Abstract
Soybean oil isolated by enzyme-assisted aqueous extraction (EAE) was subjected to molecular distillation-induced deacidification, and the effects of evaporator temperature, scraper speed, and feed flow rate on oil quality (acid value, color, peroxide value, p-anisidine value, tocopherol content, and fatty acid content) were evaluated to determine the suitable deacidification conditions. Fatty acid content was largely unaffected by evaporator temperature and scraper speed, while an increase of these parameters decreased tocopherol content as well as acid, peroxide, and p-anisidine values and resulted in Lovibond color deepening. The increase of feed flow rate had an opposite effect on the above quality indices. As a result, molecular distillation of EAE-produced soybean oil under suitable conditions (evaporator temperature = 180 °C, scraper speed = 220 rpm, feed flow rate = 4 mL/min) was found to afford a high-quality deacidified product in an environmentally friendly way.
1. Introduction
Soybean oil, commonly used for cooking and referred to as “soy salad oil,” is rich in valuable nutrients (e.g., essential fatty acids, polyunsaturated fatty acids (>80%), phospholipids, and tocopherols) that can reduce the levels of cholesterol and lipids in blood. Traditionally, soybean oil is prepared by pressing or solvent leaching methods, which are characterized by high yields (>95%) but result in severe protein denaturation and the presence of harmful solvent residues [1]. Hence, the development of new soybean oil extraction methods meeting the requirements of green clean production and comprehensive utilization is a task of high practical importance.
Recently, enzyme-assisted aqueous extraction (EAE) has emerged as a new green technology for soybean oil isolation [2]. During EAE, soybeans are mechanically disrupted, and the released oil is treated with an enzyme capable of degrading macromolecular complexes such as lipoproteins, lipopolysaccharides, and cell walls to destroy tissue structure and hydrolyze the above complexes. As a result, oil is separated from proteins based on differences in oil-water density and affinity between components [3,4,5]. EAE has been effectively used to process coconuts, soybeans, and corn germ, affording oil in 90–98% yield and good quality protein meal [6,7]. Although EAE-produced oil exhibits superior quality, it requires further refinement, as the decomposition of fatty acid esters therein into free fatty acids results in an increase of acid value and thus adversely affects oil quality. In some cases, this acidification is so severe that soybean oil becomes inedible and can only be used for soap production or has to be discarded as waste. Therefore, EAE-produced soybean oil has to be deacidified in a timely manner.
Deacidification, considered to be the most important and complex refining process in commercial manufacturing [8], is commonly performed via caustic refining and physical distillation [9]. However, caustic refining causes a significant loss of neutral oil due to the use of excess alkali, while physical distillation involves long-term exposure to high temperatures and hence results in undesirable color change and nutrient loss. Consequently, conventional deacidification methods are not well suited for highly acidic crude oils and fats, and can only be applied to high-quality oil obtained by EAE. Therefore, the optimization of these processes to ensure high oil quality and low acid content is a task of high practical significance. Fernandes et.al described the use of palm fatty-acid distillate to produce biolubricants, the contribution provided a sustainable, environmentally benign process for the production of biolubricants [10]. Papadaki et.al used the soybean cake from biodiesel production processes and very high polarity (VHP) sugar from sugarcane mills to evaluate the microbial production of fumaric acid by Rhizopus arrhizus NRRL 2582 [11].
Recently, much effort has been directed to the development of alternative deacidification processes such as molecular distillation. This method separates light molecules from heavy ones based on the difference between their average free paths [12] and exhibits the advantages of equipment simplicity, operation safety and reliability, environmental friendliness, absence of solvent residues, etc. [13]. Under high vacuum, when the distance between evaporation and condensation surfaces is between the average free paths of light and heavy molecules, mixtures can be separated at temperatures far below their boiling points [14]. Currently, molecular distillation is widely used in the industrial production of vitamins A and E, free fatty acids (FFAs), fish oil, and other products that cannot be obtained by conventional distillation. Watanabe et al. employed short-path distillation of soybean oil deodorizer distillate to achieve an FFA removal efficiency of 96% and a tocopherol recovery of 81% [15], while Martinello et al. applied this technique to obtain refined grape seed oil with a decreased FFA content and achieve high tocopherol recovery [16]. Moreover, Wu et al. optimized the molecular distillation conditions of crude low-calorie cocoa butter [17], and Ángela et al. used molecular distillation to produce monoacylglycerol-enriched ω-3 polyunsaturated fatty acids [18].
