In Vitro Propagation Protocols and Variable Cost Comparison in Commercial Production for Paulownia tomentosa × Paulownia fortunei Hybrid as a Renewable Energy Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Culture Initiation and Stabilization
2.2. Multiplication Stage
2.3. Rooting and Acclimatization Stage
2.4. Cost Analysis
3. Results and Discussion
3.1. Micropropagation of P. tomentosa × P. fortunei
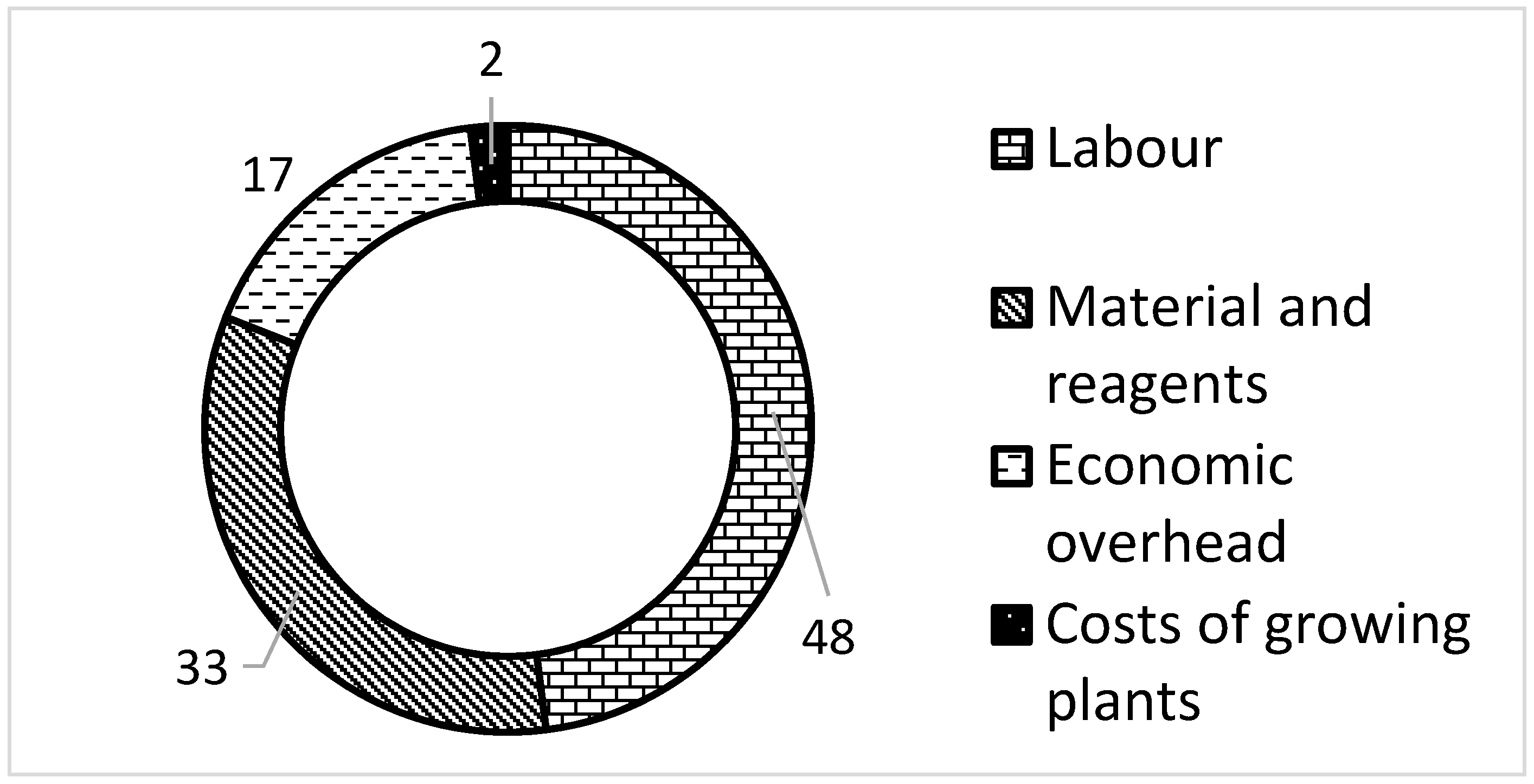
3.2. Costs of P. tomentosa × P. fortunei In Vitro Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barton, I.; Nicholas, I.; Ecroyd, C. Paulownia. For. Res. Bull. 2007, 231, 5–68. [Google Scholar]
- Bahri, B. In vitro propagation of a forest tree Paulownia tomentosa (Thunb.) Steud.—A valuable medicinal tree species. Albanian J. Agric. Sci. 2013, 12, 37–42. [Google Scholar]
- Zhu, Z.H.; Chao, C.J.; Lu, X.Y.; Xiong, Y.G. Paulownia in China: CultivationandUtilization; Asian Network for Biological Science andInternational Development Research Centre: Singapore, 1986; pp. 37–46. [Google Scholar]
- Carpenter, S.B. This “princess” heals disturbed land. Am. For. 1997, 83, 22–23. [Google Scholar]
- Azzarello, E.; Pandolfi, C.; Giordano, C.; Rossi, M.; Mugnai, S.; Mancuro, S. Ultramorphological and physiological modifications induced by high zinc levels in Paulownia tomentosa. Environ. Exp. Bot. 2012, 81, 11–17. [Google Scholar] [CrossRef]
- Bergmann, B.A.; Moon, H.K. In vitro adventitious shoot production in Paulownia. Plant Cell Rep. 1997, 16, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Ipekci, Z.; Gozukirmizi, N. Direct somatic embryogenesis and synthetic seed production from Paulownia elongata. Plant Cell Rep. 2003, 22, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ipekci, Z.; Gozukirmizi, N. Indirect somaticembryogenesis and plant regeneration from leaf and internode explants of Paulownia elongata. Plant Cell Tissue Organ Cult. 2004, 79, 341–345. [Google Scholar] [CrossRef]
