Evaluation of Quality of Eggs from Hens Kept in Caged and Free-Range Systems Using Traditional Methods and Ultra-Weak Luminescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Methodology
2.2. Raw Material
2.3. Determination of Tocopherols and Carotenoids
2.4. Quantification of Color
2.5. Haugh Unit Calculation
- : height of the albumen (mm),
- : weight of egg (g).
2.6. pH Measurement
2.7. Registration of Ultra-Weak Photonic Emission
- —calibration coefficient—if the calibration factor is in the range of (0.8–1), the measuring system is considered to be efficient and ready for measurement.
- —number of photons counted in the interval of 500 s, with reference forcing,
- —number of photons registered in an empty lightproof chamber,
- —known dose used for calibration (400 photons).
2.8. Statistical Analysis
3. Results and Discussion
3.1. Biochemical Measurement
3.2. Photon Emission
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krawczyk, J. Effect of layer age and egg production level on changes in quality of eggs from hens of conservation breeds and commercial hybrids. Ann. Anim. Sci. 2009, 9, 185–193. [Google Scholar]
- Dudek, M.; Rabsztyn, A. Egg quality of dual-purpose hens intended for small-scale farming. Acta Sci. Pol. Zootech. 2011, 10, 3–12. [Google Scholar]
- Wang, J.; Yue, H.; Wu, S.; Zhang, H.; Qi, G. Nutritional modulation of health, egg quality and environmental pollution of the layers. Anim. Nutr. 2017, 3, 91–96. [Google Scholar] [CrossRef]
- Batkowska, J.; Brodacki, A. Selected quality traits of eggs and the productivity of newly created laying hen hybrids dedicated to an extensive rearing system. Arch. Anim. Breed. 2017, 60, 87–93. [Google Scholar] [CrossRef]
- Şekeroğlu, A.; Altuntaş, E. Effects of egg weight on egg quality Characteristics. J. Sci. Food Agric. 2009, 89, 379–383. [Google Scholar] [CrossRef]
