Volatile Secondary Metabolites with Potent Antidiabetic Activity from the Roots of Prangos pabularia Lindl.—Computational and Experimental Investigations
Abstract
1. Introduction
2. Results and Discussions
2.1. Chemical Composition of Essential Oils
2.2. NMR Data of Osthole
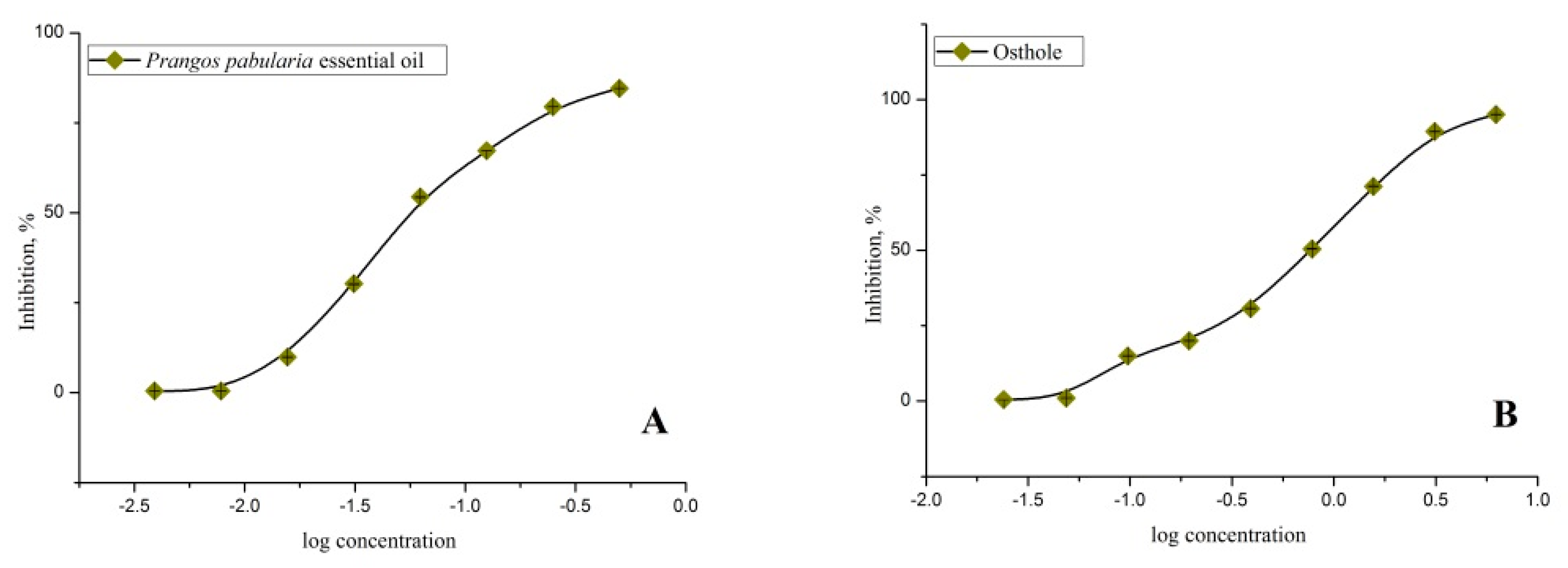
2.3. Antidiabetic Activity of Essential Oil and Isolated Compound (Osthole)
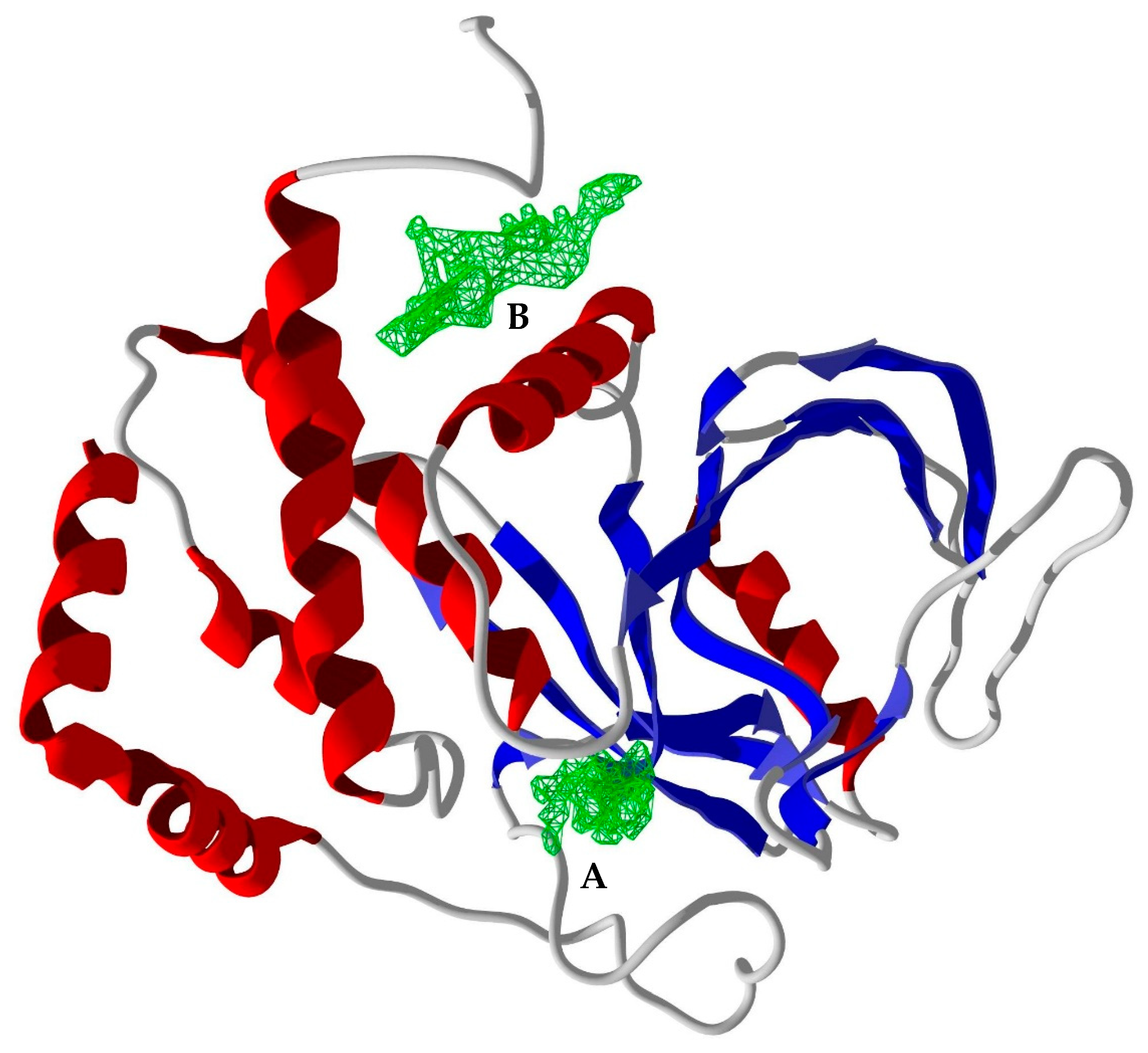
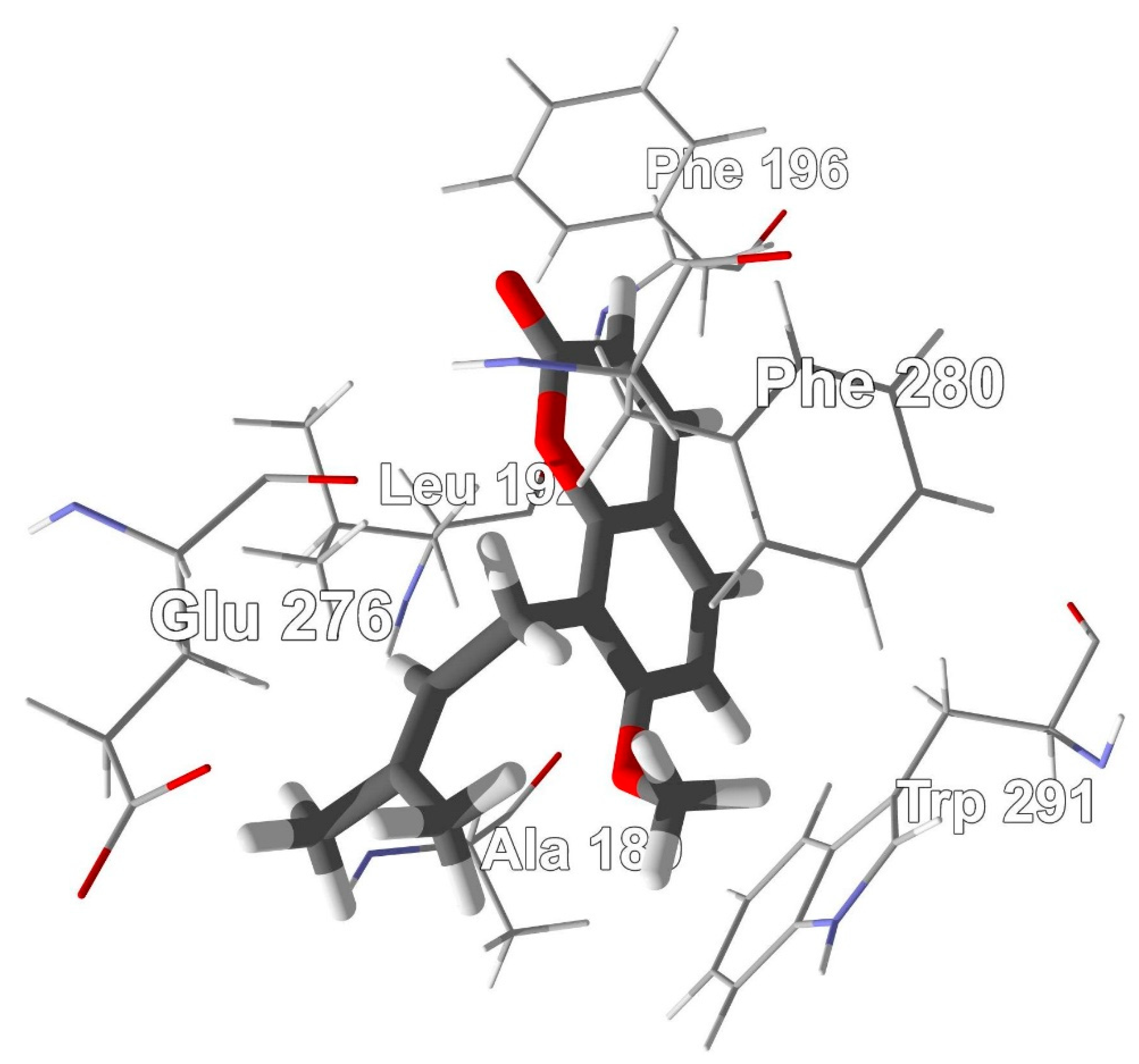
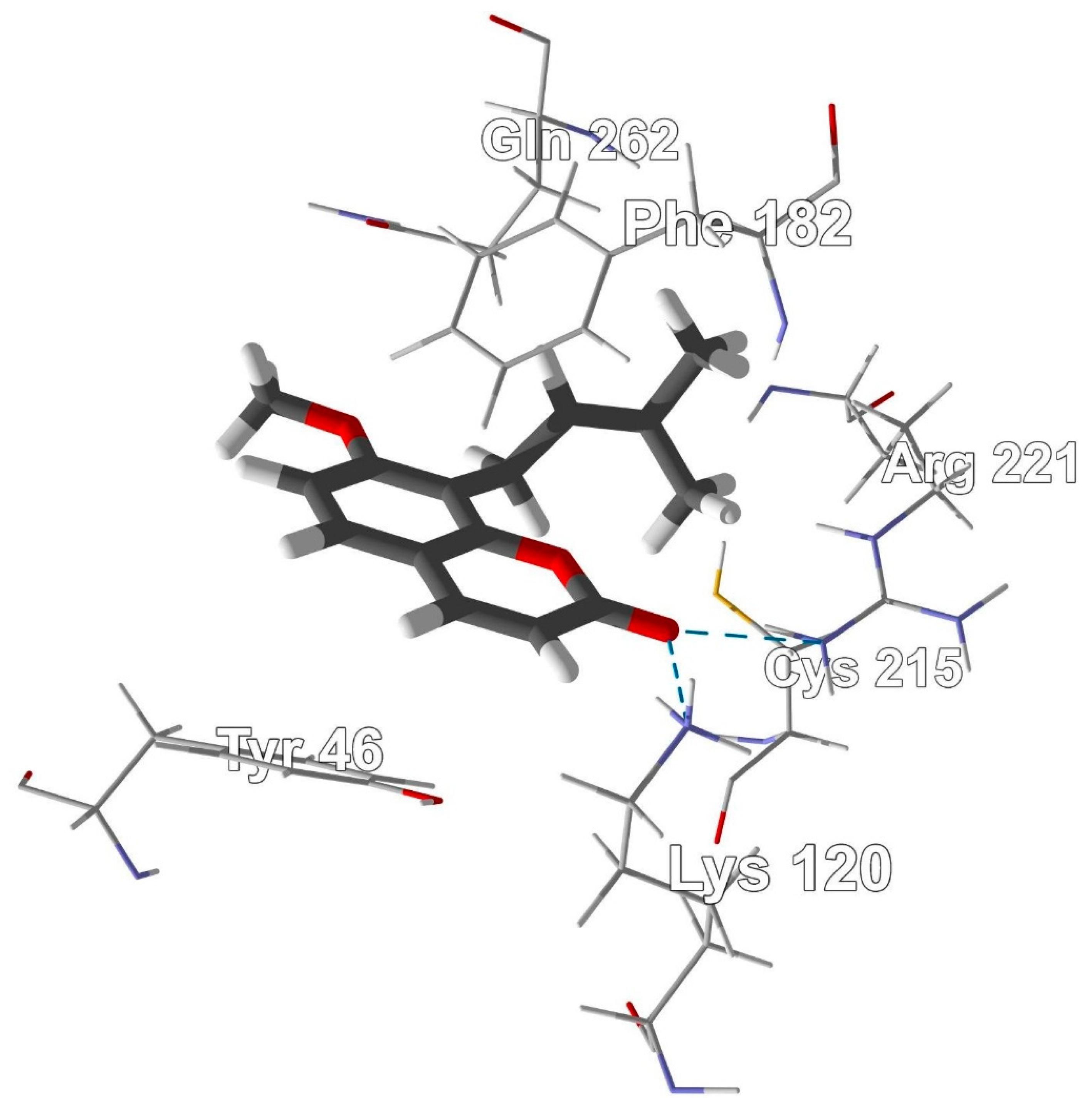
2.4. Molecular Docking
3. Materials and Methods
3.1. Plant Material
3.2. Gas Chromatography
3.3. Gas Chromatographic-Mass Spectral Analysis
3.4. NMR and HR-ESIMS Analysis
3.5. Antidiabetic Activity: PTP-1B Enzymatic Assay
3.6. Molecular Docking
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. About Diabetes; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Sharopov, F.S.; Zhang, H.; Wink, M.; Setzer, W.N. Aromatic medicinal plants from Tajikistan (Central Asia). Medicines 2015, 2, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.; Setzer, W.N. Medicinal plants of Tajikistan. In Vegetation of Central Asia and Environs; Egamberdieva, D., Öztürk, M., Eds.; Springer: Basel, Switzerland, 2018; pp. 163–210. [Google Scholar]
- Sharopov, F. Phytochemistry and Bioactivities of Selected Plant Species with Volatile Secondary Metabolites. Ph.D. Thesis, University of Heidelberg, Heidelberg, Germany, 2015. [Google Scholar]
- Yen, H.-F.; Hsieh, C.-T.; Hsieh, T.-J.; Chang, F.-R.; Wang, C.-K. In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J. Food Drug Anal. 2015, 23, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Meshkani, R.; Taghikhani, M.; Al-Kateb, H.; Larijani, B.; Khatami, S.; Sidiropoulos, G.K.; Hegele, R.A.; Adeli, K. Polymorphisms within the protein tyrosine phosphatase 1B (PTPN1) gene promoter: Functional characterization and association with type 2 diabetes and related metabolic traits. Clin. Chem. 2007, 53, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Numonov, S.; Edirs, S.; Bobakulov, K.; Qureshi, M.N.; Bozorov, K.; Sharopov, F.; Setzer, W.N.; Zhao, H.; Habasi, M.; Sharofova, M.; et al. Evaluation of the antidiabetic activity and chemical composition of Geranium collinum root extracts—Computational and experimental investigations. Molecules 2017, 22, 983. [Google Scholar] [CrossRef] [PubMed]
- Tonks, N.K.; Neel, B.G. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 2001, 13, 182–195. [Google Scholar] [CrossRef]
- Bakke, J.; Haj, F.G. Protein-tyrosine phosphatase 1B substrates and metabolic regulation. Semin. Cell Dev. Biol. 2015, 37, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Venkataraghavan, R.; Brindha, P.; Ivo, R.S. A review on protein tyrosine phosphatases—an important target for various diseases. Asian J. Pharm. Clin. Res. 2018, 11, 11–16. [Google Scholar]
- Florez, J.C.; Agapakis, C.M.; Burtt, N.P.; Sun, M.; Almgren, P.; Rastam, L.; Tuomi, T.; Gaudet, D.; Hudson, T.J.; Daly, M.J.; et al. Association testing of the protein tyrosine phosphatase 1B gene (PTPN1) with type 2 diabetes in 7,883 people. Diabetes 2005, 54, 1884–1891. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verma, M.; Gupta, S.J.; Chaudhary, A.; Garg, V.K. Protein tyrosine phosphatase 1B inhibitors as antidiabetic agents—A brief review. Bioorg. Chem. 2017, 70, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Jumaev, B.B.; Mirzoev, B.; Nigmonov, M.; Safarov, E.H.; Abdullaev, A.; Karimov, K.H. Physiological and biochemical characteristics leaf different layers of vegetative and generative shoots Prangos pabularia (Prangos pabularia Lindl.). Rep. Acad. Sci. Repub. Tajikistan 2014, 57, 695–700. [Google Scholar]
