Featured Application
Using autonomous underwater vehicles equipped with telemetry-based payload control to locate marine fish tagged with acoustic transmitters.
Abstract
An autonomous underwater vehicle (AUV) under payload control (PC) was used to map the movements of juvenile Chinook salmon (Oncorhynchus tshawytscha) tagged with acoustic transmitters. After detecting a tag, the AUV deviated from its pre-programmed route and performed a maneuver designed to enhance the location estimate of the fish and to move closer to collect proximal environmental data. Nineteen fish were released into marine waters of southeastern Alaska. Seven missions with concurrent AUV and vessel-based surveys were conducted with two to nine fish present in the area per mission. The AUV was able to repeatedly detect and estimate the location of the fish, even when multiple individuals were present. Although less effective at detecting the fish, location estimates from the vessel-based surveys helped verify the veracity of the AUV data. All of the fish left the area within 48 h of release. Most fish exhibited localized movements (milling behavior) before leaving the area. Dispersal rates calculated for the fish suggest that error associated with the location estimates was minimal. The average movement rate was 0.62 body length per second and was comparable to marine movement rates reported for other Chinook salmon stocks. These results suggest that AUV-based payload control can provide an effective method for mapping the movements of marine fish.
1. Introduction
Acoustic telemetry (defined here as the detection of fish or other aquatic fauna tagged with acoustic transmitters) is a useful tool for collecting detailed information on the distribution, movements, and habitat use of marine fish [,,]. However, the principal advantage of this method—the ability to repeatedly locate and identify tagged individuals—is often diminished by adverse marine conditions (e.g., ocean stratification, ambient noise), limited reception range (typically less than a kilometer), and the inherent tradeoffs between transmitter size, transmitting power, and operational life [,,]. Various approaches have been used to address these constraints, ranging from arrays of acoustic receivers [,,] to mobile surveys using a variety of vessel-based methods [,,,].
Autonomous underwater vehicles (AUVs) are increasingly being used to supplement the use of surface vessels for measuring the physical, chemical, and biological characteristics of the marine environment, and when equipped with acoustic receivers can be used to determine the distribution and movements of fish tagged with acoustic transmitters [,]. This approach has definite advantages over tracking fish from surface vessels, particularly for demersal or deep-water pelagic species []. Proximal data obtained from AUV-based sensors and sonar also make it possible to compare the spatial and temporal distribution of the fish with the associated environmental conditions, benthic characteristics, and the presence of other fish species and untagged con-specifics.
Despite these advantages, AUVs have their own constraints. Unlike a piloted vessel that can easily deviate from its existing course to enhance location estimates, AUVs mapping the movements of acoustically tagged fish were initially programmed to simply follow a predetermined route and record the transmitters detected. Position estimates were limited to kernel density or sound-pressure-level (SPL) estimates of proximity, which were often bimodal [,]. Positioning errors frequently resulted from the presence of tagged fish on the periphery of the pre-programmed route and the unfavorable geometry of the hyperbolic curves used to estimate fish locations []. Survey routes for unmanned vehicles (both surface and submerged) have become much more sophisticated and are typically designed to provide coverage based on the information needed and the physical features of the surrounding area, such as topography or current patterns [,,]. In the case of fish telemetry, the reception range of the transmitters is also an important consideration in planning the mission parameters and the configuration of the route [].
The use of AUVs to locate acoustically tagged fish has been enhanced by advances in payload control (PC) hardware and software, which allow the vehicle to alter its mission in response to information from an on-board sensor (i.e., reactive sampling), in this case telemetry data from an integrated acoustic receiver. This approach has been used to track the Lagrangian movements of a single acoustically tagged fish [,]. However, repeatedly locating multiple fish over a vast area remains a major challenge. In previous papers, we have discussed the development of payload control software [] and its utility for mapping the location of multiple targets (reference transmitters at known locations and depths) during a single mission []. Here, we examine the use of AUV-based payload control to synoptically map the movements of a number of highly mobile marine fish.
Chinook salmon (Oncorhynchus tshawytscha) are a wide-ranging anadromous species that spend several years in the marine environment before returning to coastal rivers throughout the northern Pacific Rim to spawn. These returns support important commercial and subsistence fisheries. Chinook salmon returns were relatively stable until the late 1990s, when dramatic declines in abundance were reported []. This trend has continued during subsequent years and resulted in severe reductions in harvest and difficulties in meeting escapement goals [,,]. Reductions in fish size and shifts in age composition to younger fish have also been reported [,,,], which may reflect significant changes in marine conditions that could have an impact on other marine species.
There is increasing evidence that the first year spent in the marine environment is a critical period for Chinook salmon [,]. Information on the spatial and temporal distribution of the fish during this period is needed to better understand and manage these populations, and can be used to parameterize assessment models for commercial or threatened stocks. When combined with environmental data, this information also provides a baseline for assessing the response of other fish species and marine communities to shifts in ocean conditions [,]. However, efforts to obtain this type of information are often hampered by the logistical challenges and severe conditions frequently encountered (e.g., vast distances, deep depths, restricted access, and turbulent seas). The goal of this study was to document the efficacy and challenges of using an AUV under payload control to collect detailed telemetry data (i.e., meter-scale resolution) for juvenile Chinook salmon in the marine environment.
2. Materials and Methods
2.1. Study Area
The study was conducted in marine waters of Port Walter (PW) and Big Port Walter (BPW) located near the southern tip of Baranof Island in southeastern Alaska (Figure 1). The entire fjord is approximately 6.1 km long, 1.2 km at its maximum width, characterized by steep rocky slopes, and surrounded by mountainous terrain. Water depths in Port Walter range from over 300 m in the central and eastern section of the fjord to less than 30 m near the entrance to Big Port Walter. Maximum depth in Big Port Walter is about 100 m near the center. Several small streams flow into the basin, and the area is used as a nursery and rearing area for juvenile salmonids (O. spp.). A research station operated by the National Marine Fisheries Service at Little Port Walter (LPW) was used to facilitate operations.
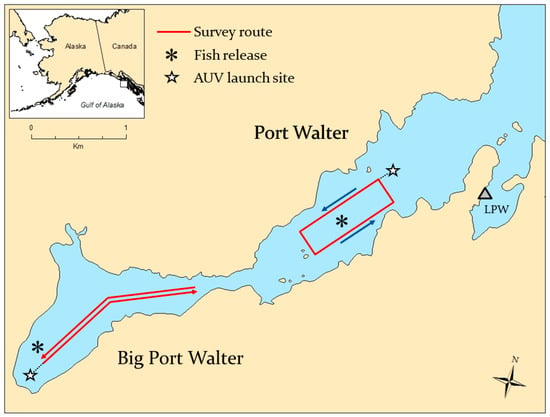
Figure 1.
Map of Port Walter and Big Port Walter, Alaska, showing the survey routes used during autonomous underwater vehicle (AUV) missions and the sites where acoustically tagged juvenile Chinook salmon were released. AUV operations were based at the Little Port Walter (LPW) research station.
2.2. Fish Tagging
Juvenile Chinook salmon were reared in vertical raceways at LPW as described by Martin and Heard []. In October 2015, 100 fish were transferred to two round ponds with free-flowing freshwater (3000 L capacity per pond). Saltwater was introduced into the round ponds in April 2016 to induce smoltification. In May, individual fish were randomly selected, sedated with an anesthetizing solution of tricaine methanesulfonate (MS-222) at a concentration of 60 mg/L of water, weighed, and measured (snout to fork of tail). The fish were surgically tagged with acoustic transmitters as described by Liedtke et al. []. Briefly, a small incision was made slightly offset and parallel to the ventral midline, and the transmitter gently inserted into the abdominal cavity. The incision was closed with simple, interrupted sutures. Handling methods were IACUC-approved under protocol 04-020 issued to T. Grothues by Rutgers University.
The transmitters (Model MM-M-8-S0, Lotek Wireless, Newmarket, ON, Canada) were cylindrical (8.5 mm in diameter, 43 mm in length), weighed 8.5 g, and emitted a unique code division multiple access (CDMA) signal burst [] every 4 s with a power output of 149 dB (referenced to 1 µPa at 1 m), transmitting frequency of 76 kHz, and 12-h on/off duty cycle with signal transmissions from 0900 to 2100 h. The CDMA signal uses an encoding scheme that relies on correlation to known patterns from a noise carrier wave and thus is highly robust against code collisions [,]. An equal number of fish were tagged with dummy tags that had the same size and weight characteristics as the transmitters. The transmitters and dummy tags were placed in separate storage pouches, sterilized with low temperature ethylene oxide gas, and sealed prior to use. All of the fish were tagged with a PIT tag (Model AVID 2028: Avid Identification Systems, Inc., Norco, CA, USA) to enable subsequent identification.
