Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience
Abstract
Featured Application
Abstract
1. Introduction
2. Modification of Physical Properties of LC Materials by Nanodopants
3. Photonic Applications
4. LC-Aided-Assembly and Self-Assembly of Nanomaterials
5. Biological Applications: Biosensors and Drug Delivery
5.1. Biosensors
5.2. Drug Delivery
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tschierske, C. Liquid Crystals: Materials Design and Self-Assembly; Springer Science & Business Media: Berlin, Germany, 2012; Volume 318. [Google Scholar]
- Bisoyi, H.K.; Kumar, S. Liquid-crystal nanoscience: An emerging avenue of soft self-assembly. Chem. Soc. Rev. 2011, 40, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew. Chem. Int. Ed. 2005, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Ohzono, T.; Fukuda, J.I. Zigzag line defects and manipulation of colloids in a nematic liquid crystal in microwrinkle grooves. Nat. Commun. 2012, 3, 701. [Google Scholar] [CrossRef] [PubMed]
- Chuang, I.; Durrer, R.; Turok, N.; Yurke, B. Cosmology in the laboratory: Defect dynamics in liquid crystals. Science 1991, 251, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Pieranski, P.; Yang, B.; Burtz, L.-J.; Camu, A.; Simonetti, F. Generation of umbilics by magnets and flows. Liq. Cryst. 2013, 40, 1593–1608. [Google Scholar] [CrossRef]
- Dierking, I.; Al-Zangana, S. Lyotropic liquid crystal phases from anisotropic nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Liquid crystalline thermotropic and lyotropic nanohybrids. Nanoscale 2013, 5, 6641–6661. [Google Scholar] [CrossRef]
- Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; et al. Discotic liquid crystals: From tailor-made synthesis to plastic electronics. Angew. Chem. Int. Ed. 2007, 46, 4832–4887. [Google Scholar] [CrossRef]
- Hegmann, T.; Qi, H.; Marx, V.M. Nanoparticles in liquid crystals: Synthesis, self-assembly, defect formation and potential applications. J. Inorg. Organomet. Polym. Mater. 2007, 17, 483–508. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Tschierske, C. Non-conventional liquid crystals—the importance of micro-segregation for self-organisation. J. Mater. Chem. 1998, 8, 1485–1508. [Google Scholar] [CrossRef]
- Takezoe, H.; Eremin, A. Bent-Shaped Liquid Crystals: Structures and Physical Properties; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Coleman, D.; Fernsler, J.; Chattham, N.; Nakata, M.; Takanishi, Y.; Körblova, E.; Link, D.; Shao, R.-F.; Jang, W.; Maclennan, J. Polarization-modulated smectic liquid crystal phases. Science 2003, 301, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Lagerwall, J.P.F.; Scalia, G. A new era for liquid crystal research: Applications of liquid crystals in soft matter nano-, bio- and microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412. [Google Scholar] [CrossRef]
- Park, H.-S.; Lavrentovich, O.D. Lyotropic chromonic liquid crystals: Emerging applications. Liq. Cryst. Beyond Disp. 2012. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Li, Y.; Bisoyi, H.K.; Wang, L.; Bunning, T.J.; Li, Q. Three-dimensional control of the helical axis of a chiral nematic liquid crystal by light. Nature 2016, 531, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Shen, Y.; Chang, Z.-N.; Li, W.-S.; Xu, Y.-C.; Fan, X.-Y.; Chen, L.-J. Dynamic cholesteric liquid crystal superstructures photoaligned by one-step polarization holography. Appl. Phys. Lett. 2017, 111, 231109. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Zola, R.S.; Bisoyi, H.K.; Wang, L.; Li, Y.; Bunning, T.J.; Li, Q. Controllable dynamic zigzag pattern formation in a soft helical superstructure. Adv. Mater. 2017, 29, 1701903. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Bunning, T.J.; Li, Q. Stimuli-driven control of the helical axis of self-organized soft helical superstructures. Adv. Mater. 2018, 30, e1706512. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, Y.C.; Ge, Y.H.; Jiang, R.G.; Wang, X.Z.; Li, S.S.; Chen, L.J. Photoalignment of dye-doped cholesteric liquid crystals for electrically tunable patterns with fingerprint textures. Opt. Express 2018, 26, 1422–1432. [Google Scholar] [CrossRef]
- Subacius, D.; Bos, P.J.; Lavrentovich, O.D. Switchable diffractive cholesteric gratings. Appl. Phys. Lett. 1997, 71, 1350–1352. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Urbas, A.M.; Li, Q. Soft materials driven by photothermal effect and their applications. Adv. Opt. Mater. 2018, 6, 1800458. [Google Scholar] [CrossRef]
- Ma, L.-L.; Li, S.-S.; Li, W.-S.; Ji, W.; Luo, B.; Zheng, Z.-G.; Cai, Z.-P.; Chigrinov, V.; Lu, Y.-Q.; Hu, W.; et al. Rationally designed dynamic superstructures enabled by photoaligning cholesteric liquid crystals. Adv. Opt. Mater. 2015, 3, 1691–1696. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y.; Shan, Y.; Gong, L.; Chen, J.; Li, S.; Chen, L. Magnetic nanoparticle-assisted tunable optical patterns from spherical cholesteric liquid crystal bragg reflectors. Nanomaterials 2017, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.; Kelly, S.M. Ordered materials for organic electronics and photonics. Adv. Mater. 2010, 23, 566–584. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Miyama, T.; Takatoh, K.; Kobayashi, S. Enhancement of the characteristics of lcds by doping nanoparticles: Reduction of the operating voltage, viscosity, and response times. In Proceedings of the Integrated Optoelectronic Devices 2006, San Jose, CA, USA, 21–26 January 2006; SPIE: Bellingham, WA, USA, 2006; p. 613501. [Google Scholar]
- Stannarius, R. Liquid crystals: More than display fillings. Nat. Mater. 2009, 8, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Ryabchun, A.; Bobrovsky, A. Cholesteric liquid crystal materials for tunable diffractive optics. Adv. Opt. Mater. 2018, 6, 1800335. [Google Scholar] [CrossRef]
- Li, W.-S.; Ma, L.-L.; Gong, L.-L.; Li, S.-S.; Yang, C.; Luo, B.; Hu, W.; Chen, L.-J. Interlaced cholesteric liquid crystal fingerprint textures via sequential uv-induced polymer-stabilization. Opt. Mater. Express 2016, 6, 19–28. [Google Scholar] [CrossRef]
- Li, W.-S.; Shen, Y.; Chen, Z.-J.; Cui, Q.; Li, S.-S.; Chen, L.-J. Demonstration of patterned polymer-stabilized cholesteric liquid crystal textures for anti-counterfeiting two-dimensional barcodes. Appl. Opt. 2017, 56, 601–606. [Google Scholar] [CrossRef]
- Huang, W.; Yuan, C.L.; Shen, D.; Zheng, Z.G. Dynamically manipulated lasing enabled by a reconfigured fingerprint texture of a cholesteric self-organized superstructure. J. Mater. Chem. C 2017, 5, 6923–6928. [Google Scholar] [CrossRef]
- Price, A.D.; Schwartz, D.K. DNA hybridization-induced reorientation of liquid crystal anchoring at the nematic liquid crystal/aqueous interface. J. Am. Chem. Soc. 2008, 130, 8188–8194. [Google Scholar] [CrossRef]
- Sivakumar, S.; Wark, K.L.; Gupta, J.K.; Abbott, N.L.; Caruso, F. Liquid crystal emulsions as the basis of biological sensors for the optical detection of bacteria and viruses. Adv. Funct. Mater. 2009, 19, 2260–2265. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yang, K.-L. Detection and quantification of DNA adsorbed on solid surfaces by using liquid crystals. Langmuir 2010, 26, 1427–1430. [Google Scholar] [CrossRef]
- Spicer, P.T. Progress in liquid crystalline dispersions: Cubosomes. Curr. Opin. Colloid Interface Sci. 2005, 10, 274–279. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Leser, M.E.; Watzke, H.J.; Michel, M. Monoglyceride self-assembly structures as delivery vehicles. Trends Food Sci. Technol. 2006, 17, 204–214. [Google Scholar] [CrossRef]
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147–148, 333–342. [Google Scholar] [CrossRef]
- Mushenheim, P.C.; Trivedi, R.R.; Weibel, D.B.; Abbott, N.L. Using liquid crystals to reveal how mechanical anisotropy changes interfacial behaviors of motile bacteria. Biophys. J. 2014, 107, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sokolov, A.; Lavrentovich, O.D.; Aranson, I.S. Living liquid crystals. Proc. Natl. Acad. Sci. USA 2014, 111, 1265. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yoon, D.K.; Jeong, H.S.; Lavrentovich, O.D.; Jung, H.-T. Smectic liquid crystal defects for self-assembling of building blocks and their lithographic applications. Adv. Funct. Mater. 2011, 21, 610–627. [Google Scholar] [CrossRef]
- Tschierske, C. Liquid crystal engineering—new complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev. 2007, 36, 1930–1970. [Google Scholar] [CrossRef]
- Zhou, S. Living liquid crystals. In Lyotropic Chromonic Liquid Crystals: From Viscoelastic Properties to Living Liquid Crystals; Zhou, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 77–92. [Google Scholar]
- Wang, L.; Ge, S.; Hu, W.; Nakajima, M.; Lu, Y. Tunable reflective liquid crystal terahertz waveplates. Opt. Mater. Express 2017, 7, 2023. [Google Scholar] [CrossRef]
- Kularatne, R.S.; Kim, H.; Boothby, J.M.; Ware, T.H. Liquid crystal elastomer actuators: Synthesis, alignment, and applications. J. Polym. Sci. B Polym. Phys. 2017, 55, 395–411. [Google Scholar] [CrossRef]
- Oldenbourg, R.; Wen, X.; Meyer, R.B.; Caspar, D.L.D. Orientational distribution function in nematic tobacco-mosaic-virus liquid crystals measured by X-ray diffraction. Phys. Rev. Lett. 1988, 61, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.S.; Ren, G.; Jenekhe, S.A. One-dimensional nanostructures of π-conjugated molecular systems: Assembly, properties, and applications from photovoltaics, sensors, and nanophotonics to nanoelectronics. Chem. Mater. 2010, 23, 682–732. [Google Scholar] [CrossRef]
- Hamley, I.W. Nanotechnologie mit weichen Materialien. Angew. Chem. 2003, 115, 1730–1752. [Google Scholar] [CrossRef]
- Anisa, M.; Abdallah, S.D.; Peter, A.S. ‘Mind the gap’: Science and ethics in nanotechnology. Nanotechnology 2003, 14, R9. [Google Scholar]
- Gupta, S.M.; Tripathi, M. A review of TO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639. [Google Scholar] [CrossRef]
- Koch, U.; Fojtik, A.; Weller, H.; Henglein, A. Photochemistry of semiconductor colloids. Preparation of extremely small ZnO particles, fluorescence phenomena and size quantization effects. Chem. Phys. Lett. 1985, 122, 507–510. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Jiang, H.; Shao, W.; Zhang, L.; He, Y. Synthesis and optical properties of TiO2 nanoparticles. Mater. Lett. 2007, 61, 79–83. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Liu, J.; He, H.; Xiao, D.; Yin, S.; Ji, W.; Jiang, S.; Luo, D.; Wang, B.; Liu, Y. Recent advances of plasmonic nanoparticles and their applications. Materials 2018, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pelligra, C.I.; Feng, X.; Osuji, C.O. Directed assembly of hybrid nanomaterials and nanocomposites. Adv. Mater. 2018, 30, 1705794. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.B.; Fu, C.H.; Einaga, Y.; Gu, Z.Z.; Fujishima, A.; Sato, O. Assembly of highly ordered three-dimensional porous structure with nanocrystalline TiO2 semiconductors. Chem. Mater. 2002, 14, 83–88. [Google Scholar] [CrossRef]
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ultrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef] [PubMed]
- Robel, I.; Kuno, M.; Kamat, P.V. Size-dependent electron injection from excited CdSe quantum dots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 4136–4137. [Google Scholar] [CrossRef] [PubMed]
- Hyun, B.-R.; Zhong, Y.-W.; Bartnik, A.C.; Sun, L.; Abruña, H.D.; Wise, F.W.; Goodreau, J.D.; Matthews, J.R.; Leslie, T.M.; Borrelli, N.F. Electron injection from colloidal PbS quantum dots into titanium dioxide nanoparticles. ACS Nano 2008, 2, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Singh, M.K.; Mathpal, M.C.; Mishra, S.K.; Agarwal, A. Study of structural transformation in TiO2 nanoparticles and its optical properties. J. Alloy Compd. 2013, 549, 114–120. [Google Scholar] [CrossRef]