Although molecular distillation is well suited for the separation of heat-sensitive and low volatile compounds, providing a new and efficient way of mitigating the problem of oil and fat rancidity in food, few studies have investigated the application of molecular distillation to EAE-produced soybean oil. To bridge this gap, we herein subjected EAE-produced soybean oil to molecular distillation and probed the effects of evaporation temperature, scraper speed, and feed flow rate on the physicochemical properties of the refined product, aiming to remove fatty acids and retain bioactive substances. As a result, molecular distillation under suitable conditions was shown to be a novel, green, and energy-efficient method for the industrial-scale refinement of EAE-produced soybean oil.
2. Materials and Methods
2.1. Materials
Mature soybeans of the Nong-Ken #42 variety (Heilongjiang Academy of Agricultural Sciences, Harbin, Heilongjiang, China) were crushed using a twin-screw extruder (Evolum EV032, Clextral, Firminy, France) at 60 °C and a screw speed of 120 rpm. Protex 6L, an alkaline protease, was purchased from Jienengke Bio-engineering Co., Ltd. (Wuxi, China). Methanol, pyridine, acetonitrile, ethanol, n-hexane, and sodium sulfate were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were of analytical grade. Deionized (DI) water was used in all experiments.
2.2. Isolation of Soybean Oil by EAE
The isolation of soybean oil by EAE refers to the method of Yang Li with some modifications [19]. Crushed soybean flour sifted through a 70-mesh sieve was dispersed in DI water (1:6, w/v), and the pH of the dispersion was adjusted to 9.0 with 2 M aqueous NaOH. The obtained mixture was supplemented with Protex 6L (2 wt%), gently stirred at 60 °C for 3 h, and heated to 100 °C for 10 min for enzyme inactivation. The resulting solution was centrifuged at 4500 g for 20 min, and the oil layer was collected and stored at −4 °C.
2.3. Molecular Distillation-Induced Deacidification
Distillation was performed using a wiped-film molecular distillator (KDL5, UIC GmbH, Alzenau, Germany), an agitation-featuring variation of a falling-film molecular distillator. The main part of the instrument was made of glass. The evaporator was surrounded by a jacket with circulating hot oil from an oil bath. The vacuum system included a diffusion pump and a mechanical pump. The surface areas of the evaporator and the internal condenser equaled 0.048 and 0.065 m2, respectively.
Distillation was performed as follows. The evaporator temperature and scraper speed were fixed at 160 °C and 220 rpm, respectively, while the feed flow rate was varied from 1.0 to 5.0 mL/min. Then, the evaporator temperature and feed flow rate were fixed at 160 °C and 3 mL/min, respectively, while the scraper speed was varied from 180 to 260 rpm. Finally, the scraper speed and feed flow rate were fixed at 220 rpm and 3 mL/min, respectively, while the evaporator temperature was varied between 120 and 200 °C. All distillations were performed at a pressure of 10−6 bar, a feed temperature of 50 °C, and a condenser temperature of 40 °C. All effluents and residues obtained after distillation were collected and stored at −18 °C for subsequent analysis.
2.4. Color
Color was determined according to American Oil Chemists’ Society (AOCS) official method Cc13b–45 (97) using a Lovibond Tintometer 181059 Model F (UK) [20].
2.5. Acid Value
The acid value (AV) was determined by titration of sample solutions in neutralized alcohol with 0.1 M NaOH in the presence of phenolphthalein as an indicator according to the AOCS official method Ca 5a-40 [21]. AV was calculated as AV (mg KOH/g) = 56.11 × VN/m, where V is the volume of KOH consumed for titration (mL), N is the molar concentration of KOH (0.1 M), and m is the sample weight.
2.6. Peroxide Value
The peroxide value (PV), used to evaluate the extent of sample oxidation, was determined according to an AOAC official method and expressed as active oxygen milliequivalents (meqO2)/kg [16]. The samples should avoid direct sunlight for determination.
2.7. p-Anisidine Value
p-Anisidine values reflected the oxidative stability of processed oil and were determined by the p-anisidine dye formation method (AOCS Cd 18-90) using glacial acetic acid. Quantitation was performed by monitoring absorbance at 350 nm (T70 UV–vis spectrometer, PG Instruments Ltd., Leicestershire, UK) [22].
