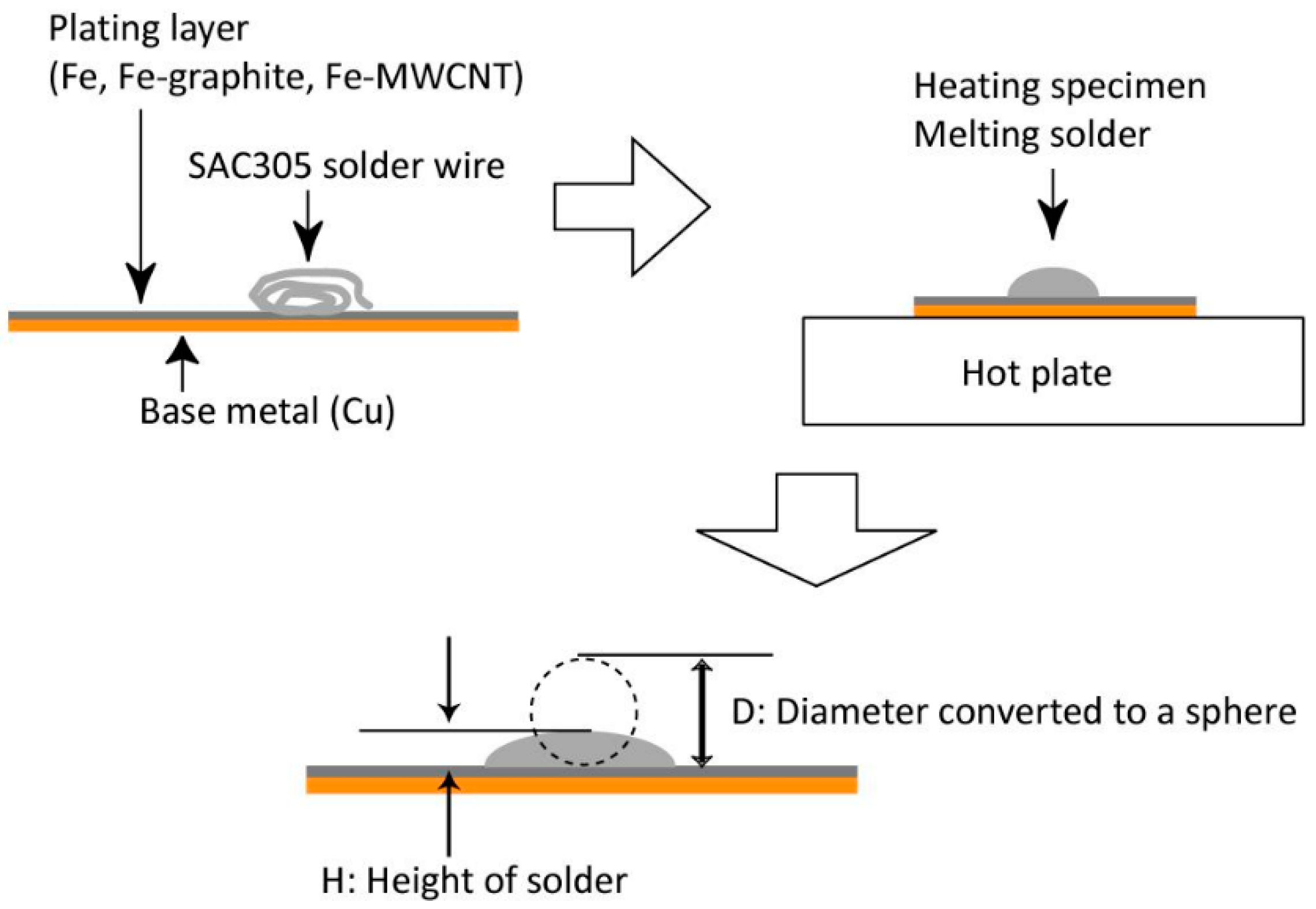
2.2. Solderability Test
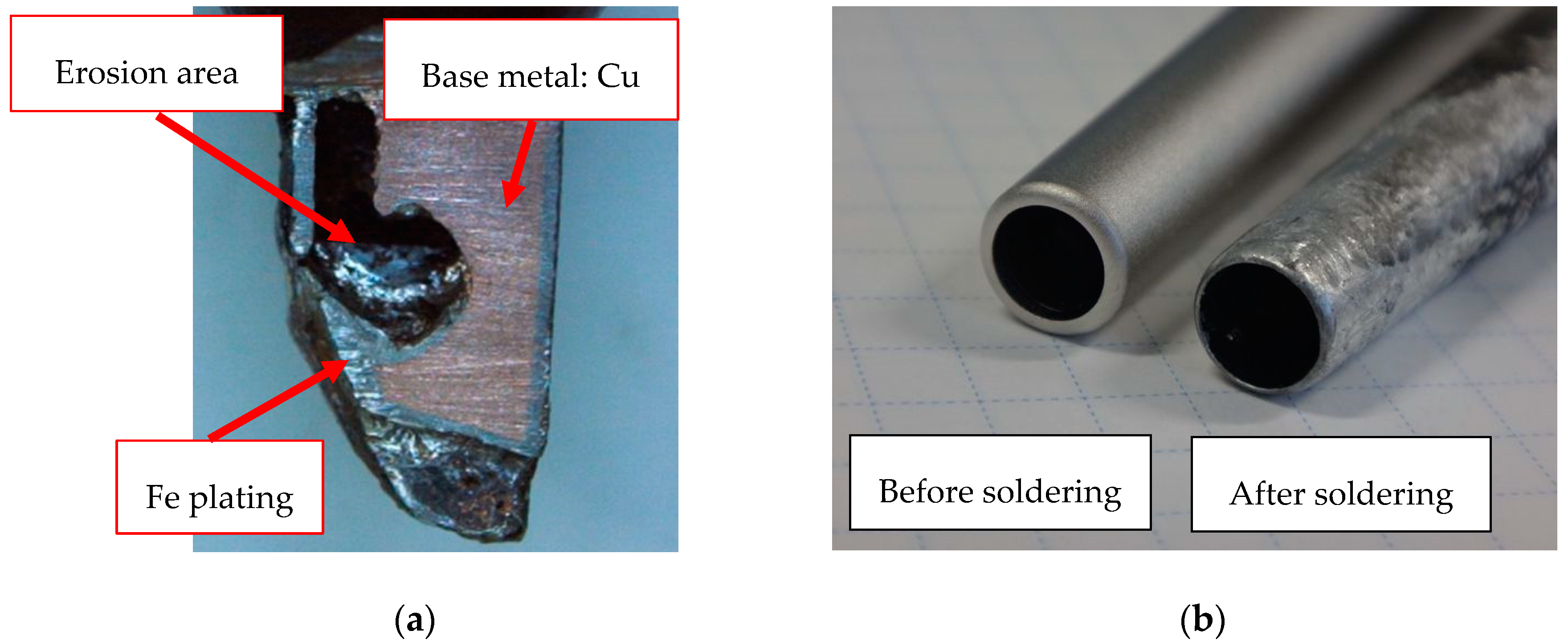
2.3. Erosion-Resistance Test