- Radojevic, L. Somatic embryos and plantlets from callus cultures Paulownia tomentosaSteud. Pflanzenphysiology 1979, 91, 57–62. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol.Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Venkateswarlu, B.; Mukhopadhyay, J.; Sreenivasan, E.; Kumar, M. Micropropagation of Paulownia fortune through in vitro axillary shoot proliferation. Indian J. Exp. Biol. 2001, 39, 594–599. [Google Scholar] [PubMed]
- Elum, Z.A.; Etowa, E.B.; Ogonda, A.U. Economics of cucumber production in rivers state, Nigeria. Agro-Science 2016, 15. [Google Scholar] [CrossRef]
- Lindeque, J.M.; van der Meschtz, A.; Slabbert, M.M.; Henn, G. Variation in phenotype and proteins in plants regenerated fromcell suspensions of potato cv. BP1. Euphytica 1991, 54, 41–44. [Google Scholar] [CrossRef]
- Mohanty, S.; Panda, M.K.; Subudhi, E.; Nayak, S. Plant regeneration from callus culture of Curcuma aromatica and in vitro detection of somaclonal variation through cytophotometric analysis. Biol. Plant. 2008, 52, 783–786. [Google Scholar] [CrossRef]
- Chunchukov, A.; Yancheva, S. Micropropagation of Paulownia species and hybrids. Annuaire de l’Université de Sofia “St. KlimentOhridski” Faculte de Biologie 2015, 100, 223–230. [Google Scholar]
- Chiachung, C. Cost analysis of plant micropropagation of Phalaenopsis. Plant Cell Tiss Organ Cult. 2016, 126, 167–175. [Google Scholar]
- Alves dos Santos Mauricio, R.; Augusto de Souza, C.; Felix da Rocha, J.; Ventura de Araujo, L.; Curitiba Espindula, M. Comparison of Economic Efficiency between in Vitro and Field Methods for Vegetative Propagation of CoffeaCanephora. Aust. J. Basic Appl. Sci. 2015, 9, 1–7. [Google Scholar]
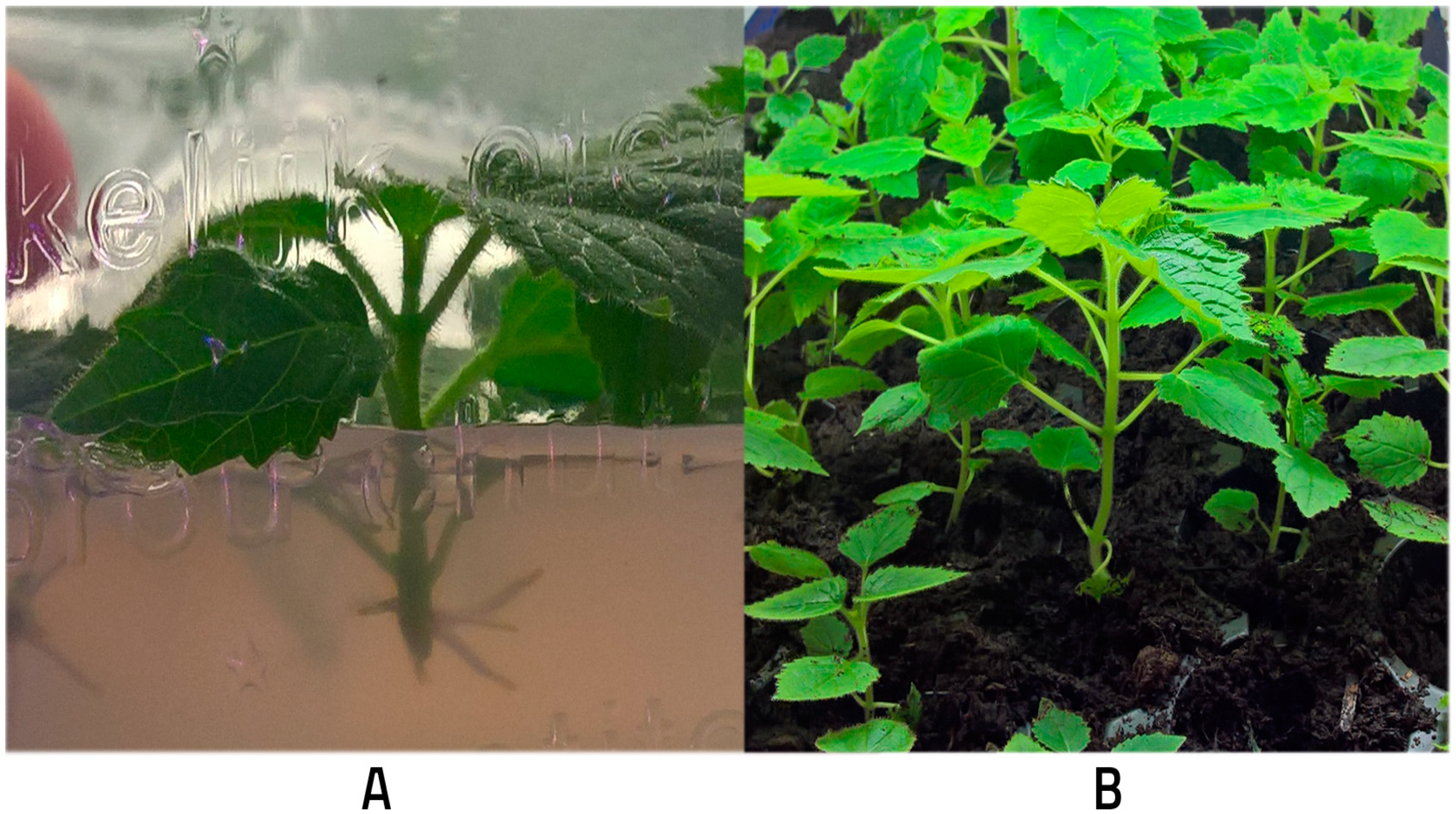
| Multiplication Stage | Rooting Stage | ||||
|---|---|---|---|---|---|
| Medium | MS1 | MS2 | MS3 | MS4 | MS5 |
| Composition | ½ MS + 0.2 mg/L BAP | ½ MS + 0.5 mg/L BAP | ½ MS + 1 mg/L BAP | ½ MS hormone free | ½ MS + 1 mg/L IBA |