2.8. Tocopherol Content
Tocopherol content was determined by normal-phase high-performance liquid chromatography (HPLC) using a modular instrument comprising a Waters 515 HPLC pump (Mildford, MA) and equipped with a Waters model 2475 multi fluorescence detector [23]. Separation was conducted on a 125-Å Porasil column with dimensions of 3.9 × 300 mm and a particle size of 10 μm (Waters, Ireland) using a 99:1 (v/v) n-hexane:isopropanol mixture as the mobile phase at a flow rate of 1.0 mL/min. Data processing was performed using Millennium 2010 Chromatography Manager software (Waters, Mildford, MA, USA). Samples (crude soybean oil, distillates, or residues) were dissolved in n-hexane (1 mg/mL) and injected into the instrument. The utilized method allowed the individual quantitation of α-, β-, γ-, and δ-tocopherols, with each chromatographic run lasting for ~10 min. Tocopherols were identified by comparing their retention times with those of tocopherol standards, and quantitation was performed using calibration curves. Standard deviations (SDs) were calculated as follows:
where X is the theoretical tocopherol concentration of a sample, Mi is the corresponding concentration determined from the calibration curve, and N is the number of injections. Total tocopherol content was obtained by summing up the contents of individual components, and the corresponding SD was determined by summing up SDs of individual components.
2.9. Fatty Acid Content
The fatty acid contents of soybean oil and diacylglycerol (DAG)-enriched oil generated by the glycerolysis reaction were analyzed by gas chromatography–mass spectrometry (GC–MS). Fatty acid methyl ester preparation was performed using a modification of the method of Liu et al. [24]. The GC–MS system comprised a gas chromatograph (Trace Ultra, Thermo Finnigan, San Jose, CA, USA), an autosampler (Trisplus, Thermo Finnigan) and a quadrupole mass spectrometer (DSQ II, Thermo Finnigan). Separation was performed on a TR-5MS capillary column (30 mm × 0.2 mm, 0.25 µm; Thermo Finnigan). Samples (1.0 µL) were injected using a split ratio of 100:1. Helium was used as the carrier gas at a flow rate of 1.0 mL/min. The injection and ion source temperatures equaled 250 and 230 °C, respectively. The following oven temperature program was employed: hold for 1 min at 40 °C, increase to 150 °C at 10 °C/min, hold for 2 min, increase to 220 °C at 10 °C/min (no holding time), increase to 280 °C at 5 °C/min, hold for 3 min. After the end of each run, the system was equilibrated at 40 °C prior to the injection of the next sample. Mass spectra were recorded at three scans per second for m/z = 50–500. Chromatograms and mass spectra were evaluated using XcaliburTM v. 2.0 software bundle (Thermo Finnigan) [21].
2.10. Statistical Analysis
All experiments were carried out at least in triplicate. The results were expressed as mean ± SD and plotted using Origin 9.1 software. Statistical analysis was performed by a one-way ANOVA test followed by Duncan’s test using SPSS 25 software. A level of p < 0.05 was considered to be statistically significant.
3. Results and Discussion
3.1. Characterization of EAE-Produced Soybean Oil
Table 1 lists the characteristics of soybean oils produced by EAE and alkaline refining, revealing that the former had an AV of 0.57 mg KOH/g, a PV of 2.98 meqO2/g, and a p-anisidine value of 5.2, while the corresponding color was determined as yellow 5.2 and red 0.4. Deacidification resulted in a decrease of acid and p-anisidine values to 0.27 mg KOH/g and 5.02, respectively, while the PV slightly increased to 3.13 meqO2/g, and the color deepened to yellow 20.7/red 14.5. Moreover, alkaline refining-based deacidification decreased the total, α-, β-, γ-, and δ-tocopherol contents from 1230.9 to 301.79, 147.3 to 72.92, 63.5 to 7.5, 703.2 to 189.06, and 316.9 to 32.31 mg/kg, respectively, that is, led to a large loss of tocopherols.

Table 1.
Properties of soybean oil prepared by enzyme-assisted aqueous extraction (EAE) and conventional alkaline deacidification (AD).
3.2. Effect of Molecular Distillation Parameters on Deacidification Performance
Figure 1A demonstrates the effect of evaporator temperature on AV and deacidification efficiency, revealing that an increase of this temperature from 120 to 200 °C decreased the AV from 0.25 to 0.10 mg KOH/g and increased deacidification efficiency from 50.93% to 79.80%, in agreement with the results of Wu et al. [17]. The rapid increase of deacidification efficiency with increasing evaporation temperature was ascribed to the concomitantly increasing efficiency of volatile fatty acid removal. In theory, molecular distillation achieves separation by utilizing differences between the average free paths of molecular motion. In turn, these free paths are proportional to the evaporator temperature [25], i.e., an increase of evaporator temperature accelerates the evaporation of materials and increases the proportion of small molecules such as free fatty acids in the light phase, while the AV of heavy-phase oil decreases.