- Chik, M.W.; Hussain, Z.; Zulkefeli, M.; Tripathy, M.; Kumar, S.; Majeed, A.B.A.; Byrappa, K. Polymer-wrapped single-walled carbon nanotubes: A transformation toward better applications in healthcare. Drug Deliv. Transl. Res. 2018, 9, 578–594. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed self-assembly of nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef]
- Goodby, J.W.; Saez, I.M.; Cowling, S.J.; Görtz, V.; Draper, M.; Hall, A.W.; Sia, S.; Cosquer, G.; Lee, S.-E.; Raynes, E.P. Transmission and amplification of information and properties in nanostructured liquid crystals. Angew. Chem. Int. Ed. 2008, 47, 2754–2787. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Hegmann, T. Impact of nanoscale particles and carbon nanotubes on current and future generations of liquid crystal displays. J. Mater. Chem. 2008, 18, 3288–3294. [Google Scholar] [CrossRef]
- Blanc, C.; Coursault, D.; Lacaze, E. Ordering nano- and microparticles assemblies with liquid crystals. Liq. Cryst. Rev. 2013, 1, 83–109. [Google Scholar] [CrossRef]
- Shivakumar, U.; Mirzaei, J.; Feng, X.; Sharma, A.; Moreira, P.; Hegmann, T. Nanoparticles: Complex and multifaceted additives for liquid crystals. Liq. Cryst. 2011, 38, 1495–1514. [Google Scholar] [CrossRef]
- Dierking, I. Nanomaterials in liquid crystals. Nanomaterials 2018, 8, 453. [Google Scholar] [CrossRef]
- Garbovskiy, Y.A.; Glushchenko, A.V. Liquid crystalline colloids of nanoparticles. In Solid State Physics; Academic Press: Cambridge, MA, USA, 2010; Volume 62, pp. 1–74. [Google Scholar]
- Poulin, P.; Stark, H.; Lubensky, T.C.; Weitz, D.A. Novel colloidal interactions in anisotropic fluids. Science 1997, 275, 1770. [Google Scholar] [CrossRef]
- Khoo, I.; Chen, K.; Williams, Y.Z. Orientational photorefractive effect in undoped and cdse nanorods-doped nematic liquid crystal—Bulk and interface contributions. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 443–450. [Google Scholar] [CrossRef]
- Khoo, I.C.; Williams, Y.Z.; Lewis, B.; Mallouk, T. + − Photorefractive CdSe and gold nanowire-doped liquid crystals and polymer-dispersed-liquid-crystal photonic crystals. Mol. Cryst. Liq. Cryst. 2006, 446, 233–244. [Google Scholar] [CrossRef]
- Kinkead, B.; Hegmann, T. Effects of size, capping agent, and concentration of CdSe and CdTe quantum dots doped into a nematic liquid crystal on the optical and electro-optic properties of the final colloidal liquid crystal mixture. J. Mater. Chem. 2010, 20, 448–458. [Google Scholar] [CrossRef]
- Bartkiewicz, S.; Matczyszyn, K.; Miniewicz, A.; Kajzar, F. High gain of light in photoconducting polymer–nematic liquid crystal hybrid structures. Opt. Commun. 2001, 187, 257–261. [Google Scholar] [CrossRef]
- Müller, J.; Sönnichsen, C.; von Poschinger, H.; von Plessen, G.; Klar, T.A.; Feldmann, J. Electrically controlled light scattering with single metal nanoparticles. Appl. Phys. Lett. 2002, 81, 171–173. [Google Scholar] [CrossRef]
- Tomohiro, M.; Jirakorn, T.; Hiroyuki, S.; Yoshio, S.; Yukihide, S.; Naoki, T.; Shunsuke, K. Fast switching of frequency modulation twisted nematic liquid crystal display fabricated by doping nanoparticles and its mechanism. Jpn. J. Appl. Phys. 2004, 43, 2580. [Google Scholar]
- Rajiv, M.; Sat Prakash, Y.; Abhishek Kumar, S.; Abhishek Kumar, M.; Kamal Kumar, P.; Prashant, K.S.; Avinash Chand, P. Zinc oxide (1% Cu) nanoparticle in nematic liquid crystal: Dielectric and electro-optical study. Jpn. J. Appl. Phys. 2009, 48, 101501. [Google Scholar]
- Li, X.; Yang, C.; Wang, Q.; Jia, D.; Hu, L.; Peng, Z.; Xuan, L. Enhanced birefringence for metallic nanoparticle doped liquid crystals. Opt. Commun. 2013, 286, 224–227. [Google Scholar] [CrossRef]
- Park, S.Y.; Stroud, D. Surface-enhanced plasmon splitting in a liquid-crystal-coated gold nanoparticle. Phys. Rev. Lett. 2005, 94, 217401. [Google Scholar] [CrossRef] [PubMed]
- Kossyrev, P.A.; Yin, A.; Cloutier, S.G.; Cardimona, D.A.; Huang, D.; Alsing, P.M.; Xu, J.M. Electric field tuning of plasmonic response of nanodot array in liquid crystal matrix. Nano Lett. 2005, 5, 1978–1981. [Google Scholar] [CrossRef] [PubMed]
- Brochard, F.; De Gennes, P. Theory of magnetic suspensions in liquid crystals. J. Phys. 1970, 31, 691–708. [Google Scholar] [CrossRef]
- Rault, J.; Cladis, P.E.; Burger, J.P. Ferronematics. Phys. Lett. A 1970, 32, 199–200. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Buchnev, O.; Nandhakumar, I. Ferroelectric nanoparticles in low refractive index liquid crystals for strong electro-optic response. Appl. Phys. Lett. 2008, 92, 103307. [Google Scholar] [CrossRef]
- Li, F.; Buchnev, O.; Cheon, C.I.; Glushchenko, A.; Reshetnyak, V.; Reznikov, Y.; Sluckin, T.J.; West, J.L. Orientational coupling amplification in ferroelectric nematic colloids. Phys. Rev. Lett. 2006, 97, 147801. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S.P.; Biradar, A.M.; Choudhary, A.; Sreenivas, K. Enhanced electro-optical properties in gold nanoparticles doped ferroelectric liquid crystals. Appl. Phys. Lett. 2007, 91, 023120. [Google Scholar] [CrossRef]
- Yada, M.; Yamamoto, J.; Yokoyama, H. Direct observation of anisotropic interparticle forces in nematic colloids with optical tweezers. Phys. Rev. Lett. 2004, 92, 185501. [Google Scholar] [CrossRef]
- Smalyukh, I.I.; Kuzmin, A.N.; Kachynski, A.V.; Prasad, P.N.; Lavrentovich, O.D. Optical trapping of colloidal particles and measurement of the defect line tension and colloidal forces in a thermotropic nematic liquid crystal. Appl. Phys. Lett. 2005, 86, 021913. [Google Scholar] [CrossRef]
- Lapanik, A.; Rudzki, A.; Kinkead, B.; Qi, H.; Hegmann, T.; Haase, W. Electrooptical and dielectric properties of alkylthiol-capped gold nanoparticle–ferroelectric liquid crystal nanocomposites: Influence of chain length and tethered liquid crystal functional groups. Soft Matter 2012, 8, 8722–8728. [Google Scholar] [CrossRef]
- Shukla, R.K.; Feng, X.; Umadevi, S.; Hegmann, T.; Haase, W. Influence of different amount of functionalized bulky gold nanorods dopant on the electrooptical, dielectric and optical properties of the flc host. Chem. Phys. Lett. 2014, 599, 80–85. [Google Scholar] [CrossRef]
- Joshi, T.; Kumar, A.; Prakash, J.; Biradar, A.M. Low power operation of ferroelectric liquid crystal system dispersed with zinc oxide nanoparticles. Appl. Phys. Lett. 2010, 96, 253109. [Google Scholar] [CrossRef]
- Lisetski, L.N.; Minenko, S.S.; Zhukov, A.V.; Shtifanyuk, P.P.; Lebovka, N.I. Dispersions of carbon nanotubes in cholesteric liquid crystals. Mol. Cryst. Liq. Cryst. 2009, 510, 43–50. [Google Scholar] [CrossRef]
- Singh, U.B.; Dhar, R.; Dabrowski, R.; Pandey, M.B. Enhanced electro-optical properties of a nematic liquid crystals in presence of BaTiO3 nanoparticles. Liq. Cryst. 2014, 41, 953–959. [Google Scholar] [CrossRef]
- San, S.E.; Okutan, M.; Köysal, O.; Yerli, Y. Carbon nanoparticles in nematic liquid crystals. Chin. Phys. Lett. 2008, 25, 212. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, L.; Shang, Y.; Li, X.; Guo, L. Facet-dependent electro-optical properties of cholesteric liquid crystals doped with Cu2O nanocrystals. Nano Res. 2018, 11, 4836–4845. [Google Scholar] [CrossRef]
- Williams, Y.; Chan, K.; Park, J.H.; Khoo, I.C.; Lewis, B.; Mallouk, T.E. Electro-Optical and Nonlinear Optical Properties of Semiconductor Nanorod Doped Liquid Crystals. In Proceedings of the Optics and Photonics 2005, San Diego, CA, USA, 22 July 2005; SPIE: Bellingham, WA, USA, 2005; p. 593613. [Google Scholar]
- Garbovskiy, Y.; Glushchenko, A. Ferroelectric nanoparticles in liquid crystals: Recent progress and current challenges. Nanomaterials 2017, 7, 361. [Google Scholar] [CrossRef]
- Shelestiuk, S.M.; Reshetnyak, V.Y.; Sluckin, T.J. Frederiks transition in ferroelectric liquid-crystal nanosuspensions. Phys. Rev. E 2011, 83, 041705. [Google Scholar] [CrossRef]
- Kurochkin, O.; Buchnev, O.; Iljin, A.; Park, S.K.; Kwon, S.B.; Grabar, O.; Yu, R. A colloid of ferroelectric nanoparticles in a cholesteric liquid crystal. J. Opt. A Pure Appl. Opt. 2009, 11, 024003. [Google Scholar] [CrossRef]
- Lopatina, L.M.; Selinger, J.V. Maier-saupe-type theory of ferroelectric nanoparticles in nematic liquid crystals. Phys. Rev. E 2011, 84, 041703. [Google Scholar] [CrossRef]
- Chen, S.-H.; Amer, N.M. Observation of macroscopic collective behavior and new texture in magnetically doped liquid crystals. Phys. Rev. Lett. 1983, 51, 2298–2301. [Google Scholar] [CrossRef]
- Martínez-Miranda, L.J.; McCarthy, K.; Kurihara, L.K.; Harry, J.J.; Noel, A. Effect of the surface coating on the magnetic nanoparticle smectic-a liquid crystal interaction. Appl. Phys. Lett. 2006, 89, 161917. [Google Scholar] [CrossRef]
- Kopčanský, P.; Tomašovičová, N.; Koneracká, M.; Závišová, V.; Timko, M.; Džarová, A.; Šprincová, A.; Éber, N.; Fodor-Csorba, K.; Tóth-Katona, T.; et al. Structural changes in the 6chbt liquid crystal doped with spherical, rodlike, and chainlike magnetic particles. Phys. Rev. E 2008, 78, 011702. [Google Scholar] [CrossRef]
- Mertelj, A.; Lisjak, D.; Drofenik, M.; Čopič, M. Ferromagnetism in suspensions of magnetic platelets in liquid crystal. Nature 2013, 504, 237. [Google Scholar] [CrossRef]
- Mertelj, A.; Osterman, N.; Lisjak, D.; Čopič, M. Magneto-optic and converse magnetoelectric effects in a ferromagnetic liquid crystal. Soft Matter 2014, 10, 9065–9072. [Google Scholar] [CrossRef]
- Hiroyuki, Y.; Yuma, T.; Kosuke, K.; Hitoshi, K.; Tetsuya, T.; Akihiko, F.; Susumu, K.; Hirotsugu, K.; Masanori, O. Nanoparticle-stabilized cholesteric blue phases. Appl. Phys. Express 2009, 2, 121501. [Google Scholar]
- Karatairi, E.; Rožič, B.; Kutnjak, Z.; Tzitzios, V.; Nounesis, G.; Cordoyiannis, G.; Thoen, J.; Glorieux, C.; Kralj, S. Nanoparticle-induced widening of the temperature range of liquid-crystalline blue phases. Phys. Rev. E 2010, 81, 041703. [Google Scholar] [CrossRef]
- Prakash, J.; Choudhary, A.; Kumar, A.; Mehta, D.S.; Biradar, A.M. Nonvolatile memory effect based on gold nanoparticles doped ferroelectric liquid crystal. Appl. Phys. Lett. 2008, 93, 112904. [Google Scholar] [CrossRef]
- Dolgov, L.O.; Yaroshchuk, O.V. Electrooptic properties of liquid crystals filled with silica nanoparticles of different sorts. Colloid Polym. Sci. 2004, 282, 1403–1408. [Google Scholar] [CrossRef]
- Basu, R. Soft memory in a ferroelectric nanoparticle-doped liquid crystal. Phys. Rev. E 2014, 89, 022508. [Google Scholar] [CrossRef]
- Kempaiah, R.; Liu, Y.; Nie, Z.; Basu, R. Giant soft-memory in liquid crystal nanocomposites. Appl. Phys. Lett. 2016, 108, 083105. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Toshima, N.; Maeda, K.; Yoshikawa, H.; Xu, J.; Kobayashi, S. Frequency modulation response of a liquid-crystal electro-optic device doped with nanoparticles. Appl. Phys. Lett. 2002, 81, 2845–2847. [Google Scholar] [CrossRef]
- Dierking, I.; Scalia, G.; Morales, P.; LeClere, D. Aligning and reorienting carbon nanotubes with nematic liquid crystals. Adv. Mater. 2004, 16, 865–869. [Google Scholar] [CrossRef]
- Özgan, Ş.; Eskalen, H.; Tapkıranlı, Y. Thermal and electro-optic properties of graphene oxide-doped hexylcyanobiphenyl liquid crystal. J. Theor. Appl. Phys. 2018, 12, 169–176. [Google Scholar] [CrossRef]
- Gardner, D.F.; Evans, J.S.; Smalyukh, I.I. Towards reconfigurable optical metamaterials: Colloidal nanoparticle self-assembly and self-alignment in liquid crystals. Mol. Cryst. Liq. Cryst. 2011, 545, 1227–1245. [Google Scholar] [CrossRef]
