Mining Sequential Patterns of Diseases Contracted and Medications Prescribed before the Development of Stevens-Johnson Syndrome in Taiwan
Abstract
:1. Introduction
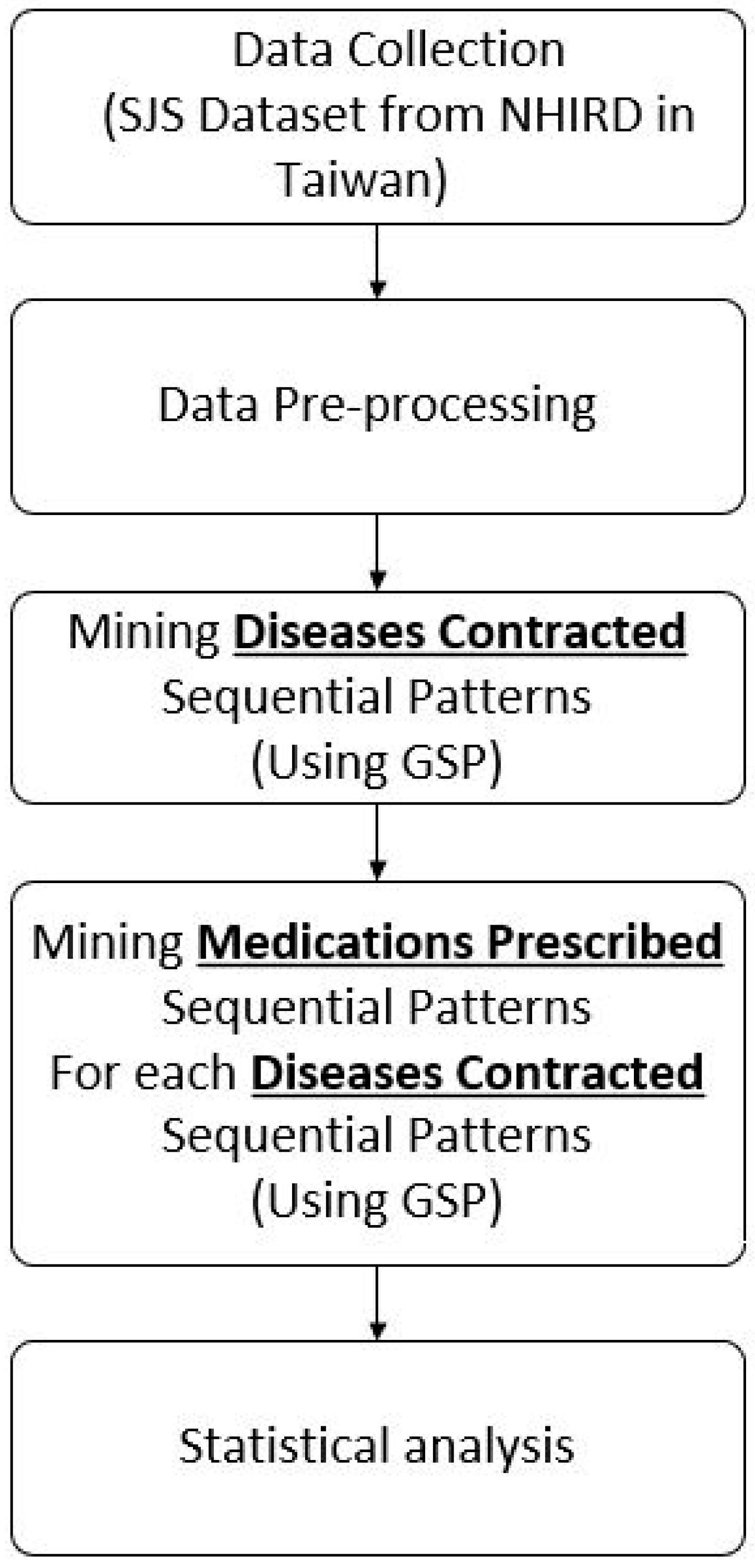
2. Methods
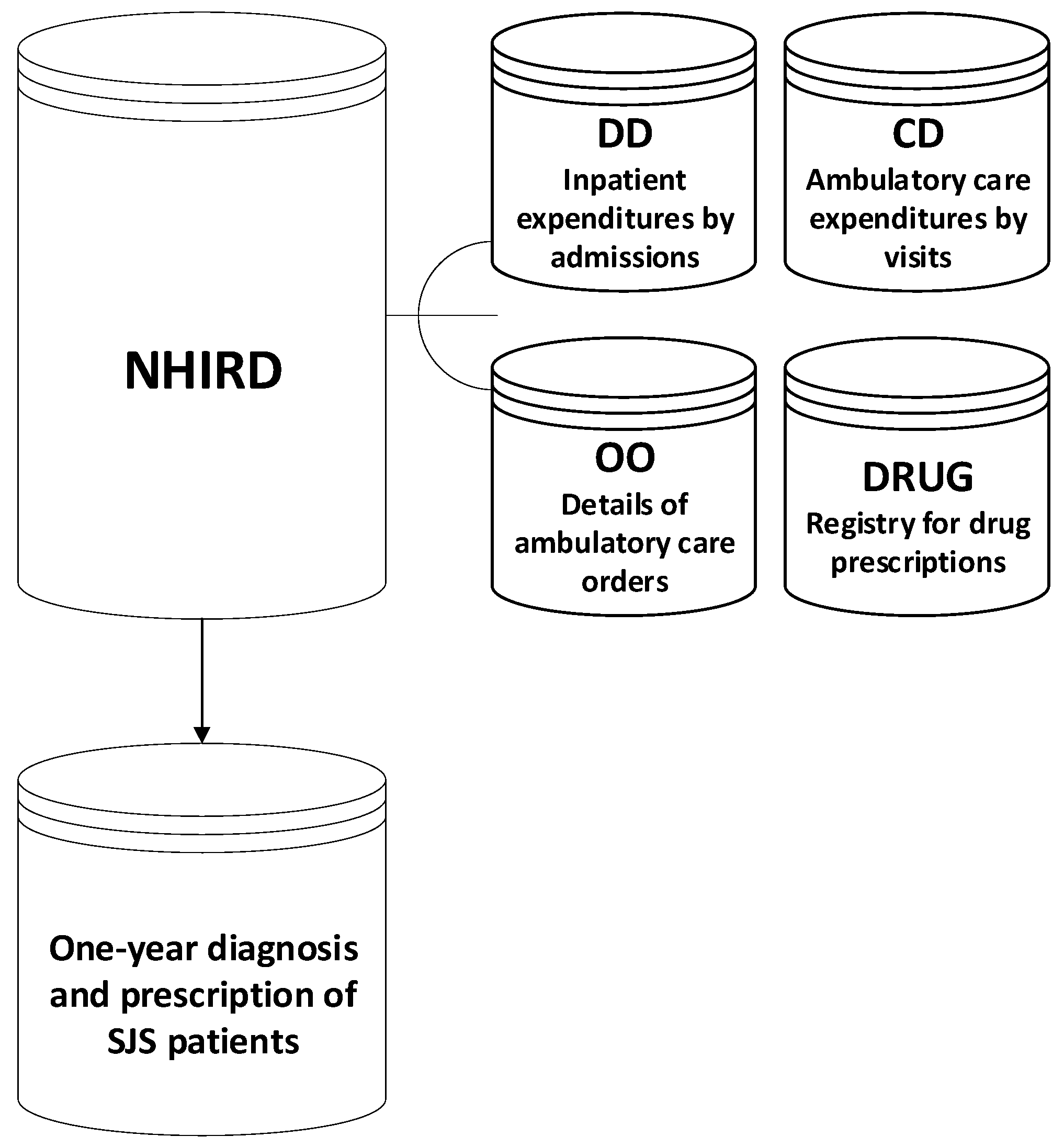
2.1. Data Collection
2.2. Data Pre-Processing
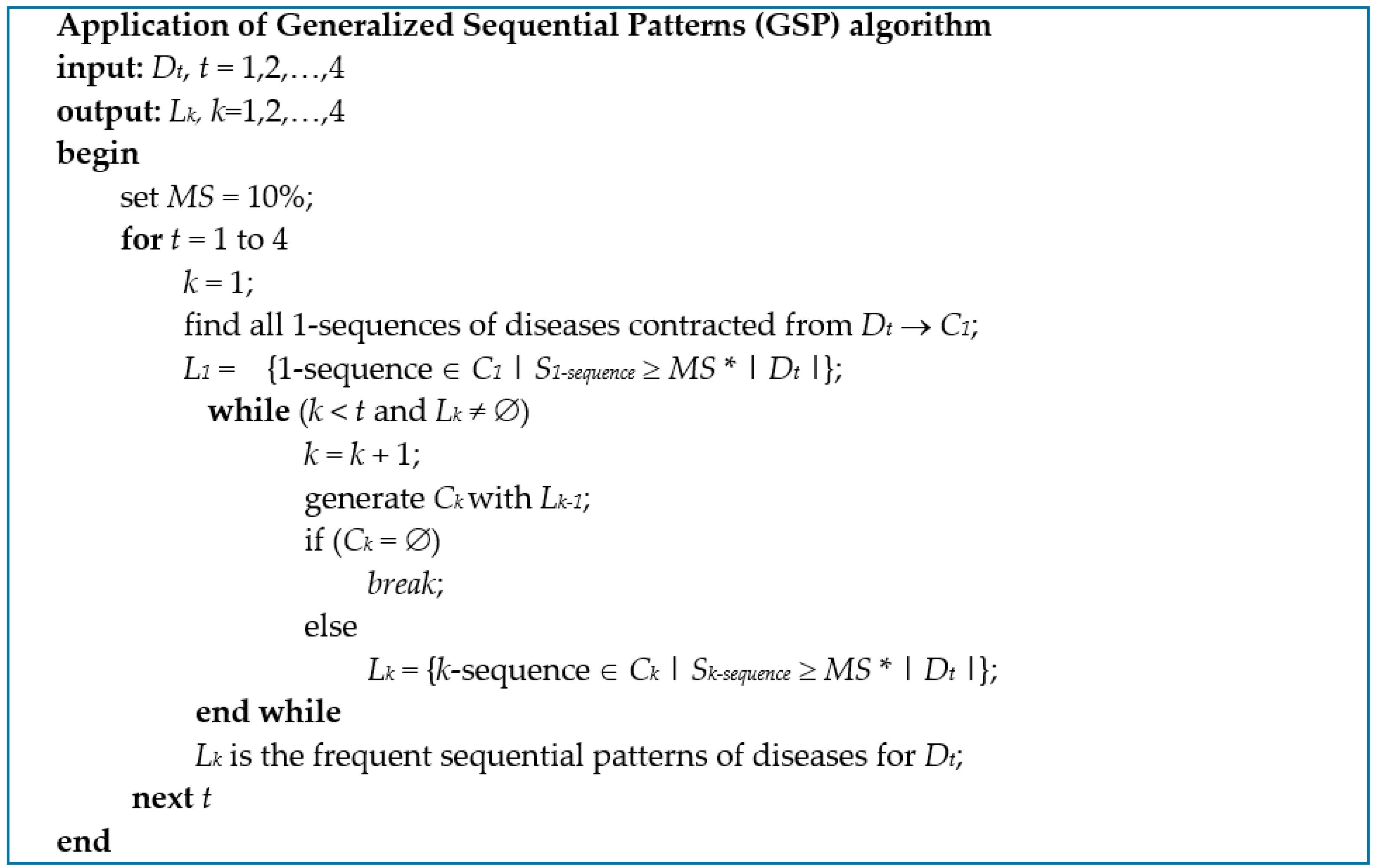
2.3. Mining Sequential Patterns of ContractedDiseases
2.4. Mining Sequential Patterns of Medications Prescribed
2.5. Statistical Analysis
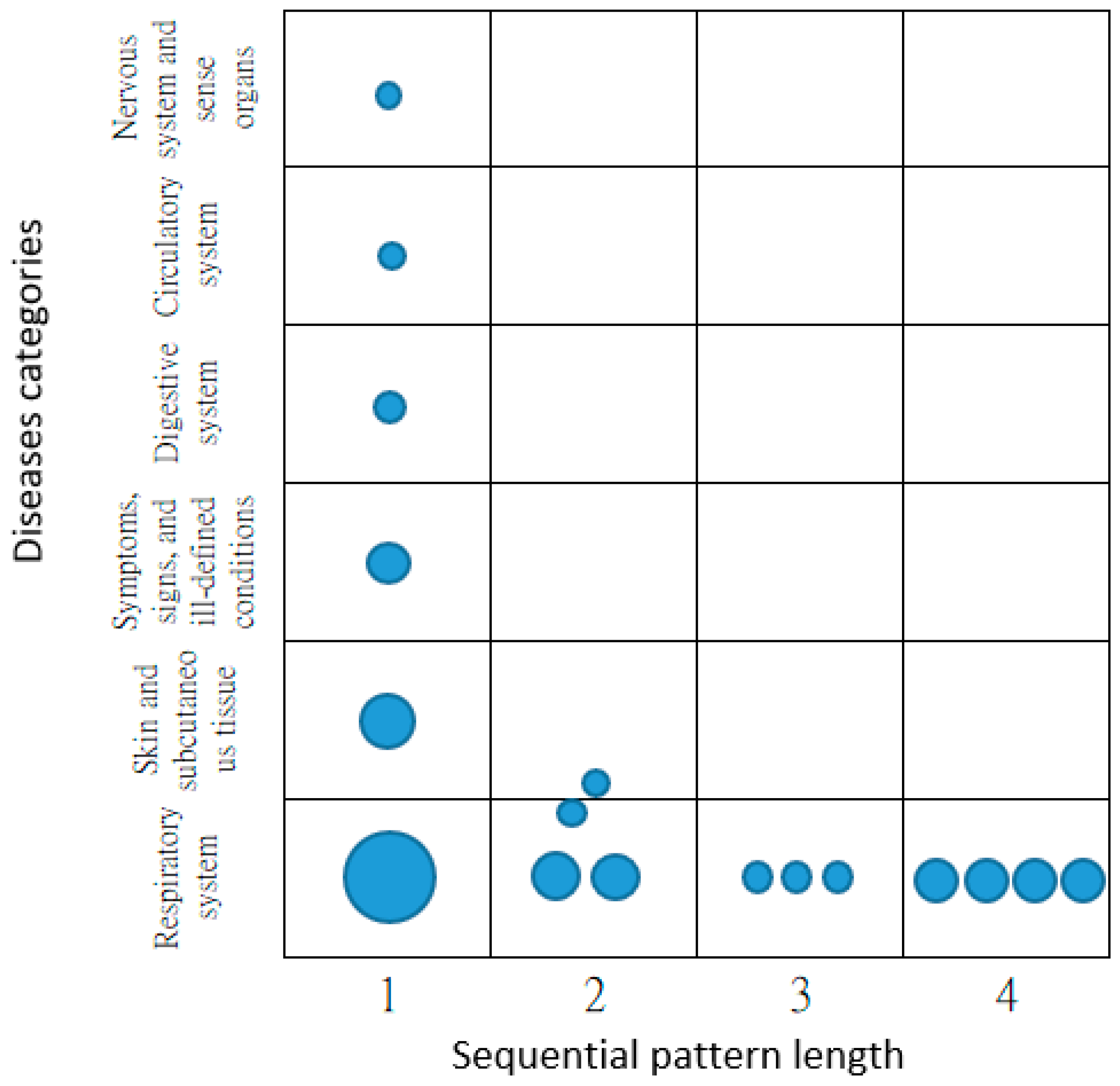
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Matheny, M.E.; Hu, Y.; Xu, H. Data mining methodologies for pharmacovigilance. ACM SIGKDD Explor. Newsl. 2012, 14, 35–42. [Google Scholar] [CrossRef]
- Budnitz, D.S.; Pollock, D.A.; Weidenbach, K.N.; Mendelsohn, A.B.; Schroeder, T.J.; Annest, J.L. National surveillance of emergency department visits for outpatient adverse drug events. JAMA 2006, 296, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Breckenridge, A.M.; Kitteringham, N.R.; Park, B.K. Adverse drug reactions. BMJ 1998, 316, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.Z.; Ding, W.; Nazeri, Z. Mining adverse drug reactions from electronic health records. In Proceedings of the 2013 IEEE 13th International Conference on Data Mining Workshops, Dallas, TX, USA, 7–10 December 2013; pp. 1137–1140. [Google Scholar]
- Trifiro, G.; Pariente, A.; Coloma, P.M.; Kors, J.A.; Polimeni, G.; Miremont-Salame, G.; Catania, M.A.; Salvo, F.; David, A.; Moore, N.; et al. Data mining on electronic health record databases for signal detection in pharmacovigilance: Which events to monitor? Pharmacoepidemiol. Drug Saf. 2009, 18, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Importance of Pharmacovifilance: Safety Monitoring of Medicinal Products. Available online: http://apps.who.int/medicinedocs/pdf/s4893e/s4893e.pdf (accessed on 12 October 2018).
- Hauben, M.; Madigan, D.; Gerrits, C.M.; Walsh, L.; van Puijenbroek, E.P. The role of data mining in pharmacovigilance. Expert Opin. Drug Saf. 2005, 4, 929–948. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, Ministry of Health and Welfare. Taiwan 2017 Drug Adverse Reaction Notification Case Analysis in Taiwan. Available online: https://www.fda.gov.tw/TC/siteList.aspx?sid=4229 (accessed on 15 October 2018).