- Shin, D.; Narciso-Gaytán, C.; Regenstein, J.M.; Sánchez-Plata, M.X. Effect of various refrigeration temperatures on quality of shell eggs. J. Sci. Food Agric. 2012, 92, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Batkowska, J.; Brodacki, A.; Knaga, S. Quality of Laying Hen Eggs During Storage Depending on Egg Weight and Type of Cage System (Conventional vs. Furnished Cages). Ann. Anim. Sci. 2014, 3, 707–719. [Google Scholar] [CrossRef]
- Wang, X.C.; Wu, S.; Zhang, H.; Yue, H.; Qi, G.; Li, J. Effect of dietary protein sources and storage temperatures on egg internal quality of stored shell eggs. Anim. Nutr. 2015, 1, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Oziemblowski, M.; Dróżdż, M.; Kiełbasa, P.; Dróżdż, T.; Gąsiorski, A.; Nawara, P.; Tabor, S. Ultra weak luminescence (USL) as a potential method for evaluating the quality of traditional food. Przeglad Elektrotechniczny 2017, 94, 104–107. [Google Scholar]
- Borc, R.; Jaśkowska, A.; Dudziak, A. Ultra-Low Photon Emission from Living Systems; Lublin University of Technology: Lublin, Poland, 2015. [Google Scholar]
- Laager, F.M.; Becker, N.M.; Park, S.H.; Soh, K.S. Effects of Lac operon activation, deletion of the Yhha gene, and the removal of oxygen on the ultraweak photon emission of Escherichia coli. Electromagn. Biol. Med. 2009, 28, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Nerudová, M.; Červinková, K.; Hašek, J.; Cifra, M. Optical spectral analysis of ultra-weak photon emission from tissue culture and yeast cells. Proc. SPIE 2015, 9450. [Google Scholar] [CrossRef]
- Van Wijk, E.P.A.; Van der Greef, J.; Van Wijk, R. Photon counts statistics in leukocyte cell dynamics. J. Phys. Conf. Ser. 2011, 329. [Google Scholar] [CrossRef]
- Salari, V.; Valian, H.; Bassereh, H.; Bókkon, I.; Barkhordari, A. Ultraweak photon emission in the brain. J. Integr. Neurosci. 2015, 14, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Kobayashi, M.; Inaba, H. Delayed Fluorescence and Ultraweak Light Emission from Isolated Chloroplasts (Comparison of Emission Spectra and Concentration Dependence). Plant Cell Physiol. 1992, 33, 689–693. [Google Scholar]