- Valentine, J.; Zhang, S.; Zentgraf, T.; Ulin-Avila, E.; Genov, D.A.; Bartal, G.; Zhang, X. Three-dimensional optical metamaterial with a negative refractive index. Nature 2008, 455, 376. [Google Scholar] [CrossRef] [PubMed]
- Zheludev, N.I.; Kivshar, Y.S. From metamaterials to metadevices. Nat. Mater. 2012, 11, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Si, G.; Leong, E.S.P.; Jiang, X.; Lv, J.; Lin, J.; Dai, H.; Liu, Y.J. All-optical, polarization-insensitive light tuning properties in silver nanorod arrays covered with photoresponsive liquid crystals. Phys. Chem. Chem. Phys. 2015, 17, 13223–13227. [Google Scholar] [CrossRef] [PubMed]
- Nemati, A.; Wang, Q.; Hong, M.; Teng, J. Tunable and reconfigurable metasurfaces and metadevices. Opto Electron. Adv. 2018, 1, 180009. [Google Scholar] [CrossRef]
- Liu, Y.J.; Hao, Q.; Smalley, J.S.T.; Liou, J.; Khoo, I.C.; Huang, T.J. A frequency-addressed plasmonic switch based on dual-frequency liquid crystals. Appl. Phys. Lett. 2010, 97, 091101. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366. [Google Scholar] [CrossRef] [PubMed]
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Chu, K.C.; Chao, C.Y.; Chen, Y.F.; Wu, Y.C.; Chen, C.C. Electrically controlled surface plasmon resonance frequency of gold nanorods. Appl. Phys. Lett. 2006, 89, 103107. [Google Scholar] [CrossRef]
- Evans, P.R.; Wurtz, G.A.; Hendren, W.R.; Atkinson, R.; Dickson, W.; Zayats, A.V.; Pollard, R.J. Electrically switchable nonreciprocal transmission of plasmonic nanorods with liquid crystal. Appl. Phys. Lett. 2007, 91, 043101. [Google Scholar] [CrossRef]
- Singh, D.P.; Gupta, S.K.; Srivastava, A.; Manohar, R. The phenomenon of induced photoluminescence in ferroelectric mesophase. J. Lumin. 2013, 139, 60–63. [Google Scholar] [CrossRef]
- Tong, X.; Zhao, Y. Liquid-crystal gel-dispersed quantum dots: Reversible modulation of photoluminescence intensity using an electric field. J. Am. Chem. Soc. 2007, 129, 6372–6373. [Google Scholar] [CrossRef] [PubMed]
- Rodarte, L.A.; Cisneros, F.; Hein, E.J.; Ghosh, S.; Hirst, S.L. Quantum dot/liquid crystal nanocomposites in photonic devices. Photonics 2015, 2, 855–864. [Google Scholar] [CrossRef]
- Danilov, V.V.; Artem’ev, M.V.; Baranov, A.V.; Ermolaeva, G.M.; Utkina, N.A.; Khrebtov, A.I. Fluorescence of semiconductor nanorods in liquid-crystal composites. Opt. Spectrosc. 2008, 105, 306–309. [Google Scholar] [CrossRef]
- Ozaki, M.; Kasano, M.; Ganzke, D.; Haase, W.; Yoshino, K. Mirrorless lasing in a dye-doped ferroelectric liquid crystal. Adv. Mater. 2002, 14, 306–309. [Google Scholar] [CrossRef]
- Chen, L.-J.; Lee, C.-R.; Chu, C.-L. Surface passivation assisted lasing emission in the quantum dots doped cholesteric liquid crystal resonating cavity with polymer template. RSC Adv. 2014, 4, 52804–52807. [Google Scholar] [CrossRef]
- Lee, C.-R.; Lin, S.-H.; Guo, J.-W.; Lin, J.-D.; Lin, H.-L.; Zheng, Y.-C.; Ma, C.-L.; Horng, C.-T.; Sun, H.-Y.; Huang, S.-Y. Electrically and thermally controllable nanoparticle random laser in a well-aligned dye-doped liquid crystal cell. Opt. Mater. Express 2015, 5, 1469–1481. [Google Scholar] [CrossRef]
- Rodarte, A.L.; Gray, C.; Hirst, L.S.; Ghosh, S. Spectral and polarization modulation of quantum dot emission in a one-dimensional liquid crystal photonic cavity. Phys. Rev. B 2012, 85, 035430. [Google Scholar] [CrossRef]
- Wu, K.-J.; Chu, K.-C.; Chao, C.-Y.; Chen, Y.-F.; Lai, C.-W.; Kang, C.-C.; Chen, C.-Y.; Chou, P.-T. CdS nanorods imbedded in liquid crystal cells for smart optoelectronic devices. Nano Lett. 2007, 7, 1908–1913. [Google Scholar] [CrossRef]
- Ruhwandl, R.W.; Terentjev, E.M. Long-range forces and aggregation of colloid particles in a nematic liquid crystal. Phys. Rev. E 1997, 55, 2958–2961. [Google Scholar] [CrossRef]
- Stark, H. Physics of colloidal dispersions in nematic liquid crystals. Phys. Rep. 2001, 351, 387–474. [Google Scholar] [CrossRef]
- Pires, D.; Fleury, J.-B.; Galerne, Y. Colloid particles in the interaction field of a disclination line in a nematic phase. Phys. Rev. Lett. 2007, 98, 247801. [Google Scholar] [CrossRef] [PubMed]
- Samitsu, S.; Takanishi, Y.; Yamamoto, J. Molecular manipulator driven by spatial variation of liquid-crystalline order. Nat. Mater. 2010, 9, 816. [Google Scholar] [CrossRef] [PubMed]
- Kuksenok, O.V.; Ruhwandl, R.W.; Shiyanovskii, S.V.; Terentjev, E.M. Director structure around a colloid particle suspended in a nematic liquid crystal. Phys. Rev. E 1996, 54, 5198–5203. [Google Scholar] [CrossRef]
- Mondain-Monval, O.; Dedieu, J.C.; Gulik-Krzywicki, T.; Poulin, P. Weak surface energy in nematic dispersions: Saturn ring defects and quadrupolar interactions. Eur. Phys. J. B Condens. Matter Complex. Syst. 1999, 12, 167–170. [Google Scholar] [CrossRef]
- Stark, H. Director field configurations around a spherical particle in a nematic liquid crystal. Eur. Phys. J. B Condens. Matter Complex. Syst. 1999, 10, 311–321. [Google Scholar] [CrossRef]
- Koenig, G.M.; de Pablo, J.J.; Abbott, N.L. Characterization of the reversible interaction of pairs of nanoparticles dispersed in nematic liquid crystals. Langmuir 2009, 25, 13318–13321. [Google Scholar] [CrossRef] [PubMed]
- Škarabot, M.; Muševič, I. Direct observation of interaction of nanoparticles in a nematic liquid crystal. Soft Matter 2010, 6, 5476–5481. [Google Scholar] [CrossRef]
- Tomar, V.; Roberts, T.F.; Abbott, N.L.; Hernández-Ortiz, J.P.; de Pablo, J.J. Liquid crystal mediated interactions between nanoparticles in a nematic phase. Langmuir 2012, 28, 6124–6131. [Google Scholar] [CrossRef]
- Kotar, J.; Vilfan, M.; Osterman, N.; Babič, D.; Čopič, M.; Poberaj, I. Interparticle potential and drag coefficient in nematic colloids. Phys. Rev. Lett. 2006, 96, 207801. [Google Scholar] [CrossRef]
- Škarabot, M.; Ravnik, M.; Žumer, S.; Tkalec, U.; Poberaj, I.; Babič, D.; Osterman, N.; Muševič, I. Interactions of quadrupolar nematic colloids. Phys. Rev. E 2008, 77, 031705. [Google Scholar] [CrossRef]
- Vilfan, M.; Osterman, N.; Čopič, M.; Ravnik, M.; Žumer, S.; Kotar, J.; Babič, D.; Poberaj, I. Confinement effect on interparticle potential in nematic colloids. Phys. Rev. Lett. 2008, 101, 237801. [Google Scholar] [CrossRef] [PubMed]
- Chernyshuk, S.B.; Lev, B.I. Elastic interaction between colloidal particles in confined nematic liquid crystals. Phys. Rev. E 2010, 81, 041701. [Google Scholar] [CrossRef] [PubMed]
- Muševič, I.; Škarabot, M.; Tkalec, U.; Ravnik, M.; Žumer, S. Two-dimensional nematic colloidal crystals self-assembled by topological defects. Science 2006, 313, 954. [Google Scholar] [CrossRef] [PubMed]
- Škarabot, M.; Ravnik, M.; Žumer, S.; Tkalec, U.; Poberaj, I.; Babič, D.; Osterman, N.; Muševič, I. Two-dimensional dipolar nematic colloidal crystals. Phys. Rev. E 2007, 76, 051406. [Google Scholar] [CrossRef] [PubMed]
- Muševič, I.; Škarabot, M. Self-assembly of nematic colloids. Soft Matter 2008, 4, 195–199. [Google Scholar] [CrossRef]
- Ognysta, U.; Nych, A.; Nazarenko, V.; Škarabot, M.; Muševič, I. Design of 2d binary colloidal crystals in a nematic liquid crystal. Langmuir 2009, 25, 12092–12100. [Google Scholar] [CrossRef]
- Prathap Chandran, S.; Mondiot, F.; Mondain-Monval, O.; Loudet, J.C. Photonic control of surface anchoring on solid colloids dispersed in liquid crystals. Langmuir 2011, 27, 15185–15198. [Google Scholar] [CrossRef]
- Nych, A.; Ognysta, U.; Škarabot, M.; Ravnik, M.; Žumer, S.; Muševič, I. Assembly and control of 3d nematic dipolar colloidal crystals. Nat. Commun. 2013, 4, 1489. [Google Scholar] [CrossRef]
- Bawden, F.C.; Pirie, N.W.; Bernal, J.D.; Fankuchen, I. Liquid crystalline substances from virus-infected plants. Nature 1936, 138, 1051. [Google Scholar] [CrossRef]
- Onsager, L. The effects of shape on the interaction of colloidal particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Li, L.S.; Walda, J.; Manna, L.; Alivisatos, A.P. Semiconductor nanorod liquid crystals. Nano Lett. 2002, 2, 557–560. [Google Scholar] [CrossRef]
- Li, L.S.; Alivisatos, A.P. Semiconductor nanorod liquid crystals and their assembly on a substrate. Adv. Mater. 2003, 15, 408–411. [Google Scholar] [CrossRef]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef]
- Shimoda, H.; Oh, S.J.; Geng, H.Z.; Walker, R.J.; Zhang, X.B.; Mcneil, L.E.; Zhou, O. Self-assembly of carbon nanotubes. Adv. Mater. 2002, 14, 899–901. [Google Scholar] [CrossRef]
- Song, W.; Kinloch, I.A.; Windle, A.H. Nematic liquid crystallinity of multiwall carbon nanotubes. Science 2003, 302, 1363. [Google Scholar] [CrossRef]
- Behabtu, N.; Lomeda, J.R.; Green, M.J.; Higginbotham, A.L.; Sinitskii, A.; Kosynkin, D.V.; Tsentalovich, D.E.; Parravasquez, A.N.G.; Schmidt, J.; Kesselman, E. Spontaneous high-concentration dispersions and liquid crystals of graphene. Nat. Nanotechnol. 2010, 5, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Han, T.H.; Lee, S.H.; Kim, J.; Ahn, C.W.; Yun, J.M.; Kim, S.O. Graphene oxide liquid crystals. Angew. Chem. 2011, 50, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, C. Aqueous liquid crystals of graphene oxide. ACS Nano 2011, 5, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional liquid crystals towards the next generation of materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Nanoscience with Liquid Crystals; Springer: Berlin, Germany, 2016. [Google Scholar]
- Reznikov, Y.; Buchnev, O.; Tereshchenko, O.; Reshetnyak, V.; Glushchenko, A.; West, J. Ferroelectric nematic suspension. Appl. Phys. Lett. 2003, 82, 1917–1919. [Google Scholar] [CrossRef]
- Glushchenko, A.; Cheon, C.I.; West, J.; Li, F.; Büyüktanir, E.; Reznikov, Y.; Buchnev, A. Ferroelectric particles in liquid crystals: Recent frontiers. Mol. Cryst. Liq. Cryst. 2006, 453, 227–237. [Google Scholar] [CrossRef]
- Blach, J.F.; Saitzek, S.; Legrand, C.; Dupont, L.; Henninot, J.F.; Warenghem, M. BaTiO3 ferroelectric nanoparticles dispersed in 5CB nematic liquid crystal: Synthesis and electro-optical characterization. J. Appl. Phys. 2010, 107, 074102. [Google Scholar] [CrossRef]
- Mishra, M.; Dabrowski, R.S.; Dhar, R. Thermodynamical, optical, electrical and electro-optical studies of a room temperature nematic liquid crystal 4-pentyl-4′-cyanobiphenyl dispersed with barium titanate nanoparticles. J. Mol. Liq. 2016, 213, 247–254. [Google Scholar] [CrossRef]
- Klein, S.; Richardson Robert, M.; Greasty, R.; Jenkins, R.; Stone, J.; Thomas Michael, R.; Sarua, A. The influence of suspended nanoparticles on the frederiks threshold of the nematic host. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120253. [Google Scholar] [CrossRef] [PubMed]
- Al-Zangana, S.; Turner, M.; Dierking, I. A comparison between size dependent paraelectric and ferroelectric BaTiO3 nanoparticle doped nematic and ferroelectric liquid crystals. J. Appl. Phys. 2017, 121, 085105. [Google Scholar] [CrossRef]
- Dubey, R.; Mishra, A.; Singh, K.N.; Alapati, P.R.; Dhar, R. Electric behaviour of a schiff’s base liquid crystal compound doped with a low concentration of BaTiO3 nanoparticles. J. Mol. Liq. 2017, 225, 496–501. [Google Scholar] [CrossRef]
- Prasad, S.K.; Kumar, M.V.; Shilpa, T.; Yelamaggad, C.V. Enhancement of electrical conductivity, dielectric anisotropy and director relaxation frequency in composites of gold nanoparticle and a weakly polar nematic liquid crystal. RSC Adv. 2014, 4, 4453–4462. [Google Scholar] [CrossRef]
- Sridevi, S.; Prasad, S.K.; Nair, G.G.; D’Britto, V.; Prasad, B.L.V. Enhancement of anisotropic conductivity, elastic, and dielectric constants in a liquid crystal-gold nanorod system. Appl. Phys. Lett. 2010, 97, 151913. [Google Scholar] [CrossRef]
