CVD Synthesis of Solid, Hollow, and Nitrogen-Doped Hollow Carbon Spheres from Polypropylene Waste Materials
Abstract
1. Introduction
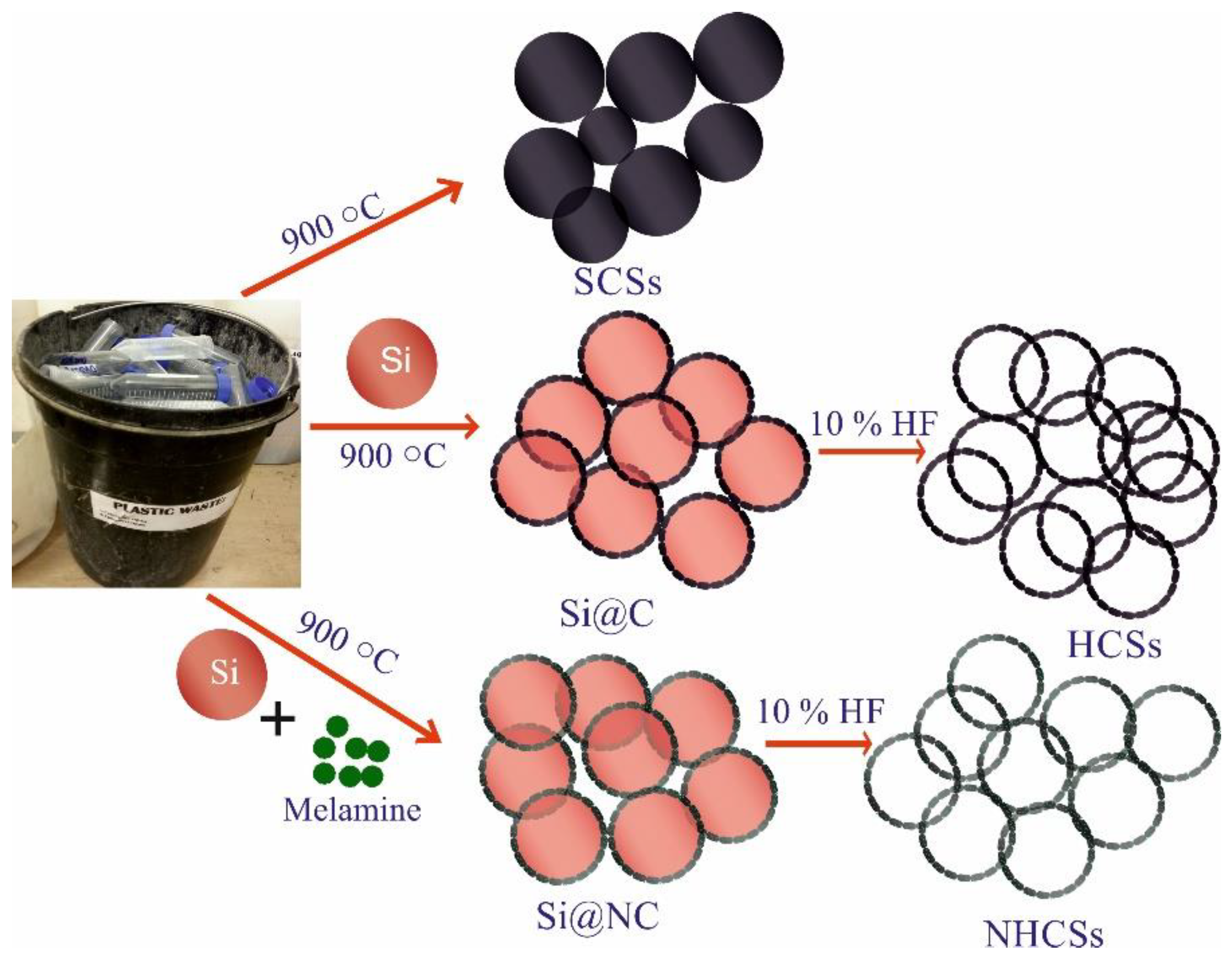
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of SCSs
2.3. Synthesis of HCSs
2.4. Nitrogen-Doped HCSs
2.5. Characterization
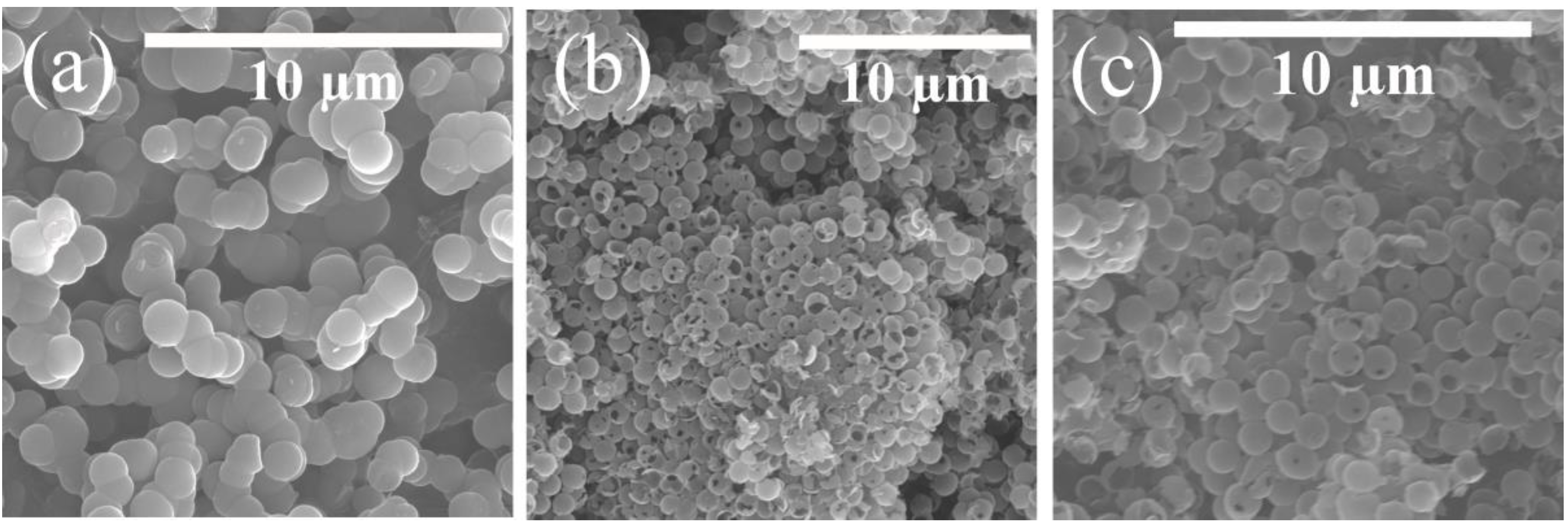
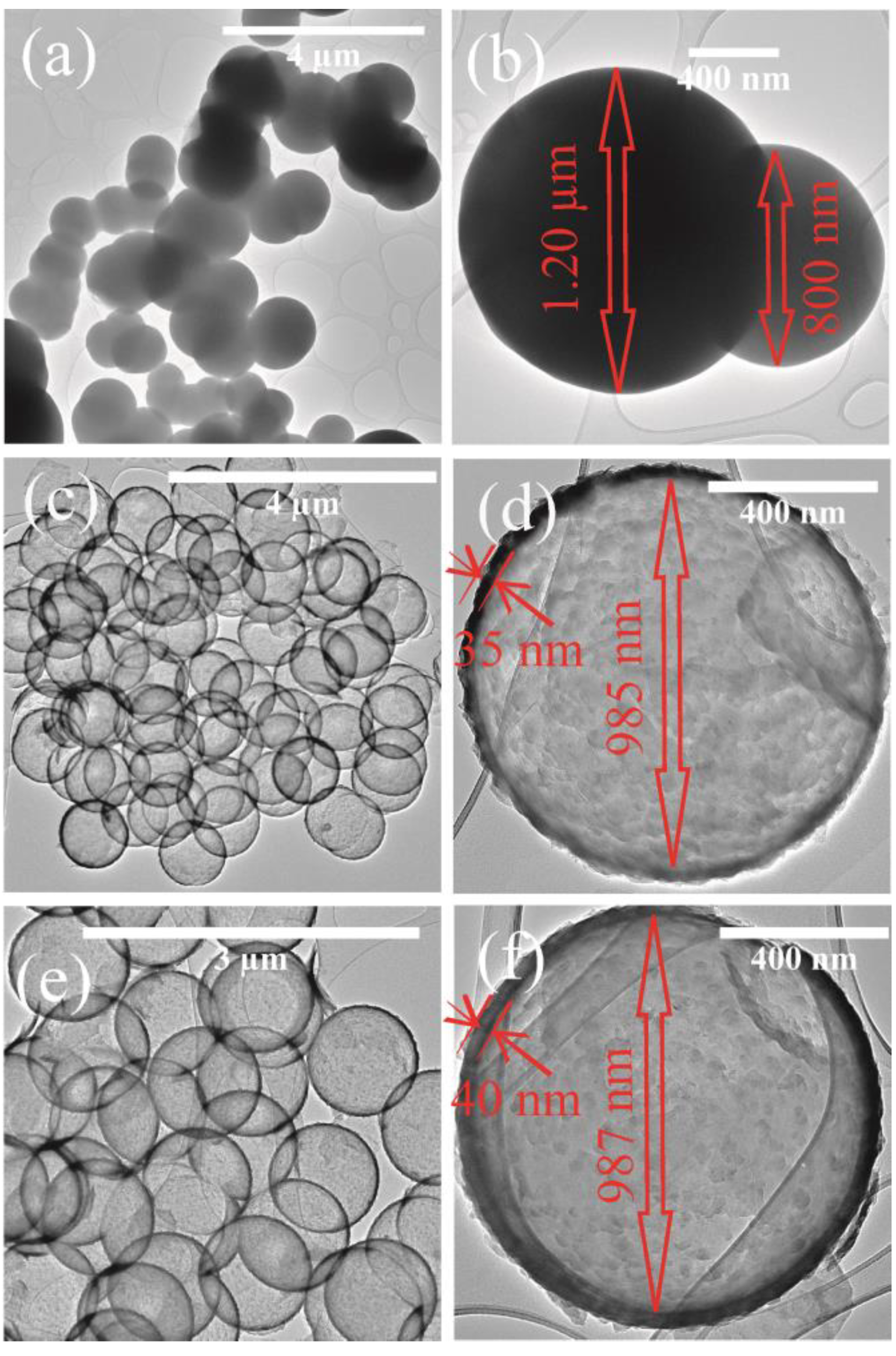
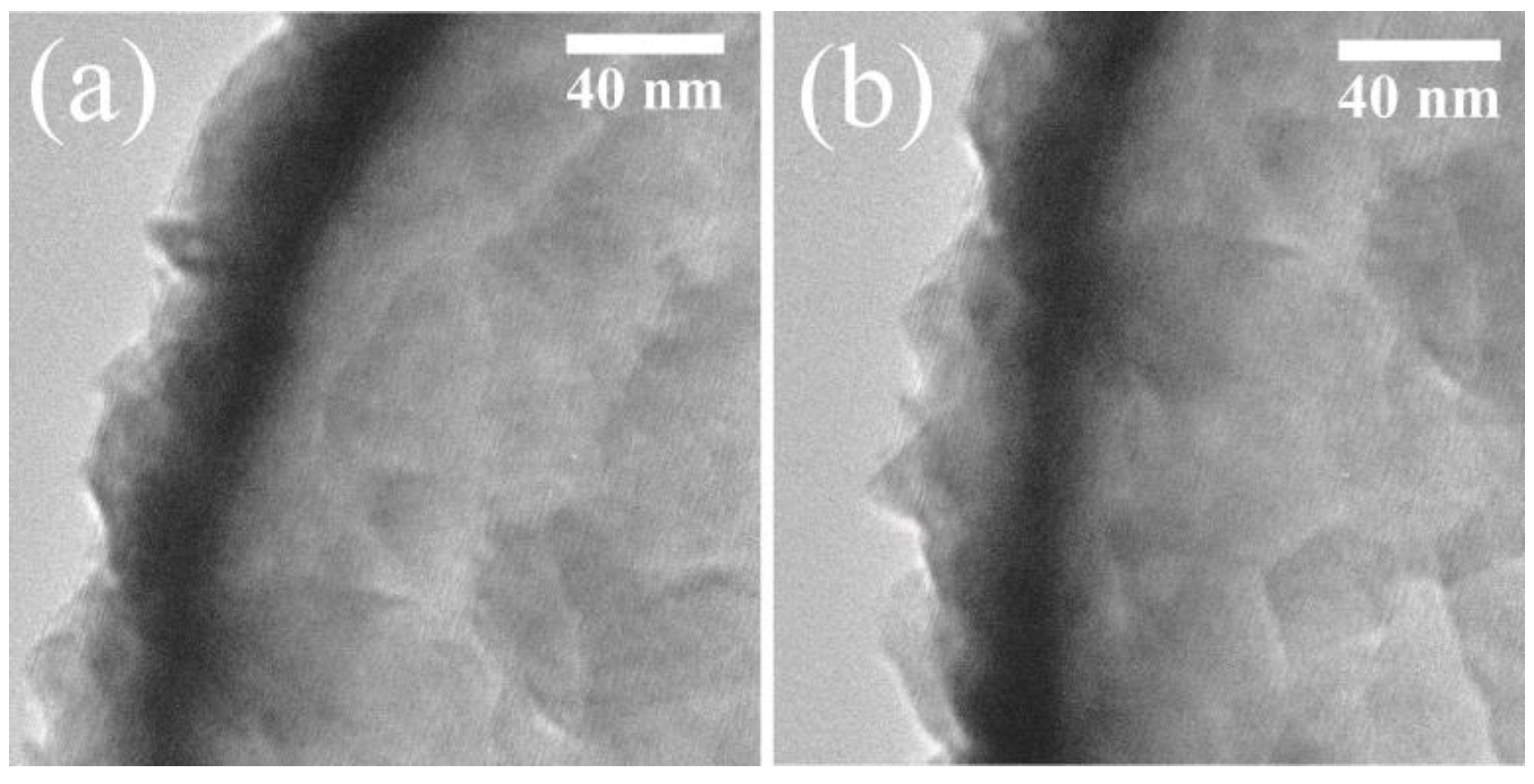
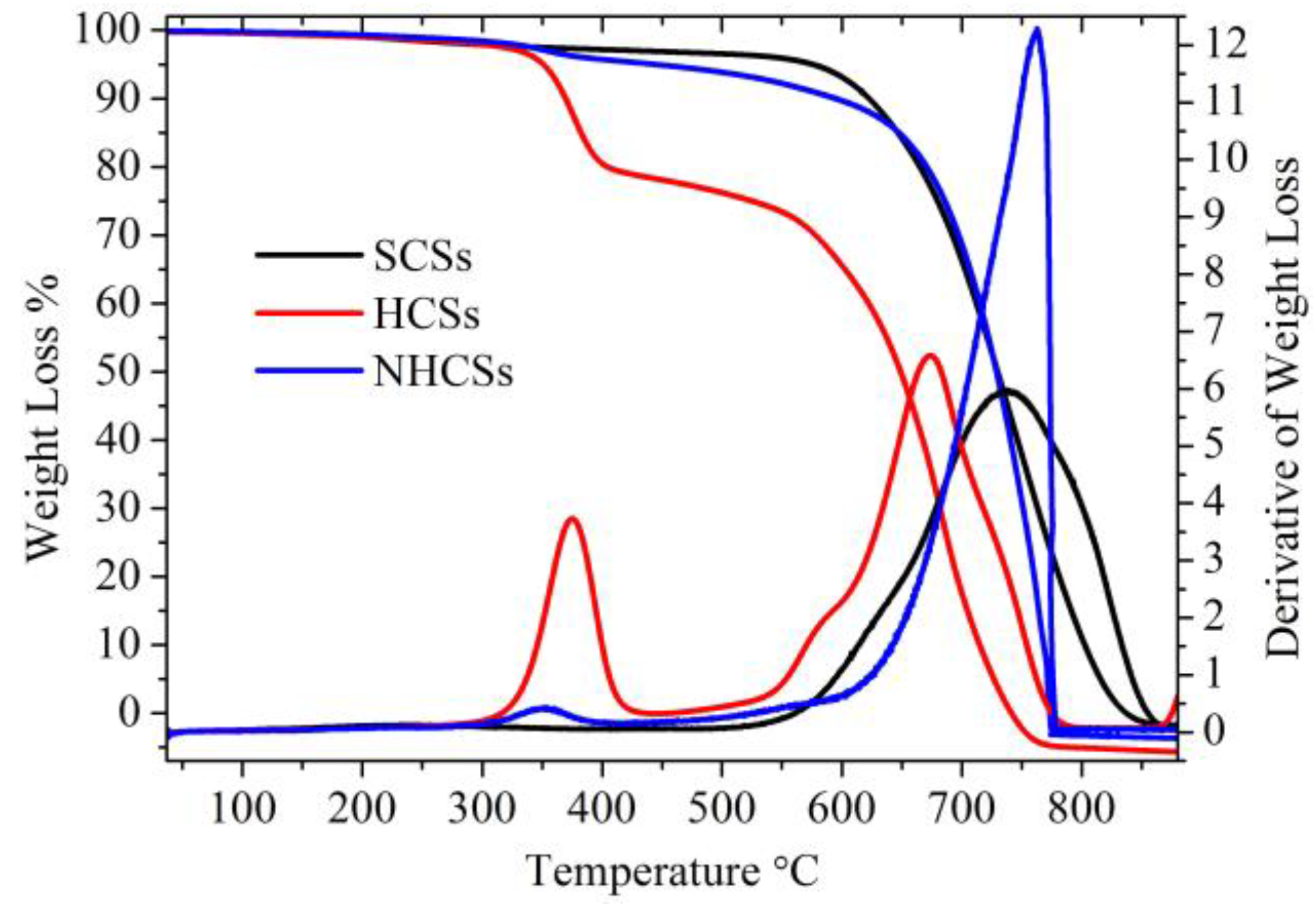
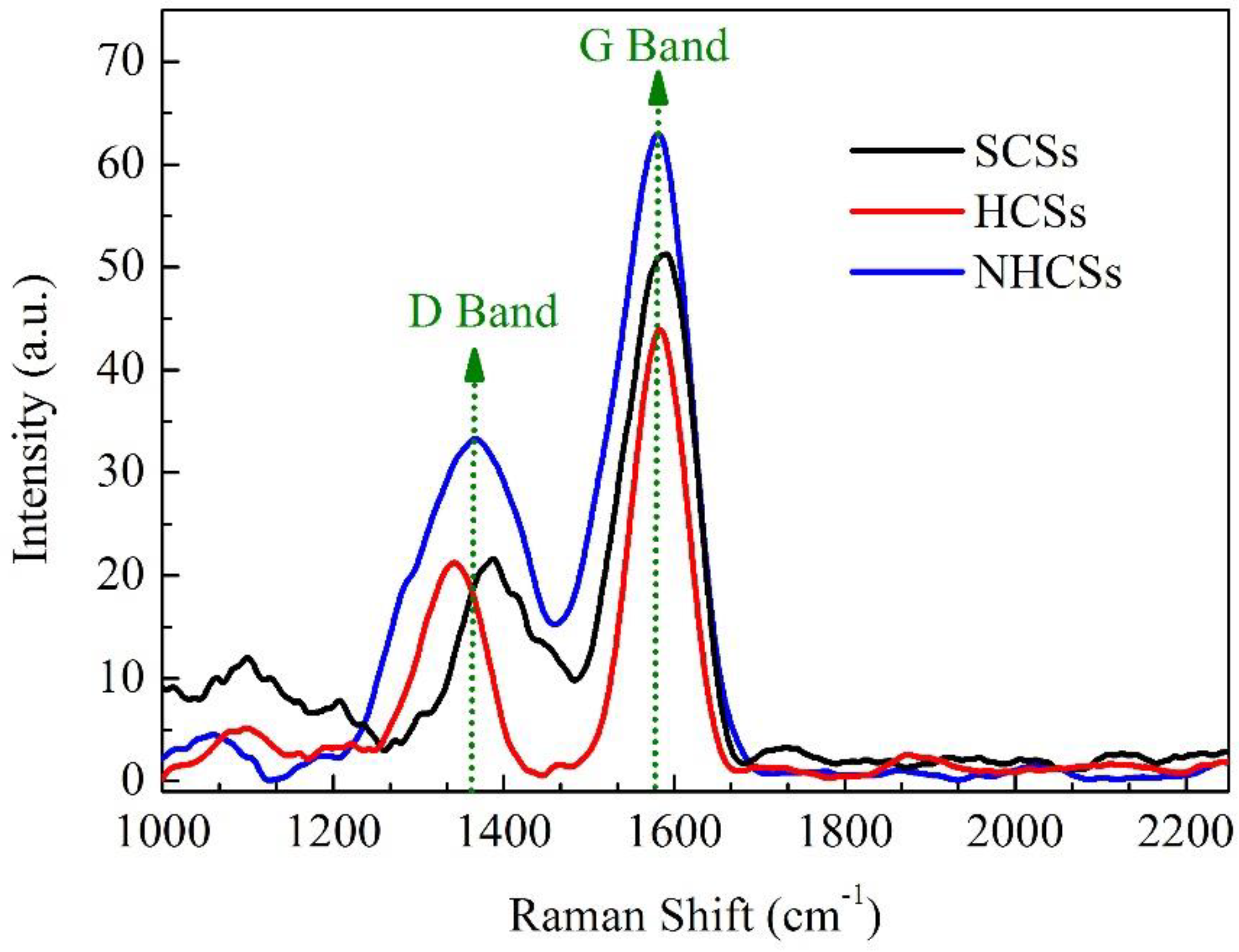
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- García, J.M. Catalyst: Design Challenges for the Future of Plastics Recycling. Chem 2016, 1, 813–815. [Google Scholar] [CrossRef]
- Helms, B.A.; Russell, T.P. Reaction: Polymer Chemistries Enabling Cradle-to-Cradle Life Cycles for Plastics. Chem 2016, 1, 816–818. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Guinée, J.B.; Cucurachi, S.; Henriksson, P.J.G.; Heijungs, R. Digesting the alphabet soup of LCA. Int. J. Life Cycle Assess. 2018, 23, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Klemchuk, P.P. Degradable plastics: A critical review. Polym. Degrad. Stab. 1990, 27, 183–202. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 1. Chemical and Physical Characterization and Isotopic Tests. Environ. Sci. Technol. 2015, 49, 12080–12086. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H. Synthesis of high-density jet fuel from plastics via catalytically integral processes. RSC Adv. 2016, 6, 6154–6163. [Google Scholar] [CrossRef]
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763–774. [Google Scholar] [CrossRef]
- Antolini, E. Nitrogen-doped carbons by sustainable N-and C-containing natural resources as nonprecious catalysts and catalyst supports for low temperature fuel cells. Renew. Sust. Energ. Rev. 2016, 58, 34–51. [Google Scholar] [CrossRef]
- Feng, S.; Li, W.; Shi, Q.; Li, Y.; Chen, J.; Ling, Y.; Asiri, A.M.; Zhao, D. Synthesis of nitrogen-doped hollow carbon nanospheres for CO2 capture. Chem. Commun. 2014, 50, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Maddah, H.A. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Wu, C.; Nahil, M.A.; Miskolczi, N.; Huang, J.; Williams, P.T. Processing Real-World Waste Plastics by Pyrolysis-Reforming for Hydrogen and High-Value Carbon Nanotubes. Environ. Sci. Technol. 2014, 48, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Pol, V.G. Upcycling: Converting Waste Plastics into Paramagnetic, Conducting, Solid, Pure Carbon Microspheres. Environ. Sci. Technol. 2010, 44, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Pol, V.G.; Thackeray, M.M. Spherical carbon particles and carbon nanotubes prepared by autogenic reactions: Evaluation as anodes in lithium electrochemical cells. Energy Environ. Sci. 2011, 4, 1904–1912. [Google Scholar] [CrossRef]
- Min, J.; Zhang, S.; Li, J.; Klingeler, R.; Wen, X.; Chen, X.; Zhao, X.; Tang, T.; Mijowska, E. From polystyrene waste to porous carbon flake and potential application in supercapacitor. Waste Manag. 2019, 85, 333–340. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Chen, X.; Wen, X.; Jiang, Z.; Mijowska, E.; Wang, Y.; Tang, T. Synthesis, characterization and growth mechanism of mesoporous hollow carbon nanospheres by catalytic carbonization of polystyrene. Microporous Mesoporous Mater. 2013, 176, 31–40. [Google Scholar] [CrossRef]