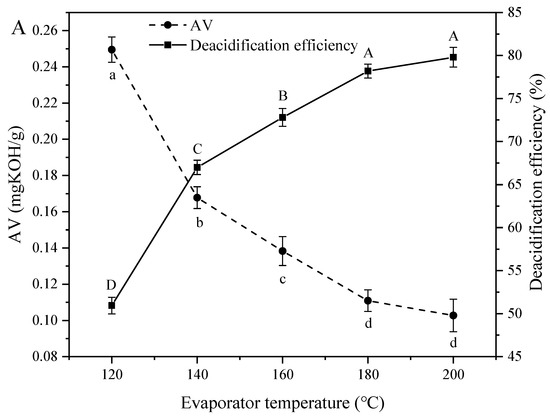
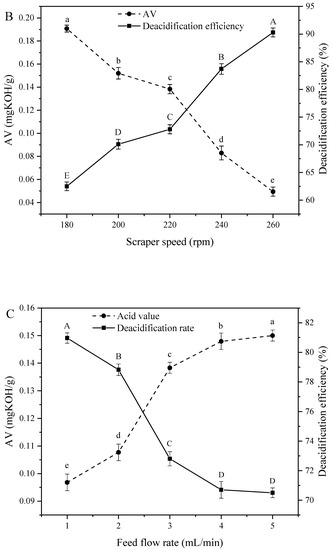
Figure 1.
Effect of (A) evaporation temperature, (B) scraper speed, and (C) feed flow rate on the acid value (AV) and deacidification efficiency of soybean oil. Note: The lowercase letters of a-e indicate that there are significant differences in acid value under different treatment conditions; the capital letter of A–E indicate that there were significant differences in deacidification rate under different treatment conditions.
Figure 1B shows the effects of scraper speed, revealing that an increase of this parameter from 180 to 260 rpm increased deacidification efficiency (from 62.50% to 90.28%) and decreased AV (from 0.19 to 0.05 mg KOH/g). This behavior was explained by the fact that high scraper speeds increase the uniformity of soybean oil distribution on the evaporation surface and thus facilitate evaporation, while saturation at a certain speed results in a slow AV decrease.
The feed flow rate influences not only heat transfer but also production capacity. As shown in Figure 1C, when the feed flow rate increased from 1 to 5 mL/min, AV increased from 0.09 to 0.15 mg KOH/g, while deacidification efficiency decreased from 80.96% to 70.50%. Low feed flow rates result in prolonged residence of materials on the evaporator surface and hence, in more efficient heat transfers and increased FFA separation efficiency [26], while the reverse is true for high flow rates. Thus, low feed flow rates are required for efficient deacidification.
3.3. Effect of Molecular Distillation Parameters on Lovibond Color
Color is a fundamental quality of edible oils. Table 2 shows the effect of molecular distillation parameters on the Lovibond color of soybean oil, revealing that yellow and red values increased with increasing evaporation temperature. For example, these color values increased approximately 4- and 21-fold, respectively, as the evaporation temperature increased from 120 to 200 °C at a feed flow rate of 3.0 mL/min and a scraper speed of 220 rpm, which agreed with previously reported results [27]. The above darkening suggested that some components (e.g., carotenoids) of EAE-produced soybean oil undergo oxidation at high temperatures to afford intensely colored products.

Table 2.
Effect of molecular distillation parameters on the Lovibond color of EAE-produced soybean oil.
Finally, as the scraper speed increased from 180 to 260 rpm, the yellow value increased 2.30-fold (from 7.7 to 17.7), while the red value increased 5.75-fold (from 1.2 to 6.9). This behavior was ascribed to the increased uniformity of oil distribution on the evaporation surface at high scraper speeds, which facilitates oxidation and results in color darkening.
Unlike evaporation temperature and scraper speed, feed flow rate was negatively correlated with Lovibond color intensity, that is, yellow and red values decreased with increasing feed flow rate, as the residence time of materials on the evaporator surface, and hence, the extent of their oxidation, decreases at high feed rates. Therefore, the use of high feed flow rates precluded the formation of deeply color products at the expense of decreased deacidification efficiency [17].