- Pandey, A.S.; Dhar, R.; Kumar, S.; Dabrowski, R. Enhancement of the display parameters of 4′-pentyl-4-cyanobiphenyl due to the dispersion of functionalised gold nano particles. Liq. Cryst. 2011, 38, 115–120. [Google Scholar] [CrossRef]
- Holt, L.A.; Bushby, R.J.; Evans, S.D.; Burgess, A.; Seeley, G. A 106-fold enhancement in the conductivity of a discotic liquid crystal doped with only 1% (w/w) gold nanoparticles. J. Appl. Phys. 2008, 103, 063712. [Google Scholar] [CrossRef]
- Singh, U.B.; Dhar, R.; Dabrowski, R.; Pandey, M.B. Influence of low concentration silver nanoparticles on the electrical and electro-optical parameters of nematic liquid crystals. Liq. Cryst. 2013, 40, 774–782. [Google Scholar] [CrossRef]
- Liu, B.; Ma, Y.; Zhao, D.; Xu, L.; Liu, F.; Zhou, W.; Guo, L. Effects of morphology and concentration of cus nanoparticles on alignment and electro-optic properties of nematic liquid crystal. Nano Res. 2017, 10, 618–625. [Google Scholar] [CrossRef]
- Khatua, S.; Manna, P.; Chang, W.-S.; Tcherniak, A.; Friedlander, E.; Zubarev, E.R.; Link, S. Plasmonic nanoparticles−liquid crystal composites. J. Phys. Chem. C 2010, 114, 7251–7257. [Google Scholar] [CrossRef]
- Elkhalgi, H.H.M.; Khandka, S.; Singh, U.B.; Pandey, K.L.; Dabrowski, R.; Dhar, R. Dielectric and electro-optical properties of a nematic liquid crystalline material with gold nanoparticles. Liq. Cryst. 2018, 45, 1795–1801. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, S.; Dhar, R. Effect of high concentration of colloidal gold nanoparticles on the thermodynamic, optical, and electrical properties of 2, 3, 6, 7, 10, 11-hexabutyloxytryphenylene discotic liquid crystalline material. Soft Matter 2017, 15, 34–44. [Google Scholar] [CrossRef]
- Gittins, D.I.; Bethell, D.; Schiffrin, D.J.; Nichols, R.J. A nanometre-scale electronic switch consisting of a metal cluster and redox-addressable groups. Nature 2000, 408, 67. [Google Scholar] [CrossRef]
- Haynes, C.L.; McFarland, A.D.; Zhao, L.; Van Duyne, R.P.; Schatz, G.C.; Gunnarsson, L.; Prikulis, J.; Kasemo, B.; Käll, M. Nanoparticle optics: The importance of radiative dipole coupling in two-dimensional nanoparticle arrays. J. Phys. Chem. B 2003, 107, 7337–7342. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef]
- Tripathi, P.; Mishra, M.; Kumar, S.; Dabrowski, R.; Dhar, R. Dependence of physical parameters on the size of silver nano particles forming composites with a nematic liquid crystalline material. J. Mol. Liq. 2018, 268, 403–409. [Google Scholar] [CrossRef]
- Mirzaei, J.; Reznikov, M.; Hegmann, T. Quantum dots as liquid crystal dopants. J. Mater. Chem. 2012, 22, 22350–22365. [Google Scholar] [CrossRef]
- Brus, L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Brus, L. Electronic wave functions in semiconductor clusters: Experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Singh, U.B.; Dhar, R.; Pandey, A.S.; Kumar, S.; Dabrowski, R.; Pandey, M.B. Electro-optical and dielectric properties of cdse quantum dots and 6chbt liquid crystals composites. AIP Adv. 2014, 4, 117112. [Google Scholar] [CrossRef]
- Konshina, E.A.; Galin, I.F.; Shcherbinin, D.P.; Gavrish, E.O. Study of dynamics and relaxation optical response of nematic liquid crystals doped with CdSe/ZnS quantum dots. Liq. Cryst. 2014, 41, 1229–1234. [Google Scholar] [CrossRef]
- Gupta, S.K.; Singh, D.P.; Tripathi, P.K.; Manohar, R.; Varia, M.; Sagar, L.K.; Kumar, S. CdSe quantum dot-dispersed DOBAMBC: An electro-optical study. Liq. Cryst. 2013, 40, 528–533. [Google Scholar] [CrossRef]
- Rastogi, A.; Pathak, G.; Srivastava, A.; Herman, J.; Manohar, R. Cd1−x ZnxS/ZnS core/shell quantum dots in nematic liquid crystals to improve material parameter for better performance of liquid crystal based devices. J. Mol. Liq. 2018, 255, 93–101. [Google Scholar] [CrossRef]
- Singh, U.B.; Pandey, M.B.; Dhar, R.; Verma, R.; Kumar, S. Effect of dispersion of cdse quantum dots on phase transition, electrical and electro-optical properties of 4pp4ob. Liq. Cryst. 2016, 43, 1075–1082. [Google Scholar] [CrossRef]
- Roy, A.; Pathak, G.; Herman, J.; Inamdar, S.R.; Srivastava, A.; Manohar, R. InP/ZnS quantum-dot-dispersed nematic liquid crystal illustrating characteristic birefringence and enhanced electro-optical parameters. Appl. Phys. A 2018, 124, 273. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Misra, A.K.; Manohar, S.; Gupta, S.K.; Manohar, R. Improved dielectric and electro-optical parameters of ZnO nano-particle (8% Cu2+) doped nematic liquid crystal. J. Mol. Struct. 2013, 1035, 371–377. [Google Scholar] [CrossRef]
- Ye, W.; Yuan, R.; Dai, Y.; Gao, L.; Pang, Z.; Zhu, J.; Meng, X.; He, Z.; Li, J.; Cai, M.; et al. Improvement of image sticking in liquid crystal display doped with γ-Fe2O3 nanoparticles. Nanomaterials 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Elkhalgi, H.H.M.; Khandka, S.; Yadav, N.; Dhar, R.; Dabrowski, R. Effects of manganese (ii) titanium oxide nano particles on the physical properties of a room temperature nematic liquid crystal 4-(trans-4′-n-hexylcyclohexyl) isothiocyanatobenzene. J. Mol. Liq. 2018, 268, 223–228. [Google Scholar] [CrossRef]
- Oh, C.-W.; Park, E.-G.; Park, H.-G. Enhanced electro-optical properties in titanium silicon oxide nanoparticle doped nematic liquid crystal system. Surf. Coat. Technol. 2019, 360, 50–55. [Google Scholar] [CrossRef]
- Li, C.-Z.; Yip, H.-L.; Jen, A.K.Y. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177. [Google Scholar] [CrossRef]
- San, S.E.; Köysal, O.; Okutan, M. Laser-induced dielectric anisotropy of a hybrid liquid crystal composite made up of methyl red and fullerene C60. J. Non-Cryst. Solids 2005, 351, 2798–2801. [Google Scholar] [CrossRef]
- Okutan, M.; Eren San, S.; Basaran, E.; Yakuphanoglu, F. Determination of phase transition from nematic to isotropic state in carbon nano-balls’ doped nematic liquid crystals by electrical conductivity-dielectric measurements. Phys. Lett. A 2005, 339, 461–465. [Google Scholar] [CrossRef]
- Shukla, R.K.; Raina, K.K.; Haase, W. Fast switching response and dielectric behaviour of fullerene/ferroelectric liquid crystal nanocolloids. Liq. Cryst. 2014, 41, 1726–1732. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, B.; Cong, H. Recent developments in fullerene-containing thermotropic liquid crystals. Curr. Org. Chem. 2017, 21, 1600–1611. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Baik, I.-S.; Jeon, S.Y.; Lee, S.H.; Park, K.A.; Jeong, S.H.; An, K.H.; Lee, Y.H. Electrical-field effect on carbon nanotubes in a twisted nematic liquid crystal cell. Appl. Phys. Lett. 2005, 87, 263110. [Google Scholar] [CrossRef]
- Chi-Yen, H.; Chao-Yuan, H.; Hung-Chih, P.; Kuang-Yao, L. Electrooptical responses of carbon nanotube-doped liquid crystal devices. Jpn. J. Appl. Phys. 2005, 44, 8077. [Google Scholar]
- Chen, H.-Y.; Lee, W. Suppression of field screening in nematic liquid crystals by carbon nanotubes. Appl. Phys. Lett. 2006, 88, 222105. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lee, W.; Clark, N.A. Faster electro-optical response characteristics of a carbon-nanotube-nematic suspension. Appl. Phys. Lett. 2007, 90, 033510. [Google Scholar] [CrossRef]
- Lee, W.; Wang, C.-Y.; Shih, Y.-C. Effects of carbon nanosolids on the electro-optical properties of a twisted nematic liquid-crystal host. Appl. Phys. Lett. 2004, 85, 513–515. [Google Scholar] [CrossRef]
- Kumar, J.; Manjuladevi, V.; Gupta, R.K.; Kumar, S. Fast response in TN liquid-crystal cells: Effect of functionalised carbon nanotubes. Liq. Cryst. 2016, 43, 488–496. [Google Scholar] [CrossRef]
- Shukla, R.K.; Chaudhary, A.; Bubnov, A.; Raina, K.K. Multi-walled carbon nanotubes-ferroelectric liquid crystal nanocomposites: Effect of cell thickness and dopant concentration on electro-optic and dielectric behaviour. Liq. Cryst. 2018, 45, 1672–1681. [Google Scholar] [CrossRef]
- Singh, D.; Bahadur Singh, U.; Bhushan Pandey, M.; Dabrowski, R.; Dhar, R. Improvement of orientational order and display parameters of liquid crystalline material dispersed with single-wall carbon nanotubes. Mater. Lett. 2018, 216, 5–7. [Google Scholar] [CrossRef]
- Singh, D.; Singh, U.B.; Pandey, M.B.; Dabrowski, R.; Dhar, R. Enhancement in electro-optical parameters of nematic liquid crystalline material with swcnts. Opt. Mater. 2018, 84, 16–21. [Google Scholar] [CrossRef]
- Schymura, S.; Scalia, G. On the effect of carbon nanotubes on properties of liquid crystals. Phil. Trans. R. Soc. A 2013, 371, 20120261. [Google Scholar] [CrossRef]
- Scalia, G.; Lagerwall, J.P.F.; Schymura, S.; Haluska, M.; Giesselmann, F.; Roth, S. Carbon nanotubes in liquid crystals as versatile functional materials. Phys. Status Solidi 2007, 244, 4212–4217. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666. [Google Scholar] [CrossRef] [PubMed]
- Gökçen, M.; Yıldırım, M.; Köysal, O. Dielectric and ac electrical conductivity characteristics of liquid crystal doped with graphene. Eur. Phys. J. Appl. Phys. 2012, 60, 30104. [Google Scholar] [CrossRef]
- Alam, T.M.; Pearce, C.J. Impact of graphene incorporation on the orientational order of graphene/liquid crystal composites. Chem. Phys. Lett. 2014, 592, 7–13. [Google Scholar] [CrossRef]
- Basu, R. Effects of graphene on electro-optic switching and spontaneous polarization of a ferroelectric liquid crystal. Appl. Phys. Lett. 2014, 105, 112905. [Google Scholar] [CrossRef]
- Basu, R.; Garvey, A.; Kinnamon, D. Effects of graphene on electro-optic response and ion-transport in a nematic liquid crystal. J. Appl. Phys. 2015, 117, 074301. [Google Scholar] [CrossRef]
- Basu, R.; Kinnamon, D.; Garvey, A. Nano-electromechanical rotation of graphene and giant enhancement in dielectric anisotropy in a liquid crystal. Appl. Phys. Lett. 2015, 106, 201909. [Google Scholar] [CrossRef]
- Javadian, S.; Dalir, N.; Kakemam, J. Non-covalent intermolecular interactions of colloidal nematic liquid crystals doped with graphene oxide. Liq. Cryst. 2017, 44, 1341–1355. [Google Scholar] [CrossRef]
- Al-Zangana, S.; Iliut, M.; Turner, M.; Vijayaraghavan, A.; Dierking, I. Properties of a thermotropic nematic liquid crystal doped with graphene oxide. Adv. Opt. Mater. 2016, 4, 1541–1548. [Google Scholar] [CrossRef]
- Al-Zangana, S.; Iliut, M.; Boran, G.; Turner, M.; Vijayaraghavan, A.; Dierking, I. Dielectric spectroscopy of isotropic liquids and liquid crystal phases with dispersed graphene oxide. Sci. Rep. 2016, 6, 31885. [Google Scholar] [CrossRef]
- Dalir, N.; Javadian, S.; Kakemam, J.; Yousefi, A. Evolution of electro-chemical and electro-optical properties of nematic liquid crystal doped with graphene oxide. J. Mol. Liq. 2018, 265, 398–407. [Google Scholar] [CrossRef]
- Lapanik, V.; Timofeev, S.; Haase, W. Electro-optic properties of nematic and ferroelectric liquid crystalline nanocolloids doped with partially reduced graphene oxide. Phase Transit. 2016, 89, 133–143. [Google Scholar] [CrossRef]
- Mrukiewicz, M.; Kowiorski, K.; Perkowski, P.; Mazur, R.; Djas, M. Threshold voltage decrease in a thermotropic nematic liquid crystal doped with graphene oxide flakes. Beilstein J. Nanotechnol. 2019, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.K.; Mirzaei, J.; Sharma, A.; Hofmann, D.; Hegmann, T.; Haase, W. Electro-optic and dielectric properties of a ferroelectric liquid crystal doped with chemically and thermally stable emissive carbon dots. RSC Adv. 2015, 5, 34491–34496. [Google Scholar] [CrossRef]
- Lapanik, V.; Lugouskiy, A.; Timofeev, S.; Haase, W. Influence of the size and the attached organic tail of modified detonation nanodiamond on the physical properties of liquid crystals. Liq. Cryst. 2014, 41, 1332–1338. [Google Scholar] [CrossRef]