- Karbowski, L.M.; Murugan, N.J.; Dotta, B.T.; Persinger, M.A. Only 1% Melanoma Proportion in Non-Malignant Cells Exacerbates Photon Emissions: Implications for Tumor Growth and Metastases. Int. J. Cancer Res. Mol. Mech. 2015, 1. [Google Scholar] [CrossRef]
- Kim, J.D.; Lim, J.; Sung, B.; Soh, K.S. Biophoton Emission from Rat Liver. J. Korean Phys. Soc. 2003, 42, 427–430. [Google Scholar]
- Zhang, J.G.; Zhong, L.F.; Zhang, M.; Xia, Y.X. Protection effects of procaine on oxidative stress and toxicities of renal cortical slices from rats caused by cisplatin in vitro. Arch. Toxicol. 1992, 66, 354–358. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takeda, M.; Sato, T.; Yamazaki, Y.; Kaneko, K.; Ito, K.-I.; Kato, H.; Inaba, H. In vivo imaging of spontaneous ultraweak photon emission from a rat’s brain correlated with cerebral energy metabolism and oxidative stress. Neurosci. Res. 1999, 34, 103–113. [Google Scholar] [CrossRef]
- Hossu, M.; Ma, L.; Zou, X.; Chen, W. Enhancement of biophoton emission of prostate cancer cells by Ag nanoparticles. Cancer Nanotechnol. 2013, 4, 21–26. [Google Scholar] [CrossRef]
- Gocławski, J.; Sekulska-Nalewajko, J.; Korzeniewska, E.; Piekarska, A. The use of optical coherence tomography for the evaluation of textural changes of grapes exposed to pulsed electric field. Comput. Electron. Agric. 2017, 142, 29–40. [Google Scholar] [CrossRef]
- Gliszczynska-Świgło, A.; Sikorska, E. Simple reversed-phase liquid chromatography method for determination of tocopherols in edible plant oils. J. Chromatogr. 2004, 1048, 195–198. [Google Scholar] [CrossRef]