3.4. Effect of Molecular Distillation Parameters on the Extent of Soybean Oil Oxidation
Hydroperoxides are the most important intermediates of unsaturated fatty acid oxidation and the main products of primary oxidation. These species are very unstable and are readily transformed to secondary oxidation products at high temperature, which increases decomposition efficiency. Therefore, PV can be viewed as an indicator of oil oxidative stability [28]. Figure 2 shows the effects of distillation parameters on the peroxide and p-anisidine values of soybean oil. All characterizations were carried out according to the recommended AOCS standard scheme for oil quality assessment.
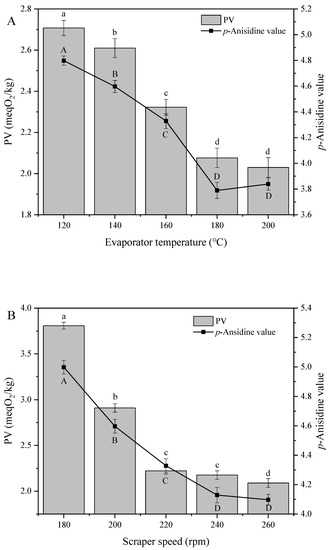
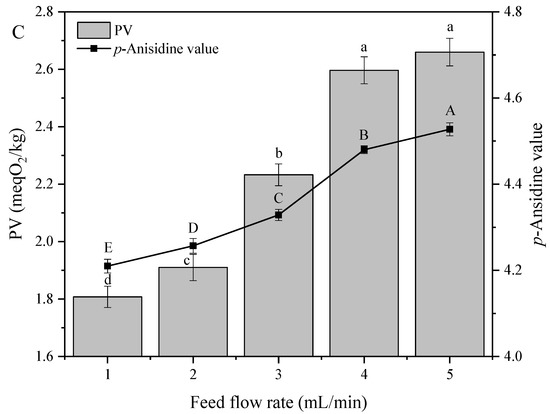
Figure 2.
Effect of (A) evaporation temperature, (B) scraper speed, and (C) feed flow rate on the peroxide (PV) and p-anisidine values of soybean oil. Note: The lowercase letters of a-d indicate that there are significant differences in peroxide value under different treatment conditions; the capital letters of A–E indicate that there were significant differences in p-anisidine value under different treatment conditions.
PV decreased with increasing evaporation temperature and scraper speed. For example, as the evaporation temperature and scraper speed increased from 120 to 200 °C and from 180 to 260 rpm, respectively, PV decreased from 2.71 to 1.99 meqO2/kg (Figure 2A) and 3.81 to 2.09 meqO2/kg (Figure 2B), respectively. The initial reduction of PV was attributed to the decomposition of the thermally unstable lipid hydroperoxide, which is the initial oxidation product of oil [29]. Since low-molecular-weight secondary oxides are distilled as the light phase, the p-anisidine value of soybean oil behaved similarly to PV as the evaporation temperature increased from 120 to 180 °C, whereas an increase of p-anisidine value was observed as the evaporation temperature further increased from 180 to 200 °C. This behavior was similar to that previously reported for other vegetable oils and was ascribed to the degradation of traces of natural bioactive substances, which generated some small molecules including aldehyde, ketone, acid, such as 2-alkenal, 2,4-adienal, and hence increased the p-anisidine value [16]. Since high distillation temperatures are believed to facilitate the loss of natural micronutrients from soybean oil, the distillation temperature of 180 °C was concluded to be suitable for practical application.
Figure 2B shows that both PV and p-anisidine value rapidly decreased as the scraper speed increased from 180 to 260 rpm. Low scraper speeds precluded the formation of a uniform liquid film on the evaporation surface, decreasing the efficiency of mass transfer and reducing separation efficiency. As the scraper speed increased, so did the centrifugal force, which brought the wiper closer to the evaporation surface and increased its effect on the liquid film. Consequently, the thickness of the liquid film decreased and became more uniform, which increased the efficiency of mass/heat transfer. At a certain speed, distribution uniformity became saturated, which resulted in a slow PV decrease.
Figure 2C shows that both PV and p-anisidine value increased with increasing feed flow rate. At low feed flow rates, materials resided on the evaporator surface for a longer time, which resulted in more efficient heat transfer and FFA separation. On the contrary, high feed flow rates decreased heat transfer efficiency and hence, increased PV and p-anisidine value.