- Chung, Y.-H.; Jou, S. Carbon nanotubes from catalytic pyrolysis of polypropylene. Mater. Chem. Phys. 2005, 92, 256–259. [Google Scholar] [CrossRef]
- Nishino, H.; Nishida, R.; Matsui, T.; Kawase, N.; Mochida, I. Growth of amorphous carbon nanotube from poly(tetrafluoroethylene) and ferrous chloride. Carbon 2003, 41, 2819–2823. [Google Scholar] [CrossRef]
- Wu, Z.J.; Kong, L.J.; Hu, H.; Tian, S.H.; Xiong, Y. Adsorption performance of hollow spherical sludge carbon prepared from sewage sludge and polystyrene foam wastes. ACS Sustain. Chem. Eng. 2015, 3, 552–558. [Google Scholar] [CrossRef]
- Liang, K.; Liu, L.; Wang, W.; Yu, Y.; Wang, Y.; Zhang, L.; Ma, C.; Chen, A. Conversion of waste plastic into orderedmesoporous carbon for electrochemical applications. J. Mater. Res. 2019, 34, 941–949. [Google Scholar] [CrossRef]
- Arena, U.; Mastellone, M.L.; Camino, G.; Boccaleri, E. An innovative process for mass production of multi-wall carbon nanotubes by means of low-cost pyrolysis of polyolefins. Polym. Degrad. Stab. 2006, 91, 763–768. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; He, J. Synthesis of carbon nanotubes and nanospheres with controlled morphology using different catalyst precursors. Nanotechnology 2008, 19, 325607. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.K.; Durbach, S.; Coville, N.J. Synthesis of Multi-Walled Carbon Nanotubes from Plastic Waste Using a Stainless-Steel CVD Reactor as Catalyst. Nanomaterials 2017, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Mohsenian, S.; Esmaeili, M.S.; Fathi, J.; Shokri, B. Synthesis of carbon nano-spheres and nano-tubes by thermal plasma processing of polypropylene. Appl. Phys. A 2018, 124, 546. [Google Scholar] [CrossRef]
- Zhang, P.; Qiao, Z.-A.; Dai, S. Recent advances in carbon nanospheres: Synthetic routes and applications. Chem. Commun. 2015, 51, 9246–9256. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures. Chem. Rev. 2016, 116, 10983–11060. [Google Scholar] [CrossRef]
- Li, S.; Pasc, A.; Fierro, V.; Celzard, A. Hollow carbon spheres, synthesis and applications—A review. J. Mater. Chem. A 2016, 4, 12686–12713. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kim, J.H.; Fang, B.; Kim, M.; Yu, J.-S. Hollow spherical carbon with mesoporous shell as a superb anode catalyst support in proton exchange membrane fuel cell. Catalys. Today 2009, 146, 25–30. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Gao, C.; Hsu, W.K.; Zhu, Y.; Huczko, A.; Bystrzejewski, M.; Roe, M.; Lee, C.Y.; Acquah, S.; Kroto, H.; et al. Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon 2005, 43, 1944–1953. [Google Scholar] [CrossRef]
- Gutiérrez-García, C.J.; Ambriz-Torres, J.M.; Contreras-Navarrete, J.d.J.; Granados-Martínez, F.G.; García-Ruiz, D.L.; García-González, L.; Zamora-Peredo, L.; Fernando Ortega-Varela, L.; Richaud, A.; Méndez, F.; et al. Synthesis of carbon spheres by atmospheric pressure chemical vapor deposition from a serial of aromatic hydrocarbon precursors. Phys. E Low-dimens. Syst. Nanostruct. 2019. [Google Scholar] [CrossRef]
- Hlekelele, L.; Franklyn, P.J.; Tripathi, P.K.; Durbach, S.H. Morphological and crystallinity differences in nitrogen-doped carbon nanotubes grown by chemical vapour deposition decomposition of melamine over coal fly ash. RSC Adv. 2016, 6, 76773–76779. [Google Scholar] [CrossRef]
- Heath, R. Chapter 25—Aldehyde Polymers: Phenolics and Aminoplastics. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 705–742. [Google Scholar] [CrossRef]