- Cho, M.-J.; Park, H.-G.; Jeong, H.-C.; Lee, J.-W.; Jung, Y.H.; Kim, D.-H.; Kim, J.-H.; Lee, J.-W.; Seo, D.-S. Superior fast switching of liquid crystal devices using graphene quantum dots. Liq. Cryst. 2014, 41, 761–767. [Google Scholar] [CrossRef]
- Raether, H. Surface excitations. In Excitation of Plasmons and Interband Transitions by Electrons; Springer: Berlin, Germany, 1980; pp. 116–171. [Google Scholar]
- Song, J.-H.; Atay, T.; Shi, S.; Urabe, H.; Nurmikko, A.V. Large enhancement of fluorescence efficiency from cdse/zns quantum dots induced by resonant coupling to spatially controlled surface plasmons. Nano Lett. 2005, 5, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Ivanov, I.A.; Qu, Y.; Huang, Y.; Duan, X. Plasmonic modulation of the upconversion fluorescence in nayf4:Yb/tm hexaplate nanocrystals using gold nanoparticles or nanoshells. Angew. Chem. Int. Ed. 2010, 49, 2865–2868. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Lakowicz, J.R. Plasmon-enhanced fluorescence from single fluorophores end-linked to gold nanorods. J. Am. Chem. Soc. 2010, 132, 5540–5541. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced raman scattering (sers). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Noginov, M.A.; Zhu, G.; Belgrave, A.M.; Bakker, R.; Shalaev, V.M.; Narimanov, E.E.; Stout, S.; Herz, E.; Suteewong, T.; Wiesner, U. Demonstration of a spaser-based nanolaser. Nature 2009, 460, 1110. [Google Scholar] [CrossRef]
- Stockman, M.I. Spasers explained. Nat. Photonics 2008, 2, 327. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (pptt) using gold nanoparticles. Lasers Med. Sci. 2007, 23, 217. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Ghaemi, H.F.; Thio, T.; Wolff, P.A. Extraordinary optical transmission through sub-wavelength hole arrays. Nature 1998, 391, 667. [Google Scholar] [CrossRef]
- Fang, N.; Lee, H.; Sun, C.; Zhang, X. Sub–diffraction-limited optical imaging with a silver superlens. Science 2005, 308, 534–537. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, H.; Xiong, Y.; Sun, C.; Zhang, X. Far-field optical hyperlens magnifying sub-diffraction-limited objects. Science 2007, 315, 1686. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhuo, X.; Wang, J. Advanced plasmonic materials for dynamic color display. Adv. Mater. 2018, 30, 1704338. [Google Scholar] [CrossRef] [PubMed]
- Kalkbrenner, T.; Ramstein, M.; Mlynek, J.; Sandoghdar, V. A single gold particle as a probe for apertureless scanning near-field optical microscopy. J. Microsc. 2001, 202, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Lazarides, A.A.; Lance Kelly, K.; Jensen, T.R.; Schatz, G.C. Optical properties of metal nanoparticles and nanoparticle aggregates important in biosensors. J. Mol. Struct. 2000, 529, 59–63. [Google Scholar] [CrossRef]
- Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzán, L.M.; García de Abajo, F.J. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef]
- Choudhary, A.; Singh, G.; Biradar, A.M. Advances in gold nanoparticle–liquid crystal composites. Nanoscale 2014, 6, 7743–7756. [Google Scholar] [CrossRef]
- Prasad, P.N. Nanophotonics; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Jensen, T.; Kelly, L.; Lazarides, A.; Schatz, G.C. Electrodynamics of noble metal nanoparticles and nanoparticle clusters. J. Clust. Sci. 1999, 10, 295–317. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Chen, B.; Zeng, X.; Baumeister, U.; Ungar, G.; Tschierske, C. Liquid crystalline networks composed of pentagonal, square, and triangular cylinders. Science 2005, 307, 96. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- De Sio, L.; Caputo, R.; Cataldi, U.; Umeton, C. Broad band tuning of the plasmonic resonance of gold nanoparticles hosted in self-organized soft materials. J. Mater. Chem. 2011, 21, 18967–18970. [Google Scholar] [CrossRef]
- Caldwell, M.E.; Yeatman, E.M. Surface-plasmon spatial light modulators based on liquid crystal. Appl. Opt. 1992, 31, 3880–3891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Voltage-induced color-selective absorption with surface plasmons. Appl. Phys. Lett. 1995, 67, 2759–2761. [Google Scholar]
- Wang, Y.; Russell, S.D.; Shimabukuro, R.L. Voltage-induced broad-spectrum reflectivity change with surface-plasmon waves. J. Appl. Phys. 2004, 97, 023708. [Google Scholar]
- Koenig, G.M.; Meli, M.-V.; Park, J.-S.; de Pablo, J.J.; Abbott, N.L. Coupling of the plasmon resonances of chemically functionalized gold nanoparticles to local order in thermotropic liquid crystals. Chem. Mater. 2007, 19, 1053–1061. [Google Scholar] [CrossRef]
- Hsu, L.-H.; Lo, K.-Y.; Huang, S.-A.; Huang, C.-Y.; Yang, C.-S. Irreversible redshift of transmission spectrum of gold nanoparticles doped in liquid crystals. Appl. Phys. Lett. 2008, 92, 181112. [Google Scholar]
- Pratibha, R.; Park, K.; Smalyukh, I.I.; Park, W. Tunable optical metamaterial based on liquid crystal-gold nanosphere composite. Opt. Express 2009, 17, 19459–19469. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, Y.; Gardner, D.; Li, X.; He, S.; Smalyukh, I.I. Self-alignment of plasmonic gold nanorods in reconfigurable anisotropic fluids for tunable bulk metamaterial applications. Nano Lett. 2010, 10, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Senyuk, B.; Tang, J.; Lee, T.; Qian, J.; He, S.; Smalyukh, I.I. Plasmonic complex fluids of nematiclike and helicoidal self-assemblies of gold nanorods with a negative order parameter. Phys. Rev. Lett. 2012, 109, 088301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yuan, Y.; Smalyukh, I.I. Electrically and optically tunable plasmonic guest–host liquid crystals with long-range ordered nanoparticles. Nano Lett. 2014, 14, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Mundoor, H.; Yuan, Y.; Smalyukh, I.I. Metal nanoparticle dispersion, alignment, and assembly in nematic liquid crystals for applications in switchable plasmonic color filters and e-polarizers. ACS Nano 2015, 9, 3097–3108. [Google Scholar] [CrossRef]
- Li, D.; Liu, F.; Ren, G.J.; Fu, P.; Yao, J.Q. Liquid crystal-modulated tunable filter based on coupling between plasmon-induced transparency and cavity mode. Opt. Eng. 2018, 57, 097101. [Google Scholar] [CrossRef]
- Rožič, B.; Fresnais, J.; Molinaro, C.; Calixte, J.; Umadevi, S.; Lau-Truong, S.; Felidj, N.; Kraus, T.; Charra, F.; Dupuis, V.; et al. Oriented gold nanorods and gold nanorod chains within smectic liquid crystal topological defects. ACS Nano 2017, 11, 6728–6738. [Google Scholar] [CrossRef]
- Jiang, L.; Mundoor, H.; Liu, Q.; Smalyukh, I.I. Electric switching of fluorescence decay in gold–silica–dye nematic nanocolloids mediated by surface plasmons. ACS Nano 2016, 10, 7064–7072. [Google Scholar] [CrossRef]
- Sheetah, G.H.; Liu, Q.; Senyuk, B.; Fleury, B.; Smalyukh, I.I. Electric switching of visible and infrared transmission using liquid crystals co-doped with plasmonic gold nanorods and dichroic dyes. Opt. Express 2018, 26, 22264–22272. [Google Scholar] [CrossRef]
- Sheetah, G.H.; Liu, Q.; Smalyukh, I.I. Self-assembly of predesigned optical materials in nematic codispersions of plasmonic nanorods. Opt. Lett. 2016, 41, 4899–4902. [Google Scholar] [CrossRef]
- Jablan, M.; Soljačić, M.; Buljan, H. Plasmons in graphene: Fundamental properties and potential applications. Proc. IEEE 2013, 101, 1689–1704. [Google Scholar] [CrossRef]
- Ju, L.; Geng, B.; Horng, J.; Girit, C.; Martin, M.; Hao, Z.; Bechtel, H.A.; Liang, X.; Zettl, A.; Shen, Y.R.; et al. Graphene plasmonics for tunable terahertz metamaterials. Nat. Nanotechnol. 2011, 6, 630. [Google Scholar] [CrossRef] [PubMed]
- Garciía de Abajo, F.J. Special Issue “2d Materials for Nanophotonics”; ACS Publications: Washington, DC, USA, 2017. [Google Scholar]
- Li, Y.; Li, Z.; Chi, C.; Shan, H.; Zheng, L.; Fang, Z. Plasmonics of 2d nanomaterials: Properties and applications. Adv. Sci. 2017, 4, 1600430. [Google Scholar] [CrossRef] [PubMed]
- Jablan, M.; Buljan, H.; Soljačić, M. Plasmonics in graphene at infrared frequencies. Phys. Rev. B 2009, 80, 245435. [Google Scholar] [CrossRef]
- García de Abajo, F.J. Graphene plasmonics: Challenges and opportunities. ACS Photonics 2014, 1, 135–152. [Google Scholar] [CrossRef]
- Reshetnyak, V.Y.; Bunning, T.J.; Evans, D.R. Using liquid crystals to control surface plasmons. Liq. Cryst. 2018, 45, 2010–2021. [Google Scholar] [CrossRef]
- Zhu, M.; Baffou, G.; Meyerbröker, N.; Polleux, J. Micropatterning thermoplasmonic gold nanoarrays to manipulate cell adhesion. ACS Nano 2012, 6, 7227–7233. [Google Scholar] [CrossRef] [PubMed]
- Stehr, J.; Hrelescu, C.; Sperling, R.A.; Raschke, G.; Wunderlich, M.; Nichtl, A.; Heindl, D.; Kürzinger, K.; Parak, W.J.; Klar, T.A.; et al. Gold nanostoves for microsecond DNA melting analysis. Nano Lett. 2008, 8, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549. [Google Scholar] [CrossRef] [PubMed]
- Huschka, R.; Neumann, O.; Barhoumi, A.; Halas, N.J. Visualizing light-triggered release of molecules inside living cells. Nano Lett. 2010, 10, 4117–4122. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Evans, J.S.; Lee, T.; Senyuk, B.; Keller, P.; He, S.; Smalyukh, I.I. Optical manipulation of shape-morphing elastomeric liquid crystal microparticles doped with gold nanocrystals. Appl. Phys. Lett. 2012, 100, 241901. [Google Scholar] [CrossRef]
- Fong, W.-K.; Hanley, T.L.; Thierry, B.; Kirby, N.; Waddington, L.J.; Boyd, B.J. Controlling the nanostructure of gold nanorod–lyotropic liquid-crystalline hybrid materials using near-infrared laser irradiation. Langmuir 2012, 28, 14450–14460. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.-K.; Hanley, T.L.; Thierry, B.; Tilley, A.; Kirby, N.; Waddington, L.J.; Boyd, B.J. Understanding the photothermal heating effect in non-lamellar liquid crystalline systems, and the design of new mixed lipid systems for photothermal on-demand drug delivery. Phys. Chem. Chem. Phys. 2014, 16, 24936–24953. [Google Scholar] [CrossRef]
- Pezzi, L.; De Sio, L.; Veltri, A.; Placido, T.; Palermo, G.; Comparelli, R.; Curri, M.L.; Agostiano, A.; Tabiryan, N.; Umeton, C. Photo-thermal effects in gold nanoparticles dispersed in thermotropic nematic liquid crystals. Phys. Chem. Chem. Phys. 2015, 17, 20281–20287. [Google Scholar] [CrossRef] [PubMed]
- Luciano De, S.; Tiziana, P.; Roberto, C.; Maria Lucia, C.; Nelson, T.; Timothy, J.B. Plasmonic photoheating of gold nanorods in thermo-responsive chiral liquid crystals. J. Opt. 2016, 18, 125005. [Google Scholar]
- Palermo, G.; Cataldi, U.; De Sio, L.; Bürgi, T.; Tabiryan, N.; Umeton, C. Optical control of plasmonic heating effects using reversible photo-alignment of nematic liquid crystals. Appl. Phys. Lett. 2016, 109, 191906. [Google Scholar] [CrossRef]
- Wang, L.; Gutierrez-Cuevas, K.G.; Urbas, A.; Li, Q. Near-infrared light-directed handedness inversion in plasmonic nanorod-embedded helical superstructure. Adv. Opt. Mater. 2016, 4, 247–251. [Google Scholar] [CrossRef]
- Wang, L.; Bisoyi, H.K.; Zheng, Z.; Gutierrez-Cuevas, K.G.; Singh, G.; Kumar, S.; Bunning, T.J.; Li, Q. Stimuli-directed self-organized chiral superstructures for adaptive windows enabled by mesogen-functionalized graphene. Mater. Today 2017, 20, 230–237. [Google Scholar] [CrossRef]
- Gutierrez-Cuevas, K.G.; Wang, L.; Xue, C.; Singh, G.; Kumar, S.; Urbas, A.; Li, Q. Near infrared light-driven liquid crystal phase transition enabled by hydrophobic mesogen grafted plasmonic gold nanorods. Chem. Commun. 2015, 51, 9845–9848. [Google Scholar] [CrossRef]