Each fish was returned to the round pond after tagging and observed until its swimming behavior could not be distinguished from the untagged fish. The fish were held and observed for over 15 days after tagging. Water temperature during this period averaged 8.3 °C (±0.6 °C). Fish tagged with acoustic transmitters were transferred to a container filled with aerated saltwater, and transported to Port Walter or Big Port Walter for release into the marine environment. Ten untagged fish were released with each group of tagged individuals to bolster the size of the release since schooling has been reported for juvenile salmonids [,,] and social behavior may influence the movement patterns exhibited by the fish. The fish with dummy tags were sacrificed and examined as described by Liedtke and Rub [].
2.3. Fish Tracking
The tagged fish were located using a REMUS 100 AUV (Hydroid Inc., Pocasset, Maine, a subsidiary of Kongsberg Maritime, Kongsberg, Norway). This torpedo-shaped, propeller-driven vehicle (Figure 2) was 2 m in length, 19 cm in diameter, weighed 31 kg, had a maximum operating depth of 100 m, and cruising speed of 2.4 ms−1. Prior to launch, a pre-programmed mission, including operational parameters and instructions (e.g., vehicle speed, depth, and survey route), was uploaded to the AUV’s on-board navigational computer. An integrated GPS receiver provided positioning information while on the surface. Since GPS fixes can’t be obtained underwater, the vehicle’s position while submerged was estimated using either trilateration based on acoustic beacons moored at known locations within the study area, or dead reckoning where the route was recorded in an analogous 2-s interval from dead reckoning computations. Position estimates were rectified during post-processing. The error between the initial position at depth and the new GPS position acquired after surfacing was smeared across the submerged point estimates of the route using the RENAV feature of the AUV’s native software.
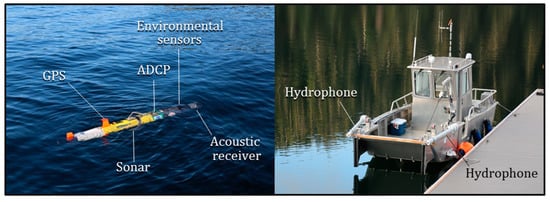
Figure 2.
Remus 100 autonomous underwater vehicle (left panel) used during field trials in marine waters of southeastern Alaska during 2016. The vehicle was equipped with payload control hardware and software (internal), a receiver and hydrophone for detecting and recording acoustic transmitters, an acoustic Doppler current profiler (ADCP), environmental sensors, a side-scan sonar, and a GPS receiver for collecting positioning data. A boat equipped with an acoustic receiver and dual hydrophones was used to conduct concurrent surveys (right panel). The hydrophone struts were lowered into the water and vertically oriented during tracking, with the hydrophones submerged to a depth of 1 m.
The AUV was equipped with an integrated omnidirectional coaxial hydrophone and acoustic receiver (Model WHS 3050: Lotek Wireless) mounted on the nose of the vehicle (Figure 2) and running the proprietary data logging software MAPHost (Lotek Wireless). The equipment was capable of detecting, identifying and recording CDMA transmitters used to tag the fish. The vehicle was also equipped with a 600 kHz side-scan sonar and suite of environmental sensors for chemical, bathymetric, and biological oceanographic studies [].
The vehicle was instrumented with a payload control hardware/software stack that operated using software called Synthetic Aperture OVerRide (SAOVR) designed to map the location of acoustically tagged fish []. The software and a series of operational scripts were uploaded to an internal guest computer that communicated with the AUV’s proprietary control system through the factory-installed RECON communications protocol (Hydroid Inc.). After detecting a transmitter and meeting certain operational criteria [], the vehicle deviated from its pre-programmed route and performed a maneuver designed to (1) enhance the location estimate of the tagged fish and (2) move the AUV closer to the fish to obtain proximal information on the environmental conditions associated with the location. Based on simulation results, a base-forward equilateral triangle was selected as the preferred maneuver []. The vehicle would begin the maneuver by turning 30° to the right of the pre-programmed route, then 60° left (crossing the default pathway), and then 60° left to return to the original deviation point and resume the pre-programmed route (Figure 3). This maneuver ensured that the target (i.e., the tagged fish) was approached from several different angles to optimize the creation of a synthetic aperture []. Travel time for the maneuver was 60 s/segment, with the vehicle traveling approximately 120 m/segment based on an average cruising speed of 2.0 ms−1.
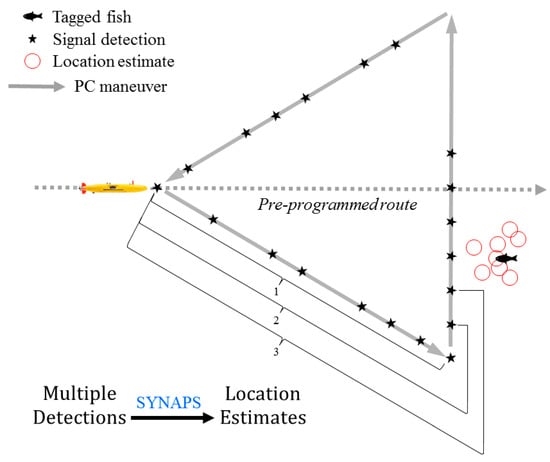
Figure 3.
Payload control (PC) maneuver, consisting of a base-forward triangle, used by an autonomous underwater vehicle to estimate the location of acoustically tagged fish. SYNAPS software was used to calculate a series of location estimates using multiple signal detections, starting with the first 8 detections (Estimate 1) and increasing incrementally (Estimate 2 based on 9 detections, Estimate 3 based on 10 detections, etc.).
A series of missions with multiple legs were conducted in Big Port Walter using the SAOVR software to override the pre-programmed route and execute a maneuver when a tagged fish was detected. The AUV traveled along a route generally running southwest to northeast (legs 1, 3, and 5) and northeast to southwest (legs 2, 4, and 6) as shown in Figure 1. The surveys were conducted at depths of 20–25 m with a flat trajectory once the AUV reached its cruising depth. A single mission with multiple legs was conducted in Port Walter at a depth of 35 m, with the AUV traveling along a rectangular route with multiple repetitions. All missions ran at a cruising speed of approximately 2 ms−1. The vehicle surfaced at the end of each leg to obtain a GPS fix for course correction.
The survey data collected by the AUV included time-stamped records identifying the fish detected, signal strength of the transmitter, environmental sensor data, and the corresponding position of the AUV. These data were also collected for any other fish detected during the maneuver. Additional maneuvers were temporarily suspended to prevent the AUV from getting locked in a positive feedback loop []. Sequential transmitter detections recorded during the maneuver (Figure 3) were used to create a series of location estimates for each fish detected using synthetic apertures [] with the stipulation that the set of detections not exceed 90 s from the first to the last detection. Multiple synchronous position solutions were calculated based on permutations of the different subsets of sequential detections (Figure 3) and filtered by several quality metrics. The proprietary software SYNAPS (Lotek Wireless) was used to calculate these estimates. The software applies a differencing engine to find the best intersection between the surfaces of the multiple detection spheres obtained, similar to the approach used by GPS [,] after removing the cumulative transmission interval from the timestamps of the set of transmitter detections. SYNAPS also provided diagnostic variables that measured the overall quality of each location solution, including residual (Resid) and dilution of precision (DOP). Resid is a stability metric based on the error estimate of the signal flight time (i.e., propagation delay associated with the signal) with smaller Resid values indicating a better location estimate []. The DOP metric is a measure of the intersection of the spheres delineating possible times of signal arrival to different hydrophone locations within the aperture, and thus is sensitive to the geometry of the aperture. Smaller DOP values result from apertures with a good parabolic shape and are associated with more accurate location estimates []. Resid was used as the primary screening metric, with DOP used as a secondary assessment variable. Location estimates with Resid values <30.0 were considered reasonable. Since multiple estimates were obtained using different combinations of signal detections (Figure 3), the estimate with the smallest Resid was selected as the best location estimate for a given tag during a particular detection event. The multiple legs of the mission typically resulted in a series of temporally distinct detection events (and associated location estimates) for the individual fish. Although the reception range for the tagged fish could not be determined, reference transmitters deployed at known locations within the study area were consistently detected at 500 m and infrequently detected at distances up to 980 m [].