| Ingredients for Medium Preparation | Wholesale Prices of Ingredients (in$) | Ingredients for 1 L of Medium Preparation | Costs of Ingredients for 1 L of Medium Preparation (in $) |
|---|---|---|---|
| MS medium Duchefa 100 L | 55.19 | MS medium Duchefa (0.5 L) | 0.27 |
| Sucrose (food sugar) 1 kg (1000 g) | 0.51 | Sucrose (20 g) | 0.01 |
| Agar Ducheffa 25 kg (25,000 g) | 2116.55 | Agar Ducheffa (7 g) | 0.59 |
| BAP Ducheffa 25 g (25,000 mg) | 121.76 | BAP Ducheffa (0.5 mg) | 0.002* |
| Total cost per 1 liter of MS2 medium | 0.88 | ||
| Medium | Effect on Explant | Possibility of Further Usage |
|---|---|---|
| MS1 | 1-2 axillary bud formation, slow growth, weak plants | NO |
| MS2 | 2 axillary bud formation, good growth | YES |
| MS3 | callus overgrowth what resulted in explants and callusing of the tissue, poor condition of plants | NO |
| Medium | Effect on Explant | Rooting Percentage | Possibility of Further Usage |
|---|---|---|---|
| 1/2MS hormone free (MS4) | rooting after 2 weeks, healthy long, branched roots | 95% | YES |
| 1/2MS + 1 mg/L IBA (MS5) | rooting after 2 weeks, short, hard roots. Easy to break when taken out from agar, needs careful treatment | 100% | YES |
| Costs in $ of Production of Container with 10 Explants | Full Light Conditions | 70% Reduced Light Conditions | Reduced Materials, Reagents and Labor Cost | |||
|---|---|---|---|---|---|---|
| by 10%, Full Light Conditions | by 10%, Reduced Light Conditions by 70% | by 20%, Full Light Conditions | by 20%, Reduced Light Conditions by 70% | |||
| Materials and reagents | 0.14 | 0.14 | 0.13 | 0.13 | 0.11 | 0.11 |
| Labor | 0.2 | 0.2 | 0.18 | 0.18 | 0.16 | 0.16 |
| Cost of growing | 0.03 | 0.01 | 0.03 | 0.01 | 0.03 | 0.01 |
| Economic overhead | 0.07 | 0.07 | 0.07 | 0.06 | 0.06 | 0.06 |
| Total cost of container with 10 unrooted plants | 0.44 | 0.42 | 0.41 | 0.38 | 0.36 | 0.34 |