3.5. Effect of Molecular Distillation Parameters on Tocopherol Content
Figure 3A shows that tocopherol content significantly decreased with increasing temperature, in agreement with the results of previous studies [23,30]. Specifically, the total tocopherol content declined from 1063.2 to 322.3 mg/kg as the temperature increased from 120 to 200 °C. The above behavior was mainly ascribed to the increased evaporation of heavy components at high temperatures. Tocopherol is unstable at the high temperature and easily degrades, causing the decrease of tocopherol content. In the initial stage of distillation, the evaporation rate of light components is higher than that of heavy components, therefore the tocopherol content in distillates increased gradually. In the initial stage of distillation, the evaporation rate of light components exceeded that of heavy components, and the distillate therefore became enriched with tocopherols as the temperature increased [31]. However, when the temperature reached a certain value, noticeable evaporation of heavy components was observed, and no significant change of tocopherol content was therefore detected.
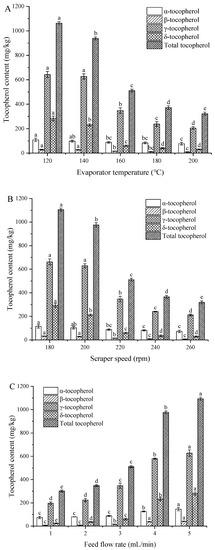
Figure 3.
Effect of (A) evaporation temperature, (B) scraper speed, and (C) feed flow rate on the tocopherol contents of soybean oil. Note: Different letters indicate significant differences between the same groups (p < 0.05).
Figure 3B shows that as scraper speed increased from 180 to 260 rpm, tocopherol content decreased from 1104.9 to 318.9 mg/kg, which was explained as follows. At low scraper speeds, no uniform liquid film was formed on the evaporation surface. As the scraper speed increased, the liquid film became thinner, and the surface renewal speed increased, which increased the tocopherol content of the distillate and greatly reduced the tocopherol content of the residue.
Figure 3C shows that tocopherol content increased with increasing feed flow rate. This behavior was ascribed to the concomitant decrease of oil residence time, which, in turn, decreased the tocopherol distillation rate and increased the tocopherol content of distillation residues.
3.6. Effect of Molecular Distillation Parameters on Fatty Acid Content
Table 3 presents the effects of distillation conditions on the GC–MS-determined contents of fatty acids, revealing that significant (and opposite) changes were observed only for oleic and linoleic acids [32]. At a constant feed flow rate, the evaporation temperature of palmitic acid was lower than that of oleic acid because of the shorter carbon chain of the former. The oleic acid content of soybean oil started to decrease when the evaporation temperature reached the oleic acid evaporation range. Thus, the evaporation of oleic acid was concluded to be suppressed by that of palmitic acid at low temperatures [33]. In addition, previous studies have shown that oleic and linoleic acids have relatively high oxidative stabilities [34]. Similarly, as the scraper speed increased, the soybean oil distribution became more uniform, which favored the evaporation of oleic acid. Notably, the content of palmitic acid could be reduced to 11.37%, while those of oleic and linoleic acids could be increased to 21.70% and 52.07%, respectively. On the contrary, an increase of feed flow rate reduced the oil-evaporator contact time, which was not conducive to the evaporation of oleic acid. Consequently, as the feed flow rate increased from 1 to 5 mL/min, the content of palmitic acid increased from 11.25% to 11.49%, while those of oleic and linoleic acids decreased from 21.70% to 21.44% and from 52.25% to 52.01%, respectively.

Table 3.
Effect of molecular distillation parameters on the fatty acid contents of EAE-produced soybean oil.
4. Conclusions
Molecular distillation was shown to be a suitable method for the deacidification of EAE-produced soybean oil, allowing one to almost completely remove FFAs while avoiding color darkening. The effects of distillation parameters (evaporation temperature, scraper speed, and feed flow rate) on the physicochemical properties of soybean oil were evaluated in detail. The use of high evaporation temperatures and scraper speeds increased deacidification efficiency, avoided product darkening, and favored the retention of tocopherols, while the use of high feed flow rates had the opposite effect. The determined suitable molecular distillation parameters (evaporation temperature = 180 °C, scraper speed = 220 rpm, feed flow rate = 4 mL/min) were concluded to be applicable to the industrial refining of EAE-produced soybean oil to ensure environmentally friendly production. Moreover, the obtained results are expected to provide a firm theoretical basis for the subsequent lipase extraction and refining processes.