3. Results and Discussion
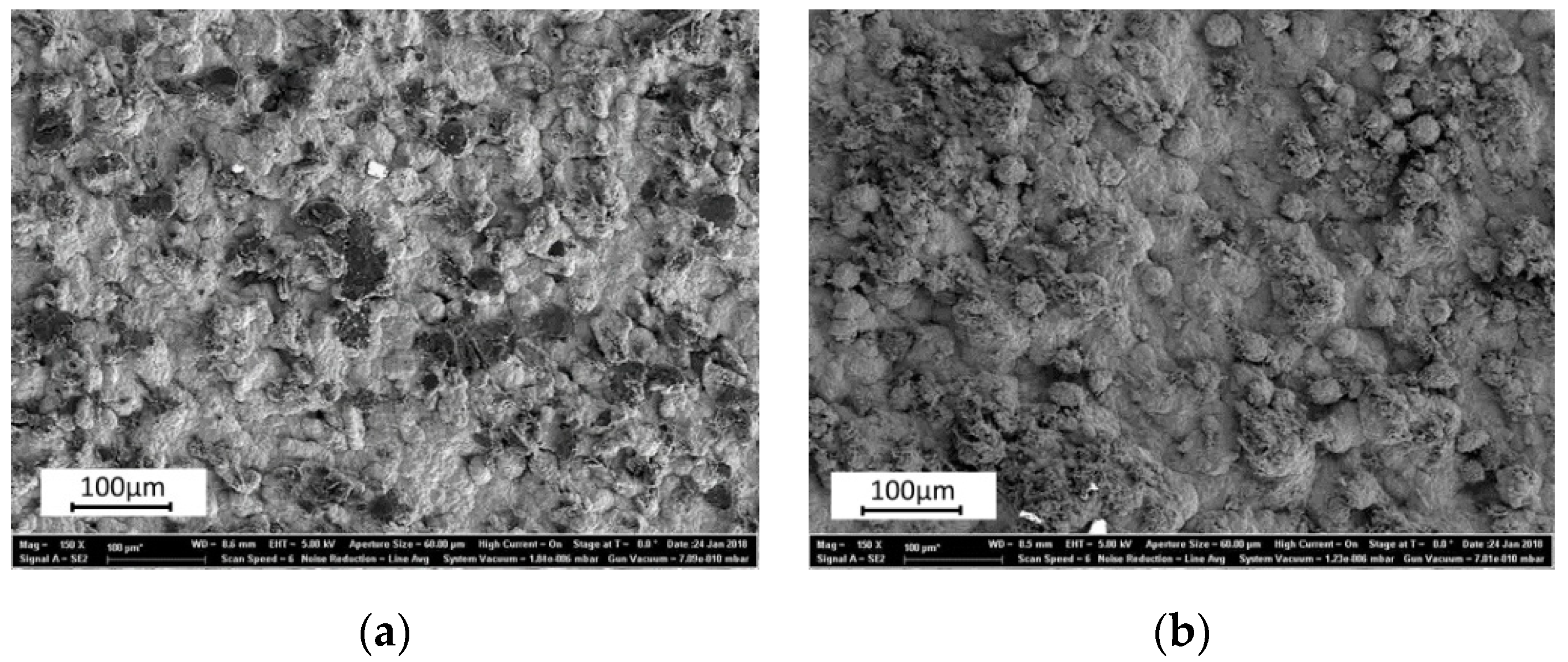
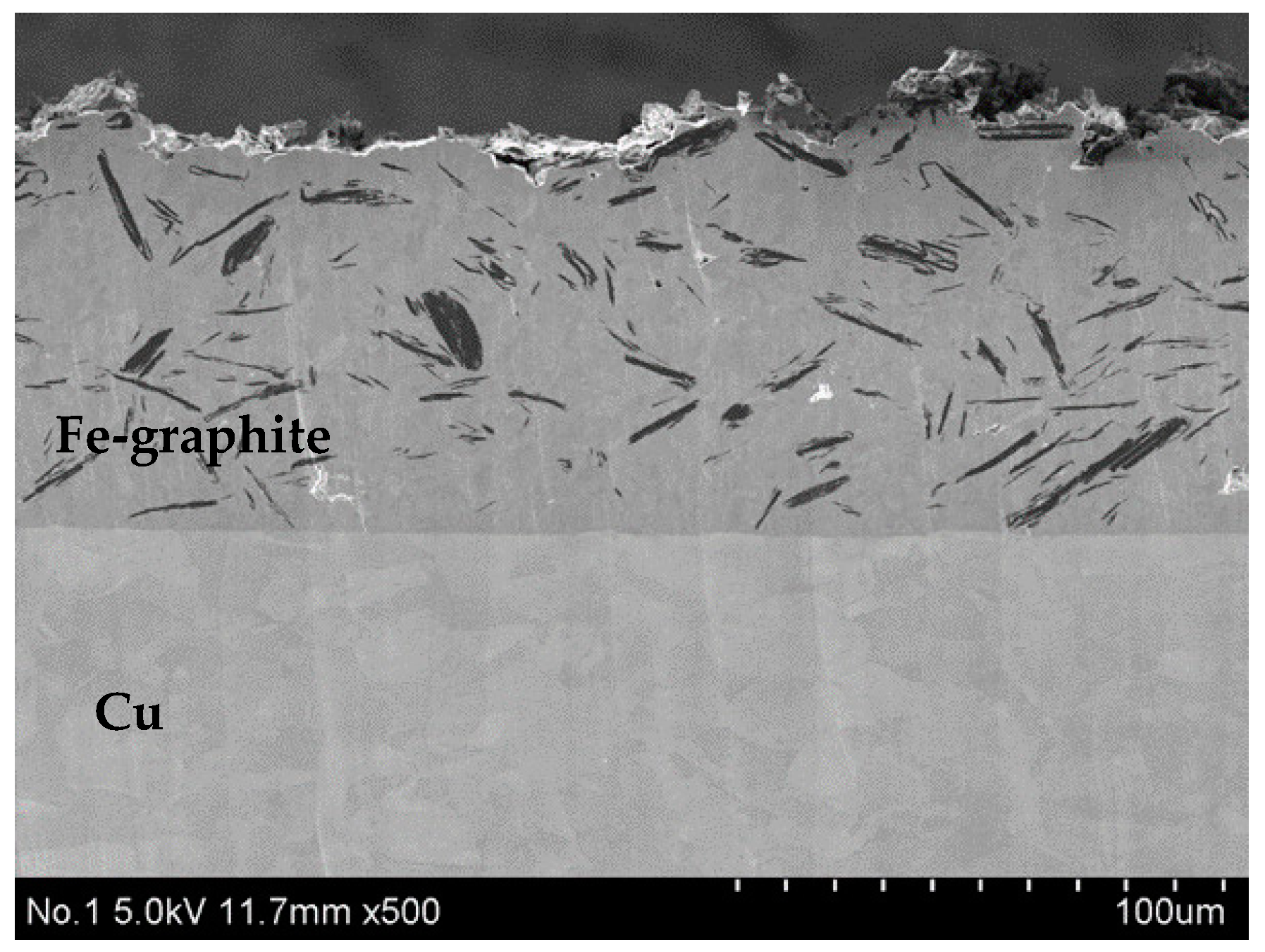