- Ferrari, C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Xiong, H.; Moyo, M.; Motchelaho, M.A.; Tetana, Z.N.; Dube, S.M.A.; Jewell, L.L.; Coville, N.J. Fischer–Tropsch synthesis: Iron catalysts supported on N-doped carbon spheres prepared by chemical vapor deposition and hydrothermal approaches. J. Catal. 2014, 311, 80–87. [Google Scholar] [CrossRef]
- Xiong, H.; Moyo, M.; Motchelaho, M.A.; Jewell, L.L.; Coville, N.J. Fischer–Tropsch synthesis over model iron catalysts supported on carbon spheres: The effect of iron precursor, support pretreatment, catalyst preparation method and promoters. Appl. Catal. A Gen. 2010, 388, 168–178. [Google Scholar] [CrossRef]
- Marzorati, S.; Vasconcelos, J.M.; Ding, J.; Longhi, M.; Colavita, P.E. Journal of Materials Chemistry. A Materiuals. Energy Sustain. 2015, 3, 18920–18927. [Google Scholar]
- Nongwe, I.; Ravat, V.; Meijboom, R.; Coville, N.J. Efficient and reusable Co/nitrogen doped hollow carbon sphere catalysts for the aerobic oxidation of styrene. Appl. Catal. A Gen. 2013, 466, 1–8. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Islam, R.U.; Witcomb, M.J.; van Otterlo, W.A.L.; Coville, N.J. Catalytic Activity of Metal Nanoparticles Supported on Nitrogen-Doped Carbon Spheres. ChemCatChem 2010, 2, 51–54. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, P.K.; Durbach, S.; Coville, N.J. CVD Synthesis of Solid, Hollow, and Nitrogen-Doped Hollow Carbon Spheres from Polypropylene Waste Materials. Appl. Sci. 2019, 9, 2451. https://doi.org/10.3390/app9122451
Tripathi PK, Durbach S, Coville NJ. CVD Synthesis of Solid, Hollow, and Nitrogen-Doped Hollow Carbon Spheres from Polypropylene Waste Materials. Applied Sciences. 2019; 9(12):2451. https://doi.org/10.3390/app9122451
Chicago/Turabian StyleTripathi, Pranav K., Shane Durbach, and Neil J. Coville. 2019. "CVD Synthesis of Solid, Hollow, and Nitrogen-Doped Hollow Carbon Spheres from Polypropylene Waste Materials" Applied Sciences 9, no. 12: 2451. https://doi.org/10.3390/app9122451
APA StyleTripathi, P. K., Durbach, S., & Coville, N. J. (2019). CVD Synthesis of Solid, Hollow, and Nitrogen-Doped Hollow Carbon Spheres from Polypropylene Waste Materials. Applied Sciences, 9(12), 2451. https://doi.org/10.3390/app9122451