- Sadikov, Y.J. Medicinal plant of Tajikistan. News Tajik Acad. Sci. 2003, 5, 41–48. [Google Scholar]
- Razavi, S.M. Chemical composition and some allelopathic aspects of essential oils of (Prangos ferulacea L.) Lindl at different stages of growth. J. Agric. Sci. Technol. 2012, 14, 349–356. [Google Scholar]
- Shokoohinia, Y.; Hosseinzadeh, L.; Alipour, M.; Mostafaie, A.; Mohammadi-Motlagh, H.-R. Comparative evaluation of cytotoxic and apoptogenic effects of several coumarins on human cancer cell lines: Osthole induces apoptosis in p53-deficient H1299 cells. Adv. Pharmacol. Sci. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, K.; Hamedeyazdan, S.; Hodaei, D.; Fathiazad, F. An in vitro ethnopharmacological study on Prangos ferulacea: A wound healing agent. BioImpacts 2017, 7, 75–82. [Google Scholar] [CrossRef][Green Version]
- Başer, K.H.; Demirci, B.; Demirci, F.; Bedir, E.; Weyerstahl, P.; Marschall, H.; Duman, H.; Aytaç, Z.; Hamann, M.T. A new bisabolene derivative from the essential oil of Prangos uechtritzii fruits. Planta Medica 2000, 66, 674–677. [Google Scholar] [CrossRef]
- Özek, G.; Bedir, E.; Tabanca, N.; Ali, A.; Khan, I.A.; Duran, A.; Başer, K.H.C.; Özek, T. Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils. Open Chem. 2018, 16, 453–467. [Google Scholar]
- Tabanca, N.; Wedge, D.E.; Li, X.C.; Gao, Z.; Ozek, T.; Bernier, U.R.; Epsky, N.D.; Baser, K.H.; Ozek, G. Biological evaluation, overpressured layer chromatography separation, and isolation of a new acetylenic derivative compound from Prangos platychlaena ssp. platychlaena fruit essential oils. J. Planar Chromatogr.—Mod. TLC 2018, 31. [Google Scholar] [CrossRef]
- Numonov, S.; Bobakulov, K.; Numonova, M.; Sharopov, F.S.; Setzer, W.N.; Khalilov, Q.; Begmatov, N.; Habasi, M.; Aisa, H.A. New coumarin from the roots of Prangos pabularia. Nat. Prod. Res. 2017, 32, 2325–2332. [Google Scholar] [CrossRef]
- Ozek, G.; Ozek, T.; Iscan, G.; Baser, K.H.C.; Hamzaoglu, E.; Duran, A. Comparison of hydrodistillation and microdistillation methods for the analysis of fruit volatiles of Prangos pabularia Lindl., and evaluation of its antimicrobial activity. S. Afr. J. Bot. 2007, 73, 563–569. [Google Scholar] [CrossRef][Green Version]
- Tabanca, N.; Tsikolia, M.; Ozek, G.; Ozek, T.; Abbas, A.; Bernier, U.R.; Duran, A.; Baser, K.H.; Khan, I.A. The identification of suberosin from Prangos pabularia essential oil and its mosquito activity against Aedes aegypti. Rec. Nat. Prod. 2016, 10, 311–325. [Google Scholar]
- Razavi, S.M. Chemical and allelopathic analyses of essential oils of Prangos pabularia Lindl. from Iran. Nat. Prod. Res. 2011, 26, 2148–2151. [Google Scholar] [PubMed]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Meshkatalsadat, M.H.; Bamoniri, A.; Batooli, H. The bioactive and volatile constituents of Prangos acaulis (DC) Bornm extracted using hydrodistillation and nanoscale injection techniques. Dig. J. Nanomater. Biostruct. 2010, 5, 263–266. [Google Scholar]