An aluminum boat (5 m in length with an enclosed cabin) was used to deploy and recover the AUV, and to conduct concurrent vessel-based surveys (Figure 2). The boat was instrumented with an acoustic receiver (Model MAP 600 RT: Lotek Wireless) and stereo hydrophones (Model LHP, Lotek Wireless) capable of detecting, identifying, and recording CDMA transmitter signals. Hydrophone struts were attached on the port and starboard side of the vessel (spaced 1.6 m apart). The struts were lowered into the water and vertically oriented during the survey with the hydrophones submerged to a depth of 1 m. The struts were hydrodynamic in shape, making it possible for the boat to maintain speeds up to 2.6 ms−1 (5 knots) without cavitation. Similar to the AUV missions, SYNAPS software (Lotek Wireless) was used to calculate a series of location estimates from the telemetry data collected for each fish detected and to provide diagnostic variables that measured the overall quality of the estimates. These data were treated the same as the information obtained by the AUV. Positioning information for the boat was collected with a GPS receiver (Model GPSMap-76CSX, Garmin, Olathe, KS, USA). Paired AUV and vessel-based location estimates with an offset time (i.e., difference in the time of detection between the two estimates) <20 min were examined and compared.
We examined the dispersal rate of the fish based on the best location estimates made by the AUV over the entire tracking period. The rate between any two points, distance moved (m) divided by time elapsed (s), represents the movement of the fish plus any error in the location estimate (combined as ϒ). These factors cannot be parsed because the actual position of the fish is unknown, but ϒ can be compared to possible swimming rates and the distribution of ϒ described. This distribution represents the movement behavior of the fish (directed movements vs milling) over time, ranging from hours to days, and helped quantify expectations related to emigration and residence within the area. Further, quantification of the distribution, especially the extent and frequency of large numbers in the distribution tails relative to possible swimming rates, indicates the extent to which error may be problematic in the filtered/selected solution set. To do this, we calculated the rate of movement between each pair of best location estimates jk for a given fish, for individuals with more than two estimates. Distance calculations were made using the Pythagorean theorem assuming a flat plane, because curvature of the geoid surface is negligible at such small distances and because sound underwater is not constrained to follow the surface. We calculated the mean and standard deviation of ϒ within and among fish and fit the distribution of all fish combined using an appropriate estimator in MATLAB (MathWorks Inc., Natick, MA, USA).
δx/t = distancejk/(Tk − Tj),
3. Results
3.1. Fish Tagging
A total of 20 fish were tagged with acoustic transmitters. The fish averaged 230 mm (sd = 12 mm, range 202–249 mm) in length and weighed an average of 164 g (sd = 28 g, range 109–212 g). The weight of the transmitter was ~5% of the fish’s body weight. Recovery after tagging was prompt, with 19 (95%) fish exhibiting swimming patterns indistinguishable from the untagged fish (i.e., upright body orientation and unlabored swimming against the current) <5 min after being returned to the round pond. The one remaining fish exhibited no discernible difference 10–15 min after being released in the tank. Twenty fish were tagged with dummy tags. These individuals exhibited a similar recovery pattern (i.e., no discernible difference from the untagged fish <5 min after release), and showed no signs of physical injury when sacrificed and examined. The transmitter of one of the tagged fish malfunctioned, and this individual was also sacrificed and examined; no adverse physical injuries were observed.
3.2. Survey Missions
Seven survey missions were conducted from 19 May to 26 May 2016 to track the movements of the juvenile salmon, including six missions (M20–M25) in Big Port Walter and one mission (M26) in Port Walter (Table 1). A total of 19 tagged fish were released, ranging from two to four individuals per mission. The fish began to disperse from the immediate release site within 10–15 min. Several fish remained in the study area over several days and were present during subsequent missions, increasing the total number of potential targets during the study to 31 fish (Table 1). The number of fish present (including those released during a previous mission) ranged from two to nine individuals per mission. All of the fish ultimately left the study area.

Table 1.
Numbers of acoustically tagged Chinook salmon located during survey missions conducted concurrently with an autonomous underwater vehicle and boat in marine waters of Big Port Walter (BPW) and Port Walter (PW), Alaska, during 2016. The total number of fish present and detected in the area (including those released as part of the mission and those remaining from previous releases) are indicated.
Of the 31 potential targets present during the study, 28 (90%) were detected by the AUV (Table 1). A comparable number of fish (29, 94%) were detected during the vessel-based surveys. In most cases, the same fish were detected by both methods (Table 2). However, in interpreting these data it is important to understand that the AUV and vessel-based surveys were spatially and temporally disjunct, and that the similar detection rates belie considerable differences in the number and pattern of detections per fish. We had considerable difficulty detecting, locating, and maintaining contact with the fish during the vessel-based surveys, particularly when greater numbers were present within the area. Due to these difficulties, we initially attempted to locate and track the movements of a particular fish, but would often lose contact as it moved through the area. To address this problem, we serendipitously used real-time output from the AUV to facilitate the vessel-based surveys. We were able to monitor the movements of the AUV on a laptop computer and could see when it was performing a maneuver. By moving the boat to that location, we were often able to detect the fish and confirm what the AUV was targeting. The number of fish detected and the number of location estimates per fish would have been substantially less if we had relied solely on the vessel-based tracking. There were no false positives (i.e., detections for a transmitter not released) in the 6760 detections recorded by the AUV.

Table 2.
Detections of acoustically tagged Chinook salmon located during survey missions conducted concurrently with an autonomous underwater vehicle (A) and boat (B) in marine waters of Big Port Walter (BPW) and Port Walter (PW), Alaska, during 2016. Bold upper case letters indicate detections made during the same mission that the fish were released; lower case letters indicate detections during subsequent missions.
A total of 107 best location estimates were obtained during the study. The movements of the fish based on these estimates are illustrated in Figure 4. Five fish were located during Mission 24 (left panel), including three fish released at the start of the mission and two fish released the previous day during Mission 22 (black line) and Mission 23 (red line). Several fish were located during successive surveys (Table 2), including Fish 12003 located during missions 21, 22, and 23 (Figure 4, right panel). Best location estimates from the other missions in Big Port Walter (Appendix A) and Port Walter (Appendix B) are also shown.
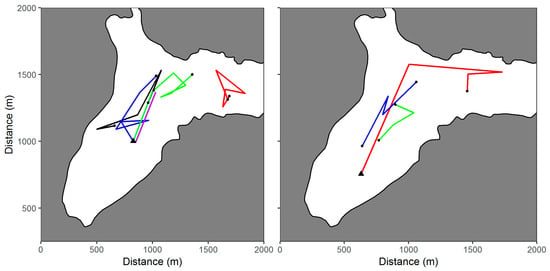
Figure 4.
Movements of juvenile Chinook salmon tagged with acoustic transmitters and located using an autonomous underwater vehicle in Big Port Walter, Alaska. The movements of five fish detected during Mission 24 are shown in the left panel. The right panel shows the movements of Fish 12003 over three successive missions. The site where the fish were initially released (black triangle) and the last location of the fish (black dot) are indicated.
We also compared the best location estimates obtained during the concurrent AUV and vessel-based surveys. Comparable vessel-based estimates were not obtained for all of the AUV locations largely due to differences in the time of detection; a total of 46 paired estimates met the established criteria (i.e., smallest Resid for each methods, time offset <20 min). Movements of the fish based on these estimates are illustrated in Figure 5, including a fish released and located during the same mission (left panel) and one locate during a subsequent mission (right panel). The vessel-based location estimates are represented by numbers that correspond to the sequential order (based on the time of detection) of the paired AUV estimates (e.g., boat estimates designated by 2 and 4 correspond to the second and fourth AUV location estimate obtained during the mission). Paired location estimates for the other missions are shown in Appendix B and Appendix C. The time offset for most of the paired estimates (35, 76%) was ≤5 min (Appendix D), suggesting that although juvenile salmon are relatively mobile, the differences observed between the AUV and vessel-based locations were probably not unduly affected by the movements of the fish.
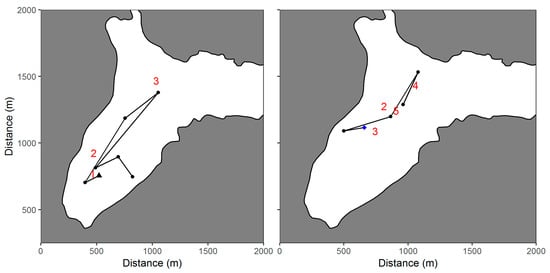
Figure 5.