- Ruth, S.; Alewijn, M.; Rogers, K.; Newton-Smith, E.; Tena, N.; Bollen, M.; Koot, A. Authentication of organic and conventional eggs by carotenoid profiling. Food Chem. 2011, 126, 1299–1305. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Aboonajmi, M.; Amir, S.; Najafabadi, T.A.; Kondo, N. Quality Assessment of Poultry Egg Based on Visible–Near Infrared Spectroscopy and Radial Basis Function Networks. Int. J. Food Prop. 2016, 19, 1163–1172. [Google Scholar] [CrossRef]
- Karadas, F.; Grammenidis, E.; Surai, P.F.; Acamovic, T.; Sparks, N.H.C. Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. Br. Poult. Sci. 2006, 47, 561–566. [Google Scholar] [CrossRef]
- Lokaewmanee, K.; Yamauchi, K.; Okuda, N. Effects of dietary red pepper on egg yolk colour and histological intestinal morphology in laying hens. J. Anim. Physiol. Anim. Nutr. 2009, 97, 986–995. [Google Scholar] [CrossRef]
- Islam, K.M.S.; Khalil, M.; Männer, K.; Raila, J.; Rawel, H.; Zentek, J.; Schweigert, F.J. Lutein specific relationships among some spectrophotometric and colorimetric parameters of chicken egg yolk. J. Poult. Sci. 2017, 54, 271–277. [Google Scholar] [CrossRef]
- Lewko, L.; Gornowicz, E. Effect of Housing System on Egg Quality in Laying Hensvol. Ann. Anim. Sci. 2011, 11, 607–616. [Google Scholar] [CrossRef]
- Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Carotenoids in avian nutrition and embryonic development. 1. Absorption, availability and levels in plasma and egg yolk. J. Poult. Sci. 2001, 38, 1–27. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, I.V.; Karadas, F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016, 1, 1–11. [Google Scholar] [CrossRef]
- Hammershøj, M.; Steenfeldt, S. Organic egg production. II: The quality of organic eggs is influenced by hen genotype, diet and forage material analyzed by physical parameters, functional properties and sensory evaluation. Anim. Feed Sci. Technol. 2015, 208, 182–197. [Google Scholar] [CrossRef]