- Sajjadi, S.E.; Mehregan, I. Chemical composition of the essential oil of Prangos asperula Boiss. subsp. haussknechtii (Boiss.) Herrnst. et Heyn fruits. DARU J. Pharm. Sci. 2003, 11, 23–28. [Google Scholar]
- Mneimne, M.; Baydoun, S.; Nemer, N.; Apostolides, N.A. Chemical composition and antimicrobial activity of essential oils isolated from aerial parts of Prangos asperula Boiss. (Apiaceae) growing wild in Lebanon. Med. Aromat. Plants 2016, 5, 2–5. [Google Scholar]
- Sajjadi, S.E.; Zeinvand, H.; Shokoohinia, Y. Isolation and identification of osthol from the fruits and essential oil composition of the leaves of Prangos asperula Boiss. Res. Pharm. Sci. 2009, 4, 19–23. [Google Scholar]
- Lingan, K. A review on major constituents of various essential oils and its application. J. Transl. Med. (Sunnyvale) 2018, 8, 1000201. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Ermin, N.; Adigüzel, N.; Aytaç, Z. Composition of the essential oil of Prangos ferulacea (L.) Lindl. J. Essent. Oil Res. 2011, 8, 297–298. [Google Scholar] [CrossRef]
- Massumi, M.A.; Fazeli, M.R.; Alavi, S.H.R.; Ajani, Y. Chemical constituents and antibacterial activity of essential oil of Prangos ferulacea (L.) Lindl. fruits. Iran. J. Pharm. Sci. 2007, 3, 171–176. [Google Scholar]
- Delnavazi, M.R.; Soleimani, M.; Hadjiakhoondi, A.; Yass, N. Isolation of phenolic derivatives and essential oil analysis of Prangos ferulacea (L.) Lindl. aerial parts. Iran. J. Pharm. Res. 2017, 16, 207–215. [Google Scholar] [PubMed]
- Ercan, S.F.; Bas, H.; Koc, M.; Pandir, D.; Öztemiz, S. Insecticidal activity of essential oil of Prangos ferulacea (Umbelliferae) against Ephestia kuehniella (Lepidoptera: Pyralidae) and Trichogramma embryophagum (Hymenoptera: Trichogrammatidae). Turkish J. Agric. For. 2013, 37, 719–725. [Google Scholar] [CrossRef]
- Mohibi, Z.; Heshmati, G.A.; Sefidkon, F.; Chahouki, Z.M.A. The influence of plant growth satge, individuals of species, and extraction methods on the essential oil content and the chemical composition of Prangos ferulacea (L.) Lindl. Appl. Ecol. Environ. Res. 2017, 15, 1765–1776. [Google Scholar] [CrossRef]
- Amiri, H. Chemical composition and antibacterial activity of essential oil of Prangos ferulacea (L.) Lindl. J. Med. Plants 2007, 1, 36–41. [Google Scholar]
- Razavi, S.M.; Nazemiyeh, H.; Zarrini, G.; Asna-Asharii, S.; Dehghan, G. Chemical composition and antimicrobial activity of essential oil of Prangos ferulaceae (L.) Lindl from Iran. Nat. Prod. Res. 2009, 24, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Başer, K.H.C.; Özek, T.; Demirci, B.; Duman, H. Composition of the essential oil of Prangos heyniae H. Duman et M. F. Watson, a new endemic from Turkey. Flavour Fragr. J. 2000, 15, 47–49. [Google Scholar] [CrossRef]
- Kiliç, Ö.; Bengü, A.Ş.; Özdemir, F.A.; Çelik, Ş. Essential oil composition of two Prangos Lindl. (Apiaceae) species from Turkey. Prog. Nutr. 2017, 19, 69–74. [Google Scholar]
- Abolghasemi, M.M.; Piryaei, M. Fast determination of Prangos uloptera essential oil by nanoporous silica-polypyrrole SPME fiber. Chemija 2012, 23, 244–249. [Google Scholar]
- Sefidkon, F.N. Chemical composition of the oil of Prangos uloptera DC. J. Essent. Oil Res. 2001, 13, 84–85. [Google Scholar] [CrossRef]
- Alikhah-Asl, M.; Azarnivand, H.; Jafari, M.; Arzani, H.; Amin, G.; Zare-Chahouki, M.A. Variations of essential oils in fresh and dried aerial parts of Prangos uloptera. J. Nat. Prod. 2012, 5, 5–9. [Google Scholar]