- Taiwan Drug Relief Foundation. Drug Relief Profile in Taiwan. Available online: http://www.tdrf.org.tw/ch/05knows/kno_07_main.asp?bull_id=6475 (accessed on 19 September 2017).
- Stevens, A.M. A New eruptive fever associated with stomatitis and ophthalmia. Am. J. Dis. Child. 1922, 24, 526–533. [Google Scholar] [CrossRef]
- Mawson, A.R.; Eriator, I.; Karre, S. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN): Could retinoids play a causative role? Med. Sci. Monit. 2015, 21, 133–143. [Google Scholar] [PubMed]
- Roujeau, J.C.; Kelly, J.P.; Naldi, L.; Rzany, B.; Stern, R.S.; Anderson, T.; Auquier, A.; Bastuji-Garin, S.; Correia, O.; Locati, F.; et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N. Engl. J. Med. 1995, 333, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Lee, J.L.; Naguwa, S.M.; Cheema, G.S.; Gershwin, M.E. Stevens-Johnson syndrome and toxic epidermal necrolysis. Autoimmun. Rev. 2008, 7, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Raucci, U.; Rossi, R.; da Cas, R.; Rafaniello, C.; Mores, N.; Bersani, G.; Reale, A.; Pirozzi, N.; Menniti-Ippolito, F.; Traversa, G.; et al. Stevens-Johnson syndrome associated with drugs and vaccines in children: A case-control study. PLoS ONE 2013, 8, e68231. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Kano, Y.; Fujita, H.; Kambara, T.; Matsukura, S.; Katayama, I.; Azukizawa, H.; Miyachi, Y.; Endo, Y.; Asada, H.; et al. Efficacy of additional i.v. immunoglobulin to steroid therapy in Stevens-Johnson syndrome and toxic epidermal necrolysis. J. Dermatol. 2015, 42, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Harr, T.; French, L.E. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J. Rare Dis. 2010, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Genetic and Rare Diseases Information Center (GARD); U.S. Department of Health & Human Services. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Available online: https://rarediseases.info.nih.gov/diseases/7700/stevens-johnson-syndrome (accessed on 14 October 2018).