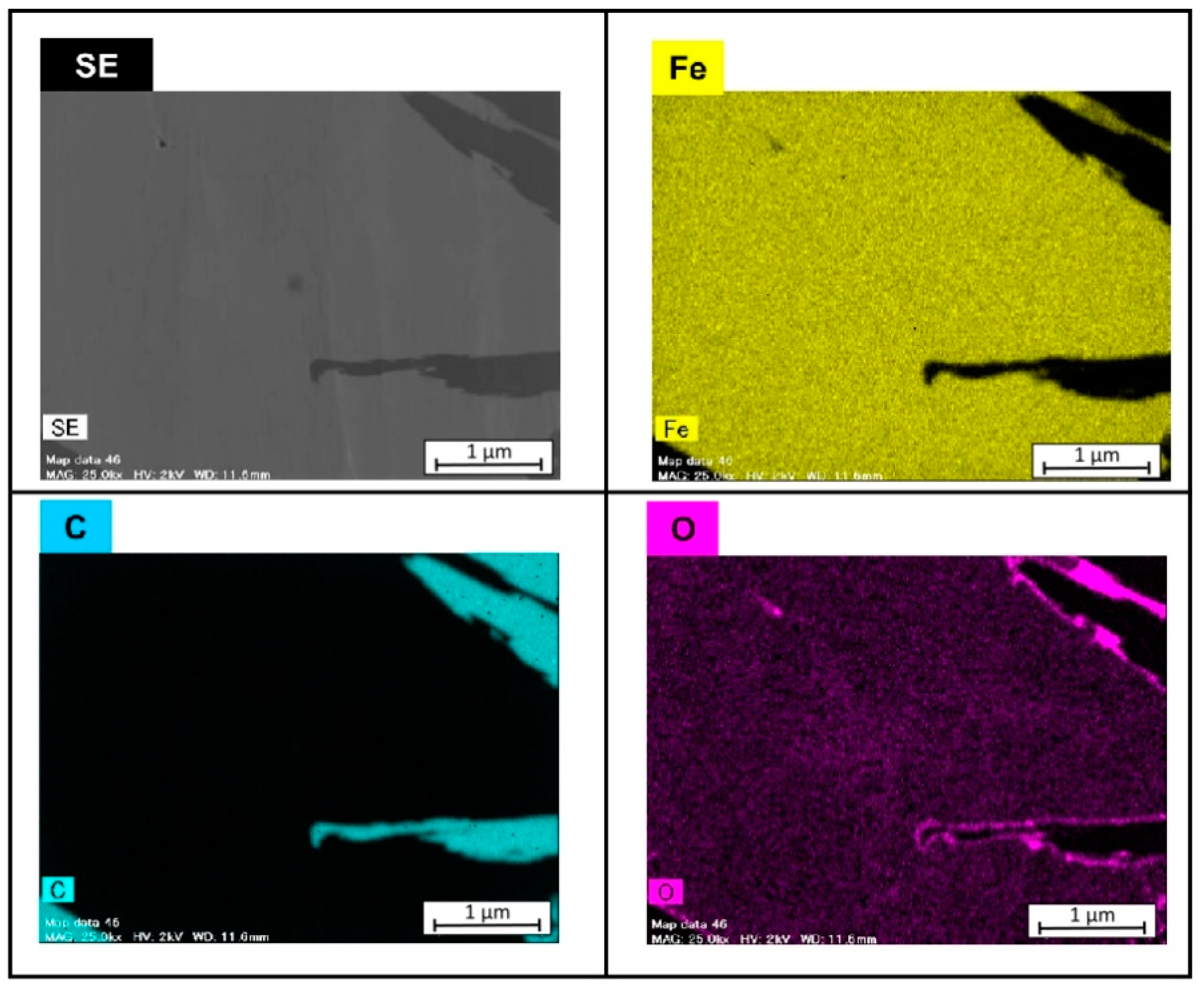
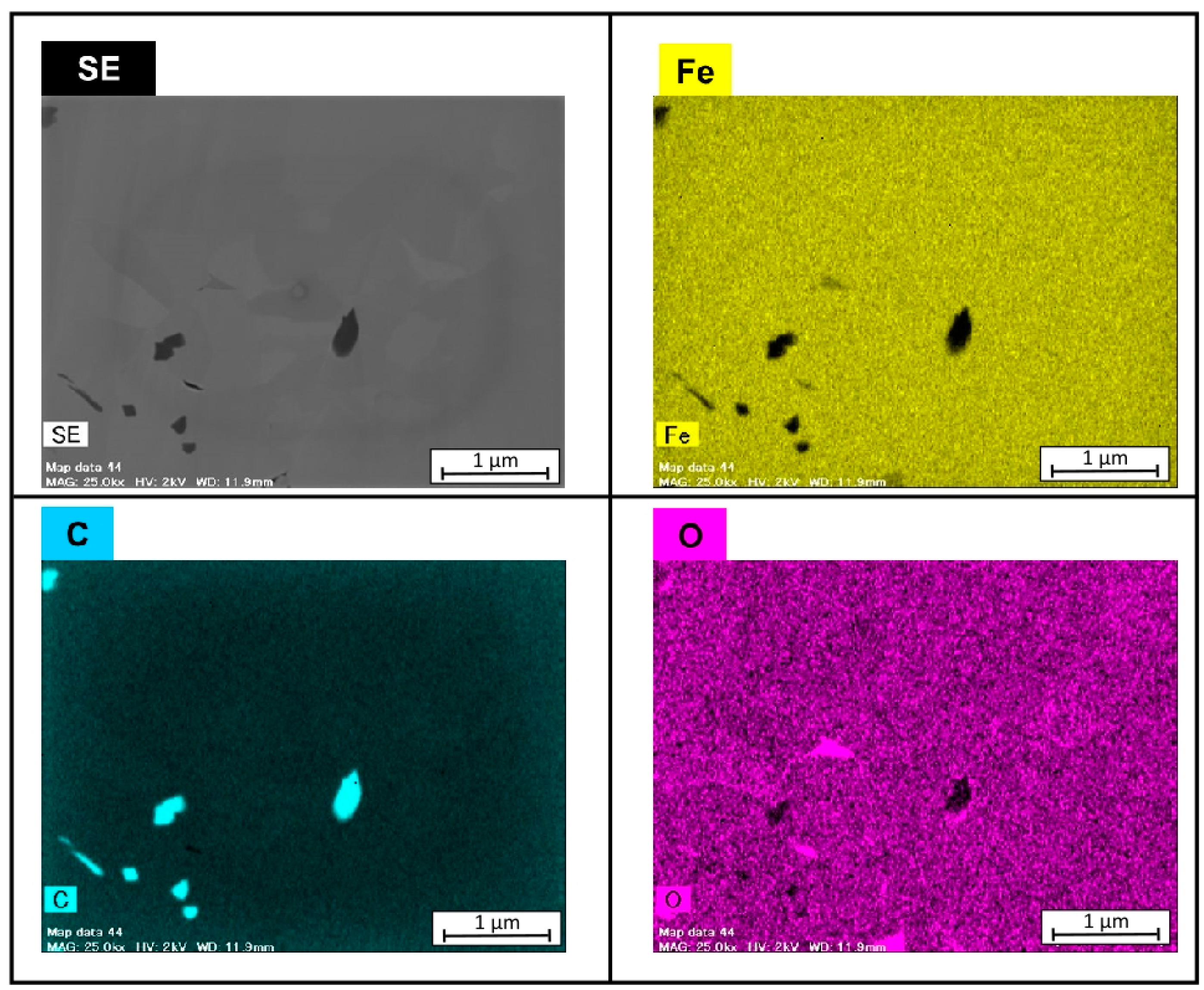
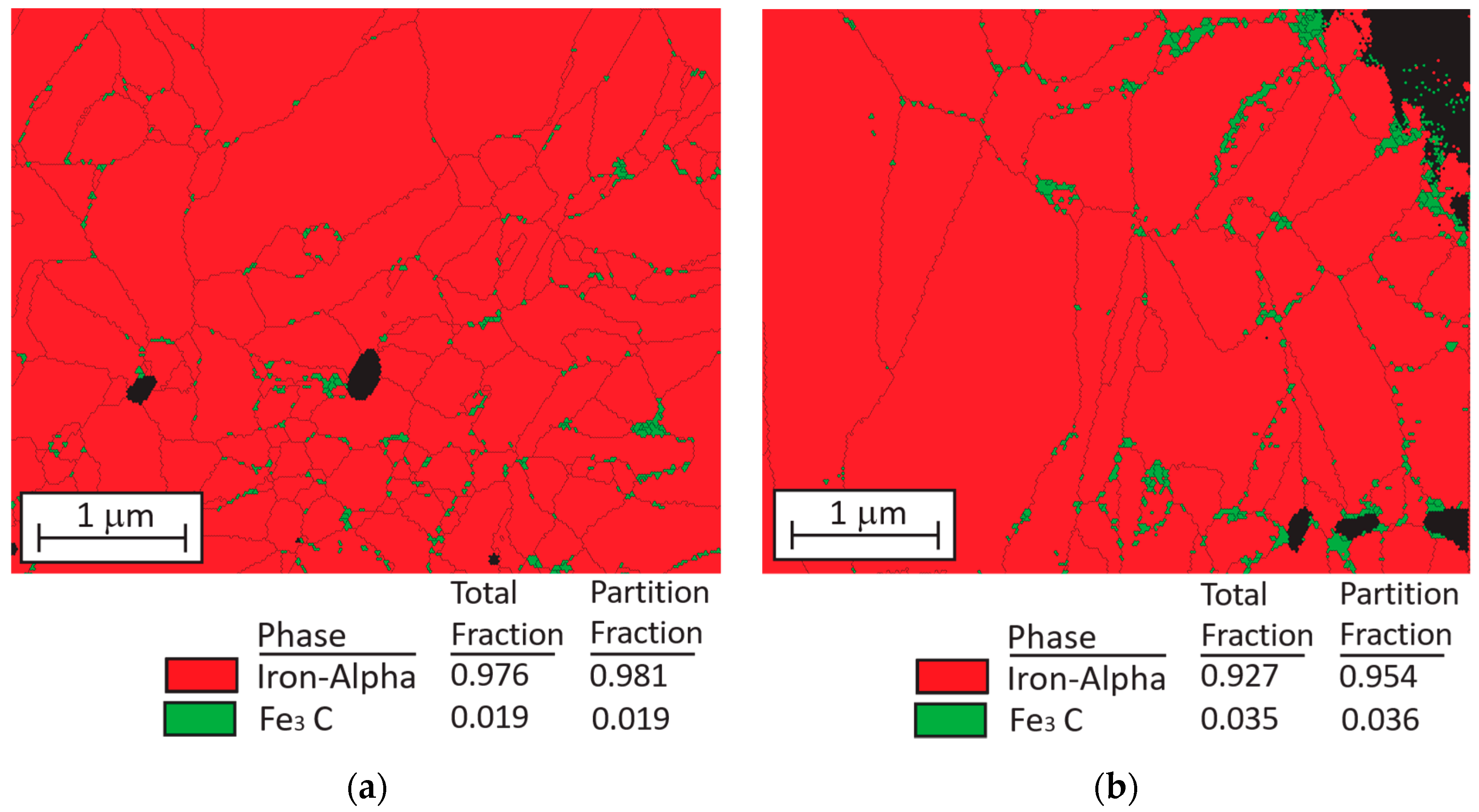