- Schlatterer, J.; Breithaupt, D.E. Xanthophylls in Commercial Egg Yolks Quantification and Identification by HPLC and LC-(APCI)MS Using a C30 Phase. J. Agric. Food Chem. 2006, 54, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Karadas, F.; Møller, A.P.; Karageçili, M.R. A comparison of fat-soluble antioxidants in wild and farm-reared chukar partridges (Alectoris chukar). Comp. Biochem. Physiol. 2017, 208, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kiełbasa, P.; Dróżdż, T.; Nawara, P.; Dróżdż, M. The use of bio-photons emission for the quality parameterization of food products. Przeglad Elektrotechniczny 2017, 93, 153–156. [Google Scholar]
- Trzyniec, K.; Kiełbasa, P.; Oziemblowski, M.; Dróżdż, M.; Nawara, P.; Posyłek, Z.; Leja, R. Using photons emission to evaluate the quality of apples. Przeglad Elektrotechniczny 2017, 93, 183–186. [Google Scholar]
- Becker, W. Fluorescence lifetime imaging by multi-dimensional time correlated single photon counting. Med. Photonics 2015, 27, 41–61. [Google Scholar] [CrossRef]
- Narayana, P.S.; Varalakshmi, D.; Pullaiah, T. Research Methodology in Plant Science; Scientific Publishers: Jodhpur, India, 2016. [Google Scholar]
- De Haas, J.T.M.; Dorenbos, P. Methods for Accurate Measurement of the Response of Photomultiplier Tubes and Intensity of Light Pulses. IEEE Trans. Nucl. Sci. 2011, 58, 1290–1296. [Google Scholar] [CrossRef]
- Usa, M.; Kobayashi, M.; Scott, R.Q.; Hiratsuka, R.; Maeda, T.; Inaba, H. Ultraweak biophoton emission and bioelectrical activity in plant tissues. In Biological Luminescence; World Scientific Publishing: Singapore, 1990; pp. 117–130. [Google Scholar]
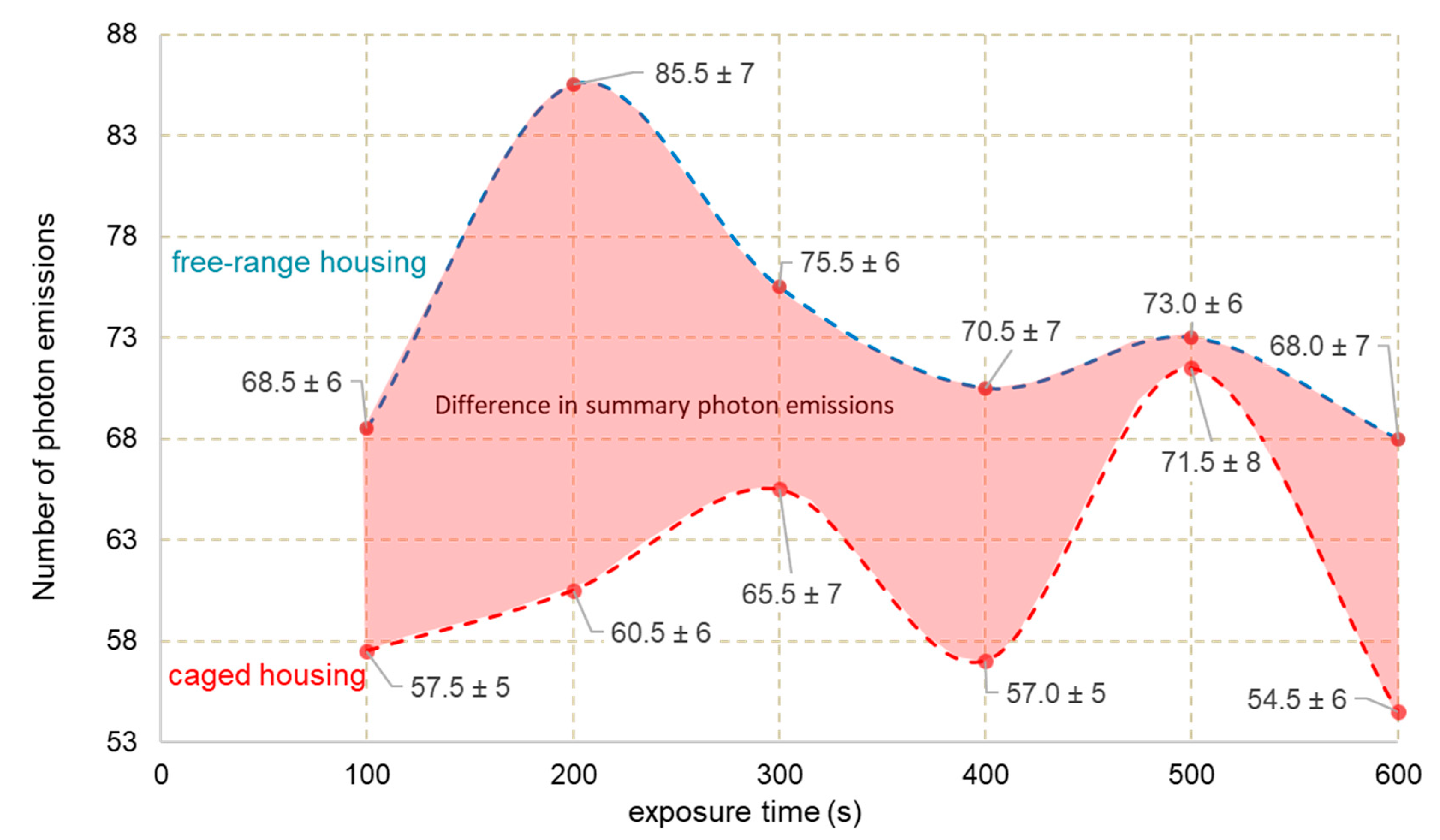
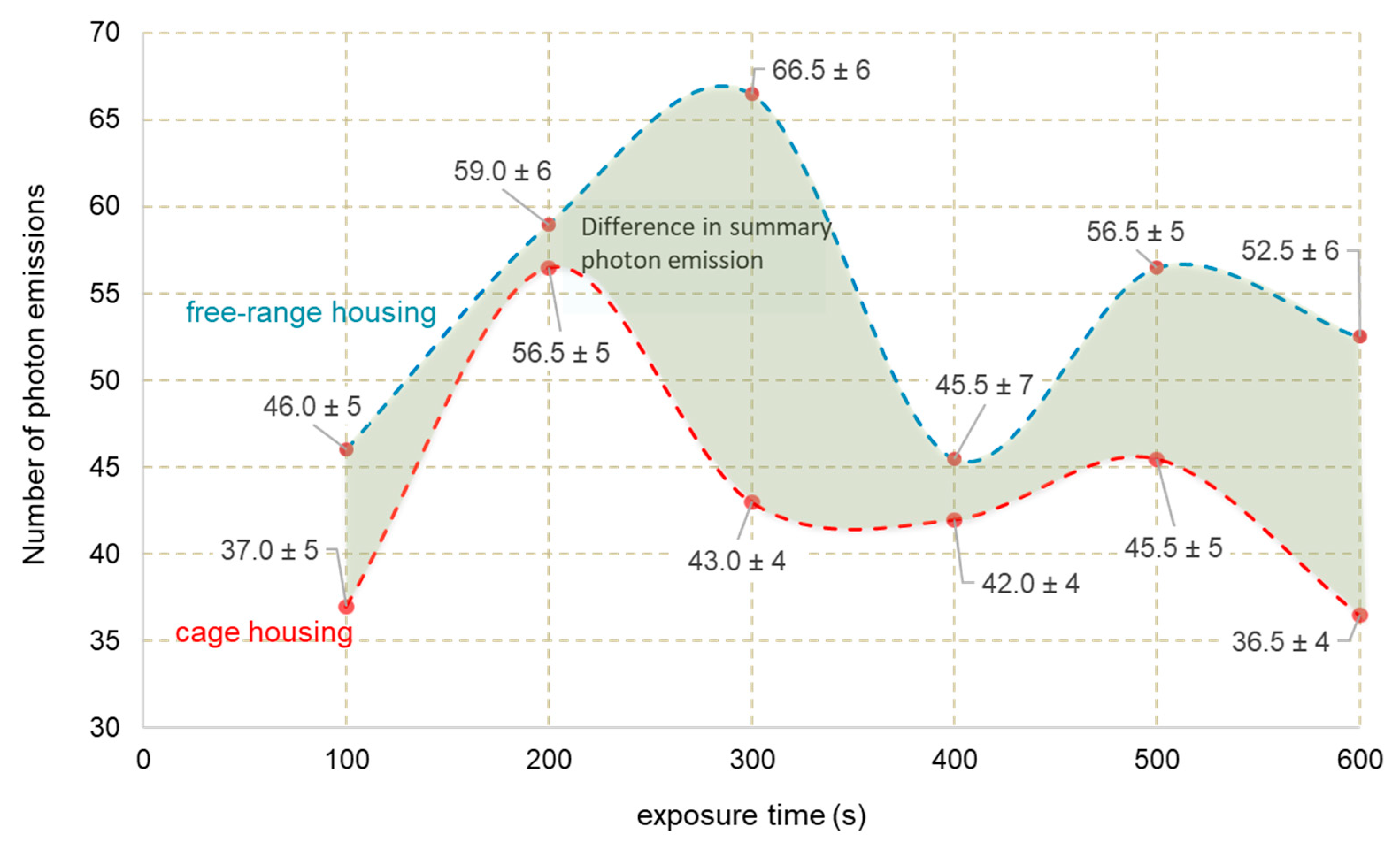
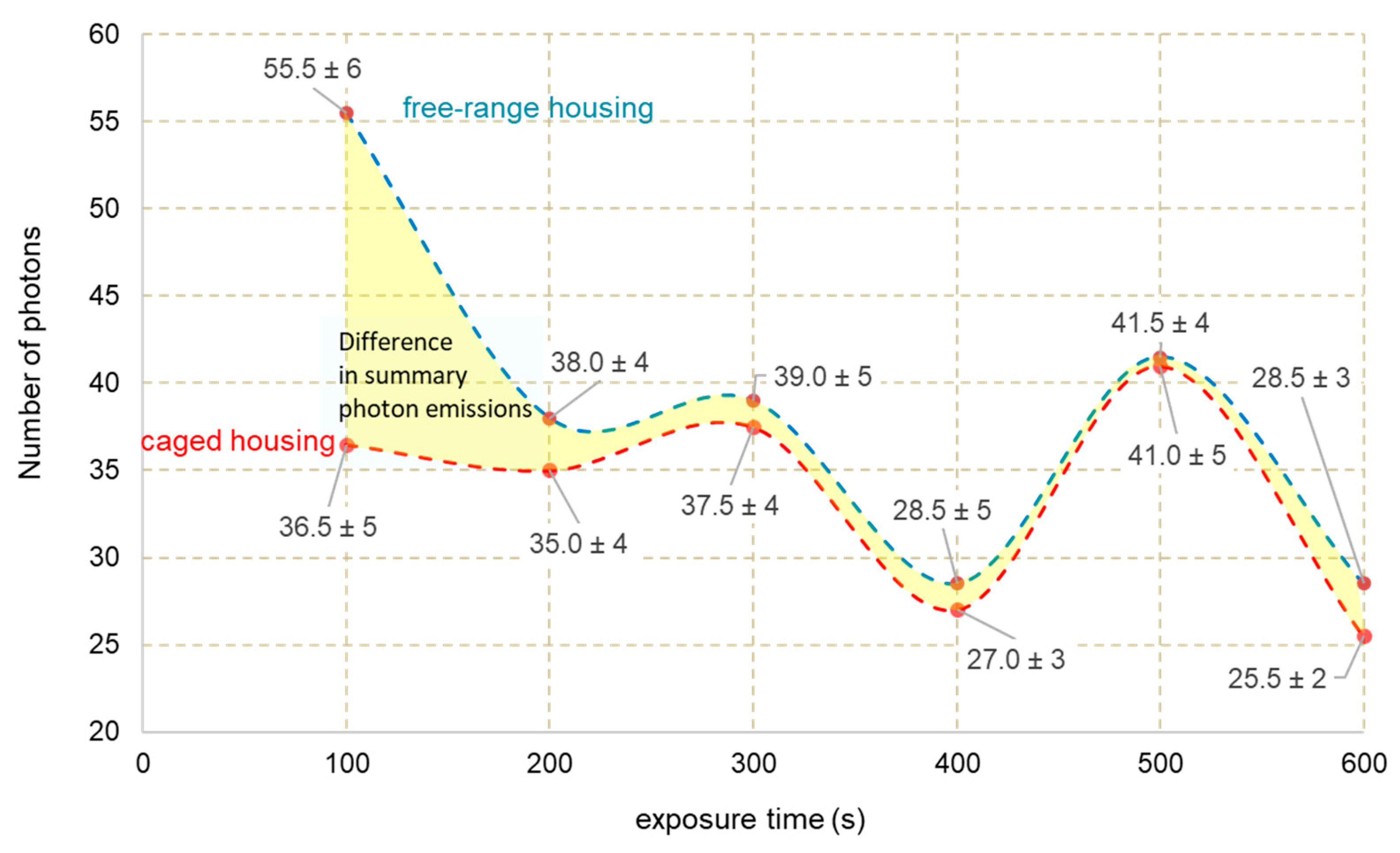
| Features | Eggs C | Eggs F |
|---|---|---|
| egg yolk | ||
| Color of yolk | 8.00 ± 0.30 | 9.00 ± 0.20 |
| pH | 6.12 ± 0.20 | 6.07 ± 0.08 |
| egg albumen | ||
| Haugh units | 84.60 ± 0.59 | 78.60 ± 0.36 |
| pH | 8.11 ± 0.22 | 8.19 ± 0.38 |
| Sample | Carotenoids | Total | |||||