Comparison of the movement of Fish 12010 (left panel) and Fish 12004 (right panel) located using an autonomous underwater vehicle and vessel-based tracking in Big Port Walter, Alaska. The site where the fish were either released (black triangle) or first located for the fish released during a previous mission (blue diamond) are indicated. Subsequent AUV locations are represented by black dots. Vessel-based location estimates are designated by numbers that correspond to the sequential order of the paired AUV estimates (e.g., the numbers 2 and 4 correspond to the second and fourth AUV location).
Most (14, 82%) of the fish released in Big Port Walter left the area within 24 h, with the three remaining fish leaving within 48 h. Residency information is not available for the two fish released in Port Walter during the last mission (M26). Estimated dispersal rates were calculated for 14 fish that met the established criteria (Figure 6), yielding a total of 485 pairwise combinations for rate estimate ϒ. The rates ranged from 0.0002 to 1.5040 ms−1, with a sample mean and standard deviation of 0.0757 ± 0.1531 ms−1. However, these rates exhibited a half-normal distribution with the mode equal to the minimum (essentially 0) and 93% of ϒ < 0.25 ms−1 (Figure 7). The mode in ϒ was not necessarily related to the mode in elapsed time, but could be depending on the particular span of observations since the fish moved independently of one another, often interspersed linear movements with milling behavior (e.g., Fish 12003, M21), or returned to a location near their initial position after a series of ostensibly stochastic movements (e.g., Fish 12004, M25) (Appendix C). The sample mean was therefore heavily biased towards fish that milled, or that made linear excursions and then returned to the initial location, because more data points were collected for these individuals and greatly weighted the average. The grand mean and standard deviation with individual fish as the sample unit was 0.1419 ± 0.1065 ms−1. Expressed as body length per second for comparative purposes, this was 0.62 BLs−1, with a maximum rate of 6.54 BLs−1.
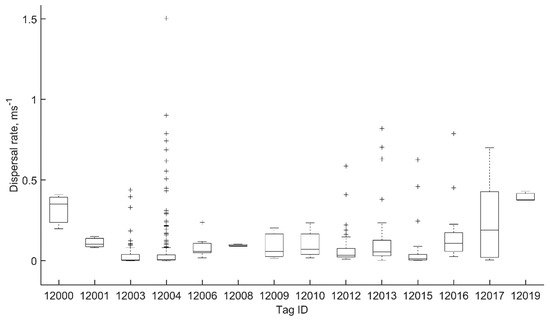
Figure 6.
Box–whisker plot of the dispersal rate distribution of acoustically tagged juvenile Chinook salmon in marine waters of Port Walter and Big Port Walter, Alaska, representing the median value (central mark), 25th and 75th percentiles (lower and upper box edges), most extreme data points (whiskers), and outliers (+).
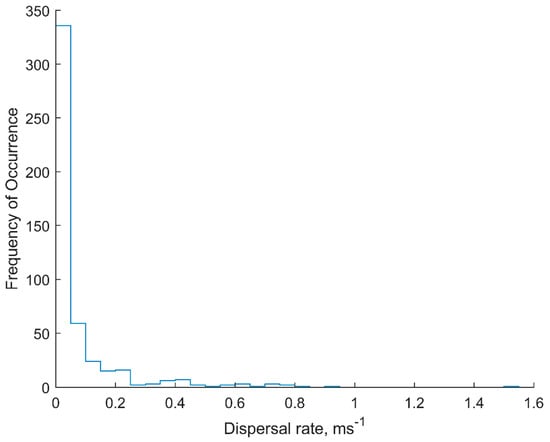
Figure 7.
Histogram of the distribution of dispersal rate plus error (ϒ) of acoustically tagged juvenile Chinook salmon in marine waters of Port Walter and Big Port Walter, Alaska.
4. Discussion
A variety of factors complicate efforts to document the movements of acoustically tagged fish in the marine environment, ranging from the technical limitations associated with acoustic telemetry to the logistical challenges often encountered particularly when working under remote or adverse conditions. These factors can severely limit the ability to repeatedly locate and identify the tagged individuals. The inherent difficulties associated with tracking multiple fish or highly mobile species over vast areas can exacerbate these problems.
Advances in AUV technology can enhance efforts to collect acoustic telemetry data on marine fish. In addition to functioning autonomously, making it possible for researchers to focus on other tasks, AUVs equipped with payload control have the potential to substantially improve the quality and expand the type of information obtained. Maneuvers conducted in response to detection events can enhance the accuracy of location estimates, and the ability of the AUV to move closer to the fish to collect proximal information on habitat use and the associated environmental conditions has obvious advantages. AUVs can also work in cooperative pairs [], and eventually swarms, to enhance the information collected [,].
During this study, the reactive sampling conducted by the AUV under payload control was effective at repeatedly detecting and estimating the location of the tagged fish. The vehicle was able to maintain contact even when multiple individuals were present, with most fish located repeatedly over the course of a mission. Although the actual locations of the fish were unknown, the general agreement between the paired (i.e., temporally similar) AUV and vessel-based estimates suggests that the information provided by the AUV was credible. Further developments in software routines and algorithms (e.g., maneuvers that further enhance the location estimates, refined methods for processing the telemetry data collected when multiple fish are present) will likely lead to further improvements in performance. Additional information and insights on the movement patterns of marine fish may also be useful to enhance the pre-programmed routes used to make initial contact with wide-ranging or highly mobile species based on a series of informed search models []. For example, large fast moving fish such as adult salmon or sturgeon (Acipenser spp.) may swim a greater distance (>10 m) during the time needed to create a synthetic aperture compared to more sedentary species such as crab (Cancer spp.) or flounder (Paralichthys spp.). As a result, the accuracy of the location estimates may be substantially less, although the estimates may still provide useful information depending on the research objectives of the study and the spatial resolution needed. Knowing something about the behavior and movements of the fish would make it possible to tailor the parameters of the search routes and maneuvers to reflect the desired scale and enhance survey results [,,].
Differences were observed between the two survey methods used during the study. The AUV was much more effective at repeatedly locating the fish, whereas we had considerable difficulty detecting and maintaining contact during the vessel-based surveys, particularly when greater numbers of fish were present within the area. Our initial approach in response to this problem was to continue to track the individuals detected from the boat, ranging out after contact was lost in search of other fish. This approach is biased toward more Lagrangian movements, which focus on more extended observations of an individual over space and time rather than a comprehensive inventory of fish present within the area. In addition, mapping efforts would tend to be concentrated in areas where the fish were detected (to increase the probability of making contact) rather than conducting a more systematic search of the surrounding area. The serendipitous approach of using real-time information from the AUV substantially enhanced the vessel-based results and helped to verify the veracity of the AUV data. The advantage of using AUVs to collect telemetry data has been demonstrated in previous AUV–boat comparisons where similar survey routes were used for both methods []. Although less definitive in shallow waters (<20 m), detection rate and tracking success were significantly greater for the AUV for both reference tags (i.e., stationary transmitters at known locations and depth) and free-ranging animals located at deeper depths. The enhanced performance is likely due to a number of factors. The AUV is quieter when running than a surface vessel, can operate below the thermocline and halocline (and avoid the sound channeling associated with these conditions), and is less affected by signal degradation caused by sea surface noise. Although a submerged hydrophone can be towed from a boat, it is susceptible to becoming entangled in marine vegetation and debris, and signal strength is reduced due to line loss. The position of the hydrophone (a factor used to estimate transmitter locations) is also less certain depending on the length of the cable, the velocity of the current, and the speed of the vessel. Further, an AUV eliminates the human bias caused by the tendency to “look where the light is better”, in other words a reluctance to leave an area where fish have been detected and search other areas that may (or may not) produce useful information. These results suggest that AUVs can provide a viable method for mapping the spatial and temporal distribution of acoustically tagged marine fish.
A basic assumption in any tagging study is that the handling methods used do not adversely affect the fish (i.e., tagged fish behave the same as untagged fish) or that any effect is limited in severity and duration and ultimately has negligible effect [,,]. During this study, the behavior of the fish prior to release, as well as the necropsy results for the fish tagged and sacrificed, suggest that the fish were not adversely affected by the handling methods. The initial movements of the fish, dispersing from the release site relatively soon after release, also suggest that the fish were not exhibiting latent effects from the tagging.