3.1. Microsturucture of Fe–Graphite and Fe–MWCNT Composite Plating Layers
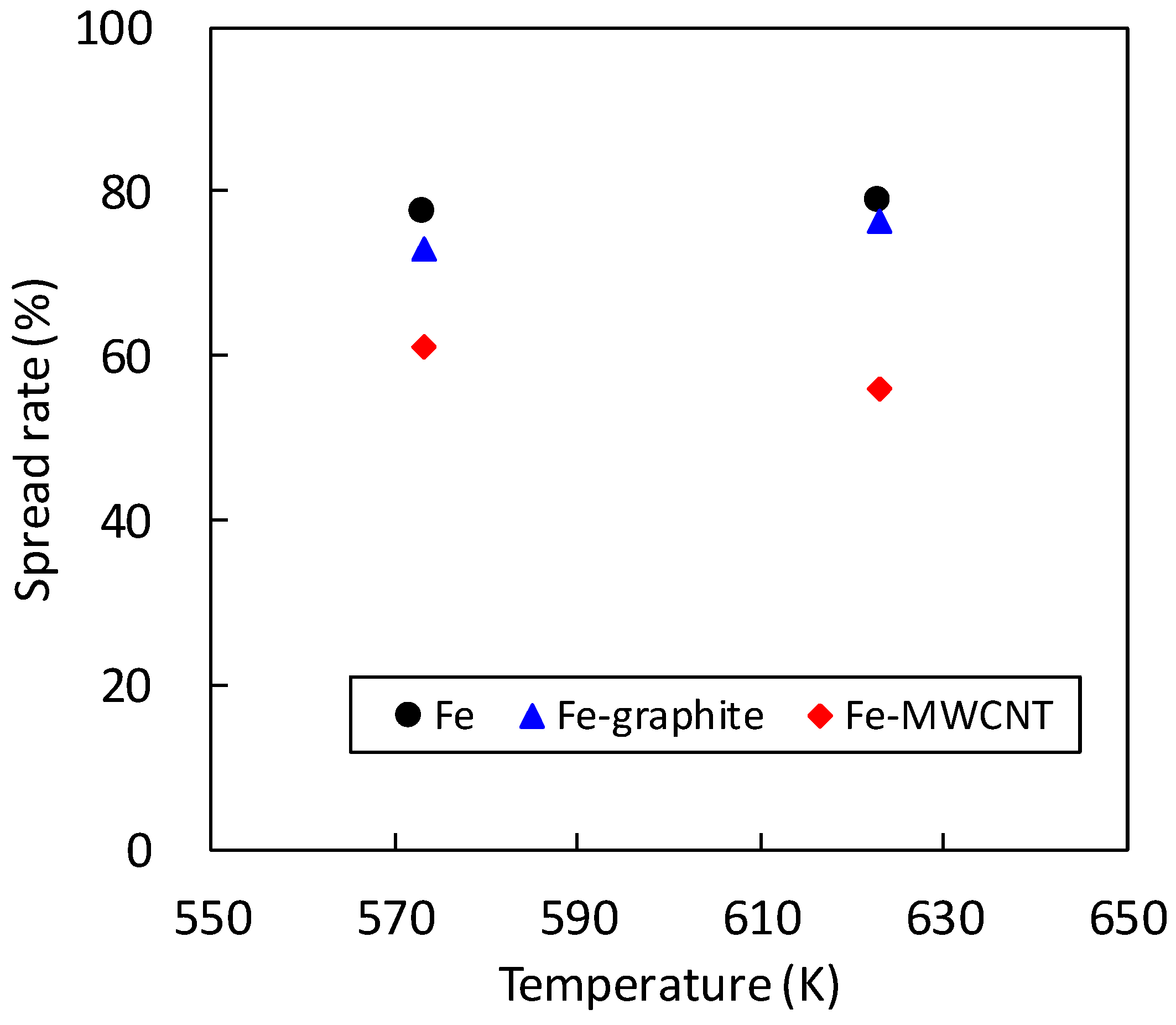
3.2. Solderability Test