- Ikeda, T.; Tsutsumi, O. Optical switching and image storage by means of azobenzene liquid-crystal films. Science 1995, 268, 1873. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, D.; Ishiguro, H.; Shimizu, Y.; Uchida, K. Thermal and photoinduced liquid crystalline phase transitions with a rod–disc alternative change in the molecular shape. J. Mater. Chem. 2012, 22, 25065–25071. [Google Scholar] [CrossRef]
- Lin, T.-H.; Li, Y.; Wang, C.-T.; Jau, H.-C.; Chen, C.-W.; Li, C.-C.; Bisoyi, H.K.; Bunning, T.J.; Li, Q. Red, green and blue reflections enabled in an optically tunable self-organized 3d cubic nanostructured thin film. Adv. Mater. 2013, 25, 5050–5054. [Google Scholar] [CrossRef]
- Fernandez-Palacio, F.; Poutanen, M.; Saccone, M.; Siiskonen, A.; Terraneo, G.; Resnati, G.; Ikkala, O.; Metrangolo, P.; Priimagi, A. Efficient light-induced phase transitions in halogen-bonded liquid crystals. Chem. Mater. 2016, 28, 8314–8321. [Google Scholar] [CrossRef] [PubMed]
- Coles, H.; Morris, S. Liquid-crystal lasers. Nat. Photonics 2010, 4, 676. [Google Scholar] [CrossRef]
- Zheng, Z.-G.; Liu, B.-W.; Zhou, L.; Wang, W.; Hu, W.; Shen, D. Wide tunable lasing in photoresponsive chiral liquid crystal emulsion. J. Mater. Chem. C 2015, 3, 2462–2470. [Google Scholar] [CrossRef]
- Furumi, S.; Yokoyama, S.; Otomo, A.; Mashiko, S. Phototunable photonic bandgap in a chiral liquid crystal laser device. Appl. Phys. Lett. 2004, 84, 2491–2493. [Google Scholar] [CrossRef]
- Lucchetta, D.E.; Criante, L.; Francescangeli, O.; Simoni, F. Light amplification by dye-doped holographic polymer dispersed liquid crystals. Appl. Phys. Lett. 2004, 84, 4893–4895. [Google Scholar] [CrossRef]
- Chen, L.-J.; Lin, J.-D.; Huang, S.-Y.; Mo, T.-S.; Lee, C.-R. Thermally and electrically tunable lasing emission and amplified spontaneous emission in a composite of inorganic quantum dot nanocrystals and organic cholesteric liquid crystals. Adv. Opt. Mater. 2013, 1, 637–643. [Google Scholar] [CrossRef]
- Chen, L.-J.; Lin, J.-D.; Lee, C.-R. An optically stable and tunable quantum dot nanocrystal-embedded cholesteric liquid crystal composite laser. J. Mater. Chem. C 2014, 2, 4388–4394. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, C.; Liu, Y.; Su, H.; Wei, L.; Huang, T.; Dellas, N.; Shang, S.; Mohney, S.E.; Wang, J.; et al. Lasing from colloidal inp/zns quantum dots. Opt. Express 2011, 19, 5528–5535. [Google Scholar] [CrossRef] [PubMed]
- Mingxuan, C.; Yating, Z.; Xiaoxian, S.; Yongli, C.; Haiting, Z.; Chao, Y.; Haitao, D.; Guang, L.; Guizhong, Z.; Jianquan, Y. Enhanced amplified spontaneous emission in a quantum dot-doped polymer-dispersed liquid crystal. Nanotechnology 2016, 27, 26LT01. [Google Scholar]
- Mingxuan, C.; Siwei, Y.; Yating, Z.; Xiaoxian, S.; Yongli, C.; Haiting, Z.; Yu, Y.; Guqiao, D.; Guizhong, Z.; Jianquan, Y. Tunable amplified spontaneous emission in graphene quantum dots doped cholesteric liquid crystals. Nanotechnology 2017, 28, 245202. [Google Scholar]
- Cuiqing, W.; Dairong, C.; Xiuling, J. Lyotropic liquid crystal directed synthesis of nanostructured materials. Sci. Technol. Adv. Mater. 2009, 10, 023001. [Google Scholar]
- Chernyshuk, S.B.; Lev, B.I. Theory of elastic interaction of colloidal particles in nematic liquid crystals near one wall and in the nematic cell. Phys. Rev. E 2011, 84, 011707. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, Z.; Silvestre, N.M.; Tasinkevych, M.; Telo da Gama, M.M. Interactions of distinct quadrupolar nematic colloids. Soft Matter 2012, 8, 10100–10106. [Google Scholar] [CrossRef]
- West, J.L.; Glushchenko, A.; Liao, G.; Reznikov, Y.; Andrienko, D.; Allen, M.P. Drag on particles in a nematic suspension by a moving nematic-isotropic interface. Phys. Rev. E 2002, 66, 012702. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Asakura, K.; Fukuda, J.; Ozaki, M. Three-dimensional positioning and control of colloidal objects utilizing engineered liquid crystalline defect networks. Nat. Commun. 2015, 6, 7180. [Google Scholar] [CrossRef] [PubMed]
- Loudet, J.-C.; Barois, P.; Poulin, P. Colloidal ordering from phase separation in a liquid-crystalline continuous phase. Nature 2000, 407, 611. [Google Scholar] [CrossRef]
- Voloschenko, D.; Pishnyak, O.P.; Shiyanovskii, S.V.; Lavrentovich, O.D. Effect of director distortions on morphologies of phase separation in liquid crystals. Phys. Rev. E 2002, 65, 060701. [Google Scholar] [CrossRef]
- Senyuk, B.; Evans, J.S.; Ackerman, P.J.; Lee, T.; Manna, P.; Vigderman, L.; Zubarev, E.R.; van de Lagemaat, J.; Smalyukh, I.I. Shape-dependent oriented trapping and scaffolding of plasmonic nanoparticles by topological defects for self-assembly of colloidal dimers in liquid crystals. Nano Lett. 2012, 12, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Senyuk, B.; Smalyukh, I.I. Elastic interactions between colloidal microspheres and elongated convex and concave nanoprisms in nematic liquid crystals. Soft Matter 2012, 8, 8729–8734. [Google Scholar] [CrossRef]
- Jose, R.; Skačej, G.; Sastry, V.S.S.; Žumer, S. Colloidal nanoparticles trapped by liquid-crystal defect lines: A lattice monte carlo simulation. Phys. Rev. E 2014, 90, 032503. [Google Scholar] [CrossRef] [PubMed]
- Lagerwall, J.P.; Scalia, G. Liquid Crystals with Nano and Microparticles; World Scientific: Singapore, 2017. [Google Scholar]
- Milette, J.; Cowling, S.J.; Toader, V.; Lavigne, C.; Saez, I.M.; Bruce Lennox, R.; Goodby, J.W.; Reven, L. Reversible long range network formation in gold nanoparticle-nematic liquid crystal composites. Soft Matter 2012, 8, 173–179. [Google Scholar] [CrossRef]
- Coursault, D.; Grand, J.; Zappone, B.; Ayeb, H.; Lévi, G.; Félidj, N.; Lacaze, E. Linear self-assembly of nanoparticles within liquid crystal defect arrays. Adv. Mater. 2012, 24, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Bitar, R.; Agez, G.; Mitov, M. Cholesteric liquid crystal self-organization of gold nanoparticles. Soft Matter 2011, 7, 8198–8206. [Google Scholar] [CrossRef]
- Stratford, K.; Henrich, O.; Lintuvuori, J.S.; Cates, M.E.; Marenduzzo, D. Self-assembly of colloid-cholesteric composites provides a possible route to switchable optical materials. Nat. Commun. 2014, 5, 3954. [Google Scholar] [CrossRef]
- Coursault, D.; Blach, J.-F.; Grand, J.; Coati, A.; Vlad, A.; Zappone, B.; Babonneau, D.; Lévi, G.; Félidj, N.; Donnio, B.; et al. Tailoring anisotropic interactions between soft nanospheres using dense arrays of smectic liquid crystal edge dislocations. ACS Nano 2015, 9, 11678–11689. [Google Scholar] [CrossRef]
- Milette, J.; Relaix, S.; Lavigne, C.; Toader, V.; Cowling, S.J.; Saez, I.M.; Lennox, R.B.; Goodby, J.W.; Reven, L. Reversible long-range patterning of gold nanoparticles by smectic liquid crystals. Soft Matter 2012, 8, 6593–6598. [Google Scholar] [CrossRef]
- Yoon, D.K.; Choi, M.C.; Kim, Y.H.; Kim, M.W.; Lavrentovich, O.D.; Jung, H.-T. Internal structure visualization and lithographic use of periodic toroidal holes in liquid crystals. Nat. Mater. 2007, 6, 866. [Google Scholar] [CrossRef]
- Scalia, G.; von Bühler, C.; Hägele, C.; Roth, S.; Giesselmann, F.; Lagerwall, J.P.F. Spontaneous macroscopic carbon nanotube alignment via colloidal suspension in hexagonal columnar lyotropic liquid crystals. Soft Matter 2008, 4, 570–576. [Google Scholar] [CrossRef]
- Schymura, S.; Enz, E.; Roth, S.; Scalia, G.; Lagerwall, J.P.F. Macroscopic-scale carbon nanotube alignment via self-assembly in lyotropic liquid crystals. Synth. Met. 2009, 159, 2177–2179. [Google Scholar] [CrossRef]
- Sousa, M.; Cloutier, S.; Jian, K.; Weissman, B.; Hurt, R.; Crawford, G. Patterning lyotropic liquid crystals as precursors for carbon nanotube arrays. Appl. Phys. Lett. 2005, 87, 173115. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M.; Osuji, C.O. Nanocomposites of vertically aligned single-walled carbon nanotubes by magnetic alignment and polymerization of a lyotropic precursor. ACS Nano 2010, 4, 6651–6658. [Google Scholar] [CrossRef]
- Schymura, S.; Dölle, S.; Yamamoto, J.; Lagerwall, J. Filament formation in carbon nanotube-doped lyotropic liquid crystals. Soft Matter 2011, 7, 2663–2667. [Google Scholar] [CrossRef]
- Kasprzak, C.R.; Scherzinger, E.T.; Sarkar, A.; Miao, M.; Porcincula, D.H.; Madriz, A.M.; Pennewell, Z.M.; Chau, S.S.; Fernando, R.; Stefik, M.; et al. Ordered nanostructures of carbon nanotube–polymer composites from lyotropic liquid crystal templating. Macromol. Chem. Phys. 2018, 219, 1800197. [Google Scholar] [CrossRef]
- Scalia, G. Alignment of carbon nanotubes in thermotropic and lyotropic liquid crystals. ChemPhysChem 2010, 11, 333–340. [Google Scholar] [CrossRef]
- Ji, Y.; Huang, Y.Y.; Terentjev, E.M. Dissolving and aligning carbon nanotubes in thermotropic liquid crystals. Langmuir 2011, 27, 13254–13260. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.D.; Patrick, D.L. Organizing carbon nanotubes with liquid crystals. Nano Lett. 2002, 2, 1197–1201. [Google Scholar] [CrossRef]
- Tie, W.; Yang, G.H.; Bhattacharyya, S.S.; Lee, Y.H.; Lee, S.H. Electric-field-induced dispersion of multiwalled carbon nanotubes in nematic liquid crystal. J. Phys. Chem. C 2011, 115, 21652–21658. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Kumar, S. Carbon nanotubes in triphenylene and rufigallol-based room temperature monomeric and polymeric discotic liquid crystals. J. Mater. Chem. 2008, 18, 3032–3039. [Google Scholar] [CrossRef]
- Ahir, S.V.; Huang, Y.Y.; Terentjev, E.M. Polymers with aligned carbon nanotubes: Active composite materials. Polymer 2008, 49, 3841–3854. [Google Scholar] [CrossRef]
- Lavrentovich, O.D. Liquid crystals, photonic crystals, metamaterials, and transformation optics. Proc. Natl. Acad. Sci. USA 2011, 108, 5143. [Google Scholar] [CrossRef]
- Ravnik, M.; Alexander, G.P.; Yeomans, J.M.; Žumer, S. Three-dimensional colloidal crystals in liquid crystalline blue phases. Proc. Natl. Acad. Sci. USA 2011, 108, 5188. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, Q.; Wei, Z.; Chen, Y.; Chen, F.; Huang, Z.; Li, X.; Wang, H.; Wang, X.; Cheng, Z. All-inorganic perovskite quantum dots stabilized blue phase liquid crystals. Opt. Express 2018, 26, 18310–18319. [Google Scholar] [CrossRef] [PubMed]
- Mitov, M.; Portet, C.; Bourgerette, C.; Snoeck, E.; Verelst, M. Long-range structuring of nanoparticles by mimicry of a cholesteric liquid crystal. Nat. Mater. 2002, 1, 229. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Christian, B.; François de, G. Fingerprint patterning of solid nanoparticles embedded in a cholesteric liquid crystal. J. Phys. Condens. Matter 2004, 16, S1981. [Google Scholar]
- Pendery, J.S.; Merchiers, O.; Coursault, D.; Grand, J.; Ayeb, H.; Greget, R.; Donnio, B.; Gallani, J.-L.; Rosenblatt, C.; Félidj, N.; et al. Gold nanoparticle self-assembly moderated by a cholesteric liquid crystal. Soft Matter 2013, 9, 9366–9375. [Google Scholar] [CrossRef]
- Rahimi, M.; Roberts, T.F.; Armas-Pérez, J.C.; Wang, X.; Bukusoglu, E.; Abbott, N.L.; de Pablo, J.J. Nanoparticle self-assembly at the interface of liquid crystal droplets. Proc. Natl. Acad. Sci. USA 2015, 112, 5297. [Google Scholar] [CrossRef]
- Chang, C.; Zhao, Y.; Liu, Y.; An, L. Liquid crystallinity of carbon nanotubes. RSC Adv. 2018, 8, 15780–15795. [Google Scholar] [CrossRef]
- Bravo-Sanchez, M.; Simmons, T.J.; Vidal, M.A. Liquid crystal behavior of single wall carbon nanotubes. Carbon 2010, 48, 3531–3542. [Google Scholar] [CrossRef]
- Lu, L.; Chen, W. Large-scale aligned carbon nanotubes from their purified, highly concentrated suspension. ACS Nano 2010, 4, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Windle, A.H. Isotropic−nematic phase transition of dispersions of multiwall carbon nanotubes. Macromolecules 2005, 38, 6181–6188. [Google Scholar] [CrossRef]