The residency time exhibited during the study was limited, with all of the fish leaving the area within 48 h. Although one fish (Fish 12019, M20) displayed directed movements (leaving the area ~2 h after release), most exhibited a series of localized movements within the area prior to departure. The average movement rate observed (0.62 BLs−1) was well within the range of what would be considered reasonable for juvenile Chinook salmon. Movement rates reported for juvenile Chinook salmon in the Salish Sea (Washington, USA) averaged 0.86 BLs−1 with a maximum individual rate of 2.88 BLs−1 []. Ocean movements of juvenile Chinook salmon in coastal waters of southern British Columbia (Canada) averaged 0.33 BLs−1 []. Brett estimated that the optimal swimming speed (i.e., minimum cost per distance traveled) for sockeye salmon (O. nerka) was approximately one body length per second [], and this metric is often applied to other salmonids. The dispersal rate for 93% of the fish during this study (≤0.25 ms−1, 1.1 BLs−1) was typically ≤ the optimal swimming speed, likely due to the milling behavior exhibited by the fish. Although not egregious, the maximum movement rate observed (6.54 BLs−1) was unrealistic and more closely approximates burst swimming speeds exhibited by adult salmonids [,]. Although this result demonstrates the potential error associated with telemetry-based location estimates, the small number of outliers with a movement rate of >2 BLs−1 (0.45 ms−1) suggest that this approach is reasonable even with occasional error. The largest errors likely occur when a relatively linear synthetic aperture produces a bimodal set of solutions with equally good Resid and DOP values for location estimates on both sides of the AUV’s route, making it difficult to determine the “correct” estimate. If the wrong estimate is selected, the error would be approximately twice the distance from the actual position of the fish. With additional work, it would be possible to automatically screen out or devalue estimates made from such linear synthetic apertures.
Marine movement rates undoubtedly depend on a number of factors, including the size and migratory behavior of the fish, as well as local conditions (e.g., currents and topography). Consequently, it is uncertain whether our results are analogous to the movements of wild Chinook salmon entering marine waters. During this study the fish were transported to a release site distant from their natal stream. The fish were also substantially larger in size (averaging 230 mm in length) than similarly aged fish from wild stocks. Average length of Chinook salmon smolts from rivers along the northwestern coast of North America range from 68 to 133 mm []. Likewise, the average length of juvenile Chinook salmon released at LPW since 2011 averaged 125 mm (J. Eiler, National Marine Fisheries Service, unpublished data). However, acoustically tagged juveniles released at LPW (average length of 133 mm) exhibited localized movements near the mouth of Port Walter for over 7 days after entering the marine environment, and general observations of untagged fish suggest that juvenile salmonids utilize Port Walter as a nursery and rearing area (J. Eiler, National Marine Fisheries Service, personal communication).
5. Conclusions
This study demonstrated that AUVs operating under payload control can be an effective method for mapping the spatial and temporal distribution of juvenile salmon in the marine environment. The AUV was able to repeatedly detect and estimate the location of the fish even when multiple individuals were present. The first year in saltwater is a critical period for juvenile salmon, and telemetry information can provide useful insights into this life stage. Additional work is needed to determine the suitability of using AUVs under payload control for mapping the movements of more mobile and wide-ranging life stages of salmon and other marine species.
Author Contributions
Conceptualization, J.E. and T.G.; methodology, J.E., T.G., J.D., and R.S.; formal analysis, J.E. and T.G.; investigation, J.E., T.G., J.D. and R.S.; funding acquisition, J.E. and T.G.; writing, J.E. and T.G.
Funding
Financial support for this study was provided by the North Pacific Research Board (NPRB Project 1529). In-kind support was provided by the National Marine Fisheries Service, Auke Bay Laboratories and Rutgers University Institute of Marine and Coastal Sciences.
Acknowledgments
We thank J. Beeman, T. Liedke, and R. Tomka for their assistance tagging juvenile salmon. D. Kehoe provided technical support for the telemetry portion of the project. We are particularly grateful to M. Adams, R. Bare, M. DeLuca, and B. Weinlaeder for their assistance with field operations. We also thank J. Watson for assistance with figures. The manuscript was critically reviewed by A. Gray, J. Lee, and A. Marinelli. The findings and conclusions in the paper are those of the authors and do not necessarily represent the views of the National Marine Fisheries Service, NOAA. Reference to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA, or Rutgers University.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
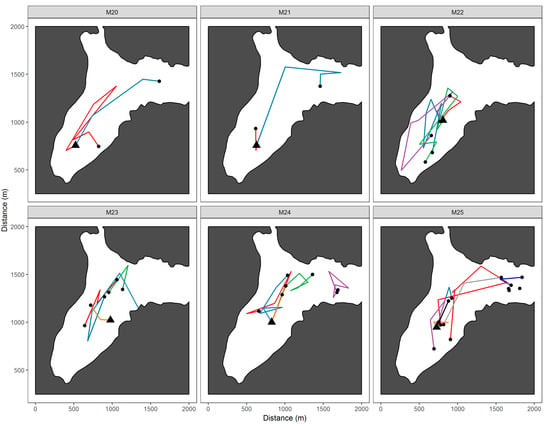
Figure A1.
Movements of juvenile Chinook salmon tagged with acoustic transmitters and located using an autonomous underwater vehicle during a series of survey missions in Big Port Walter, Alaska. The site where the fish were initially released (black triangle) and the last location of the fish (black dot) are indicated.
Appendix B
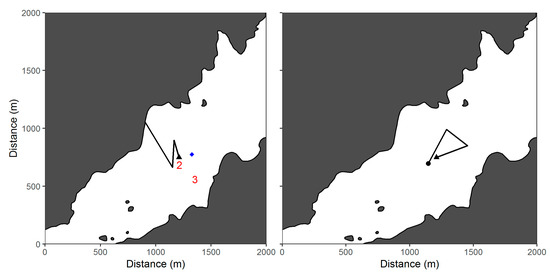
Figure A2.
Movements of Fish 12000 (left panel) and Fish 12001 (right panel) tagged with acoustic transmitters and located using an autonomous underwater vehicle and vessel-based tracking in Port Walter, Alaska. The site where the fish were released (black triangle) and the last location of the fish (black dot) are indicated. Vessel-based location estimates are designated by numbers that correspond to the sequential order of the paired AUV estimates (e.g., the second and third AUV location). One location estimate was obtained for Fish 12011 (blue diamond) released during a previous mission.
Appendix C
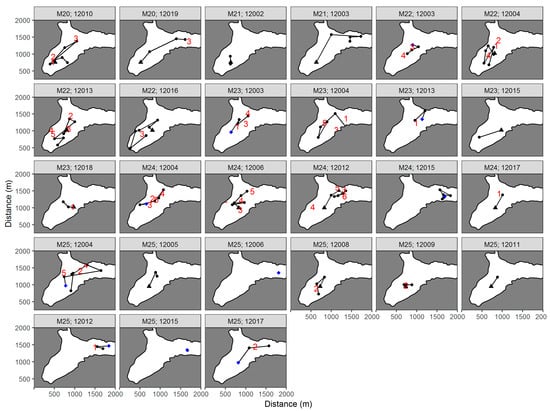
Figure A3.
Comparison of the location estimates of juvenile Chinook salmon tagged with acoustic transmitters and located using an autonomous underwater vehicle and vessel-based tracking in Big Port Walter, Alaska. The site where the fish were either released (black triangle) or first located for fish released during a previous mission (blue diamond) are indicated. Subsequent AUV locations are represented by black dots. Vessel-based location estimates are designated by numbers that correspond to the sequential order of the paired AUV estimates (e.g., the numbers 2 and 4 correspond to the second and fourth AUV location).
Appendix D

Table A1.
Detections of acoustically tagged Chinook salmon located during survey missions conducted concurrently with an autonomous underwater vehicle and surface vessel in marine waters of Big Port Walter (BPW) and Port Walter (PW), Alaska, during 2016. The diagnostic variable used to measure the overall quality of the location solution (Resid) and the difference in time between the AUV and paired vessel-based location estimates (offset) are provided.
Table A1.