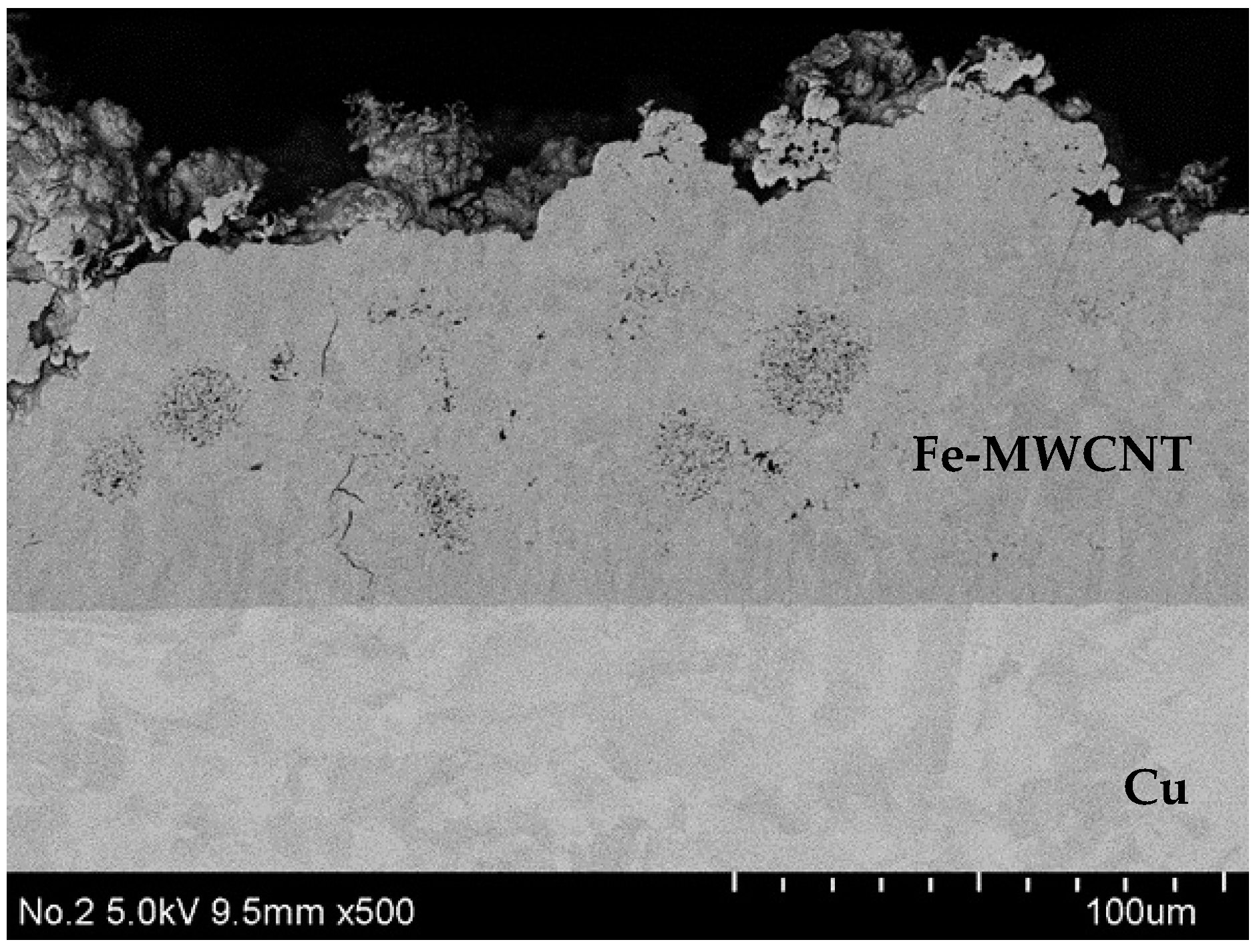
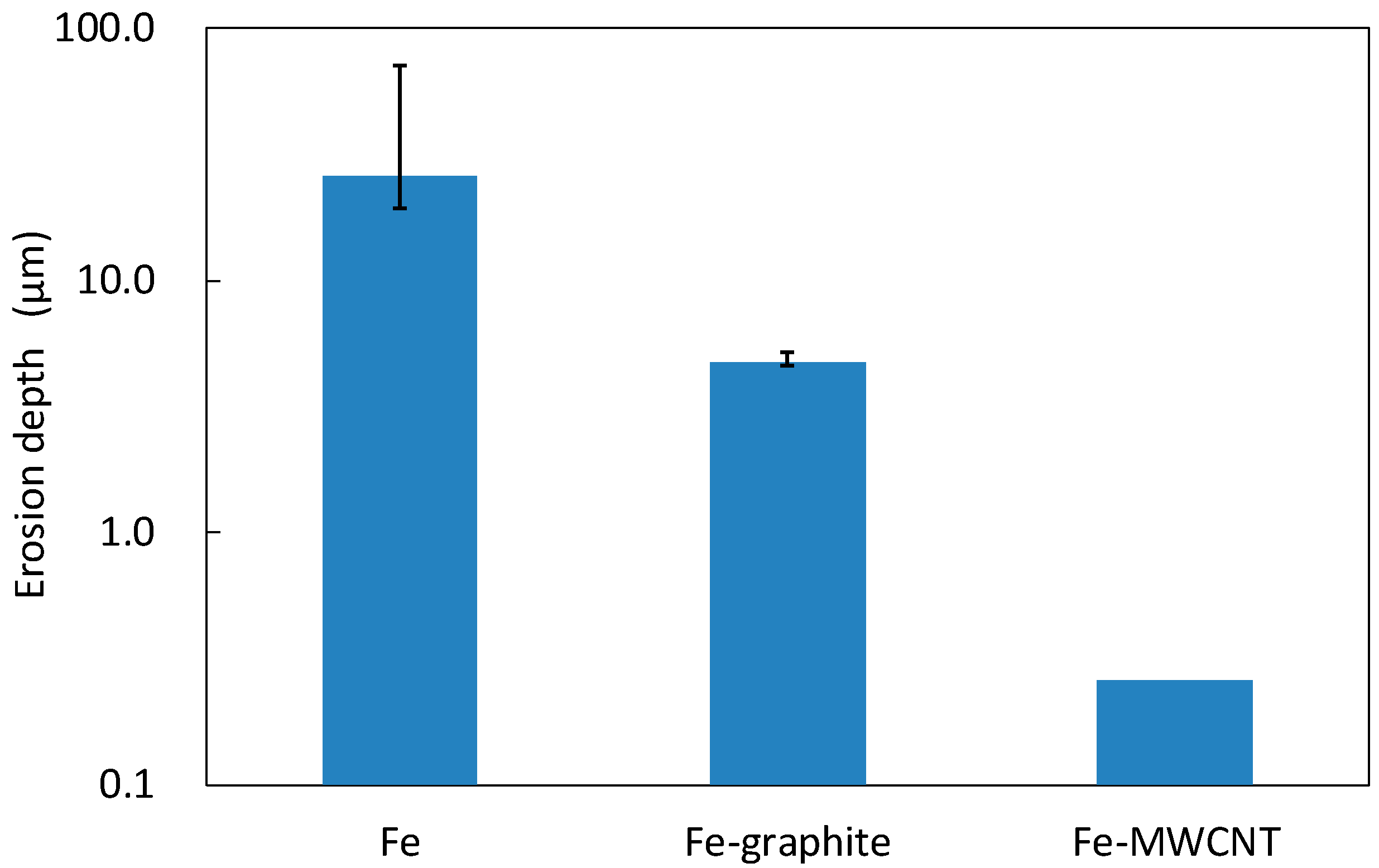
3.3. Erosion-Resistance Test
4. Conclusions
- (1)
- Graphite and MWCNT were compounded not only on the Fe plating surface but also inside the plating film. Fibrous graphite can be uniformly dispersed in the Fe plating layer. MWCNTs are grouped together in small patches in the Fe plating layer.