- Garcia-Doval, I.; LeCleach, L.; Bocquet, H.; Otero, X.L.; Roujeau, J.C. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch. Dermatol. 2000, 136, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Sweileh, W.M. Bibliometric analysis of literature on toxic epidermal necrolysis and Stevens-Johnson syndrome: 1940–2015. Orphanet J. Rare Dis. 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.E.; Archambault, R.; Mersfelder, T.L. Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: A review of the literature. Am. J. Health Syst. Pharm. 2010, 67, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Umetsu, R.; Mataki, K.; Kato, Y.; Ueda, N.; Nakayama, Y.; Hane, Y.; Matsui, T.; Hatahira, H.; Sasaoka, S. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese Adverse Drug Event Report database. J. Pharm. Health Care Sci. 2016, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.A.; Mudgil, A.V.; Rosmarin, D.M. Toxic epidermal necrolysis. J. Am. Acad. Dermatol. 2007, 56, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Suwarsa, O.; Yuwita, W.; Dharmadji, H.P.; Sutedja, E. Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 2009–2013. Asia Pac. Allergy 2016, 6, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Tay, Y.K. Profile and pattern of Stevens-Johnson syndrome and toxic epidermal necrolysis in a general hospital in Singapore: Treatment outcomes. Acta Derm. Venereol. 2012, 92, 62–66. [Google Scholar] [CrossRef]
- Aroke, D.; Tchouakam, D.N.; Awungia, A.T.; Mapoh, S.Y.; Ngassa, S.N.; Kadia, B.M. Ivermectin induced Steven-Johnsons syndrome: Case report. BMC Res. Notes 2017, 10, 179. [Google Scholar] [CrossRef]
- Abe, R. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Soluble Fas ligand involvement in the pathomechanisms of these diseases. J. Dermatol. Sci. 2008, 52, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Haddad, C.; Sidoroff, A.; Kardaun, S.H.; Mockenhaupt, M.; Creamer, D.; Dunant, A.; Roujeau, J.C. Stevens-Johnson syndrome/toxic epidermal necrolysis: Are drug dictionaries correctly informing physicians regarding the risk? Drug Saf. 2013, 36, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Hung, S.I.; Yang, J.Y.; Su, S.C.; Huang, S.P.; Wei, C.Y.; Chin, S.W.; Chiou, C.C.; Chu, S.C.; Ho, H.C.; et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat. Med. 2008, 14, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, C.; Agustianty, S.; Wang, H.C. Developing a data mining approach to investigate association between physician prescription and patient outcome—A study on re-hospitalization in Stevens-Johnson Syndrome. Comput. Methods Progr. Biomed. 2013, 112, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Brook, U. Stevens-Johnson syndrome and abnormal root development: A case report. Int. J. Paediatr. Dent. 1994, 4, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Brook, U.; Singer, L.; Fried, D. Development of severe Stevens-Johnson syndrome after administration of slow-release theophylline. Pediatr. Dermatol. 1989, 6, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Rzany, B.; Mockenhaupt, M.; Holländer, N.; Stocker, U.; Mueller, J.; Baur, S.; Schöpf, E. Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN): Evaluation for drug risk based on sale numbers in defined daily doses (DDD). Example of the H2-antagonists. Pharmacoepidemiol. Drug Saf. 1995, 4, 207–212. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, J.C.; Huang, D.F.; Huang, Y.S.; Huang, Y.H.; Huo, T.I.; Chang, F.Y.; Lee, S.D. Ranitidine-related Stevens–Johnson syndrome in patients with severe liver diseases: A report of two cases. J. Gastroenterol. Hepatol. 2001, 16, 481–483. [Google Scholar] [CrossRef]
- Mockenhaupt, M.; Viboud, C.; Dunant, A.; Naldi, L.; Halevy, S.; Bavinck, J.N.B.; Sidoroff, A.; Schneck, J.; Roujeau, J.C.; Flahault, A. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J. Investig. Dermatol. 2008, 128, 35–44. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Hong, H.S.; Hsih, M.S.; Yang, L.C.; Ho, H.C.; Wu, J.Y.; Chen, Y.T. Medical genetics: A marker for Stevens-Johnson syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef]
- Hung, S.-I.; Chung, W.-H.; Jee, S.-H.; Chen, W.-C.; Chang, Y.-T.; Lee, W.-R.; Hu, S.-L.; Wu, M.-T.; Chen, G.-S.; Wong, T.-W.; et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet. Genom. 2006, 16, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, Ministry of Health and Welfare. Taiwan International Quality Management Strategy for Drug Quality. Available online: https://www.fda.gov.tw/TC/siteList.aspx?sid=2013 (accessed on 8 March 2017).