- Hayta, Ş. Essential oil composition of the fruit of Prangos uloptera (Apiaceae) DC. from Turkey. Pharm. Chem. J. 2018, 5, 1–5. [Google Scholar]
- Figueroa, M.; Rivero-Cruz, I.; Rivero-Cruz, B.; Bye, R.; Navarrete, A.; Mata, R. Constituents, biological activities and quality control parameters of the crude extract and essential oil from Arracacia tolucensis var. multifida. J. Ethnopharmacol. 2007, 113, 125–131. [Google Scholar] [CrossRef]
- Wang, H.; Gu, D.; Wang, M.; Guo, H.; Wu, H.; Tian, G.; Li, Q.; Yang, Y.; Tian, J. A strategy based on gas chromatography-mass spectrometry and virtual molecular docking for analysis and prediction of bioactive composition in natural product essential oil. J. Chromatogr. A 2017, 1501, 128–133. [Google Scholar] [CrossRef]
- Saifudin, A.; Tanaka, K.; Kadota, S.; Tezuka, Y. Chemical constituents of Blumea balsamifera of Indonesia and their protein tyrosine phosphatase 1B inhibitory activity. Nat. Prod. Commun. 2012, 7, 815–818. [Google Scholar] [CrossRef]
- Wu, W.B.; Huan, Z.; Dong, S.; Yue, M. New triterpenoids with protein tyrosine phosphatase 1B inhibition from Cedrela odorata. Asian Nat. Prod. Res. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Bharti, S.K.; Kumar, A.; Prakash, O.; Krishnan, S.; Gupta, A.K. Essential oil of Cymbopogon citratus against diabetes: Validation by in vivo experiments and computational studies. J. Bioanal. Biomed. 2013, 5, 194–203. [Google Scholar]
- Zhang, Z.-R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid.-Based Complement. Altern. Med. 2015, 2015, 919616. [Google Scholar] [CrossRef]
- Liang, H.-J.; Suk, F.-M.; Wang, C.-K.; Hung, L.-F.; Liu, D.-Z.; Chen, N.-Q.; Chen, Y.-C.; Chang, C.-C.; Liang, Y.-C. Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem.-Biol. Interact. 2009, 181, 309–315. [Google Scholar] [CrossRef]
- Lee, W.-H.; Lin, R.-J.; Lin, S.-Y.; Chen, Y.-C.; Lin, H.-M.; Liang, Y.-C. Osthole enhances glucose uptake through activation of AMP-activated protein kinase in skeletal muscle cells. J. Agric. Food Chem. 2011, 59, 12874–12881. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, X.; Xin, J.; Wu, Y.; Li, H. Coumarins improved type 2 diabetes induced by high-fat diet and streptozotocin in mice via antioxidation. Can. J. Physiol. Pharm. 2018, 96, 765–771. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Ala, P.J.; Gonneville, L.; Hillman, M.C.; Becker-Pasha, M.; Wei, M.; Reid, B.G.; Klabe, R.; Yue, E.W.; Wayland, B.; Douty, B.; et al. Structural basis for inhibition of protein-tyrosine phosphatase 1B by isothiazolidinone heterocyclic phosphonate mimetics. J. Biol. Chem. 2006, 281, 32784–32795. [Google Scholar] [CrossRef]
- Wiesmann, C.; Barr, K.J.; Kung, J.; Zhu, J.; Erlanson, D.A.; Shen, W.; Fahr, B.J.; Zhong, M.; Taylor, L.; Randal, M.; et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat. Struct. Mol. Biol. 2004, 11, 730–737. [Google Scholar] [CrossRef]
- Black, E.; Breed, J.; Breeze, A.L.; Embrey, K.; Garcia, R.; Gero, T.W.; Godfrey, L.; Kenny, P.W.; Morley, A.D.; Minshull, C.A.; et al. Structure-based design of protein tyrosine phosphatase-1B inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 2503–2507. [Google Scholar] [CrossRef]