Detections of acoustically tagged Chinook salmon located during survey missions conducted concurrently with an autonomous underwater vehicle and surface vessel in marine waters of Big Port Walter (BPW) and Port Walter (PW), Alaska, during 2016. The diagnostic variable used to measure the overall quality of the location solution (Resid) and the difference in time between the AUV and paired vessel-based location estimates (offset) are provided.
| Previous | Time of Location | Resid | Location | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area | Mission | Fish ID | Release | AUV | Vessel | Offset | AUV | Vessel | Sequence 1 |
| BPW | M20 | 12010 | -- | 13:43 | 13:48 | 5:00 | 5.8 | 6.3 | 1 |
| 14:35 | 14:37 | 2:00 | 6.0 | 26.1 | 2 | ||||
| 15:01 | 15:05 | 4:00 | 6.8 | 5.6 | 3 | ||||
| 15:59 | 4.5 | ||||||||
| 16:45 | 5.1 | ||||||||
| 17:41 | 5.1 | ||||||||
| BPW | M20 | 12019 | -- | 14:33 | 4.6 | ||||
| 15:07 | 5.1 | ||||||||
| 15:15 | 15:25 | 10:00 | 6.1 | 8.0 | 3 | ||||
| BPW | M21 | 12002 | -- | 14:02 | 5.4 | ||||
| 14:16 | 5.0 | ||||||||
| BPW | M21 | 12003 | -- | 13:37 | 4.9 | ||||
| 14:42 | 14:44 | 2:00 | 5.2 | 4.5 | 2 | ||||
| 14:52 | 4.8 | ||||||||
| 14:58 | 4.5 | ||||||||
| BPW | M22 | 12003 | M21 | 10:30 | |||||
| 11:31 | |||||||||
| 12:03 | 12:07 | 4:00 | 6.1 | 7.3 | 3 | ||||
| 12:40 | 12:40 | 0:00 | 5.3 | 8.8 | 4 | ||||
| BPW | M22 | 12004 | -- | 9:52 | 9:48 | 4:00 | 5.8 | 7.2 | 1 |
| 9:58 | 10:01 | 3:00 | 5.9 | 5.2 | 2 | ||||
| 10:41 | 5.1 | ||||||||
| 10:50 | 10:51 | 1:00 | 5.0 | 5.5 | 4 | ||||
| 11:30 | 6.7 | ||||||||
| 11:46 | 5.9 | ||||||||
| BPW | M22 | 12013 | -- | 9:51 | 6.7 | ||||
| 10:00 | 10:08 | 8:00 | 6.1 | 11.6 | 2 | ||||
| 10:33 | 10:38 | 5:00 | 6.3 | 6.7 | 3 | ||||
| 10:49 | 10:54 | 5:00 | 4.3 | 6.8 | 4 | ||||
| 11:42 | 11:46 | 4:00 | 4.9 | 8.0 | 5 | ||||
| 11:53 | 5.4 | ||||||||
| BPW | M22 | 12016 | -- | 9:52 | 4.7 | ||||
| 10:33 | 4.2 | ||||||||
| 10:53 | 10:53 | 0:00 | 5.1 | 5.6 | 3 | ||||
| 11:41 | 5.6 | ||||||||
| 11:52 | 5.4 | ||||||||
| 12:42 | 4.1 | ||||||||
| BPW | M23 | 12003 | M21 | 15:22 | 15:39 | 17:00 | 7.0 | 4.5 | 1 |
| 16:29 | 4.3 | ||||||||
| 16:48 | 16:52 | 4:00 | 5.1 | 8.0 | 3 | ||||
| 18:04 | 18:06 | 2:00 | 4.9 | 4.9 | 4 | ||||
| BPW | M23 | 12004 | M22 | 15:52 | 15:58 | 6:00 | 6.5 | 12.1 | 1 |
| 16:23 | 4.3 | ||||||||
| 16:44 | 16:51 | 7:00 | 5.0 | 5.1 | 3 | ||||
| 17:33 | 5.9 | ||||||||
| 18:12 | 18:12 | 0:00 | 5.7 | 7.0 | 5 | ||||
| BPW | M23 | 12013 | M22 | 15:52 | 15:53 | 1:00 | 5.3 | 6.6 | 1 |
| 16:20 | 4.9 | ||||||||
| 16:30 | 6.2 | ||||||||
| BPW | M23 | 12015 | -- | 16:48 | 6.6 | ||||
| BPW | M23 | 12018 | -- | 15:36 | 15:36 | 0:00 | 5.9 | 8.1 | 1 |
| 15:45 | 5.9 | ||||||||
| BPW | M24 | 12004 | M22 | 9:40 | 5.1 | ||||
| 9:45 | 9:47 | 2:00 | 5.2 | 16.4 | 2 | ||||
| 10:35 | 10:37 | 2:00 | 5.4 | 9.9 | 3 | ||||
| 11:35 | 11:37 | 2:00 | 5.7 | 4.2 | 4 | ||||
| 12:03 | 12:06 | 3:00 | 4.6 | 8.8 | 5 | ||||
| BPW | M24 | 12006 | -- | 9:45 | 4.6 | ||||
| 10:30 | 10:37 | 7:00 | 5.8 | 10.3 | 2 | ||||
| 10:51 | 10:50 | 1:00 | 5.0 | 6.9 | 3 | ||||
| 11:42 | 11:44 | 2:00 | 4.9 | 11.3 | 4 | ||||
| 12:10 | 12:13 | 3:00 | 5.9 | 6.2 | 5 | ||||
| BPW | M24 | 12012 | -- | 9:54 | 4.9 | ||||
| 10:00 | 10:05 | 5:00 | 5.5 | 12.3 | 2 | ||||
| 10:20 | 10:26 | 6:00 | 5.4 | 4.6 | 3 | ||||
| 10:55 | 10:55 | 0:00 | 5.6 | 6.2 | 4 | ||||
| 11:03 | 11:07 | 4:00 | 5.6 | 7.3 | 5 | ||||
| 11:29 | 11:30 | 1:00 | 5.0 | 18.7 | 6 | ||||
| BPW | M24 | 12015 | M23 | 10:09 | 4.6 | ||||
| 10:16 | 6.9 | ||||||||
| 11:14 | 4.9 | ||||||||
| 11:20 | 5.6 | ||||||||
| 12:19 | 4.6 | ||||||||
| 12:25 | 5.7 | ||||||||
| BPW | M24 | 12017 | -- | 9:54 | 9:54 | 0:00 | 4.4 | 6.1 | 1 |
| BPW | M25 | 12004 | M22 | 15:38 | 5.5 | ||||
| 15:43 | 15:55 | 12:00 | 4.3 | 14.5 | 2 | ||||
| 16:14 | 5.5 | ||||||||
| 16:21 | 16:21 | 0:00 | 5.7 | 6.1 | 4 | ||||
| 16:53 | 16:53 | 0:00 | 6.5 | 5.3 | 5 | ||||
| 17:21 | 6.1 | ||||||||
| 17:26 | 5.3 | ||||||||
| BPW | M25 | 12005 | -- | 15:46 | 5.0 | ||||
| 17:22 | 5.2 | ||||||||
| BPW | M25 | 12006 | M24 | 16:09 | 5.7 | ||||
| BPW | M25 | 12008 | -- | 15:47 | 5.2 | ||||
| 16:36 | 16:37 | 1:00 | 6.3 | 6.3 | 2 | ||||
| 17:25 | 6.9 | ||||||||
| BPW | M25 | 12009 | -- | 15:40 | 6.1 | ||||
| 16:35 | 16:35 | 0:00 | 5.5 | 5.9 | 2 | ||||
| 16:45 | 4.4 | ||||||||
| BPW | M25 | 12011 | -- | 15:45 | 6.4 | ||||
| BPW | M25 | 12012 | M24 | 16:10 | 16:18 | 8:00 | 4.8 | 7.7 | 1 |
| 17:06 | 5.7 | ||||||||
| 17:12 | 5.7 | ||||||||
| BPW | M25 | 12015 | M23 | 16:14 | 4.1 | ||||
| 17:13 | 5.0 | ||||||||
| BPW | M25 | 12017 | M24 | 15:40 | 6.4 | ||||
| 15:52 | 15:57 | 5:00 | 6.4 | 8.6 | 2 | ||||
| 16:14 | 4.4 | ||||||||
| PW | M26 | 12000 | -- | 10:07 | 5.0 | ||||
| 10:18 | 10:24 | 6:00 | 6.2 | 5.5 | 2 | ||||
| 10:42 | 10:48 | 6:00 | 6.7 | 6.2 | 3 | ||||
| PW | M26 | 12001 | -- | 10:00 | 5.1 | ||||
| 10:35 | 5.7 | ||||||||
| 11:11 | 6.6 | ||||||||
| PW | M26 | 12011 | M25 | 11:48 | 4.5 | ||||
1 Sequential order of the AUV estimates used to represent the paired vessel-based location.
References
- Arnold, G.; Dewar, H. Electronic Tags in Marine Fisheries Research: A 30-Year Perspective. In Electronic Tagging and Tracking in Marine Fisheries, Proceedings of the Symposium on Tagging and Tracking Marine Fish with Electronic Devices, Honolulu, HI, USA, 7–11 February 2000; Sibert, J.R., Nielsen, J.L., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 7–64. [Google Scholar] [CrossRef]
- Childs, A.-R.; Cowley, P.; Næsje, T.; Booth, A.; Potts, W.; Thorstad, E.; Økland, F. Do environmental factors influence the movement of estuarine fish? A case study using acoustic telemetry. Estuar. Coast. Shelf Sci. 2008, 78, 227–236. [Google Scholar] [CrossRef]
- Marshall, A.; Mills, J.S.; Rhodes, K.L.; McIlwain, J. Passive acoustic telemetry reveals highly variable home range and movement patterns among unicornfish within a marine reserve. Coral Reefs 2011, 30, 631–642. [Google Scholar] [CrossRef]
- Pincock, D.G.; Johnston, S.V. Acoustic telemetry overview. In Telemetry Techniques: A User Guide for Fisheries Research; Adams, N.S., Beeman, J.W., Eiler, J.H., Eds.; American Fisheries Society: Besthesda, MD, USA, 2012; pp. 305–337. [Google Scholar]
- Grothues, T. A review of acoustic telemetry technology and a perspective on diversification in coastal tracking arrays. In Tagging and Tracking of Marine Animals with Electronic Devices; Nielson, J.L., Arrizabalaga, H., Fragoso, N., Hobday, A., Lutcavage, M., Sibert, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 9, pp. 77–90. ISBN 978-1-4020-9639-6. [Google Scholar]
- Welch, D.W.; Boehlert, G.W.; Ward, B.R. POST—The Pacific Ocean salmon tracking project. Oceanol. Acta 2003, 25, 243–253. [Google Scholar] [CrossRef]
- Heupel, M.; Semmens, J.; Hobday, A. Automated acoustic tracking of aquatic animals: Scales, design and deployment of listening station arrays. Mar. Freshw. Res. 2006, 57, 1–13. [Google Scholar] [CrossRef]