| Total cost of container with 10 rooted plants | 0.84 | 0.82 | 0.81 | 0.78 | 0.76 | 0.74 |
| Week | Number of Plants Cultured in Full Light | Number of Containers Cultured in Full Light | Total Cost of Production in Full Light ($) | Number of Plants Cultured in 70% Reduced Light | Number of Containers Cultured in 70% Reduced Light | Total Cost of Production in 70% Reduced Light ($) |
|---|---|---|---|---|---|---|
| 1 | 10 | 1 | 0.45 | 10 | 1 | 0.42 |
| 4 | 70 | 7 | 3.58 | 120 | 12 | 5.45 |
| 8 | 490 | 49 | 25.50 | 1440 | 144 | 65.79 |
| 12 | 3430 | 343 | 178.93 | 17,280 | 1728 | 789.90 |
| 16 | 24,010 | 2401 | 1252.98 | 207,360 | 20,736 | 9479.17 |
| 20 Rooting | 48,020 | 4802 | 3173.78 | 414,720 | 41,472 | 26,067.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pożoga, M.; Olewnicki, D.; Jabłońska, L. In Vitro Propagation Protocols and Variable Cost Comparison in Commercial Production for Paulownia tomentosa × Paulownia fortunei Hybrid as a Renewable Energy Source. Appl. Sci. 2019, 9, 2272. https://doi.org/10.3390/app9112272
Pożoga M, Olewnicki D, Jabłońska L. In Vitro Propagation Protocols and Variable Cost Comparison in Commercial Production for Paulownia tomentosa × Paulownia fortunei Hybrid as a Renewable Energy Source. Applied Sciences. 2019; 9(11):2272. https://doi.org/10.3390/app9112272
Chicago/Turabian StylePożoga, Mariusz, Dawid Olewnicki, and Lilianna Jabłońska. 2019. "In Vitro Propagation Protocols and Variable Cost Comparison in Commercial Production for Paulownia tomentosa × Paulownia fortunei Hybrid as a Renewable Energy Source" Applied Sciences 9, no. 11: 2272. https://doi.org/10.3390/app9112272
APA StylePożoga, M., Olewnicki, D., & Jabłońska, L. (2019). In Vitro Propagation Protocols and Variable Cost Comparison in Commercial Production for Paulownia tomentosa × Paulownia fortunei Hybrid as a Renewable Energy Source. Applied Sciences, 9(11), 2272. https://doi.org/10.3390/app9112272