- Klopfenstein, S.R.; Evdokimov, A.G.; Colson, A.-O.; Fairweather, N.T.; Neuman, J.J.; Maier, M.B.; Gray, J.L.; Gerwe, G.S.; Stake, G.E.; Howard, B.W.; et al. 1,2,3,4-Tetrahydroisoquinolinyl sulfamic acids as phosphatase PTP1B inhibitors. Bioorganic Med. Chem. Lett. 2006, 16, 1574–1578. [Google Scholar] [CrossRef]
- Wan, Z.-K.; Lee, J.; Xu, W.; Erbe, D.V.; Joseph-McCarthy, D.; Follows, B.C.; Zhang, Y.-L. Monocyclic thiophenes as protein tyrosine phosphatase 1B inhibitors: Capturing interactions with Asp48. Bioorg. Med. Chem. Lett. 2006, 16, 4941–4945. [Google Scholar] [CrossRef]
- Han, Y.; Belley, M.; Bayly, C.I.; Colucci, J.; Dufresne, C.; Giroux, A.; Lau, C.K.; Leblanc, Y.; McKay, D.; Therien, M.; et al. Discovery of [(3-bromo-7-cyano-2-naphthyl)(difluoro)methyl]phosphonic acid, a potent and orally active small molecule PTP1B inhibitor. Bioorg. Med. Chem. Lett. 2008, 18, 3200–3205. [Google Scholar] [CrossRef]
- Byler, K.G.; Ogungbe, I.V.; Setzer, W.N. In-silico screening for anti-Zika virus phytochemicals. J. Mol. Graph. Model. 2016, 69, 78–91. [Google Scholar] [CrossRef]
- Setzer, M.S.; Byler, K.G.; Ogungbe, I.V.; Setzer, W.N. Natural products as new treatment options for trichomoniasis: A molecular docking investigation. Sci. Pharm. 2017, 85, 5. [Google Scholar] [CrossRef]
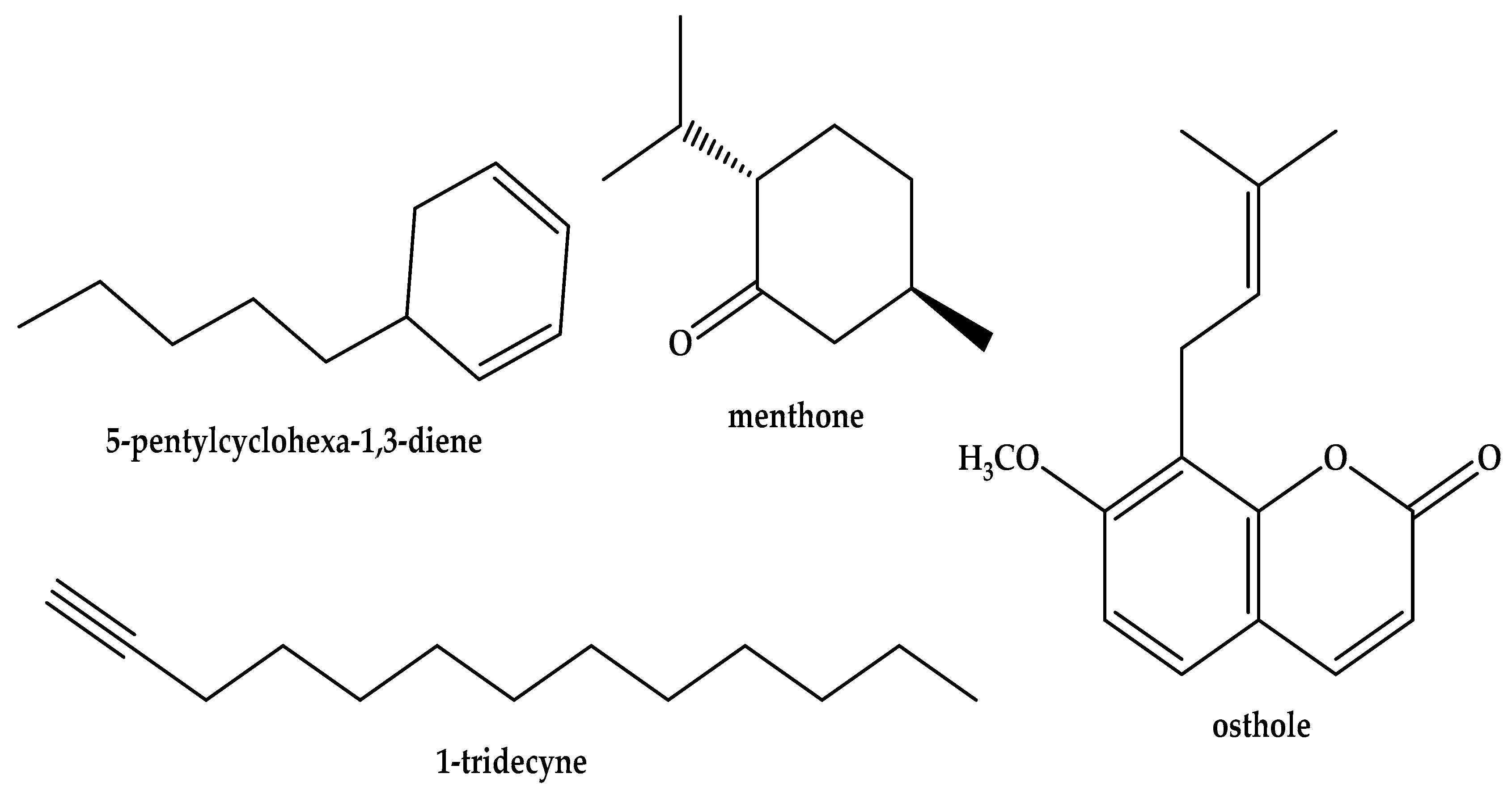
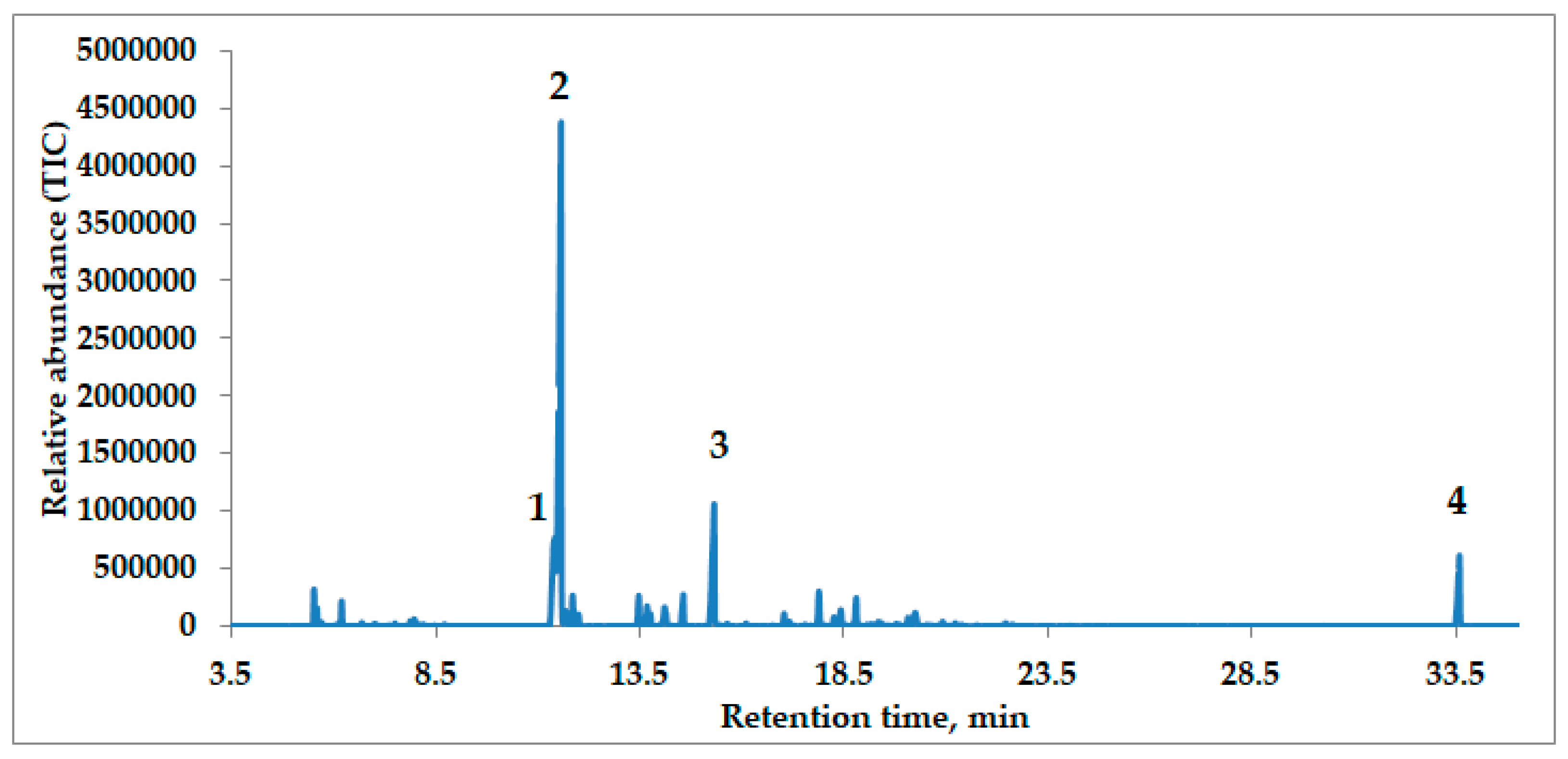
| RT | RI | Compound | MS | Fragmentations, m/z (%) | % |
|---|---|---|---|---|---|
| 5.487 | 934 | (E)-1,3-Nonadiene | 124.13 | 67 (100%); 54 (82.37%); 124 (41.49%); 68 (36.07%); 81 (35.96%) | 1.5 |
| 5.556 | 937 | 1-Nonen-4-yne | 122.11 | 79 (100%); 77 (49.67%); 78 (21.32%); 80 (19.82%) | 0.8 |
| 5.674 | 941 | α-Pinene | 136.13 | 93 (100%); 91 (74.23%); 79 (52.77%); 77 (51.98%); 92 (42.0%) | 0.1 |
| 6.006 | 953 | Camphene | 136.13 | 93 (100%); 91 (81.41%); 77 (56.16%); 79 (54.03%); 92 (31.5%) | Tr |
| 6.106 | 957 | Propylbenzene | 120.09 | 91 (100%); 65 (18.7%); 120 (17.78%); 92 (10.48%); 78 (8.87%) | 0.2 |
| 6.162 | 959 | (3E)-2-Methylocten-5-yne | 122.11 | 79 (100%); 91 (86.08%); 77 (84.95%); 93 (59.7%); 122 (35.51%) | 1.1 |
| 6.656 | 977 | Sabinene | 136.14 | 93 (100%); 77 (69.92%); 79 (68.13%); 91 (58.51%); 53 (30.37%) | 0.2 |
| 6.974 | 989 | β-Pinene | 136.13 | 93 (100%); 91 (28.2%); 69 0(26.9%); 79 (23.51%); 77.1 (22.1%) | 0.1 |
| 7.068 | 993 | 3-Octanol | 130.14 | 59 (100%); 83 (81.4%); 55 (78.3%); 101 (29.96); 57 (23.3%) | Tr |
| 7.324 | 1002 | α-Phellandrene | 136.13 | 93 (100%); 122 (84.73%); 91 (81.47%); 79 (75.95%); 107 (52.5%) | 0.1 |
| 7.468 | 1008 | δ-3-Carene | 136.13 | 93 (100%); 91 (95.41%); 77(56.16%); 79 (54.03%); 92 (31.5%) | 0.1 |
| 7.849 | 1022 | 1,9-Decadiyne | 134.11 | 79 (100%); 77 (52.95%); 67 (51.87%); 91 (49.70%); 81 (37.7%) | 0.3 |
| 7.943 | 1025 | Limonene | 136.13 | 93 (100%); 68 (80.91%); 67 (80.89%); 79 (80.07%); 91 (73.16%) | 0.4 |
| 7.999 | 1027 | 1,8-Cineole | 154.14 | 81 (100%); 111 (90%); 67 (87.77%); 108 (87.37%); 55 (85.72%) | 0.2 |
| 8.162 | 1033 | (Z)-β-Ocimene | 136.13 | 93 (100%); 91 (86.50%); 79 (63.32%); 77 (43.30%); 92 (36.2%) | 0.1 |
| 8.437 | 1044 | (E)-β-Ocimene | 136.13 | 93 (100%); 91 (86.50%); 79 (63.32%); 77 (43.30%); 80 (37.2%) | 0.1 |
| 8.693 | 1053 | 5-Butylcyclohexa-1,3-diene | 136.13 | 79 (100%); 91 (84.32%); 77 (79.63%); 93 (49.2%); 136 (36.48%) | 0.1 |
| 11.399 | 1154 | Menthone | 154.14 | 112 (100%); 55 (77.25%); 69 (69.36%); 139 (44.49%); 97 (34.09%) | 12.6 |