- (2)
- Although the solderability of molten SAC305 decreased in the Fe–graphite and the Fe–MWCNT composite plating layers, the spread rates of SAC305 were more than 50%, which is a practical level. Therefore, they can be applied to the solder iron tip, the point soldering nozzle and so on.
- (3)
- From the results of the erosion-resistance test, it was shown that the Fe–graphite and the Fe–MWCNT composite plating layers have the effect of reducing erosion in molten SAC305 compared to the Fe plating layer.
- (4)
- EBSD analysis results revealed that a small amount of Fe3C compounds form in grain boundaries of α-Fe in both Fe–graphite and Fe–MWCNT plating layers. Since Fe3C particles suppress the diffusion of Fe into SAC305 solder, erosion-resistance characteristics can be further improved by forming Fe3C in the plating film efficiently.
- (5)
- By using not only a solder iron tip but also soldering bath made of Fe–MWCNT composite plating, it is expected that erosion can be suppressed, and it can be used for a longer time.
Author Contributions
Funding
Conflicts of Interest
References
- Morris, J.; O’Keefe, M.J. Equipment impacts of lead free wave soldering. Proc. APEX 2003 2003, 1, 1–9. [Google Scholar]
- Nishikawa, H.; Takemoto, T.; Kifune, K.; Uetani, T.; Sekimori, N. Effect of iron plating conditions on reaction in molten lead-free solder. Mater. Trans. 2004, 3, 741–746. [Google Scholar] [CrossRef][Green Version]
- Takemoto, T.; Takemoto, M. Dissolution of stainless steels in molten lead-free solders. Solder. Surf. Mt. Technol. 2006, 18, 24–30. [Google Scholar] [CrossRef]
- Serizawa, K.; Takemoto, T.; Yamamoto, K. Analysis of erosion mechanism and development of evaluation method for lead-free soldering equipment. In Proceedings of the 15th Symposium on Microjoining and Assembly Technology in Electronics, Yokohama, Japan, 29–30 January 2009; pp. 379–382. [Google Scholar]
- Takemoto, T.; Nishikawa, H.; Serizawa, K.; Yamamoto, K. Mechanism of damage of flow soldering bath by molten lead-free solder and its prevention methods. In Proceedings of the 15th Symposium on Microjoining and Assembly Technology in Electronics, Yokohama, Japan, 29–30 January 2009; pp. 383–386. [Google Scholar]
- Nishikawa, H.; Sabase, K.; Takemoto, T. Effect of some factor on erosion of stainless steel by molten lead-free solder. In Proceedings of the 15th Symposium on Microjoining and Assembly Technology in Electronics, Yokohama, Japan, 29–30 January 2009; pp. 387–390. [Google Scholar]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Miyazaki, M.; Watanabe, J.; Hatsuzawa, K.; Arai, S. Wettability and erosion resistance of Ni-MWCNT composite film for lead-free solder. In Proceedings of the 16th Symposium on Microjoining and Assembly Technology in Electronics, Yokohama, Japan, 2–3 February 2010; pp. 235–238. [Google Scholar]
- Yamauchi, A.; Sekito, Y.; Kurokawa, K.; Tanaka, J. Corrosion behavior of Fe-based alloys in molten lead-free solders. In Proceedings of the 17th Micro Electronics Symposium, Kobe, Japan, 13–14 September 2007; pp. 107–110. [Google Scholar]
- Kawamoto, T.; Yamauchi, A.; Kawakubo, S.; Irisawa, A.; Kurokawa, K.; Tanaka, J. Relationship between growth rate of interfacial reaction layer and carbon content in solder/carbon steel system. In Proceedings of the Japan Institute of Electronics Packaging, Yokohama, Japan, 8–10 March 2011; pp. 165–166. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, J.; Hatsuzawa, K.; Ogata, S.; Yoshida, S.; Shohji, I. Erosion Resistance Properties of Iron–Carbon Composite Plating to Molten Lead-Free Solder. Appl. Sci. 2019, 9, 2724. https://doi.org/10.3390/app9132724
Watanabe J, Hatsuzawa K, Ogata S, Yoshida S, Shohji I. Erosion Resistance Properties of Iron–Carbon Composite Plating to Molten Lead-Free Solder. Applied Sciences. 2019; 9(13):2724. https://doi.org/10.3390/app9132724
Chicago/Turabian StyleWatanabe, Jun, Kenji Hatsuzawa, Shigeyuki Ogata, Shinichi Yoshida, and Ikuo Shohji. 2019. "Erosion Resistance Properties of Iron–Carbon Composite Plating to Molten Lead-Free Solder" Applied Sciences 9, no. 13: 2724. https://doi.org/10.3390/app9132724
APA StyleWatanabe, J., Hatsuzawa, K., Ogata, S., Yoshida, S., & Shohji, I. (2019). Erosion Resistance Properties of Iron–Carbon Composite Plating to Molten Lead-Free Solder. Applied Sciences, 9(13), 2724. https://doi.org/10.3390/app9132724