- Bishop, M.A.; Eiler, J.H. Migration patterns of post-spawning Pacific herring in a subarctic sound. Deep-Sea Res. II Top. Stud. Oceanogr. 2018, 147, 108–115. [Google Scholar] [CrossRef]
- Holland, K.N.; Brill, R.W.; Ferguson, J.S.; Chang, R.K.; Yost, R. A small vessel technique for tracking pelagic fish. Mar. Fish. Rev. 1985, 47, 26–32. [Google Scholar]
- Ng, C.L.; Able, K.W.; Grothues, T.M. Habitat use, site fidelity, and movement of adult striped bass in a southern New Jersey estuary based on mobile acoustic telemetry. Trans. Am. Fish. Soc. 2007, 136, 1344–1355. [Google Scholar] [CrossRef]
- Sackett, D.K.; Able, K.W.; Grothues, T.M. Habitat dynamics of summer flounder Paralichthys dentatus within a shallow USA estuary, based on multiple approaches using acoustic telemetry. Mar. Ecol. Prog. Ser. 2008, 364, 199–212. [Google Scholar] [CrossRef]
- Nielsen, J.K.; Niezgoda, G.H.; Taggart, S.J.; Cooke, S.J.; Anson, P.; Hassler, C.T.; Hanson, K.C. Mobile positioning of tagged aquatic animals using acoustic telemetry with a synthetic hydrophone array (SYNAPS, Synthetic Aperture Positioning System). In Advances in Fish Tagging and Marking Technology; McKenzie, J., Parsons, B., Seitz, A.C., Kopf, R.K., Mesa, M., Phelps, Q., Eds.; American Fisheries Society Symposium 76: Bethseda, MD, USA, 2012; pp. 223–250. [Google Scholar]
- Grothues, T.M.; Dobarro, J.; Eiler, J. Collecting, interpreting, and merging fish telemetry data from an AUV: Remote sensing from an already remote platform. In Proceedings of the 2010 IEEE/OES Autonomous Underwater Vehicles, Monterey, CA, USA, 1–3 September 2010; pp. 1–9. [Google Scholar]
- Clark, C.M.; Forney, C.; Manii, E.; Shinzaki, D.; Gage, C.; Farris, M.; Lowe, C.G.; Moline, M. Tracking and following a tagged leopard shark with an autonomous underwater vehicle. J. Field Robot. 2013, 30, 309–322. [Google Scholar] [CrossRef]
- Eiler, J.H.; Grothues, T.M.; Dobarro, J.A.; Masuda, M.M. Comparing autonomous underwater vehicle (AUV) and vessel-based tracking performance for locating acoustically tagged fish. Mar. Fish. Rev. 2014, 75, 27–42. [Google Scholar] [CrossRef]
- Grothues, T.M.; Dobarro, J.; Ladd, J.; Higgs, A.; Niezgoda, G.; Miller, D. Use of a multi-sensored AUV to telemeter tagged Atlantic sturgeon and map their spawning habitat in the Hudson River, USA. In Proceedings of the 2008 IEEE/OES Autonomous Underwater Vehicles, Woods Hole, MA, USA, 13–14 October 2008; pp. 1–7. [Google Scholar]
- Grothues, T.M.; Davis, W.C. Sound pressure level weighting of the center of activity method to approximate sequential fish positions from acoustic telemetry. Can. J. Fish. Aquat. Sci. 2013, 70, 1359–1371. [Google Scholar] [CrossRef]
- Newhall, A.E.; Lin, Y.; Grothues, T.M.; Lynch, J.F.; Gawarkiewicz, G.G. A method of observing acoustic scattering and absorbtion by fish schools using autonomous underwater vehicles. IEEE J. Ocean. Eng. 2016, 42, 29–36. [Google Scholar] [CrossRef]
- Singh, Y.; Sharma, S.; Sutton, R.; Hutton, D.; Khan, A. A constrained A * approach toward optimal path planning for an unmanned surface vehicle in a maritime environment containing dynamic obstacles and ocean currents. Ocean Eng. 2018, 169, 187–201. [Google Scholar] [CrossRef]
- Singh, Y.; Sharma, S.; Sutton, R.; Hutton, D.; Sutton, R. Optimal path planning of unmanned surface vehicles. Indian J. Geo. Mar. Sci. 2018, 47, 1325–1334. [Google Scholar]
- Skomal, G.B.; Hoyos-Padilla, E.M.; Kukulya, A.; Stokey, R. Subsurface observations of white shark Carcharodon carcharias predatory behaviour using an autonomous underwater vehicle. J. Fish Biol. 2015, 87, 1293–1312. [Google Scholar] [CrossRef]
- Dodson, T.; Grothues, T.M.; Eiler, J.H.; Dobarro, J.A.; Shome, R. Acoustic-telemetry payload control of an autonomous underwater vehicle for mapping the distribution of tagged fish. Limnol. Oceanogr. Methods 2018, 16, 760–772. [Google Scholar] [CrossRef]
- Eiler, J.H.; Grothues, T.M. Developing Telemetry-Based Payload Control for Determining the Distribution and Movements of Marine Fish Using Autonomous Underwater Vehicles; NPRB Project 1529 Final Report; North Pacific Research Board: Anchorage, AK, USA, 2018. [Google Scholar]
- Heard, W.R.; Shevlyakov, E.; Zikunova, O.V.; McNicol, R.E. Chinook salmon-trends in abundance and biological characteristics. North Pac. Anadromous Fish Comm. 2007, 4, 77–91. [Google Scholar]
- Evenson, D.F.; Hayes, S.J.; Sandone, G.; Bergstrom, D.J. Yukon River Chinook salmon: Stock status, harvest, and management. In Pacific Salmon: Ecology and Management of Western Alaska’s Populations; Krueger, C.C., Zimmerman, C.E., Eds.; American Fisheries Society Symposium 70: Bethesda, MD, USA, 2009; pp. 675–701. [Google Scholar]
- Heinl, S.C.; Jones, E.L.; Piston, A.W.; Richards, P.; Shaul, L.D.; Elliot, B.W.; Miller, S.E.; Brenner, R.E.; Nichols, J.V. Review of Salmon Escapement Goals in Southeast Alaska, 2014; Alaska Department of Fish and Game Fishery Manuscript: Anchorage, AK, USA, 2014. [Google Scholar]
- Richerson, K.; Holland, D.S. Quantifying and predicting responses to a US West Coast salmon fishery closure. ICES J. Mar. Sci. 2017, 74, 2364–2378. [Google Scholar] [CrossRef]
- Bigler, B.S.; Welch, D.W.; Helle, J.H. A review of size trends among North Pacific salmon (Oncorhynchus spp.). Can. J. Fish. Aquat. Sci. 1996, 53, 455–456. [Google Scholar] [CrossRef]
- Lewis, B.; Grant, W.S.; Brenner, R.E.; Hamazaki, T. Changes in size and age of Chinook salmon Oncorhynchus tshawytscha returning to Alaska. PLoS ONE 2015, 10, e0130184. [Google Scholar] [CrossRef]
- Jeffrey, K.M.; Côté, I.M.; Irvine, J.R.; Reynolds, J.D. Changes in body size of Canadian Pacific salmon over six decades. Can. J. Fish. Aquat. Sci. 2017, 74, 191–201. [Google Scholar] [CrossRef]
- Ohlberger, J.; Ward, E.J.; Schindler, D.E.; Lewis, B. Demographic changes in Chinook salmon across the Northeast Pacific Ocean. Fish. 2018, 19, 533–546. [Google Scholar] [CrossRef]
- Beamish, R.J.; Mahnken, C. A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Prog. Oceanogr. 2001, 49, 423–437. [Google Scholar] [CrossRef]
- Duffy, E.J.; Beauchamp, D.A. Rapid growth in the early marine period improves the marine survival of Chinook salmon (Oncorhynchus tshawytscha) in Puget Sound, Washington. Can. J. Fish. Aquat. Sci. 2011, 68, 232–240. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2011, 4, 11–37. [Google Scholar] [CrossRef]
- Aschan, M.; Fossheim, M.; Greenacre, M.; Primicerio, R. Change in fish community structure in the Barents Sea. PLoS ONE 2013, 8, e62748. [Google Scholar] [CrossRef]
- Martin, R.M.; Heard, W.R. Floating vertical raceways to culture salmon (Oncorhynchus spp.). Aquaculture 1987, 61, 295–302. [Google Scholar] [CrossRef]
- Liedtke, T.L.; Beeman, J.W.; Gee, L.P. A Standard Operating Procedure for the Surgical Implantation of Transmitters in Juvenile Salmonids; Open-File Report 2012–1267; U.S. Geological Survey: Reston, VA, USA, 2012; p. 50.