| 11.543 | 1160 | 5-Pentylcyclohexa-1,3-diene | 150.14 | 79 (100%); 91 (65.34%); 77 (58.97%); 93 (58.47%); 94 (35.13%) | 44.6 |
| 11.612 | 1162 | Viridene | 150.14 | 138 (100%); 124 (39.8%); 93 (25.5%); 137 (26.1) | 0.3 |
| 11.649 | 1164 | iso-Menthone | 154.14 | 112 (100%); 55 (91.02%); 69 (65.32%); 95 (37.37%); 139 (35.89%) | 1.0 |
| 11.831 | 1170 | 2-Methoxy-3-(1-methylpropyl)pyrazine | 166.11 | 138 (100%); 124 (39.81%); 151 (37.35%); 93 (35.52%); 137 (26.1%) | 1.8 |
| 11.974 | 1176 | neoiso-Pulegol | 150.14 | 67 (100%); 55 (73.02%); 53 (62.77%); 69 (56.57%); 79 (53.38%) | 0.8 |
| 13.449 | 1231 | Unidentified | - | 55 (100%); 57 (73.89%); 56 (68.30%); 71 (65.04%); 69 (59.13%) | 1.7 |
| 13.649 | 1239 | Pulegone | 152.12 | 81 (100%); 67 (93.29%); 109 (57.3%); 152 (48.89%); 82. (38.27%) | 1.3 |
| 13.731 | 1242 | Unidentified | - | 138 (100%); 108 (27.65%); 95 (19.27%); 109 (17.38%); 54 (16.08%) | 0.7 |
| 14.018 | 1252 | Unidentified | - | 138 (100%); 108 (35.28%); 95 (27.12%); 109 (15.38%); 54 (10.2%) | 0.2 |
| 14.081 | 1255 | cis-Piperitone epoxide | 168.12 | 55 (100%); 69 (80.71%); 67 (49.99%); 125 (44.00%); 53 (43.72%) | 1.3 |
| 14.118 | 1256 | (4Z)-Decen-1-ol | 156.15 | 55 (100%); 70 (64.85%); 69 (62.42%); 56 (57.42%); 83 (49.55%) | 0.5 |
| 14.543 | 1272 | 1-Decanol | 158.17 | 55 (100%); 70 (64.85%); 69 (62.42%); 56 (57.42%); 83 (49.55%) | 2.0 |
| 15.299 | 1300 | 1-Tridecyne | 180.19 | 81 (100%); 55 (79.41%); 67 (78.15%); 69 (47.9%); 68 (40.50%) | 10.9 |
| 17.012 | 1365 | Piperitenone oxide | 166.10 | 67 (100%); 138 (63.48%); 68 (62.28%); 53 (38.29%); 79 (36.54%) | 0.7 |
| 17.143 | 1371 | 1-Undecanol | 172.18 | 55 (100%); 69 (73.24%); 56 (61.43%); 83 (55.86%); 70 (49.46%) | 0.3 |
| 17.868 | 1399 | 3-Dodecyn-2-ol | 182.17 | 55 (100%); 67 (99.69%); 69 (76.48%); 95 (72.69%); 68 (56.92%) | 2.1 |
| 18.237 | 1414 | β-Longipinene | 190.17 | 91 (100%); 77 (72.64%); 93 (62.14%); 161 (58.85%); 105 (57.88%) | 0.6 |
| 18.406 | 1421 | β-Caryophyllene | 204.19 | 91 (100%); 79 (86.59%); 93 (67.92%); 77 (66.1%); 105 (55.12%) | 1.0 |
| 18.718 | 1433 | γ-Elemene | 204.19 | 121 (100%); 177 (98.85%); 91 (58.5%); 93 (57.4%); 107 (43.1%) | 0.1 |
| 18.781 | 1436 | trans-α-Bergamotene | 204.19 | 93 (100%); 91 (80.84%); 119 (75.05%); 77 (57.71%); 69 (48.76%) | 1.6 |
| 19.331 | 1459 | (7Z)-Dodecen-1-ol | 184.18 | 67 (100%); 55 (62.01%); 81 (56.91%); 54 (49.91%); 82 (47.42%) | 0.2 |
| 19.768 | 1477 | γ-Gurjunene | 204.19 | 91 (100%); 77 (87.25%); 79 (83.86%); 93 (79.86%); 161 (76.27%) | 0.1 |
| 20.056 | 1489 | β-Selinene | 204.19 | 79 (100%); 91 (75.67%); 67 (75.38%); 93 (63.4%); 105 (61.85%) | 0.5 |
| 20.193 | 1494 | (3Z,6E)-α-Farnesene | 204.19 | 119 (100%); 91 (69.2%); 79 (60.9%); 81 (58.5%); 77 (55.6%) | 0.5 |
| 20.231 | 1496 | Valencene | 204.19 | 91 (100%); 79 (97.61%); 105 (83.71%); 93 (80.93%); 77 (75.81%) | 0.8 |
| 20.274 | 1498 | α-Selinene | 204.19 | 93 (100%); 91 (83.3%); 69 (68.3%); 105 (50.3%); 77 (49.2%) | 0.3 |
| 33.581 | 2141 | Osthole | 244.11 | 244 (100%); 201 (93.94%); 229 (93.95%); 131 (65.60%); 189 (63.98%) | 6.0 |
| Monoterpene hydrocarbons | 1.3 | ||||
| Oxygenated monoterpenoids | 17.8 | ||||
| Sesquiterpene hydrocarbons | 5.5 | ||||
| Fatty acid derived | 19.8 | ||||
| Others | 53.0 | ||||
| Total Identified | 97.3 |
| Prangos Species | Plant Part | Major Components of the Essential Oil | Ref. |
|---|---|---|---|