- Srikant, R.; Agrawal, R. Mining Sequential Patterns: Generalizations and Performance Improvements; Springer: Berlin/Heidelberg, Germany, 1996; pp. 1–17. [Google Scholar]
- Strom, B.L.; Carson, J.L.; Halpern, A.C.; Schinnar, R.; Snyder, E.S.; Stolley, P.D.; Shaw, M.; Tilson, H.H.; Joseph, M.; Dai, W.S.; et al. Using a claims database to investigate drug-induced Stevens-Johnson syndrome. Stat. Med. 1991, 10, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, F.Y.; Yang, C.L.; Huang, Y.T.; Huang, W.F. Using Taiwan’s national health insurance research databases for pharmacoepidemiology research. J. Food Drug Anal. 2007, 15, 99–108. [Google Scholar]
- Ministry of the Interior, R.O.C. (Taiwan). Monthly Bulletin of Interior Statistics. Available online: https://www.moi.gov.tw/files/site_stuff/321/1/month/month.html (accessed on 6 February 2018).
- Centers for Disease Control and Prevention, U.S. Department of Health & Human Services. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Available online: https://www.cdc.gov/nchs/icd/icd9cm.htm (accessed on 29 June 2016).
- Agrawal, R.; Srikant, R. Mining sequential patterns. In Proceedings of the Eleventh International Conference on Data Engineering, Taipei, Taiwan, 6–10 March 1995; pp. 3–14. [Google Scholar]
- Fournier-Viger, P.; Lin, J.C.W.; Gomariz, A.; Gueniche, T.; Soltani, A.; Deng, Z.H.; Lam, H.T. The SPMF Open-Source Data Mining Library Version 2. In Machine Learning and Knowledge Discovery in Databases, Proceedings of the European Conference, ECML PKdd 2016, Pt. III, Riva del Garda, Italy, 19–23 September 2016; Springer: New York, NY, USA, 2016; Volume 9853, pp. 36–40. [Google Scholar]
| SJS Patients | Population of Taiwan | |||
|---|---|---|---|---|
| Gender | ||||
| Male | 266 | 48.81% | 11,626,351 | 50.47% |
| Female | 279 | 51.19% | 11,410,680 | 49.53% |
| Age | ||||
| 0–14 | 176 | 32.29% | 3,905,203 | 16.95% |
| 15–64 | 194 | 35.60% | 16,729,608 | 72.62% |
| ≧65 | 175 | 32.11% | 2,402,220 | 10.43% |
| 545 | 100.00% | 23,037,031 | 100.00% | |
| Disease Category Code | Disease Category Name |
|---|---|
| 1 | Infectious and parasitic diseases |
| 2 | Neoplasms |
| 3 | Endocrine, nutritional and metabolic diseases and immunity disorders |
| 4 | Diseases of the blood and blood-forming organs |
| 5 | Mental disorders |
| 6 | Diseases of the nervous system and sense organs |
| 7 | Diseases of the circulatory system |
| 8 | Diseases of the respiratory system |
| 9 | Diseases of the digestive system |
| 10 | Diseases of the genitourinary system |
| 11 | Complications of pregnancy, childbirth, and the puerperium |
| 12 | Diseases of the skin and subcutaneous tissue |
| 13 | Diseases of the musculoskeletal system and connective tissue |
| 14 | Congenital anomalies |
| 15 | Certain conditions originating in the perinatal period |
| 16 | Symptoms, signs, and ill-defined conditions |
| 17 | Injury and poisoning |
| 18 | Supplementary classification of external causes of injury and poisoning |
| 19 | Supplementary classification of factors influencing health status and contact with health services |
| Medication Category Code | Medication Category Name |
|---|---|
| A | Aminoglycoside antibiotics |
| B | Carbonic anhydrase inhibitors (CAI) diuretics |
| C | Fluoroquinolones antibiotics |
| D | H2-antagonists for peptic ulcer |
| E | Lincosamides antibiotics |
| F | Nitroimidazole antibiotics |
| G | Penicillin antibiotics |