|---|---|---|---|---|---|---|---|
| Unknown Pigment | Lutein | Zeaxanthin | β-Carotene | ||||
| Eggs F | 0.7 ± 0.1 | 34.2 a ± 1.3 | 4.3 a ± 0.1 | nd | 39.1 a ± 1.4 | ||
| Eggs C | nd | 5.7 b ± 0.2 | 4.4 a ± 0.1 | 1.1 ± 0.0 | 11.2 b ± 0.2 | ||
| Tocopherol | |||||||
| α | β + γ | Δ | |||||
| Eggs F | 77.8 a ± 1.5 | 5.2 a ± 0.1 | 0.8 a ± 0.0 | 83.7 a ± 1.7 | |||
| Eggs C | 89.9 b ± 2.4 | 1.7 b ± 0.0 | 0.3 b ± 0.0 | 91.3 b ± 2.5 | |||
| Sample | Yolk Color Parameters | ||
|---|---|---|---|
| L* (C) | a* (C) | b* (C) | |
| Eggs F | 51.61 a ± 2.45 | 3.36 a ± 0.15 | 45.69 a ± 1.79 |
| Eggs C | 49.60 a ± 2.27 | 2.93 b ± 0.74 | 40.05 b ± 1.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałązka-Czarnecka, I.; Korzeniewska, E.; Czarnecki, A.; Sójka, M.; Kiełbasa, P.; Dróżdź, T. Evaluation of Quality of Eggs from Hens Kept in Caged and Free-Range Systems Using Traditional Methods and Ultra-Weak Luminescence. Appl. Sci. 2019, 9, 2430. https://doi.org/10.3390/app9122430
Gałązka-Czarnecka I, Korzeniewska E, Czarnecki A, Sójka M, Kiełbasa P, Dróżdź T. Evaluation of Quality of Eggs from Hens Kept in Caged and Free-Range Systems Using Traditional Methods and Ultra-Weak Luminescence. Applied Sciences. 2019; 9(12):2430. https://doi.org/10.3390/app9122430
Chicago/Turabian StyleGałązka-Czarnecka, Ilona, Ewa Korzeniewska, Andrzej Czarnecki, Michał Sójka, Paweł Kiełbasa, and Tomasz Dróżdź. 2019. "Evaluation of Quality of Eggs from Hens Kept in Caged and Free-Range Systems Using Traditional Methods and Ultra-Weak Luminescence" Applied Sciences 9, no. 12: 2430. https://doi.org/10.3390/app9122430
APA StyleGałązka-Czarnecka, I., Korzeniewska, E., Czarnecki, A., Sójka, M., Kiełbasa, P., & Dróżdź, T. (2019). Evaluation of Quality of Eggs from Hens Kept in Caged and Free-Range Systems Using Traditional Methods and Ultra-Weak Luminescence. Applied Sciences, 9(12), 2430. https://doi.org/10.3390/app9122430