| P. acaulis | aerial parts | δ-3-carene (25.5%), α-terpinolene (14.8%), α-pinene (13.6%), limonene (12.9%), myrcene (8.1%) | [27] |
| P. asperula | fruits | δ-3-carene (16.1%), β-phellandrene (14.7%), α-pinene (10.5%), α-humulene (7.8%), germacrene-D (5.4%) | [28] |
| P. asperula | fresh aerial parts | sabinene (43.5%), β-phellandrene (36.1%), (E)-nerolidol (15.2%), p-menth-3-ene (14.9%), (E)-nerolidol (14.7%), p-menth-3-ene (13.3%) in stem and leaves, α-phellandrene (11.9%) in fruits, β-myrcene (9.2%) in stem and leaves; α-terpinene (8.3%) in fruits, β-phellandrene (7.9%) in flowers | [29] |
| P. asperula | aerial parts | 2,3,6-trimethyl benzaldehyde (18.4%), δ-3-carene (18.0%) and α-pinene (17.4%) | [30] |
| P. asperula | fruit | sabinene (43.5%) | [31] |
| P. ferulacea | fruits | α-pinene (57%) (vegetative stage), γ-terpinene (30.2–33.3%) and α-pinene (16.7–12.8%) | [32] |
| P. ferulacea | aerial parts | (E)-anethol (95.5%) (flowering stage) | [16] |
| P. ferulacea | fruits | chrysanthenyl acetate (26.5%), limonene (19.6%), α-pinene (19.5%) | [33] |
| P. ferulacea | aerial parts | β-pinene (43.1%), α-pinene (22.1%) and δ-3-carene (16.9%) | [34] |
| P. ferulacea | aerial parts | 2,3,6-trimethyl benzaldehyde (66.6%) | [35] |
| P. ferulacea | aerial parts | β-caryophyllene (48.2%), α-humulene (10.3%) and spathulenol (9.4%) | [36] |
| P. ferulacea | aerial parts | α-pinene (36.6%) and β-pinene (31.1%) | [37] |
| P. ferulacea | roots | β-phellandrene (32.1%), m-tolualdehyde (26.2%), and δ-3-carene (25.8%) | [18] |
| P. ferulaceae | fruits and umbels | α-pinene and (Z)-β-ocimene | [38] |
| P. heyniae | fruits | β-bisabolenal (18.0–53.3%), β-bisabolenol (2.3–14.6%) and β-bisabolene (10.1–12.1%) | [39] |
| P. heyniae | aerial parts | β-bisabolenal (1.4–70.7%), (8.2%), elemol (3.4–46.9%), kessane (26.9%), β-bisabolene (14.4%), germacrene D (10.3–12.1%), germacrene B 3,7(11)-eudesmadien-2-one (16.1%) and β-bisabolenol (8.4%) | [20] |
| P. latiloba | aerial parts | geranial (26.8%) | [31] |
| P. pabularia | flowering aerial parts | α-pinene (32.4%), δ-3-carene (12.4%), germacrene D (8.1%), limonene (6.4%) and bicyclogermacrene (6.2%) | [40] |
| P. pabularia | fruit | bicyclogermacrene (21%), (Z)-β-ocimene (19%), α-humulene (8%), α-pinene (8%), spathulenol (6%), suberosin (2%) | [24] |
| P. peucedanifolia | flowering aerial parts | α-pinene (38.1%), bicyclogermacrene (11.3%) and δ-3-carene (9.2%) | [40] |
| P. uloptera | aerial parts | δ-3-carene (26.3%), α-pinene (15.4%), β-myrcene (9.0%), p-cymene (8.6%) | [41] |
| P. uloptera | aerial parts | β-caryophyllene (18.2%), germacrene D (17.2%) and limonene (8.7%) | [42] |
| P. uloptera | seed | α-pinene (41.9%) and β-cedrene (4.0%) | [42] |
| P. uloptera | aerial parts | α-pinene (20.3%), (E)-β-ocimene (19.6%), β-caryophyllene in fresh aerial parts; β-caryophyllene (13.9%), α-pinene (13.6%), caryophyllene-oxide (11.6%) in dried aerial parts; (9.9%), δ-3-carene (8.0%), germacrene D (6.0%) in fresh aerial parts; spathulenol (7.8%) and germacrene D (4.7%) in dried aerial parts | [43] |
| P. uloptera | fruits | germacrene D (17.6%), acorenone (16.9%), α-pinene (14.9%), and α-humulene (8.2%) | [44] |
| 1T48 | 1T49 | 2BGE | 2CMB | 2F71 | 2HB1 | 3CWE | |
|---|---|---|---|---|---|---|---|
| Ligand | Allosteric Site | Allosteric Site | Active Site | Active Site | Active Site | Active Site | Active Site |