| H | Theophylline bronchodilators |
| I | Tetracycline antibiotics |
| J | Muscle relaxants |
| K | Anti-tuberculous agents |
| L | Anti-fungal medications |
| M | Anticonvulsant agents |
| P | Erythromycin antibiotics |
| Q | Lipid-lowering medications |
| R | Antihypertensive medications |
| S | Sulfonamides |
| T | Non-steroidal anti-inflammatory agents (NSAIDs) |
| U | Cephalosporin antibiotics |
| Patient ID | Number of Outpatient Visits | Disease Category Codes | Medication Category Codes |
|---|---|---|---|
| P1 | 1 | 8 | K |
| 2 | 3,7,16 | M,T | |
| 3 | 8,10,12 | E | |
| 4 | 8,16,17 | C,T | |
| P2 | 1 | 8,17 | M,T |
| 2 | 8 | T | |
| P3 | 1 | 6,8,15 | H,T |
| 2 | 12 | G | |
| P4 | 1 | 8,12 | D,T |
| 2 | 3,8 | H | |
| 3 | 6,7,9 | T,U | |
| 4 | 8,12 | T |
| Patient ID | Disease Sequence | Medication Sequence |
|---|---|---|
| P1 | <(8) (3 7 16) (8 10 12) (8 16 17)> | <(K) (M T) (E) (C T)> |
| P2 | <(8 17) (8)> | <(M T) (T)> |
| P3 | <(6 8 15) (12)> | <(H T) (G)> |
| P4 | <(8 12) (3 8) (6 7 9) (8 12)> | <(D T) (H) (T U) (T)> |
| Number of Outpatient Visits (Sequential Pattern Length) | Number of SJS Patients |
|---|---|
| 1 | 129 * |
| 2 | 115 * |
| 3 | 107 * |
| 4 | 71 * |
| 5 | 44 |
| 6 | 20 |
| 7 | 22 |
| 8 | 11 |
| 9 | 8 |
| 10 | 6 |
| 11 | 5 |
| 12 | 3 |
| 13 | 2 |
| 15 | 1 |
| 19 | 1 |
| Total | 545 |
| Number of Outpatient Visits | N (%) | Stratified by Age, n (%) | |||
|---|---|---|---|---|---|
| 0–14 | 15–64 | ≥ 65 | p Value | ||
| Pattern length = 1 | 129 | 56 (43.41) | 46 (35.66) | 27 (20.93) | |
| <(8)> | 51 (39.53) | 34 (66.67) | 14 (27.45) | 3 (5.88) | * 0.000 |
| <(12)> | 30 (23.26) | 11 (36.67) | 12 (40.00) | 7 (23.33) | 0.594 |
| <(16)> | 23 (17.83) | 10 (43.48) | 7 (30.43) | 6 (26.09) | 0.654 |
| <(9)> | 17 (13.18) | 5 (29.41) | 7 (41.18) | 5 (29.41) | 0.867 |
| <(7)> | 15 (11.63) | 6 (40.00) | 9 (60.00) | NC | |
| <(6)> | 14 (10.85) | 3 (21.43) | 7 (50.00) | 4 (28.57) | 0.499 |
| Pattern length = 2 | 115 | 39 (33.91) | 43 (37.39) | 33 (28.70) | |
| <(8) (8)> | 23 (20.00) | 16 (69.57) | 6 (26.09) | 1 (4.35) | * 0.000 |
| <(8) (12)> | 14 (12.17) | 12 (85.71) | 2 (14.29) | NC | |
| Pattern length = 3 | 107 | 37 (34.58) | 33 (30.84) | 37 (34.58) | |
| <(8) (8) (8)> | 14 (13.08) | 12 (85.71) | 2 (14.29) | NC | |
| Pattern length = 4 | 71 | 24 (33.80) | 21 (29.58) | 26 (36.62) | |
| <(8) (8) (8) (8)> | 13 (18.31) | 11 (84.62) | 2 (15.38) | NC | |
| Drug-Taken | N (%) | Stratified by Age n (%) | |||
|---|---|---|---|---|---|
| 0–14 | 15–64 | ≥65 | p Value | ||
| Pattern length = 1 Disease patterns: | |||||
| <(8)> Respiratory system | 51 | 34 (66.67) | 14 (27.45) | 3 (5.88) | * 0.000 |
| <(T)> | 32 (62.75) | 23 (71.88) | 8 (25.00) | 1 (3.13) | * 0.000 |
| <(H)> | 9 (17.65) | 6 (66.67) | 2 (22.22) | 1 (11.11) | 0.166 |
| <(G)> | 6 (11.76) | 5 (83.33) | 1 (16.67) | NC | |
| <(12)> Skin and subcutaneous tissue | 30 | 11 (36.67) | 12 (40.00) | 7 (23.33) | 0.594 |
| <(T)> | 11 (36.67) | 2 (18.18) | 7 (63.64) | 2 (18.18) | 0.134 |
| <(U)> | 7 (23.33) | 2 (28.57) | 2 (28.57) | 3 (42.86) | 1 |
| <(L)> | 6 (20.00) | 4 (66.67) | 2 (33.33) | NC | |
| <(G)> | 5 (16.66) | 2 (40.00) | 3 (60.00) | NC | |
| <(D)> | 4 (13.33) | 3 (75.00) | 1 (25.00) | NC | |
| <(I)> | 4 (13.33) | 2 (50.00) | 2 (50.00) | NC | |
| <(T U)> | 3 (10.00) | 2 (66.67) | 1 (33.33) | NC | |
| <(16)> Symptoms, signs, and ill-defined conditions | 23 | 10 (43.48) | 7 (30.43) | 6 (26.09) | 0.654 |