- Niezgoda, G.; Benfield, M.; Sisak, M.; Anson, P. Tracking acoustic transmitters by code division multiple access (CDMA)-based telemetry. Hydrobiologia 2002, 483, 275–286. [Google Scholar] [CrossRef]
- Heard, W.R. Life history of pink salmon. In Pacific Salmon Life Histories; Groot, C., Margolis, L., Eds.; University of British Columbia Press: Vancouver, BC, Canada, 1991; pp. 121–230. [Google Scholar]
- Ryer, C.H.; Olla, G.L. Agonistic behavior in a schooling fish: Form, function and ontogeny. Environ. Biol. Fish. 1991, 31, 355–363. [Google Scholar] [CrossRef]
- Riley, W.D.; Ibbotson, A.T.; Maxwell, D.L.; Davison, P.I.; Beaumont, W.R.; Ives, M.J. Development of schooling behaviour during the downstream migration of Atlantic salmon Salmo salar smolts in a chalk stream. J. Fish. Biol. 2014, 85, 1042–1059. [Google Scholar] [CrossRef]
- Liedtke, T.L.; Rub, A.M.W. Techniques for telemetry transmitter attachment and evaluation of transmitter effects on fish performance. In Telemetry Techniques: A User Guide for Fisheries Research; Adams, N.S., Beeman, J.W., Eiler, J.H., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 45–87. [Google Scholar]
- Moline, M.A.; Blackwell, S.M.; von Alt, C.; Allen, B.; Austin, T.; Case, J.; Forrester, N.; Goldsborough, R.; Purcell, M.; Stokey, R.; et al. Remote environmental monitoring units: An autonomous vehicle for characterizing coastal environments. J. Atmos. Ocean. Technol. 2005, 22, 1797–1808. [Google Scholar] [CrossRef]
- Blewitt, G. Basics of the GPS Technique: Observation Equations; Geodetic Applications of GPS; Swedish Land Survey: Bastad, Sweden, 1997; p. 46. [Google Scholar]
- Meckley, T.D.; Holbrook, C.M.; Wagner, C.M.; Binder, T.R. An approach for filtering hyperbolically positioned underwater acoustic telemetry data with position precision estimates. Anim. Biotelemetry 2014, 2. [Google Scholar] [CrossRef]
- Lin, Y.; Hsiung, J.; Piersall, R.; White, C.; Lowe, C.G.; Clark, C.M. A multi-autonomous underwater vehicle system for autonomous tracking of marine life. J. Field Robot. 2016, 34, 623–829. [Google Scholar] [CrossRef]
- Lennox, R.J.; Aarestrup, K.; Cooke, S.J.; Cowley, P.D.; Deng, Z.D.; Fisk, A.T.; Harcourt, R.G.; Heupel, M.; Hinch, S.G.; Holland, K.N.; et al. Envisioning the future of aquatic animal tracking: Technology, science, and application. BioScience 2017, 67, 884–896. [Google Scholar] [CrossRef]
- Zolich, A.; Johansen, T.A.; Alfredsen, J.A. A formation of unmanned vehicles for tracking of an acoustic fish tag. In Proceedings of the 2017-Anchorage, Anchorage, AK, USA, 18–21 September 2017; pp. 1–6. [Google Scholar]
- Russell, S.; Norvig, P. Artificial Intelligence: A Modern Approach, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; ISBN 978-0-13-604259-4. [Google Scholar]
- Conner, L.M.; Plowman, B.W. Using Euclidean distances to assess nonrandom habitat use. In Radio-Tracking and Animal Populations; Millspaugh, J.J., Marzluff, J.M., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 275–290. [Google Scholar]
- Papastamatiou, Y.P.; Cartamil, D.P.; Lowe, C.G.; Meyer, C.G.; Wetherbee, B.M.; Holland, K.N. Scales of orientation, directed walks and movement path structure in sharks. J. Anim. Ecol. 2011, 80, 864–874. [Google Scholar] [CrossRef]
- Mashintonio, A.F.; Pimm, S.L.; Harris, G.M.; van Aarde, R.J.; Russell, G.J. Data-driven discovery of the spatial scales of habitat choice by elephants. PeerJ 2014, 2, e504. [Google Scholar] [CrossRef]
- Schreck, C.B. Accumulation and long-term effects of stress in fish. In The Biology of Animal Stress; Moberg, G.P., Mench, J.A., Eds.; CAB International: Wallingford, UK, 2000; pp. 147–158. [Google Scholar]
- Bridger, C.J.; Booth, R.K. The effects of biotelemetry transmitter presence and attachment procedures on fish physiology and behavior. Rev. Fish. Sci. 2003, 11, 13–34. [Google Scholar] [CrossRef]
- Chamberlin, J.W.; Kagley, A.N.; Fresh, K.L.; Quinn, T.P. Movements of yearling Chinook salmon during the first summer in marine waters of Hood Canal, Washington. Trans. Am. Fish. Soc. 2011, 140, 429–439. [Google Scholar] [CrossRef]
- Welch, D.W.; Melnychuk, M.C.; Payne, J.C.; Rechisky, E.L.; Porter, A.D.; Jackson, G.D.; Ward, B.R.; Vincent, S.P.; Wood, C.C.; Semmens, J.; et al. In situ measurement of coastal ocean movements and survival of juvenile Pacific salmon. Proc. Natl. Acad. Sci. USA 2011, 108, 8708–8713. [Google Scholar] [CrossRef]
- Brett, J.R. Energy expenditure of sockeye salmon, Oncorhynchus nerka, during sustained performance. J. Fish. Res. Board Can. 1973, 30, 1799–1809. [Google Scholar] [CrossRef]
- Colavecchia, M.; Katopodis, C.; Goosney, R.; Scruton, D.A.; McKinley, R.S. Measurement of burst swimming performance in wild Atlantic salmon (Salmo salar L.) using digital telemetry. Regul. Rivers Res. Manag. 1998, 14, 41–51. [Google Scholar] [CrossRef]
- Healey, M.C. Life history of Chinook salmon (Oncorhynchus tshawytscha). In Pacific Salmon Life Histories; Groot, C., Margolis, L., Eds.; University of British Columbia Press: Vancouver, BC, Canada, 1991; pp. 311–393. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).