- Steinert, B.W.; Dean, D.R. Magnetic field alignment and electrical properties of solution cast pet–carbon nanotube composite films. Polymer 2009, 50, 898–904. [Google Scholar] [CrossRef]
- Hobbie, E.K.; Fry, D.J. Nonequilibrium phase diagram of sticky nanotube suspensions. Phys. Rev. Lett. 2006, 97, 036101. [Google Scholar] [CrossRef] [PubMed]
- Davis, V.A.; Ericson, L.M.; Parra-Vasquez, A.N.G.; Fan, H.; Wang, Y.; Prieto, V.; Longoria, J.A.; Ramesh, S.; Saini, R.K.; Kittrell, C.; et al. Phase behavior and rheology of swnts in superacids. Macromolecules 2004, 37, 154–160. [Google Scholar] [CrossRef]
- Rai, P.K.; Pinnick, R.A.; Parra-Vasquez, A.N.G.; Davis, V.A.; Schmidt, H.K.; Hauge, R.H.; Smalley, R.E.; Pasquali, M. Isotropic−nematic phase transition of single-walled carbon nanotubes in strong acids. J. Am. Chem. Soc. 2006, 128, 591–595. [Google Scholar] [CrossRef]
- Pénicaud, A.; Poulin, P.; Derré, A.; Anglaret, E.; Petit, P. Spontaneous dissolution of a single-wall carbon nanotube salt. J. Am. Chem. Soc. 2005, 127, 8–9. [Google Scholar] [CrossRef]
- Baskaran, D.; Mays, J.W.; Bratcher, M.S. Noncovalent and nonspecific molecular interactions of polymers with multiwalled carbon nanotubes. Chem. Mater. 2005, 17, 3389–3397. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Rahatekar, S.S.; Fagan, J.A.; Chun, J.; Becker, M.L.; Naik, R.R.; Krauss, T.; Carlson, L.; Kadla, J.F.; Trulove, P.C.; et al. Comparison of the quality of aqueous dispersions of single wall carbon nanotubes using surfactants and biomolecules. Langmuir 2008, 24, 5070–5078. [Google Scholar] [CrossRef]
- Zhang, S.; Kinloch, I.A.; Windle, A.H. Mesogenicity drives fractionation in lyotropic aqueous suspensions of multiwall carbon nanotubes. Nano Lett. 2006, 6, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Q.; Kinloch, I.A.; Windle, A.H. Ordering in a droplet of an aqueous suspension of single-wall carbon nanotubes on a solid substrate. Langmuir 2010, 26, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Blanc, C.; Maugey, M.; Zakri, C.; Poulin, P.; Anglaret, E. Anisotropic thin films of single-wall carbon nanotubes from aligned lyotropic nematic suspensions. Nano Lett. 2008, 8, 4103–4107. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Windle, A.H. Size-dependence and elasticity of liquid-crystalline multiwalled carbon nanotubes. Adv. Mater. 2008, 20, 3149–3154. [Google Scholar] [CrossRef]
- Fu, W.; Liu, L.; Jiang, K.; Li, Q.; Fan, S. Super-aligned carbon nanotube films as aligning layers and transparent electrodes for liquid crystal displays. Carbon 2010, 48, 1876–1879. [Google Scholar] [CrossRef]
- Roussel, F.; Brun, J.-F.; Allart, A.; Huang, L.; O’Brien, S. Horizontally-aligned carbon nanotubes arrays and their interactions with liquid crystal molecules: Physical characteristics and display applications. AIP Adv. 2012, 2, 012110. [Google Scholar] [CrossRef]
- Park, H.-G.; Lee, M.-J.; Kim, K.; Seo, D.-S. Transparent conductive single wall carbon nanotube network films for liquid crystal displays. ECS Solid State Lett. 2012, 1, R31–R33. [Google Scholar] [CrossRef]
- Rajasekharan, R.; Dai, Q.; Wilkinson, T.D. Electro-optic characteristics of a transparent nanophotonic device based on carbon nanotubes and liquid crystals. Appl. Opt. 2010, 49, 2099–2104. [Google Scholar] [CrossRef]
- Yu, L.; Shearer, C.; Shapter, J. Recent development of carbon nanotube transparent conductive films. Chem. Rev. 2016, 116, 13413–13453. [Google Scholar] [CrossRef]
- Song, Y.I.; Lee, J.W.; Kim, T.Y.; Jung, H.J.; Jung, Y.C.; Suh, S.J.; Yang, C.-M. Performance-determining factors in flexible transparent conducting single-wall carbon nanotube film. Carbon Lett. 2013, 14, 255–258. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.-H.; Huynh, C.P.; Hawkins, S.C.; Musameh, M.; Kim, D.H.; Lee, S.H.; Choi, J. Orientational and electro-optical properties of liquid crystal aligned with a directly spinnable carbon nanotube web. Liq. Cryst. 2015, 42, 322–327. [Google Scholar] [CrossRef]
- Russell, J.M.; Oh, S.; LaRue, I.; Zhou, O.; Samulski, E.T. Alignment of nematic liquid crystals using carbon nanotube films. Thin Solid Film 2006, 509, 53–57. [Google Scholar] [CrossRef]
- Liu, Y.; Lim, Y.J.; Kundu, S.; Lee, S.H.; Lee, G.-D. Super-fast switching of twisted nematic liquid crystals with a single-wall-carbon-nanotube-doped alignment layer. J. Korean Phys. Soc. 2015, 66, 952–958. [Google Scholar] [CrossRef]
- Ren, L.; Pint, C.L.; Booshehri, L.G.; Rice, W.D.; Wang, X.; Hilton, D.J.; Takeya, K.; Kawayama, I.; Tonouchi, M.; Hauge, R.H.; et al. Carbon nanotube terahertz polarizer. Nano Lett. 2009, 9, 2610–2613. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Suzuki, H.; Zaccaria, R.P.; Sekkat, Z.; Kawata, S. Optical polarizer made of uniaxially aligned short single-wall carbon nanotubes embedded in a polymer film. Phys. Rev. B 2008, 77, 153407. [Google Scholar] [CrossRef]
- Byeong Gyun, K.; Young Jin, L.; Kwang-Un, J.; Kyu, L.; Young Hee, L.; Seung Hee, L. A tunable carbon nanotube polarizer. Nanotechnology 2010, 21, 405202. [Google Scholar]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; ter Waarbeek, R.F.; de Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science 2013, 339, 182. [Google Scholar] [CrossRef]
- Zhang, S.; Koziol, K.K.K.; Kinloch, I.A.; Windle, A.H. Macroscopic fibers of well-aligned carbon nanotubes by wet spinning. Small 2008, 4, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Ericson, L.M.; Fan, H.; Peng, H.; Davis, V.A.; Zhou, W.; Sulpizio, J.; Wang, Y.; Booker, R.; Vavro, J.; Guthy, C.; et al. Macroscopic, neat, single-walled carbon nanotube fibers. Science 2004, 305, 1447. [Google Scholar] [CrossRef]
- Li, P.; Wong, M.; Zhang, X.; Yao, H.; Ishige, R.; Takahara, A.; Miyamoto, M.; Nishimura, R.; Sue, H.-J. Tunable lyotropic photonic liquid crystal based on graphene oxide. ACS Photonics 2014, 1, 79–86. [Google Scholar] [CrossRef]
- Dan, B.; Behabtu, N.; Martinez, A.; Evans, J.S.; Kosynkin, D.V.; Tour, J.M.; Pasquali, M.; Smalyukh, I.I. Liquid crystals of aqueous, giant graphene oxide flakes. Soft Matter 2011, 7, 11154–11159. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Gudarzi, M.M.; Zheng, Q.B.; Kim, J.-K. Spontaneous formation of liquid crystals in ultralarge graphene oxide dispersions. Adv. Funct. Mater. 2011, 21, 2978–2988. [Google Scholar] [CrossRef]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Konstantinov, K.; Moulton, S.E.; Razal, J.M.; Wallace, G.G. Organic solvent-based graphene oxide liquid crystals: A facile route toward the next generation of self-assembled layer-by-layer multifunctional 3d architectures. ACS Nano 2013, 7, 3981–3990. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, C. Graphene chiral liquid crystals and macroscopic assembled fibres. Nat. Commun. 2011, 2, 571. [Google Scholar] [CrossRef]
- Tkacz, R.; Oldenbourg, R.; Mehta, S.B.; Miansari, M.; Verma, A.; Majumder, M. Ph dependent isotropic to nematic phase transitions in graphene oxide dispersions reveal droplet liquid crystalline phases. Chem. Commun. 2014, 50, 6668–6671. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.-Z.; Hong, S.-H.; Song, J.-K. Electro-optical switching of graphene oxide liquid crystals with an extremely large kerr coefficient. Nat. Mater. 2014, 13, 394. [Google Scholar] [CrossRef]
- Arenas-Guerrero, P.; Delgado, Á.V.; Jiménez, M.L. Analysis of the electro-optical response of graphene oxide dispersions under alternating fields. Carbon 2018, 144, 395–401. [Google Scholar] [CrossRef]
- Hong, S.-H.; Shen, T.-Z.; Song, J.-K. Shear-induced assembly of graphene oxide particles into stripes near surface. Liq. Cryst. 2018, 45, 1303–1311. [Google Scholar] [CrossRef]
- Guo, F.; Kim, F.; Han, T.H.; Shenoy, V.B.; Huang, J.; Hurt, R.H. Hydration-responsive folding and unfolding in graphene oxide liquid crystal phases. ACS Nano 2011, 5, 8019–8025. [Google Scholar] [CrossRef] [PubMed]
- Senyuk, B.; Behabtu, N.; Pacheco, B.G.; Lee, T.; Ceriotti, G.; Tour, J.M.; Pasquali, M.; Smalyukh, I.I. Nonlinear photoluminescence imaging of isotropic and liquid crystalline dispersions of graphene oxide. ACS Nano 2012, 6, 8060–8066. [Google Scholar] [CrossRef]
- Qi, B.; Yuan, Z.; Lu, S.; Liu, K.; Li, S.; Yang, L.; Yu, J. Mechanical and thermal properties of epoxy composites containing graphene oxide and liquid crystalline epoxy. Fibers Polym. 2014, 15, 326–333. [Google Scholar] [CrossRef]
- Lu, S.; Li, S.; Yu, J.; Yuan, Z.; Qi, B. Epoxy nanocomposites filled with thermotropic liquid crystalline epoxy grafted graphene oxide. RSC Adv. 2013, 3, 8915–8923. [Google Scholar] [CrossRef]
- Naficy, S.; Jalili, R.; Aboutalebi, S.H.; Gorkin Iii, R.A.; Konstantinov, K.; Innis, P.C.; Spinks, G.M.; Poulin, P.; Wallace, G.G. Graphene oxide dispersions: Tuning rheology to enable fabrication. Mater. Horiz. 2014, 1, 326–331. [Google Scholar] [CrossRef]
- Wu, X.; Hou, K.; Huang, J.; Wang, J.; Yang, S. Graphene-based cellular materials with extremely low density and high pressure sensitivity based on self-assembled graphene oxide liquid crystals. J. Mater. Chem. C 2018, 6, 8717–8725. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Puech, N.; Zakri, C.; Grelet, E.; Moulton, S.E.; Wallace, G.G.; Gambhir, S.; Blanc, C.; Anglaret, E.; Poulin, P. Liquid crystallinity and dimensions of surfactant-stabilized sheets of reduced graphene oxide. J. Phys. Chem. Lett. 2012, 3, 2425–2430. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.H.; Kim, Y.B.; Ambade, S.B.; Noh, S.H.; Eom, W.; Hwang, J.Y.; Lee, W.J.; Huang, J.; Han, T.H. Dynamic assembly of liquid crystalline graphene oxide gel fibers for ion transport. Sci. Adv. 2018, 4, eaau2104. [Google Scholar] [CrossRef] [PubMed]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Shepherd, R.L.; Chen, J.; Aminorroaya-Yamini, S.; Konstantinov, K.; Minett, A.I.; Razal, J.M.; Wallace, G.G. Scalable one-step wet-spinning of graphene fibers and yarns from liquid crystalline dispersions of graphene oxide: Towards multifunctional textiles. Adv. Funct. Mater. 2013, 23, 5345–5354. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. Graphene in macroscopic order: Liquid crystals and wet-spun fibers. Acc. Chem. Res. 2014, 47, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Yun, T.; Kim, J.-E.; Yu, H.; Sasikala, S.P.; Lee, K.E.; Koo, S.H.; Hwang, H.; Jung, H.J.; Park, J.Y.; et al. Mussel-inspired defect engineering of graphene liquid crystalline fibers for synergistic enhancement of mechanical strength and electrical conductivity. Adv. Mater. 2018, 30, 1803267. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Jalili, R.; Esrafilzadeh, D.; Salari, M.; Gholamvand, Z.; Aminorroaya Yamini, S.; Konstantinov, K.; Shepherd, R.L.; Chen, J.; Moulton, S.E.; et al. High-performance multifunctional graphene yarns: Toward wearable all-carbon energy storage textiles. ACS Nano 2014, 8, 2456–2466. [Google Scholar] [CrossRef]
- Mahalingam, D.K.; Wang, S.; Nunes, S.P. Graphene oxide liquid crystal membranes in protic ionic liquid for nanofiltration. ACS Appl. Nano Mater. 2018, 1, 4661–4670. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.W.; Vasagar, V.; Ha, H.; Nazarenko, S.; Ellison, C.J. Polydopamine-graphene oxide flame retardant nanocoatings applied via an aqueous liquid crystalline scaffold. Adv. Funct. Mater. 2018, 28, 1803172. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.H.; Shahini, S.; Yamamoto, J.; Campidelli, S.; Kim, Y.S.; Scalia, G. Graphene: A new liquid crystal for high performance electro-optic applications. In Liquid Crystals XXII; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; p. 107350N. [Google Scholar]
- Huang, X.; He, J.; Sun, K.; Chen, Y.; Zha, Z.; Zhou, C. Liquid crystal behavior and cytocompatibility of graphene oxide dispersed in sodium alginate solutions. Carbon 2018, 129, 258–269. [Google Scholar] [CrossRef]