| (3E)-2-Methylocten-5-yne | −64.6 (−93.9) | −60.2 (−87.4) | −62.5 (−90.8) | −61.1 (−88.7) | −60.0 (−87.2) | −57.8 (−84.1) | −62.0 (−90.1) |
| (E)-1,3-Nonadiene | −67.8 (−97.9) | −58.5 (−84.5) | −63.0 (−91.1) | −63.5 (−91.8) | −65.9 (−95.2) | −62.2 (−89.9) | −64.1 (−92.7) |
| 1-Decanol | −76.5 (−102.0) | −66.8 (−89.0) | −71.0 (−94.6) | −73.0 (−97.4) | −72.7 (−96.9) | −69.8 (−93.1) | −70.4 (−93.9) |
| 1-Tridecyne | −86.4 (−110.4) | −72.7 (−92.8) | −73.2 (−93.4) | −79.0 (−100.9) | −76.7 (−97.9) | −71.8 (−91.6) | −73.4 (−93.7) |
| 2-Methoxy-3-(1-methylpropyl)pyrazine | −76.5 (−100.4) | −64.3 (−84.4) | −65.6 (−86.0) | −66.2 (−86.8) | −62.9 (−82.5) | −66.0 (−86.5) | −71.7 (−94.0) |
| 3-Dodecyn-2-ol | −84.2 (−107.1) | −72.4 (−92.1) | −79.2 (−100.8) | −74.2 (−94.4) | −80.5 (−102.4) | −77.3 (−98.3) | −77.4 (−98.4) |
| 5-Pentyl-1,3-cyclohexadiene | −72.3 (−98.1) | −66.2 (−89.9) | −70.7 (−95.9) | −68.9 (−93.5) | −68.5 (−92.9) | −67.8 (−92.0) | −68.7 (−93.2) |
| cis-Piperitone epoxide | −76.0 (−99.3) | −65.5 (−85.6) | −65.8 (−86.0) | −67.4 (−88.1) | −69.0 (−90.2) | −73.1 (−95.4) | −75.0 (−98.0) |
| Menthone | −72.3 (−97.2) | −60.6 (−81.5) | −59.0 (−79.3) | −55.2 (−74.2) | −59.7 (−80.3) | −65.6 (−88.3) | −68.6 (−92.2) |
| Osthole | −103.4 (−119.3) | −87.1 (−100.5) | −79.3 (−91.5) | −81.5 (−94.0) | −85.4 (−98.6) | −89.3 (−103.0) | −85.9 (−99.1) |
| Pulegone | −71.8 (−97.0) | −63.6 (−85.8) | −43.4 (−58.6) | −57.3 (−77.3) | −59.0 (−79.7) | −69.1 (−93.4) | −70.6 (−95.4) |
| trans-α-Bergamotene | −74.9 (−91.7) | −65.4 (−80.1) | −67.2 (−82.3) | −68.6 (−84.1) | −62.9 (−77.0) | −81.5 (−99.8) | −68.2 (−83.5) |
| Co-crystallized ligand | −142.7 (−127.1) | −137.2 (−127.7) | −105.6 (−127.7) | −162.0 (−132.0) | −147.8 (−138.3) | −97.2 (−107.3) | −129.2 (−117.9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numonov, S.; Sharopov, F.S.; Atolikhshoeva, S.; Safomuddin, A.; Bakri, M.; Setzer, W.N.; Musoev, A.; Sharofova, M.; Habasi, M.; Aisa, H.A. Volatile Secondary Metabolites with Potent Antidiabetic Activity from the Roots of Prangos pabularia Lindl.—Computational and Experimental Investigations. Appl. Sci. 2019, 9, 2362. https://doi.org/10.3390/app9112362
Numonov S, Sharopov FS, Atolikhshoeva S, Safomuddin A, Bakri M, Setzer WN, Musoev A, Sharofova M, Habasi M, Aisa HA. Volatile Secondary Metabolites with Potent Antidiabetic Activity from the Roots of Prangos pabularia Lindl.—Computational and Experimental Investigations. Applied Sciences. 2019; 9(11):2362. https://doi.org/10.3390/app9112362
Chicago/Turabian StyleNumonov, Sodik, Farukh S. Sharopov, Sunbula Atolikhshoeva, Abduahad Safomuddin, Mahinur Bakri, William N. Setzer, Azizullo Musoev, Mizhgona Sharofova, Maidina Habasi, and Haji Akber Aisa. 2019. "Volatile Secondary Metabolites with Potent Antidiabetic Activity from the Roots of Prangos pabularia Lindl.—Computational and Experimental Investigations" Applied Sciences 9, no. 11: 2362. https://doi.org/10.3390/app9112362
APA StyleNumonov, S., Sharopov, F. S., Atolikhshoeva, S., Safomuddin, A., Bakri, M., Setzer, W. N., Musoev, A., Sharofova, M., Habasi, M., & Aisa, H. A. (2019). Volatile Secondary Metabolites with Potent Antidiabetic Activity from the Roots of Prangos pabularia Lindl.—Computational and Experimental Investigations. Applied Sciences, 9(11), 2362. https://doi.org/10.3390/app9112362