| <(T)> | 16 (69.57) | 7 (43.75) | 6 (37.5) | 3 (18.75) | 0.541 |
| <(U)> | 4 (17.39) | 1 (25.00) | 3 (75.00) | NC | |
| <(9)> Digestive system | 17 | 5 (29.41) | 7 (41.18) | 5 (29.41) | 0.867 |
| <(T)> | 9 (52.94) | 2 (22.22) | 5 (55.56) | 2 (22.22) | 0.531 |
| <(U)> | 3 (17.65) | 3 (100.00) | NC | ||
| <(T U)> | 3 (17.65) | 3 (100.00) | NC | ||
| <(D)> | 2 (11.76) | 1 (50.00) | 1 (50.00) | NC | |
| <(G)> | 2 (11.76) | 1 (50.00) | 1 (50.00) | NC | |
| <(H)> | 2 (11.76) | 1 (50.00) | 1 (50.00) | NC | |
| <(M)> | 2 (11.76) | 1 (50.00) | 1 (50.00) | NC | |
| <(7)> Circulatory system | 15 | 6 (40.00) | 9 (60.00) | NC | |
| <(R)> | 9 (60.00) | 1 (11.11) | 8 (88.89) | NC | |
| <(T)> | 8 (53.33) | 4 (50.00) | 4 (50.00) | NC | |
| <(R T)> | 4 (26.67) | 4 (100.00) | NC | ||
| <(M)> | 3 (20.00) | 2 (66.67) | 1(33.33) | NC | |
| <(J)> | 2 (13.33) | 2 (100.00) | NC | ||
| <(6)> Nervous system and sense organs | 14 | 3 (21.43) | 7 (50.00) | 4 (28.57) | 0.499 |
| <(M)> | 6 (42.86) | 1 (16.67) | 4 (66.67) | 1 (16.67) | 0.383 |
| <(T)> | 6 (42.86) | 1 (16.67) | 2 (33.33) | 3 (50.00) | 0.877 |
| <(U)> | 3 (21.43) | 3 (100.00) | NC | ||
| <(T U)> | 2 (14.29) | 2 (100.00) | NC | ||
| Pattern length = 2 Disease patterns: | |||||
| <(8) (8)> Respiratory system | 23 | 16 (69.57) | 6 (26.09) | 1 (4.35) | * 0.000 |
| <(T) (T)> | 13 (56.52) | 10 (76.92) | 3 (23.08) | NC | |
| <(U) (T)> | 4 (17.39) | 3 (75.00) | 1 (25.00) | NC | |
| <(T U) (T)> | 3 (13.04) | 2 (66.67) | 1 (33.33) | NC | |
| <(8) (12)> Respiratory system, Skin and subcutaneous tissue | 14 | 12 (85.71) | 2 (14.29) | NC | |
| <(T) (T)> | 5 (35.71) | 5 (100.00) | NC | ||
| <(T) (D)> | 3 (21.43) | 2 (66.67) | 1 (33.33) | NC | |
| Pattern length = 3 Disease pattern: | |||||
| <(8) (8) (8)> Respiratory system | 14 | 12 (85.71) | 2 (14.29) | NC | |
| <(T) (T) (T)> | 7 (50.00) | 7 (100.00) | NC | ||
| <(H) (H) (H)> | 2 (15.38) | 2 (100.00) | NC | ||
| Pattern length = 4 Disease pattern: | |||||
| <(8) (8) (8) (8)> Respiratory system | 13 | 11 (84.62) | 2 (15.38) | NC | |
| <(T) (T) (T) (T)> | 3 (23.08) | 3 (100.00) | NC | ||
| <(H) (H) (H) (H)> | 2 (15.38) | 2 (100.00) | NC | ||
| <(H) (H) (T) (T)> | 2 (15.38) | 2 (100.00) | NC | ||
| <(G T) (T) (T) (T)> | 2 (15.38) | 2 (100.00) | NC | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou-Yang, C.; Chou, S.-C.; Juan, Y.-C.; Wang, H.-C. Mining Sequential Patterns of Diseases Contracted and Medications Prescribed before the Development of Stevens-Johnson Syndrome in Taiwan. Appl. Sci. 2019, 9, 2434. https://doi.org/10.3390/app9122434
Ou-Yang C, Chou S-C, Juan Y-C, Wang H-C. Mining Sequential Patterns of Diseases Contracted and Medications Prescribed before the Development of Stevens-Johnson Syndrome in Taiwan. Applied Sciences. 2019; 9(12):2434. https://doi.org/10.3390/app9122434
Chicago/Turabian StyleOu-Yang, Chao, Shih-Chung Chou, Yeh-Chun Juan, and Han-Cheng Wang. 2019. "Mining Sequential Patterns of Diseases Contracted and Medications Prescribed before the Development of Stevens-Johnson Syndrome in Taiwan" Applied Sciences 9, no. 12: 2434. https://doi.org/10.3390/app9122434
APA StyleOu-Yang, C., Chou, S.-C., Juan, Y.-C., & Wang, H.-C. (2019). Mining Sequential Patterns of Diseases Contracted and Medications Prescribed before the Development of Stevens-Johnson Syndrome in Taiwan. Applied Sciences, 9(12), 2434. https://doi.org/10.3390/app9122434