Author Contributions
Conceptualization, B.-K.Q., F.-Y.X. and Y.L.; Funding acquisition, Y.L.; Investigation, L.H. and H.L.; Supervision, Y.L.; Writing—original draft, L.H.; Writing—review & editing, S.Z. and B.-K.Q.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2016YFD0401402, Northeast Agricultural University, grant number SB17C01.
Acknowledgments
The authors would like to acknowledge the support of the above sponsors of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosenthal, A.; Pyle, D.L.; Niranjan, K.; Gilmour, S.; Trinca, L. Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzym. Microb. Technol. 2001, 28, 499–509. [Google Scholar] [CrossRef]
- Purkrtova, Z.; Jolivet, P.; Miquel, M.; Chardot, T. Structure and function of seed lipid body-associated proteins. C. R. Biol. 2008, 331, 746–754. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and enzymatic processes for edible oil extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Campbell, K.A.; Glatz, C.E.; Johnson, L.A.; Jung, S.; De Moura, J.M.N.; Kapchie, V.; Murphy, P. Advances in aqueous extraction processing of soybeans. J. Am. Oil Chem. Soc. 2011, 88, 449–465. [Google Scholar] [CrossRef]
- Bocevska, M.; Karlović, D.; Turkulov, J.; Pericin, D. Quality of corn germ oil obtained by aqueous enzymatic extraction. J. Am. Oil Chem. Soc. 1993, 70, 1273–1277. [Google Scholar] [CrossRef]
- Kim, I.H.; Yoon, S.H. Effect of extraction solvents on oxidative stability of crude soybean oil. J. Am. Oil Chem. Soc. 1990, 67, 165–167. [Google Scholar] [CrossRef]
- McGlone, O.C.; Canales, L.M.; Carter, J.V. Coconut Oil Extraction by a New Enzymatic Process. J. Food Sci. 2010, 51, 695–697. [Google Scholar] [CrossRef]
- Rodrigues, C.E.C.; Goncalves, C.B.; Batista, E.; Meirelles, A.J. Deacidification of Vegetable Oils by Solvent Extraction. Recent Pat. Eng. 2007, 1, 95–102. [Google Scholar] [CrossRef]
- Cvengros, J. Physical refining of edible oils. J. Am. Oil Chem. Soc. 1995, 72, 1193–1196. [Google Scholar] [CrossRef]
- Fernandes, K.V.; Papadaki, A.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Koutinas, A.A.; Freire, D.M.G. Enzymatic esterification of palm fatty-acid distillate for the production of polyol esters with biolubricant properties. Ind. Crop. Prod. 2018, 116, 90–96. [Google Scholar] [CrossRef]
- Papadaki, A.; Papapostolou, H.; Alexandri, M.; Kopsahelis, N.; Papanikolaou, S.; Castro, A.M.D.; Freire, D.M.G.; Koutinas, A.A. Fumaric acid production using renewable resources from biodiesel and cane sugar production processes. Environ. Sci. Pollut. Res. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mezza, G.N.; Borgarello, A.V.; Grosso, N.R.; Fernandez, H.; Pramparo, M.C.; Gayol, M.F.J.F.c. Antioxidant activity of rosemary essential oil fractions obtained by molecular distillation and their effect on oxidative stability of sunflower oil. Food Chem. 2018, 242, 9–15. [Google Scholar] [CrossRef]
- Lutišan, J.; Cvengroš, J.; Micov, M. Heat and mass transfer in the evaporating film of a molecular evaporator. Chem. Eng. J. 2002, 85, 225–234. [Google Scholar] [CrossRef]
- Ping, S.; He, J.; Sun, P.; Jiang, S. Process optimization for the production of biodiesel from rapeseed soapstock by a novel method of short path distillation. Biosyst. Eng. 2009, 102, 285–290. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagao, T.; Hirota, Y.; Kitano, M.; Shimada, Y. Purification of tocopherols and phytosterols by a two-step in situ enzymatic reaction. J. Am. Oil Chem. Soc. 2004, 81, 339–345. [Google Scholar] [CrossRef]
- Martinello, M.; Hecker, G.; Pramparo, M.D.C. Grape seed oil deacidification by molecular distillation: Analysis of operative variables influence using the response surface methodology. J. Food Eng. 2007, 81, 60–64. [Google Scholar] [CrossRef]