- Sasikala, S.P.; Lim, J.; Kim, I.H.; Jung, H.J.; Yun, T.; Han, T.H.; Kim, S.O. Graphene oxide liquid crystals: A frontier 2d soft material for graphene-based functional materials. Chem. Soc. Rev. 2018, 47, 6013–6045. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Shoda, M.; Sugano, Y. Recent advances in bacterial cellulose production. Biotechnol. Bioprocess. Eng. 2005, 10, 1. [Google Scholar] [CrossRef]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Li, Y.; Jun-Yan Suen, J.; Prince, E.; Larin, E.M.; Klinkova, A.; Thérien-Aubin, H.; Zhu, S.; Yang, B.; Helmy, A.S.; Lavrentovich, O.D.; et al. Colloidal cholesteric liquid crystal in spherical confinement. Nat. Commun. 2016, 7, 12520. [Google Scholar] [CrossRef]
- Marchessault, R.; Morehead, F.; Walter, N. Liquid crystal systems from fibrillar polysaccharides. Nature 1959, 184, 632–633. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure–properties relationship at the single fibril level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Inui, O.; Horii, F.; Tsuji, M. Phase separation behavior in aqueous suspensions of bacterial cellulose nanocrystals prepared by sulfuric acid treatment. Langmuir 2009, 25, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Agthe, M.; Fall, A.B.; Gordeyeva, K.; Guccini, V.; Salajková, M.; Plivelic, T.S.; Lagerwall, J.P.F.; Salazar-Alvarez, G.; Bergström, L. Rod packing in chiral nematic cellulose nanocrystal dispersions studied by small-angle x-ray scattering and laser diffraction. Langmuir 2015, 31, 6507–6513. [Google Scholar] [CrossRef] [PubMed]
- Shafeiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Influence of degree of sulfation on the rheology of cellulose nanocrystal suspensions. Rheol. Acta 2013, 52, 741–751. [Google Scholar] [CrossRef]
- Dong, X.M.; Kimura, T.; Revol, J.-F.; Gray, D.G. Effects of ionic strength on the isotropic−chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 1996, 12, 2076–2082. [Google Scholar] [CrossRef]
- Araki, J.; Kuga, S. Effect of trace electrolyte on liquid crystal type of cellulose microcrystals. Langmuir 2001, 17, 4493–4496. [Google Scholar] [CrossRef]
- Song, W.; Lee, J.-K.; Gong, M.S.; Heo, K.; Chung, W.-J.; Lee, B.Y. Cellulose nanocrystal-based colored thin films for colorimetric detection of aldehyde gases. ACS Appl. Mater. Interfaces 2018, 10, 10353–10361. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef]
- Bruckner, J.R.; Kuhnhold, A.; Honorato-Rios, C.; Schilling, T.; Lagerwall, J.P.F. Enhancing self-assembly in cellulose nanocrystal suspensions using high-permittivity solvents. Langmuir 2016, 32, 9854–9862. [Google Scholar] [CrossRef]
- Beck, S.; Bouchard, J.; Berry, R. Controlling the reflection wavelength of iridescent solid films of nanocrystalline cellulose. Biomacromolecules 2011, 12, 167–172. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Vignolini, S. Controlling the photonic properties of cholesteric cellulose nanocrystal films with magnets. Adv. Mater. 2017, 29, 1701469. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-m.; Mao, W.; Wang, H.-w.; Zhao, Y.-q.; Li, X.-j.; Bi, D.-x.; Yang, L.-l.; Ge, Q.; Fang, X. Measurement of critical concentration for mesophase formation of chitosan derivatives in both aqueous and organic solutions. Polym. Int. 2006, 55, 1444–1449. [Google Scholar] [CrossRef]
- Parker, R.M.; Guidetti, G.; Williams, C.A.; Zhao, T.; Narkevicius, A.; Vignolini, S.; Frka-Petesic, B. The self-assembly of cellulose nanocrystals: Hierarchical design of visual appearance. Adv. Mater. 2018, 30, 1704477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Chodavarapu, V.P.; Kirk, A.G.; Andrews, M.P. Structured color humidity indicator from reversible pitch tuning in self-assembled nanocrystalline cellulose films. Sens. Actuators B Chem. 2013, 176, 692–697. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chodavarapu, V.P.; Kirk, A.G.; Andrews, M.P. Nanocrystalline cellulose for covert optical encryption. J. Nanophotonics 2012, 6, 063516. [Google Scholar] [CrossRef]
- Bardet, R.; Roussel, F.; Coindeau, S.; Belgacem, N.; Bras, J. Engineered pigments based on iridescent cellulose nanocrystal films. Carbohydr. Polym. 2015, 122, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Majoinen, J.; Hassinen, J.; Haataja, J.S.; Rekola, H.T.; Kontturi, E.; Kostiainen, M.A.; Ras, R.H.A.; Törmä, P.; Ikkala, O. Chiral plasmonics using twisting along cellulose nanocrystals as a template for gold nanoparticles. Adv. Mater. 2016, 28, 5262–5267. [Google Scholar] [CrossRef]
- Almeida, P.L.; Kundu, S.; Borges, J.P.; Godinho, M.H.; Figueirinhas, J.L. Electro-optical light scattering shutter using electrospun cellulose-based nano- and microfibers. Appl. Phys. Lett. 2009, 95, 043501. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Qi, H.; Hamad, W.Y.; MacLachlan, M.J. Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 2010, 468, 422. [Google Scholar] [CrossRef]
- Rofouie, P.; Alizadehgiashi, M.; Mundoor, H.; Smalyukh, I.I.; Kumacheva, E. Self-assembly of cellulose nanocrystals into semi-spherical photonic cholesteric films. Adv. Funct. Mater. 2018, 28, 1803852. [Google Scholar] [CrossRef]
- Majoinen, J.; Kontturi, E.; Ikkala, O.; Gray, D.G. Sem imaging of chiral nematic films cast from cellulose nanocrystal suspensions. Cellulose 2012, 19, 1599–1605. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Stahl, A.; Hamad, W.Y.; MacLachlan, M.J. Hard templating of nanocrystalline titanium dioxide with chiral nematic ordering. Angew. Chem. 2012, 124, 6992–6996. [Google Scholar] [CrossRef]
- Li, Y.; Prince, E.; Cho, S.; Salari, A.; Mosaddeghian Golestani, Y.; Lavrentovich, O.D.; Kumacheva, E. Periodic assembly of nanoparticle arrays in disclinations of cholesteric liquid crystals. Proc. Natl. Acad. Sci. USA 2017, 114, 2137. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Skaife, J.J.; Dubrovsky, T.B.; Abbott, N.L. Optical amplification of ligand-receptor binding using liquid crystals. Science 1998, 279, 2077. [Google Scholar] [CrossRef] [PubMed]
- Brake, J.M.; Daschner, M.K.; Luk, Y.-Y.; Abbott, N.L. Biomolecular interactions at phospholipid-decorated surfaces of liquid crystals. Science 2003, 302, 2094. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Sung, Y.-C.; Lee, M.-J.; Lee, W. Highly sensitive color-indicating and quantitative biosensor based on cholesteric liquid crystal. Biomed. Opt. Express 2015, 6, 5033–5038. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lee, M.-J.; Lee, W. Bovine serum albumin detection and quantitation based on capacitance measurements of liquid crystals. Appl. Phys. Lett. 2016, 109, 093703. [Google Scholar] [CrossRef]
- Lee, M.-J.; Chang, C.-H.; Lee, W. Label-free protein sensing by employing blue phase liquid crystal. Biomed. Opt. Express 2017, 8, 1712–1720. [Google Scholar] [CrossRef]
- Qi, L.; Hu, Q.; Kang, Q.; Yu, L. Fabrication of liquid-crystal-based optical sensing platform for detection of hydrogen peroxide and blood glucose. Anal. Chem. 2018, 90, 11607–11613. [Google Scholar] [CrossRef]
- Zhang, J.; Su, X.; Yang, D.; Luan, C. Label-free liquid crystal biosensor for cecropin b detection. Talanta 2018, 186, 60–64. [Google Scholar] [CrossRef]
- Lin, C.-M.; Wu, P.-C.; Lee, M.-J.; Lee, W. Label-free protein quantitation by dielectric spectroscopy of dual-frequency liquid crystal. Sens. Actuators B Chem. 2019, 282, 158–163. [Google Scholar] [CrossRef]
- Tan, H.; Yang, S.; Shen, G.; Yu, R.; Wu, Z. Signal-enhanced liquid-crystal DNA biosensors based on enzymatic metal deposition. Angew. Chem. Int. Ed. 2010, 49, 8608–8611. [Google Scholar] [CrossRef] [PubMed]
- Hartono, D.; Qin, W.J.; Yang, K.-L.; Yung, L.-Y.L. Imaging the disruption of phospholipid monolayer by protein-coated nanoparticles using ordering transitions of liquid crystals. Biomaterials 2009, 30, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Qiao, Y.; Han, W.; Xie, Z.; Wu, Z.; Shen, G.; Yu, R. Acetylcholinesterase liquid crystal biosensor based on modulated growth of gold nanoparticles for amplified detection of acetylcholine and inhibitor. Anal. Chem. 2012, 84, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Tan, H.; Wu, C.; Wu, Z.; Shen, G.; Yu, R. Gold nanoparticle based signal enhancement liquid crystal biosensors for DNA hybridization assays. Chem. Commun. 2012, 48, 2861–2863. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Peng, Y.; Xu, L.; Zhou, W.; Wang, Q.; Guo, L. Liquid-crystal biosensor based on nickel-nanosphere-induced homeotropic alignment for the amplified detection of thrombin. ACS Appl. Mater. Interfaces 2015, 7, 23418–23422. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jang, C.-H. Visualization of cholylglycine hydrolase activities through nickel nanoparticle-assisted liquid crystal cells. Sens. Actuators B Chem. 2017, 239, 1268–1274. [Google Scholar] [CrossRef]
- Choudhary, A.; George, F.T.; Li, G. Conjugation of nanomaterials and nematic liquid crystals for futuristic applications and biosensors. Biosensors 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Wang, X.; Mondkar, P.; Bukusoglu, E.; Abbott, N.L. Self-reporting and self-regulating liquid crystals. Nature 2018, 557, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Barauskas, J.; Johnsson, M.; Tiberg, F. Self-assembled lipid superstructures: Beyond vesicles and liposomes. Nano Lett. 2005, 5, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Vallamkondu, J.; Corgiat, E.; Buchaiah, G.; Kandimalla, R.; Reddy, P. Liquid crystals: A novel approach for cancer detection and treatment. Cancers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Soft drug delivery systems. Soft Matter 2006, 2, 760–769. [Google Scholar] [CrossRef]
- Nikam, S.; Chavan, M.; Sharma, P.H. Solid lipid nanoparticles: A lipid based drug delivery. Nanotechnology 2014, 1, 5. [Google Scholar]
- Volkhard Jenning, S.H.G. Encapsulation of retinoids in solid lipid nanoparticles (sln). J. Microencapsul. 2001, 18, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Müller-Goymann, C.C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm. 2004, 58, 343–356. [Google Scholar] [CrossRef] [PubMed]
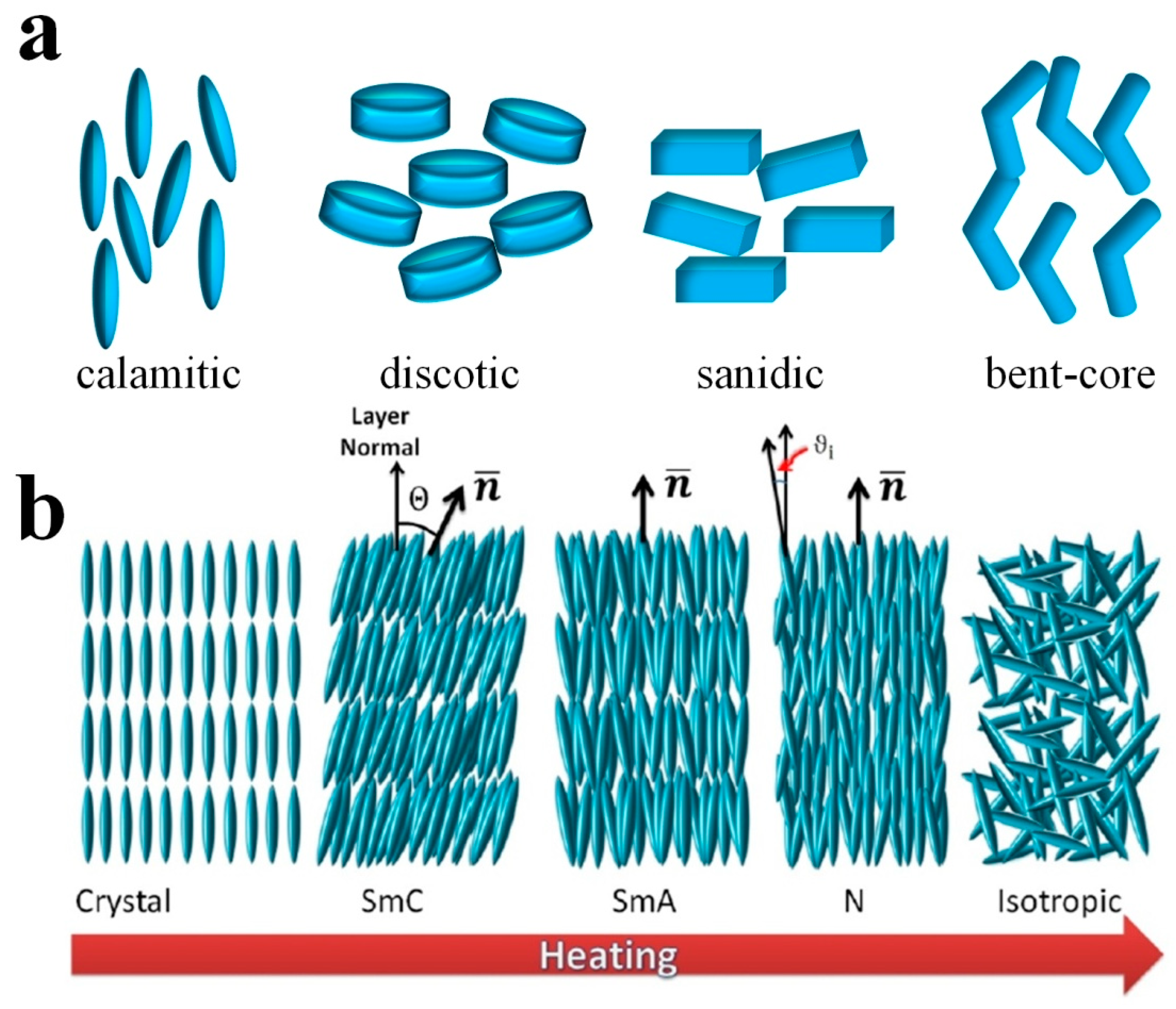
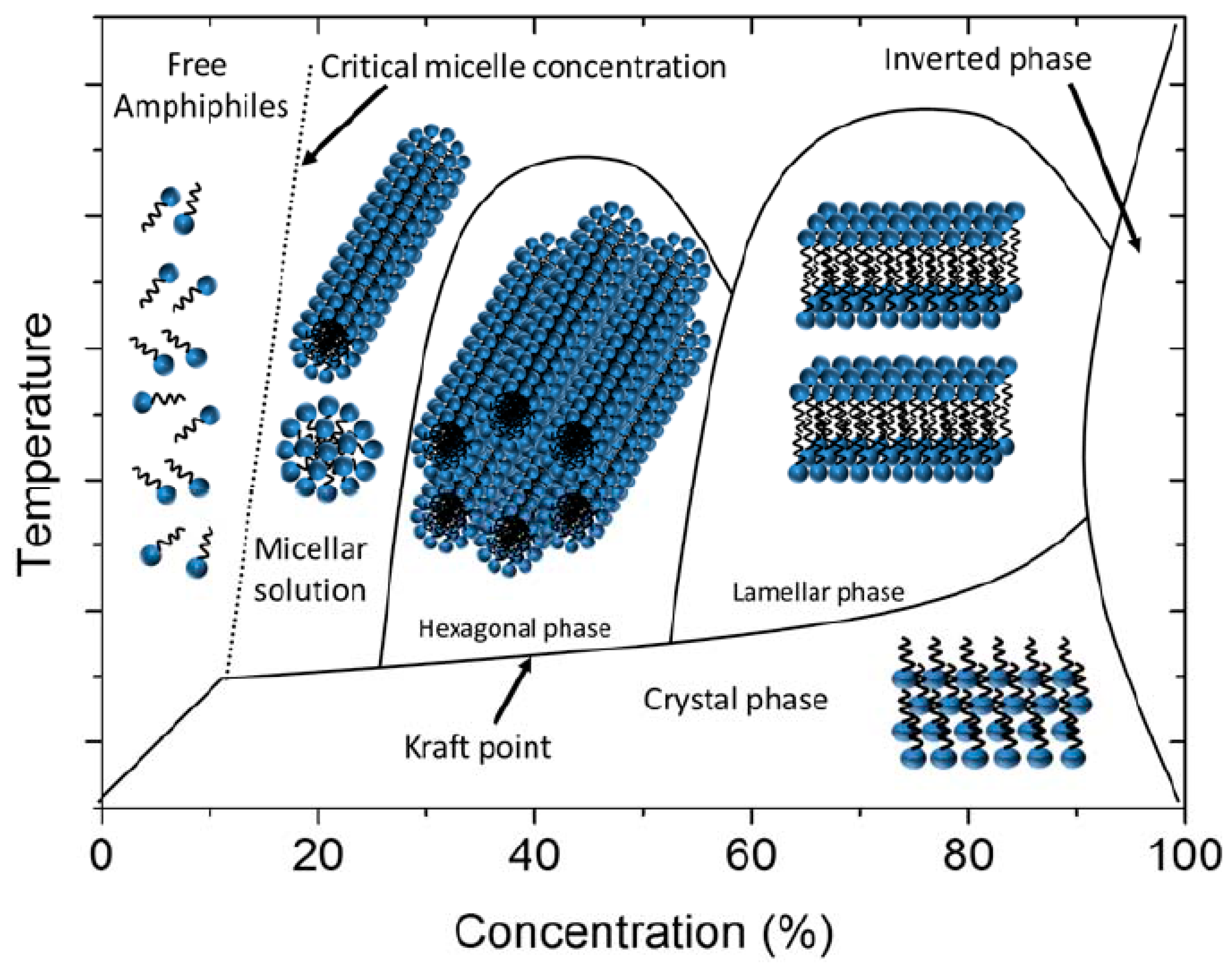
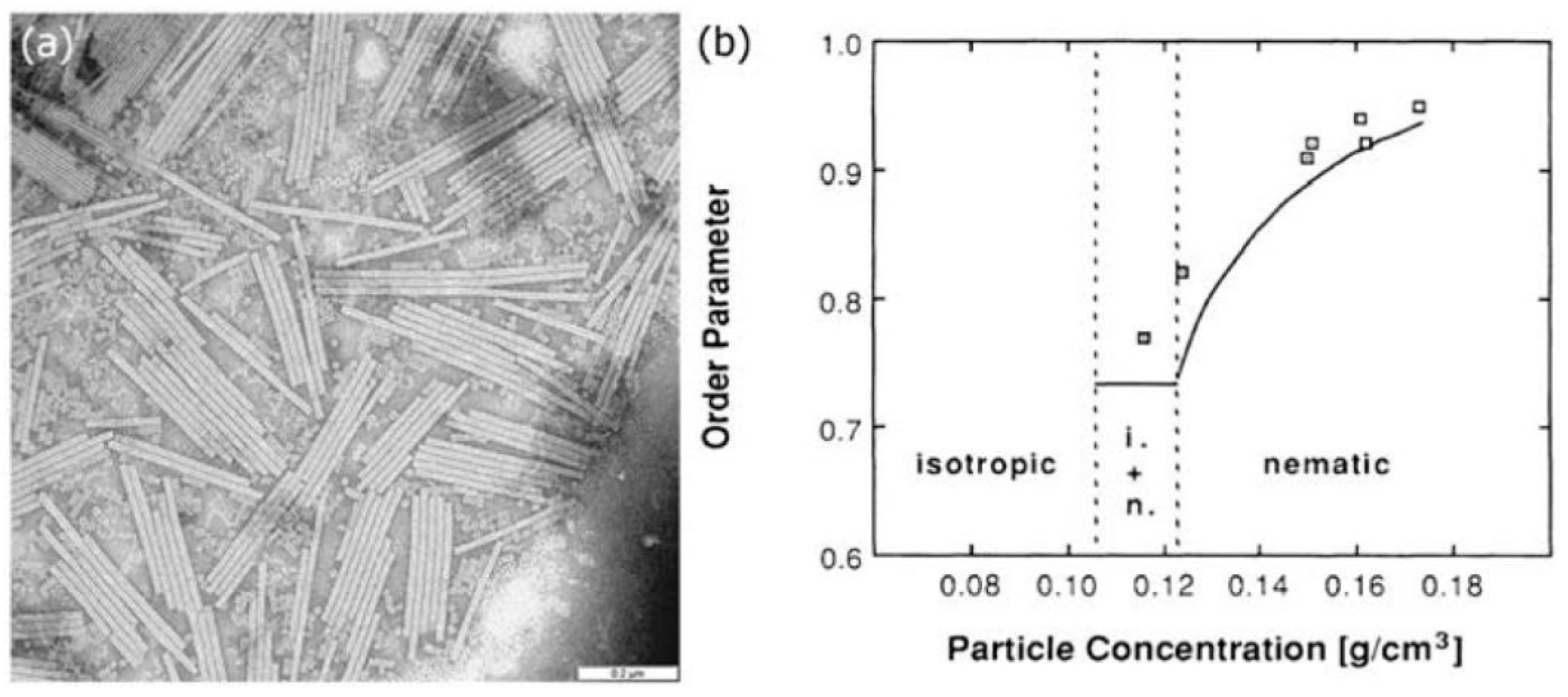
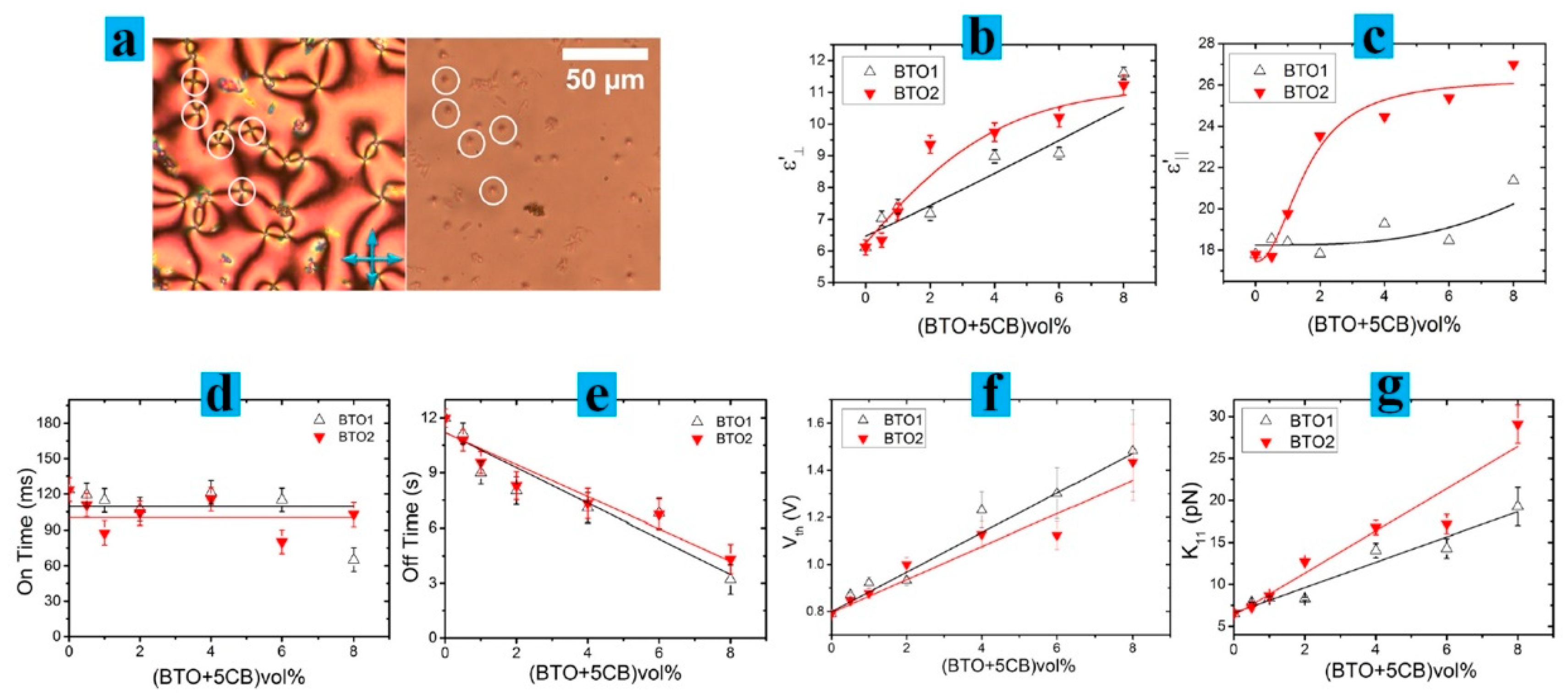
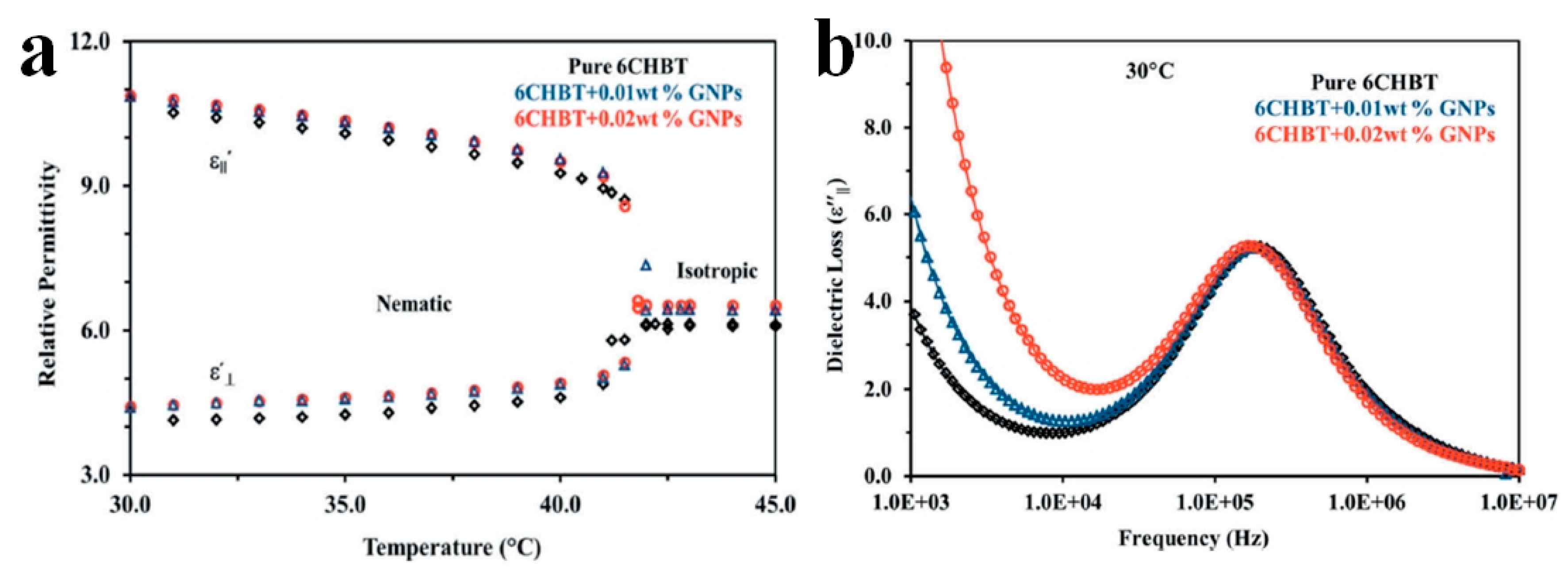
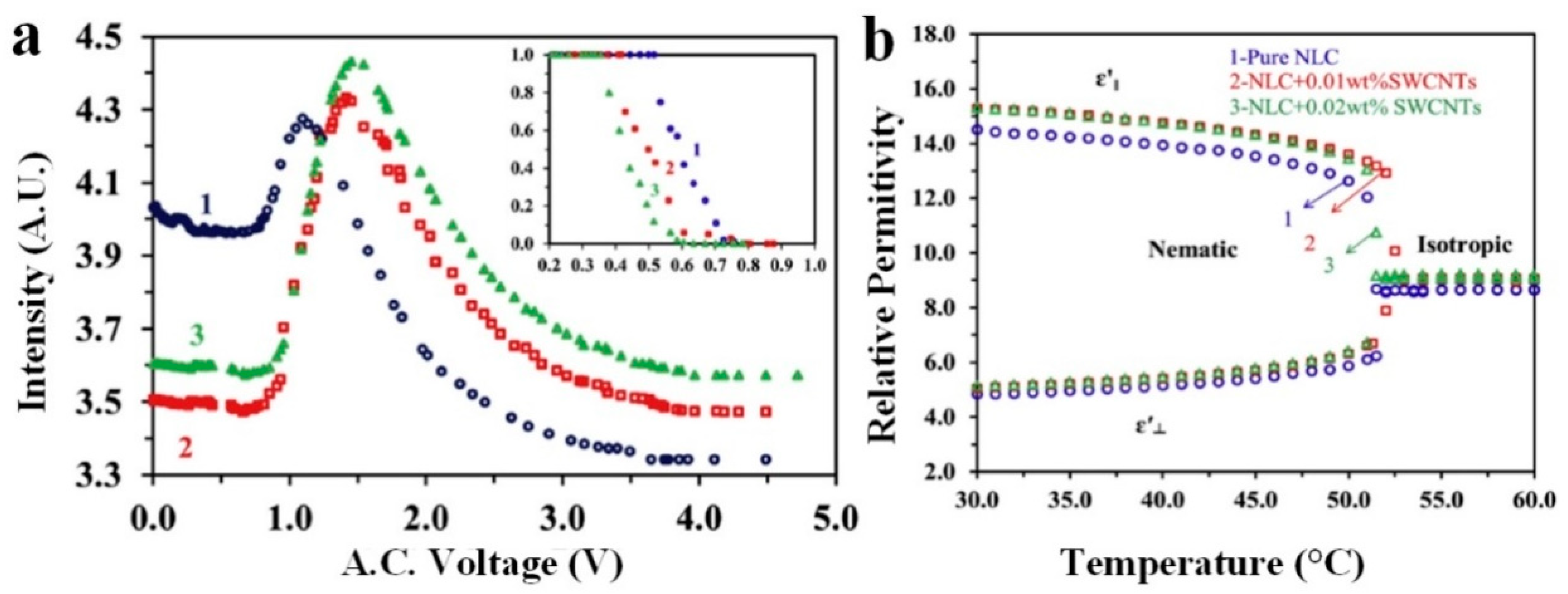
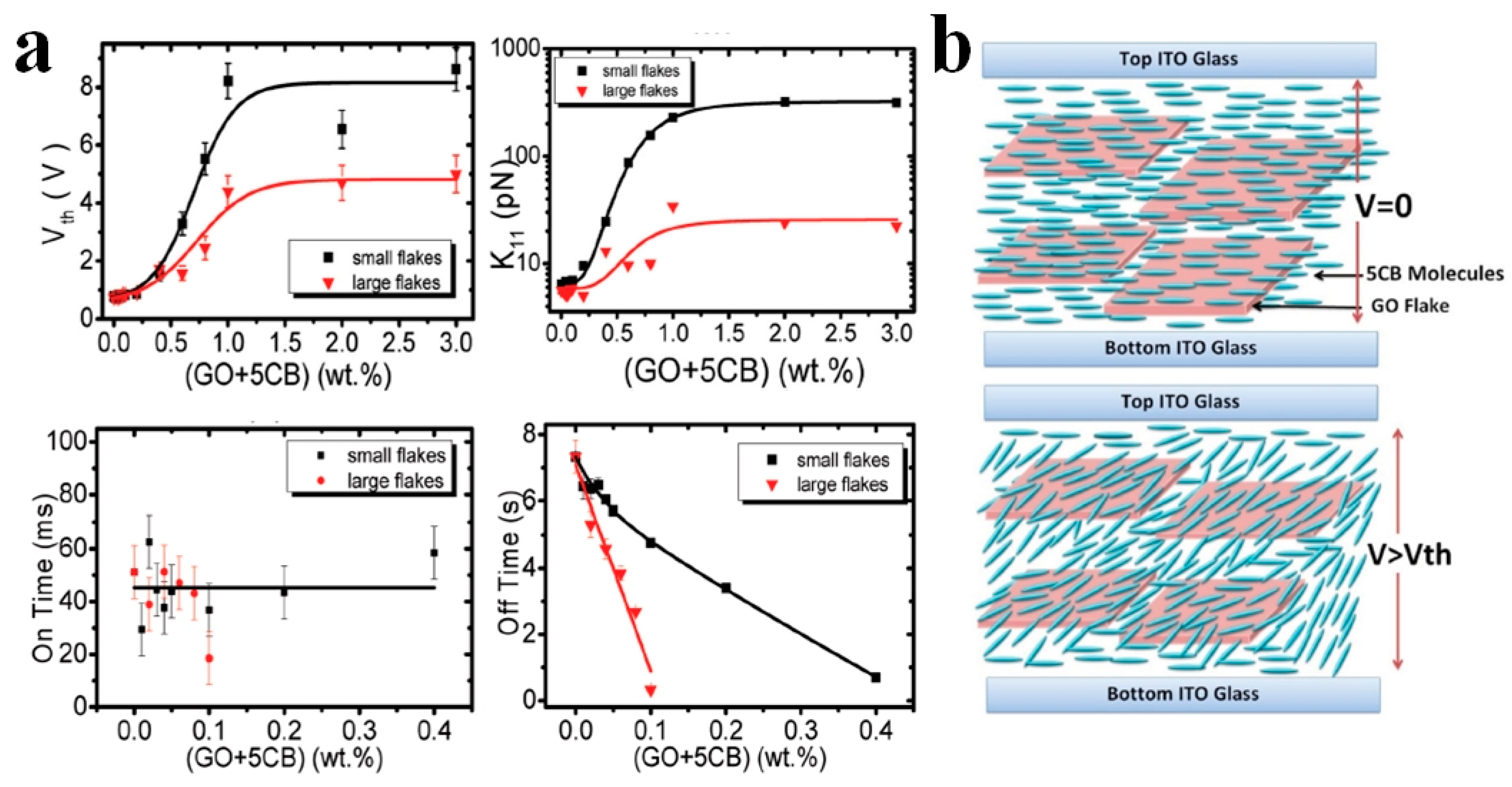
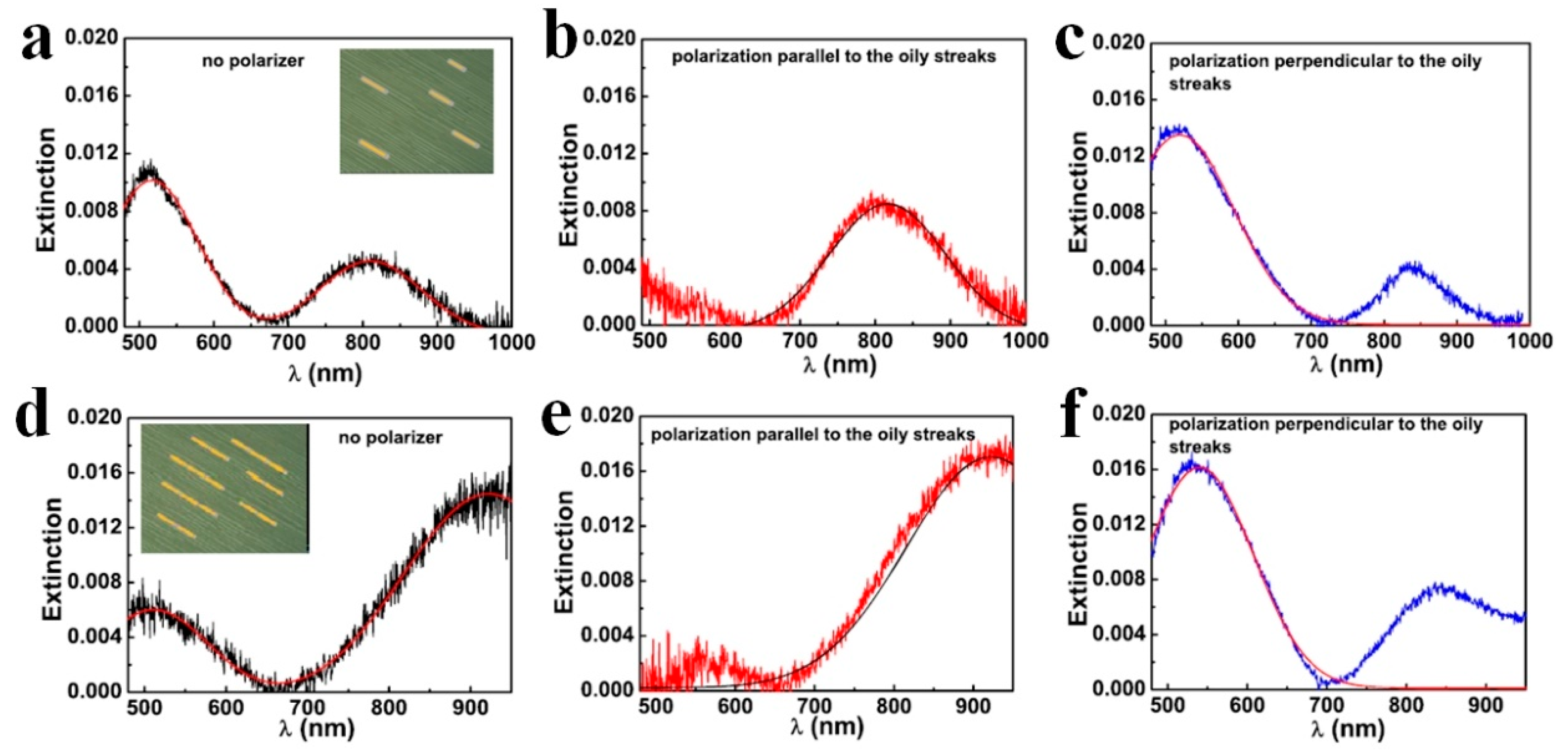
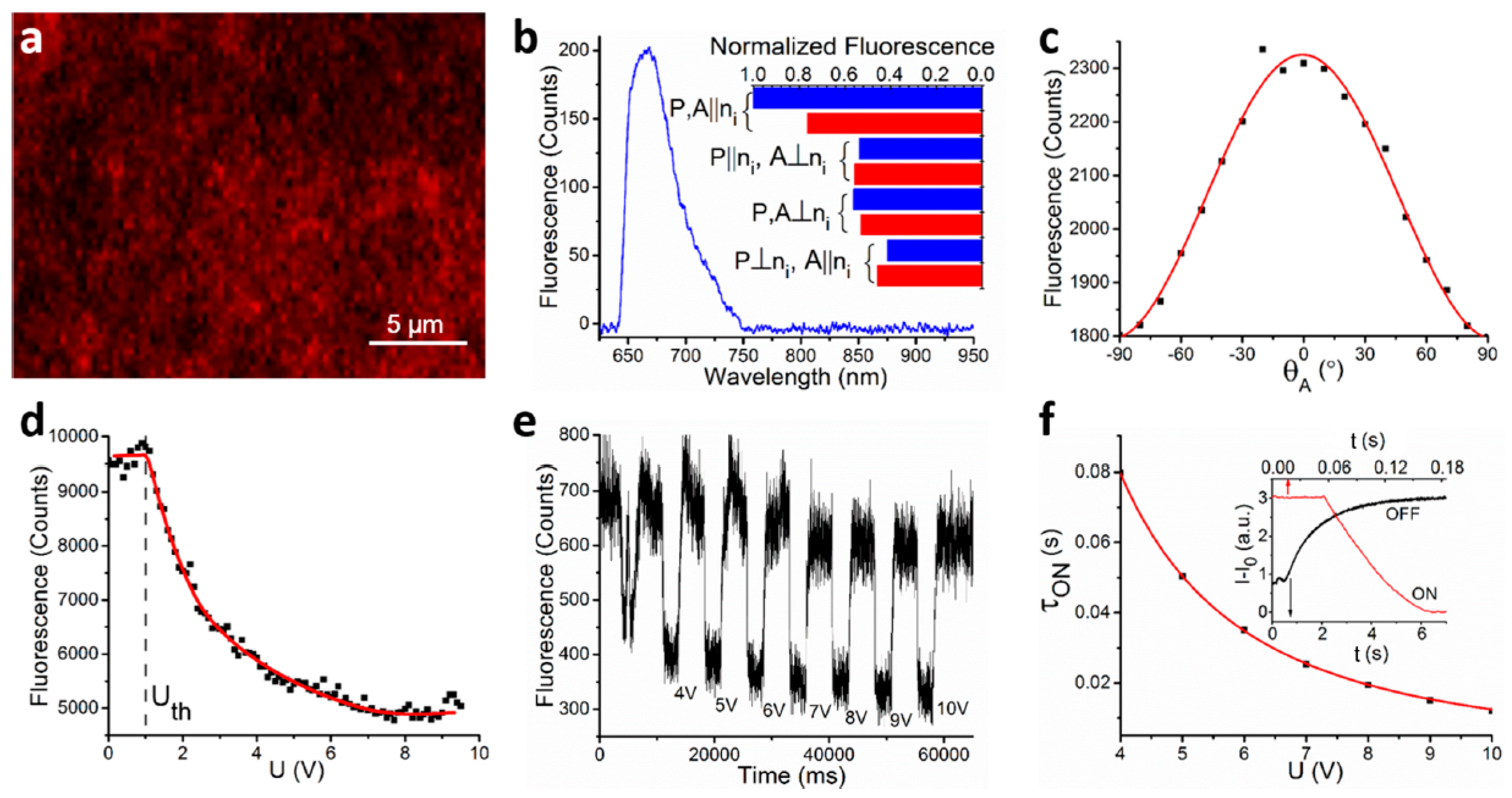
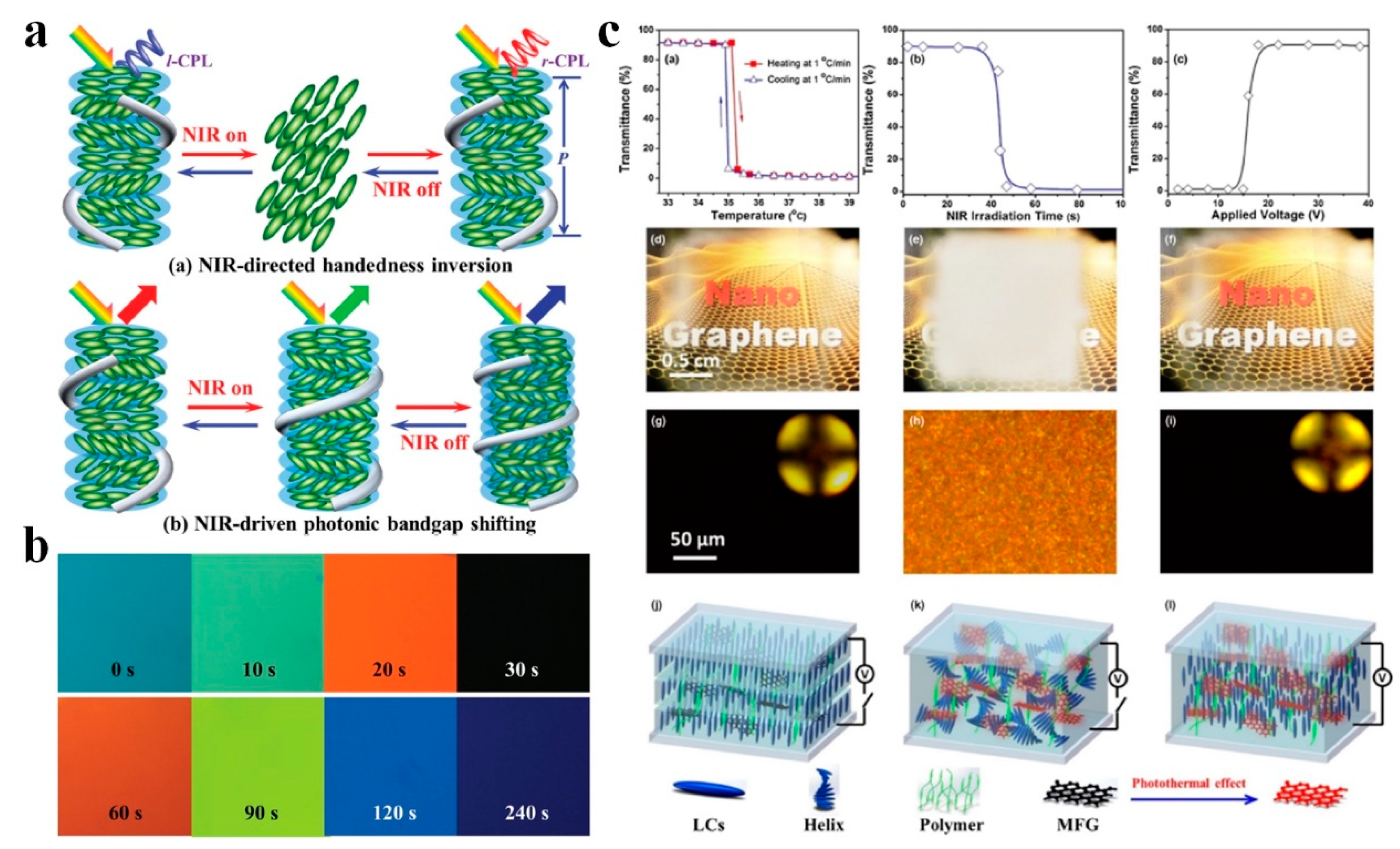
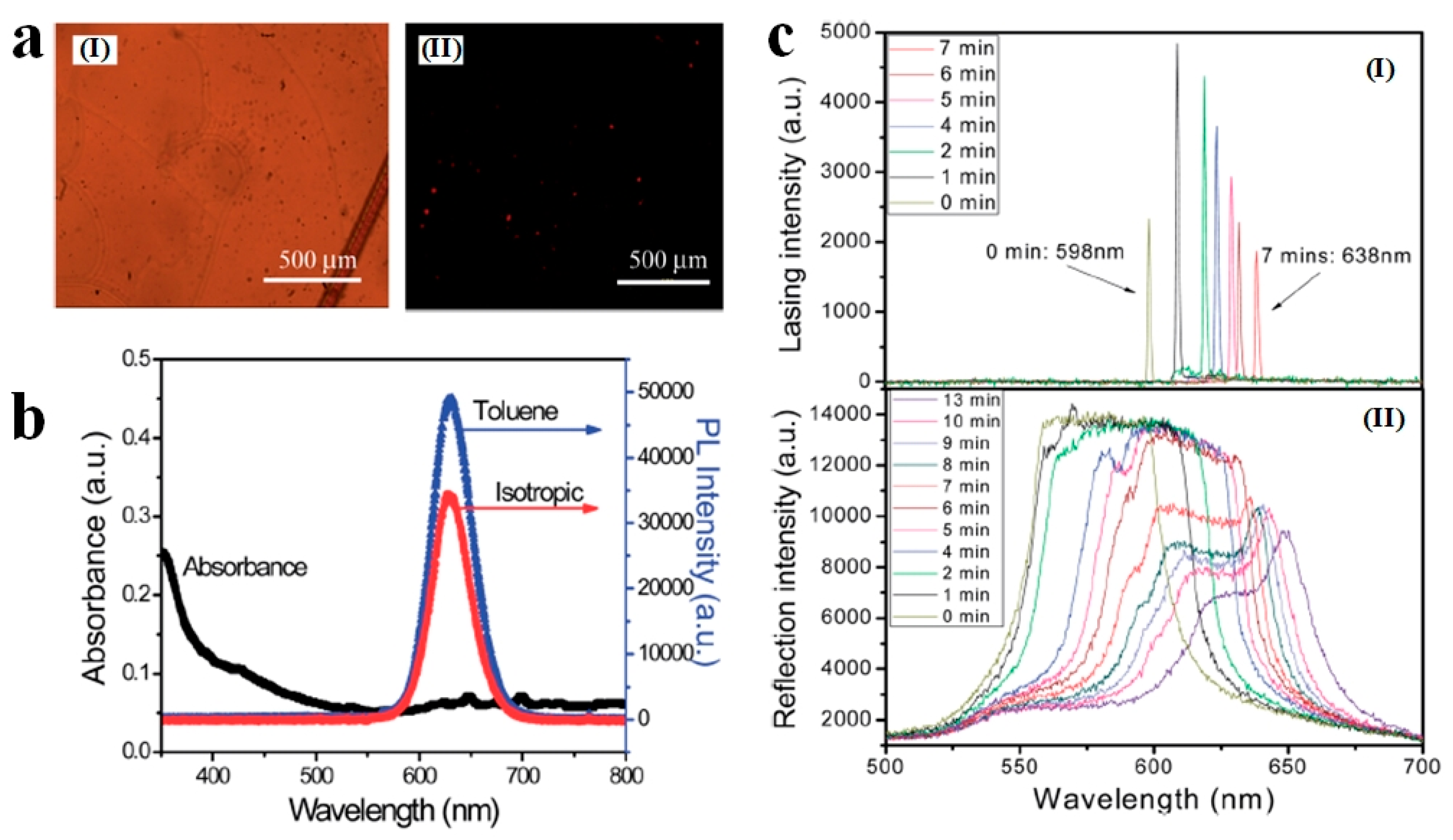
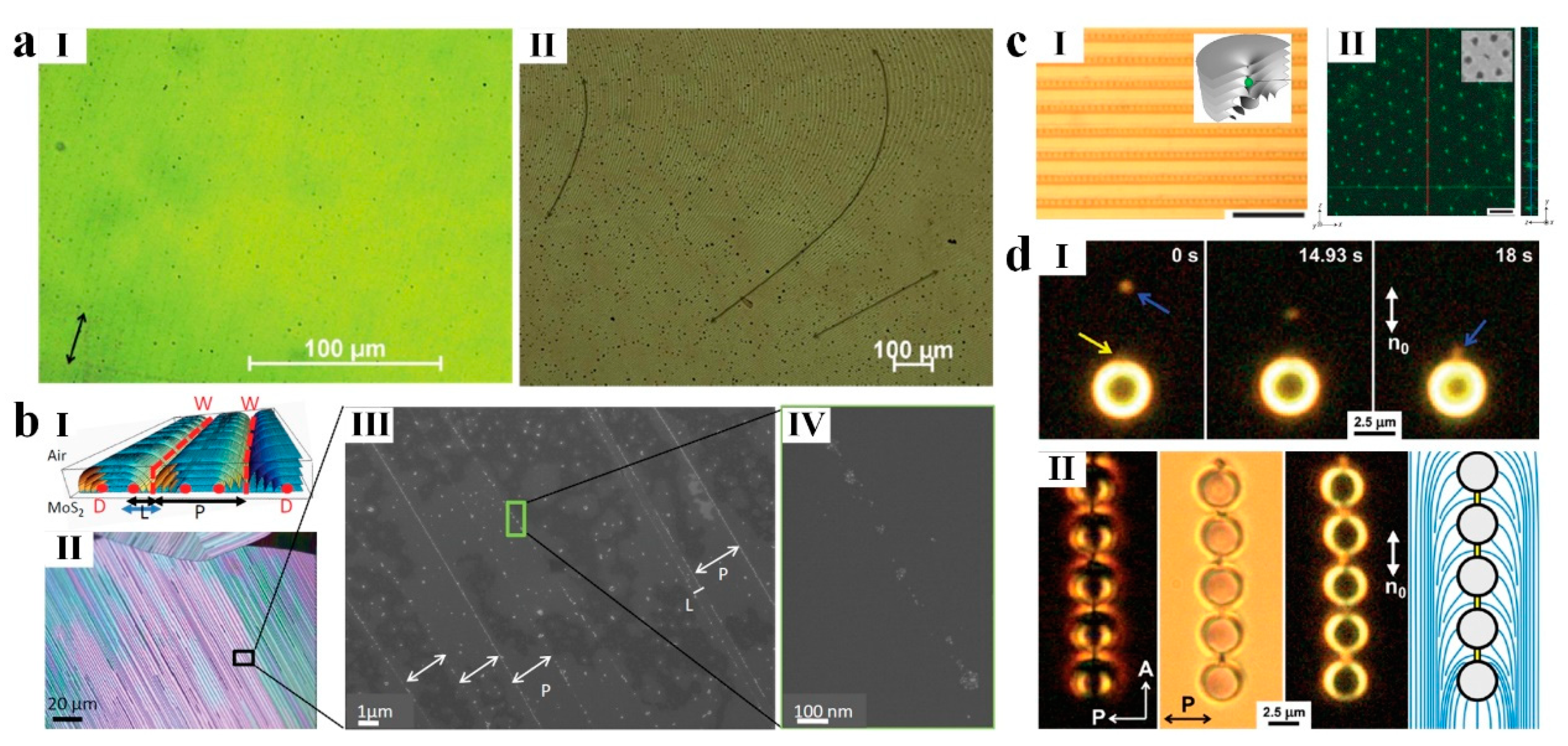
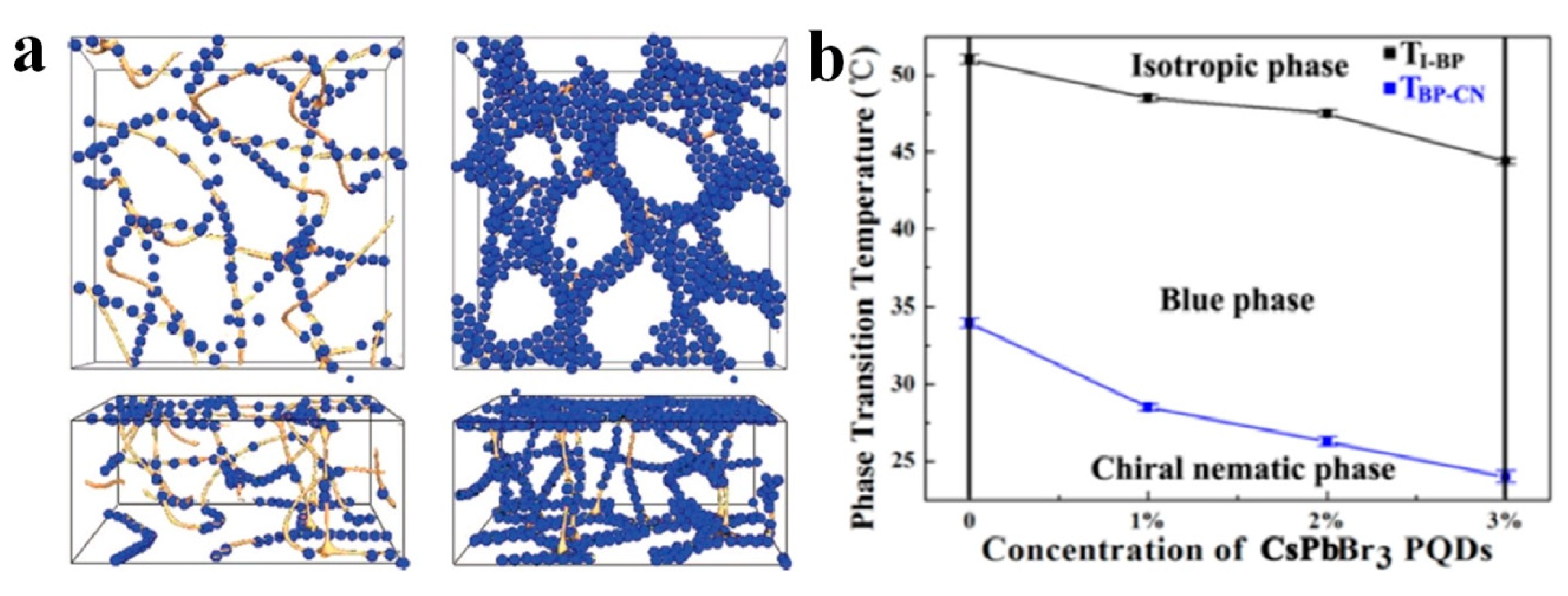
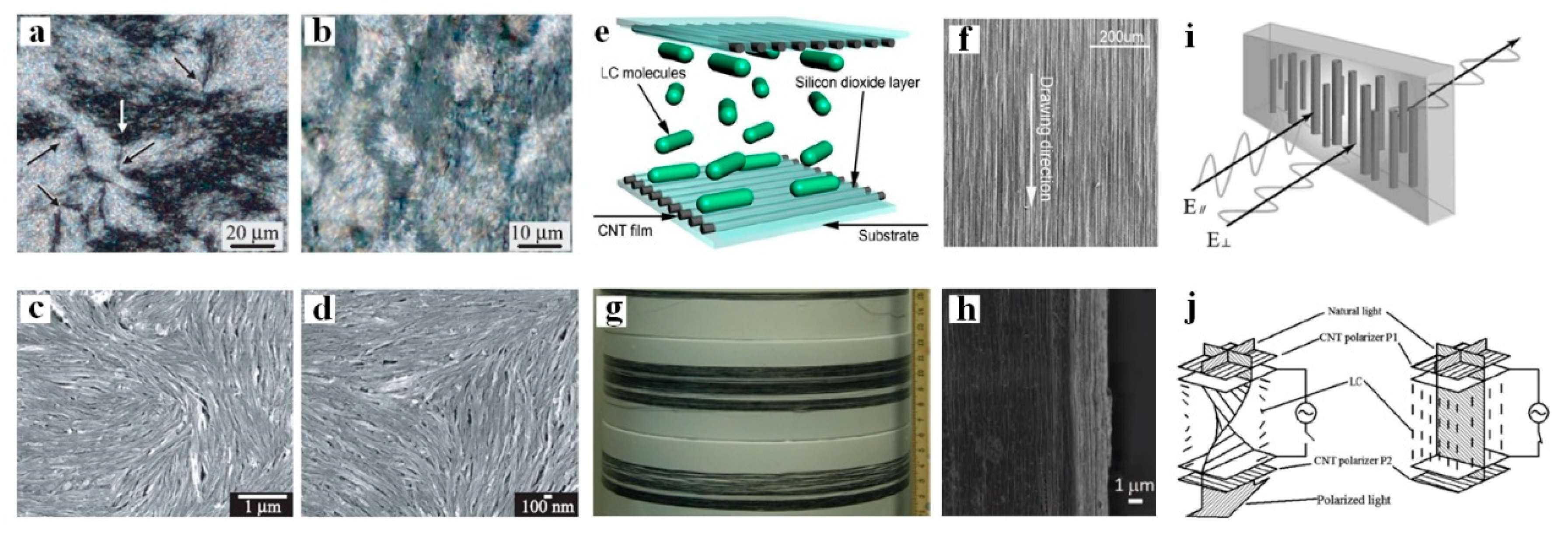
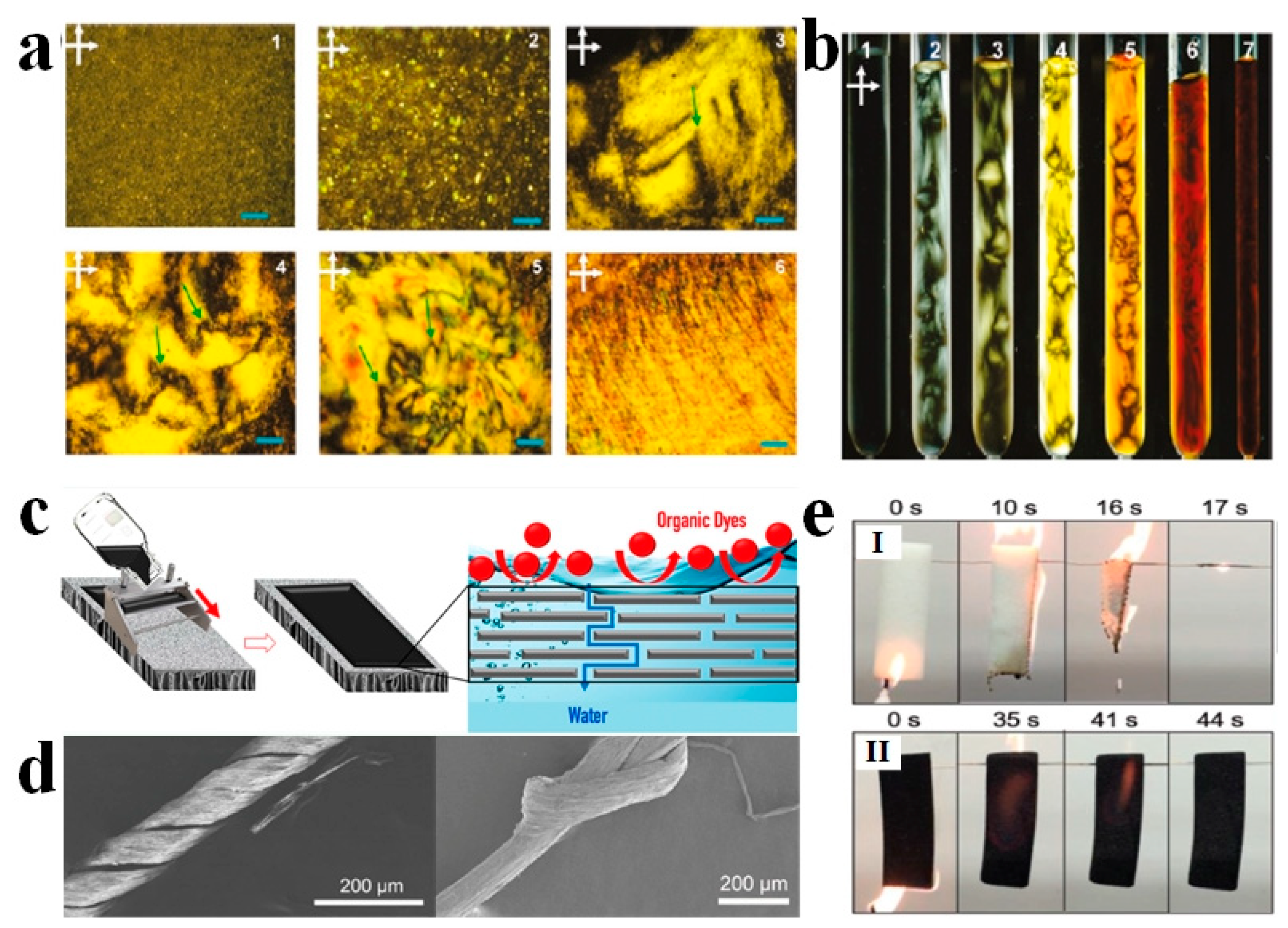
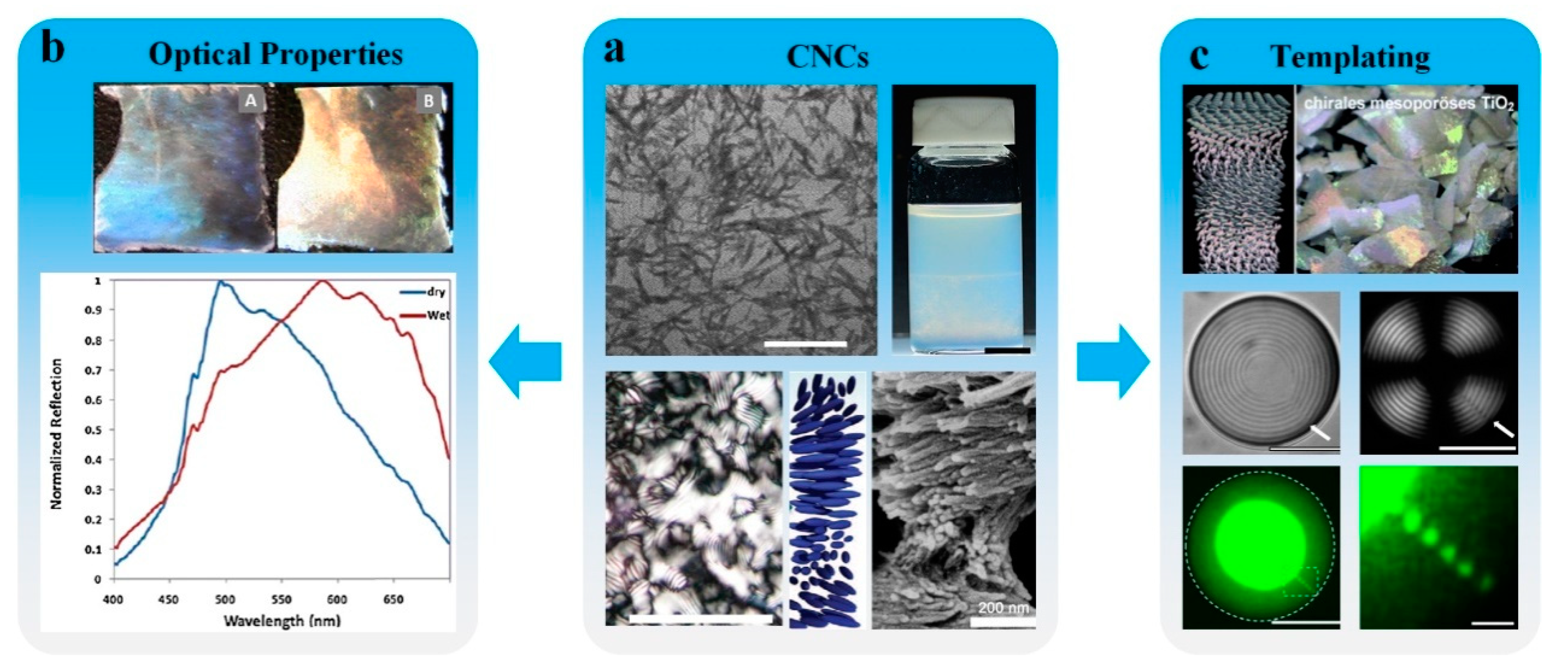
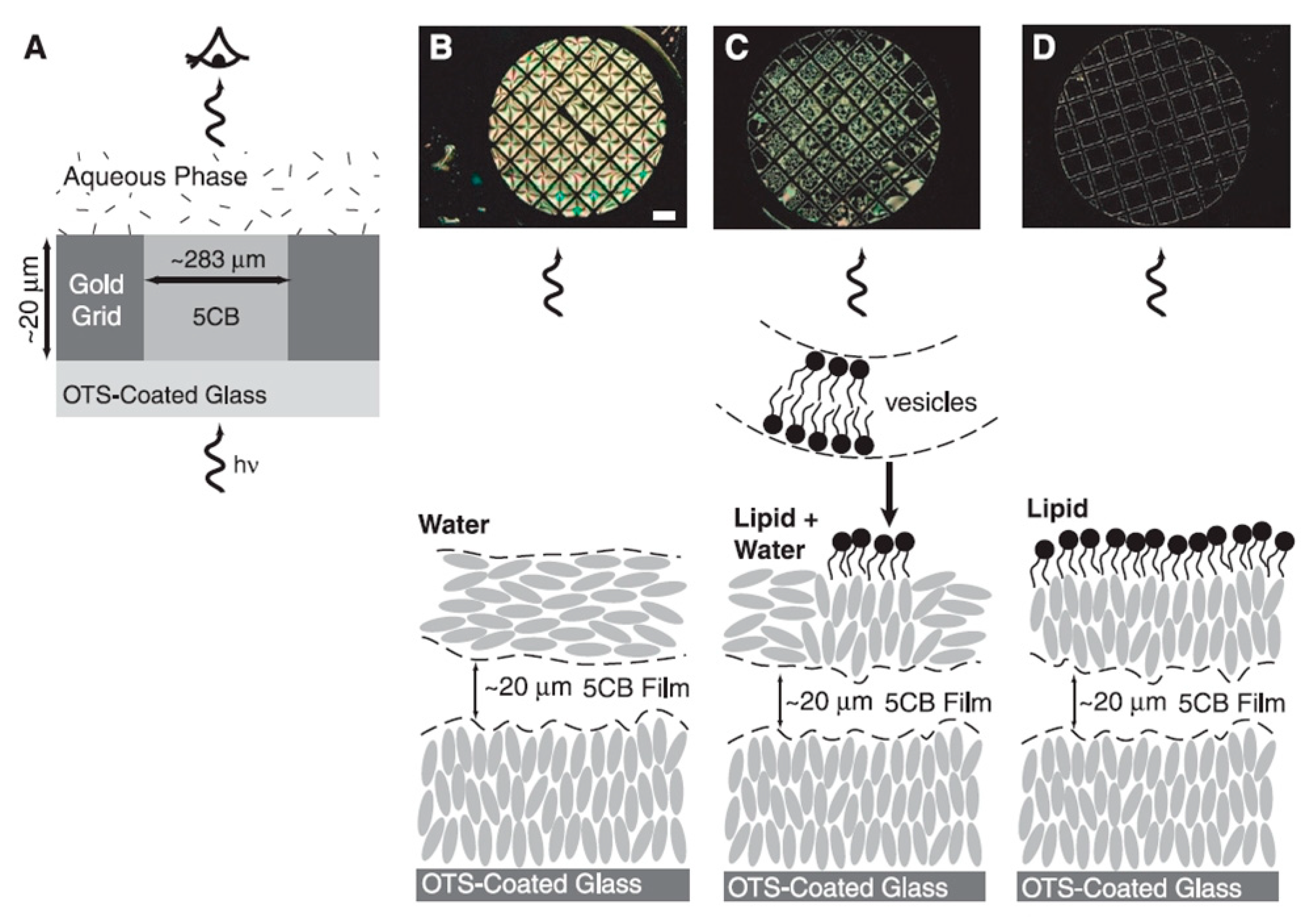
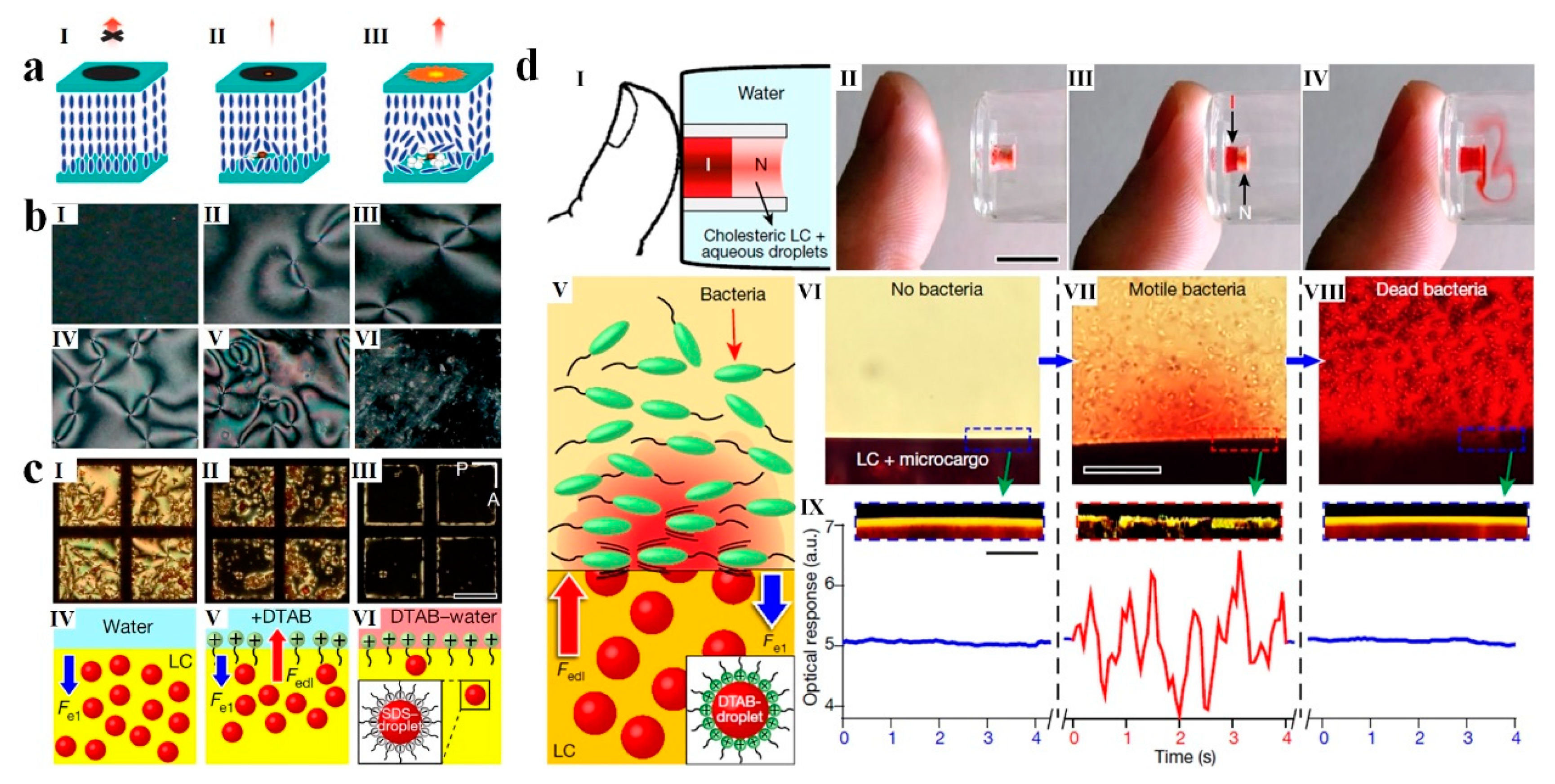
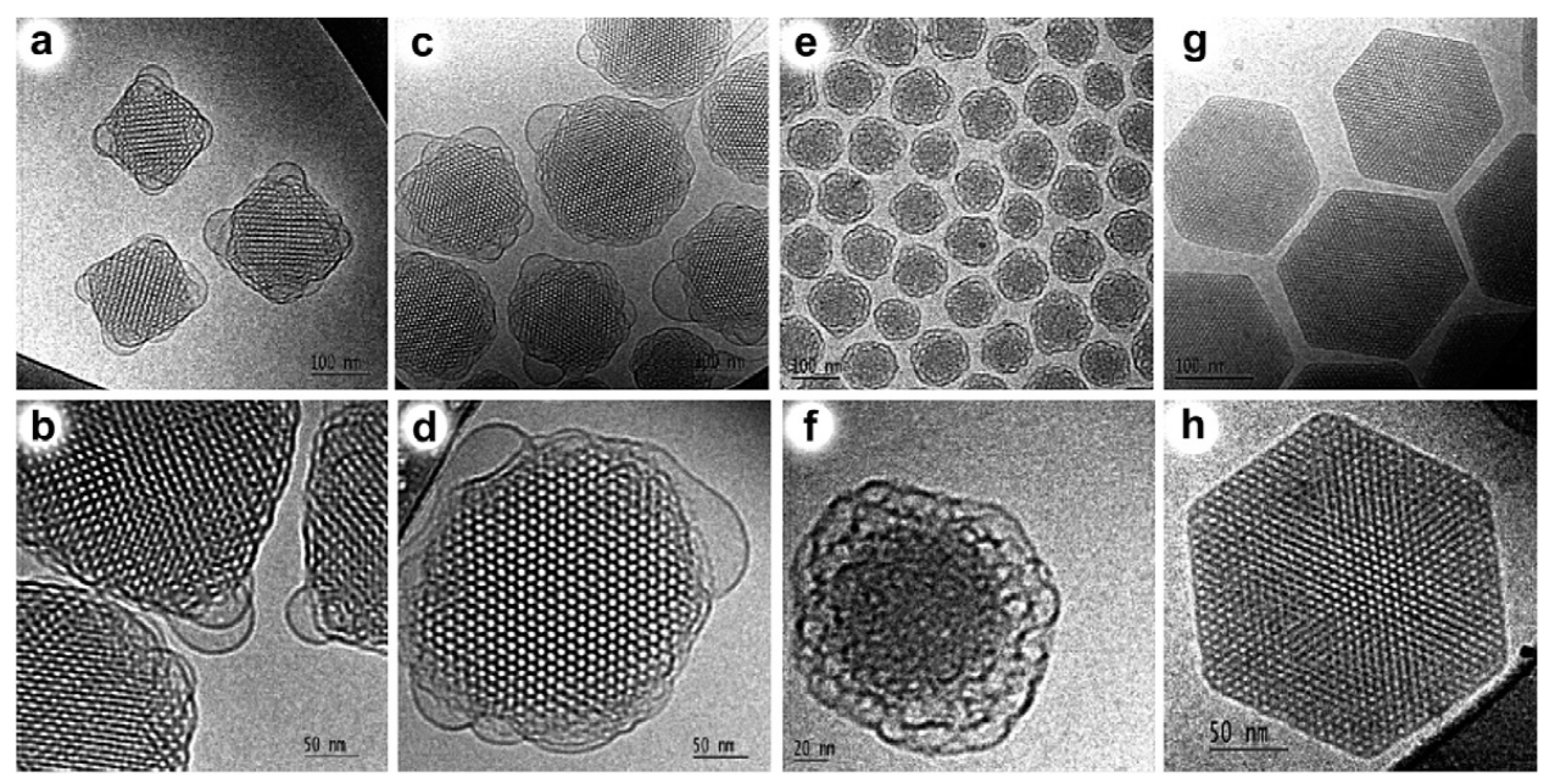
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Dierking, I. Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Appl. Sci. 2019, 9, 2512. https://doi.org/10.3390/app9122512
Shen Y, Dierking I. Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Applied Sciences. 2019; 9(12):2512. https://doi.org/10.3390/app9122512
Chicago/Turabian StyleShen, Yuan, and Ingo Dierking. 2019. "Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience" Applied Sciences 9, no. 12: 2512. https://doi.org/10.3390/app9122512
APA StyleShen, Y., & Dierking, I. (2019). Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Applied Sciences, 9(12), 2512. https://doi.org/10.3390/app9122512