- Wu, W.L.; Wang, C.; Zheng, J.X. Optimization of deacidification of low-calorie cocoa butter by molecular distillation. LWT Food Sci. Technol. 2012, 46, 563–570. [Google Scholar] [CrossRef]
- Ángela García, S.; Sanz, M.T.; Falkeborg, M.; Beltrán, S.; Guo, Z. Production and concentration of monoacylglycerols rich in omega-3 polyunsaturated fatty acids by enzymatic glycerolysis and molecular distillation. Food Chem. 2016, 190, 960–967. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, M.; Jiang, L.; Sui, X. Simplex-Centroid Mixture Design Applied to the Aqueous Enzymatic; Extraction of Fatty Acid-Balanced Oil from Mixed Seeds. J. Am. Oil Chem. Soc. 2013, 90, 349–357. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. Official Method Cc 13b-45 Color Determination by Tintometer; American Oil Chemists’ Society Press: Champaign, IL, USA, 1973. [Google Scholar]
- Yong, W.; Zhao, M.; Song, K.; Wang, L.; Xue, H.; Tang, S.; Ying, W. Separation of diacylglycerols from enzymatically hydrolyzed soybean oil by molecular distillation. Sep. Purif. Technol. 2010, 75, 114–120. [Google Scholar] [CrossRef]
- More, N.S.; Gogate, P.R. Ultrasound assisted enzymatic degumming of crude soybean oil. Ultrason. Sonochem. 2018, 42, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.F.; Ito, V.M.; Batistella, C.B.; Maciel, M.R.W. Free fatty acid separation from vegetable oil deodorizer distillate using molecular distillation process. Sep. Purif. Technol. 2006, 48, 78–84. [Google Scholar] [CrossRef]
- Ning, L.; Yong, W.; Zhao, Q.; Zhao, M. Production of palm oil-based diacylglycerol using Lecitase Ultra-catalyzed glycerolysis and molecular distillation. Food Sci. Biotechnol. 2014, 23, 365–371. [Google Scholar] [CrossRef]
- Cvengroš, J.; Lutišan, J.; Micov, M. Feed temperature influence on the efficiency of a molecular evaporator. Chem. Eng. J. 2000, 78, 61–67. [Google Scholar] [CrossRef]
- Cermak, S.C.; John, A.L.; Evangelista, R.L. Enrichment of decanoic acid in cuphea fatty acids by molecular distillation. Ind. Crop. Prod. 2007, 26, 93–99. [Google Scholar] [CrossRef]
- Compton, D.L.; Laszlo, J.A.; Eller, F.J.; Taylor, S.L. Purification of 1,2-diacylglycerols from vegetable oils: Comparison of molecular distillation and liquid CO2 extraction. Ind. Crop. Prod. 2008, 28, 113–121. [Google Scholar] [CrossRef]
- Mishra, R.; Sharma, H.K.; Singh, C. Thermal oxidation of rice bran oil during oven test and microwave heating. J. Food Sci. Technol. 2012, 49, 221–227. [Google Scholar] [CrossRef]
- Li, J.; Sun, D.; Qian, L.; Liu, Y. Subcritical Butane Extraction of Wheat Germ Oil and Its Deacidification by Molecular Distillation. Molecules 2016, 21, 1675. [Google Scholar] [CrossRef]
- Moraes, E.B.; Batistella, C.B.; Alvarez, M.E.T.; Filho, R.M.; Maciel, M.R.W. Evaluation of tocopherol recovery through simulation of molecular distillation process. Appl. Biochem. Biotechnol. 2004, 114, 689–711. [Google Scholar] [CrossRef]
- Martinello, M.A.; Villegas, M.; Pramparo, M.D.C. Retaining maximum antioxidative potency of wheat germ oil refined by molecular distillation. J. Sci. Food Agric. 2010, 87, 1559–1563. [Google Scholar] [CrossRef]
- Japir, A.A.-W.; Salimon, J.; Derawi, D.; Bahadi, M.; Yusop, M.R. Separation of free fatty acids from high free fatty acid crude palm oil using short-path distillation. AIP Conf. Proc. 2016, 1784. [Google Scholar] [CrossRef]
- Ketenoğlu, O.; Erdoğdu, F.; Tekin, A. Multi-objective Optimization of Molecular Distillation Conditions for Oleic Acid from a Rich-in-Fatty Acid Model Mixture. J. Oleo Sci. 2017, 67, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Qi, B.; Rokayya, S.; Ma, W.; Liang, J.; Sui, X.; Zhang, Y.; Jiang, L. Heating quality and stability of aqueous enzymatic extraction of fatty acid-balanced oil in comparison with other blended oils. J. Chem. 2014, 2090–9063. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).