1. Introduction
Implant osseointegration is a concept that now enjoys wide support. In 1999, Alberktsson and Zarb defined osseointegration from the clinical standpoint as a rigid and clinically asymptomatic fixation process of an alloplastic material, in bone loaded functionally [
1]. The most important aspects for successful osseointegration are the biological characteristics of the host site (the patient) and the macro- and micro-structure of the titanium implant [
1,
2]. Dental implant surfaces have now achieved outstanding performances, which were previously unimaginable. This ensures an extremely high percentage of osseointegration, even in the most complex situations [
3]. However, this meant that the margins for the further improvement of modern surfaces, through mechanical or chemical procedures, are very small. Improvement can be achieved biologically, although, through the addition of autologous growth factors, obtained by processing the patient’s venous blood to the implant surface.
The study on tissue reparative processes has highlighted the fundamental role played by platelets (in this context), which are physiological reservoirs of growth factors and proteins. There are various platelet concentrates, such as platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF), which reconstruct bone defects [
2]. Numerous studies have shown that PRF provides positive results in tissue engineering [
4]. A research by Sohn et al. has demonstrated the greater regeneration capacity of the CGF and its multi-purpose use [
5]. After a long phase of study, our therapeutic choice was the use of the CGF, for the following reasons. It is 100% autologous and biocompatible, requires a simple preparation, is easily identifiable, has a very high concentration of platelets in a fibrin network, has a presence of growth factors and no manipulation of the product is necessary when exclusively using autologous blood products, without the addition of other substances. Platelets, in particular, contain biologically active proteins at high concentrations and support cell healing, growth, and morphogenesis [
6]. In addition to platelets, CGF contains fibroblasts, leukocytes, and endothelial cells for angiogenesis and tissue remodeling; and provides a matrix for cell migration [
7,
8]. CGF is a fibrin biomaterial rich in the growth factors obtained by centrifugation of venous blood, at alternating speeds, as set on the Silfradent device [
8].
CGF, associated with guided bone regeneration, has been used to accelerate new bone formation. Due to its special characteristics, including lack of immune reaction, capability of accelerating tissue healing and vascularization, and anti-swelling properties, CGF is widely used in implant surgery [
9,
10,
11]. However, the interaction between CGF and dental implant is not clear. The addition of autologous growth factors to the implant surface is hindered by titanium’s characteristics of extremely low wettability [
12]. This means that to simply wet the implant with autologous growth factors is of little use, unless it is left in immersion for more than 30 min [
13]. This makes the procedure difficult to include in the clinical routine. In view of these difficulties, the challenge of producing a biologically active surface still remains. The present study reports a protocol that could produce a biologically active implant surface. The growth factors are incorporated onto the implant surface, using a dedicated implant ampoule, which enables the procedure to be carried out in a closed field. A centrifuge device (Round up) made by a Silfradent related to the ampoule enables autologous fibrin and growth factors to be incorporated onto the implant surface, within five seconds. We verified the adhesion of CGF on the titanium implant surface and then quantified the release of the vascular endothelial growth factor (VEGF) from CGF, the liquid phase of CGF (LPCGF), and CGF- or LPCGF-permeated implants.
2. Materials and Methods
2.1. Preparation of CGF and LPCGF
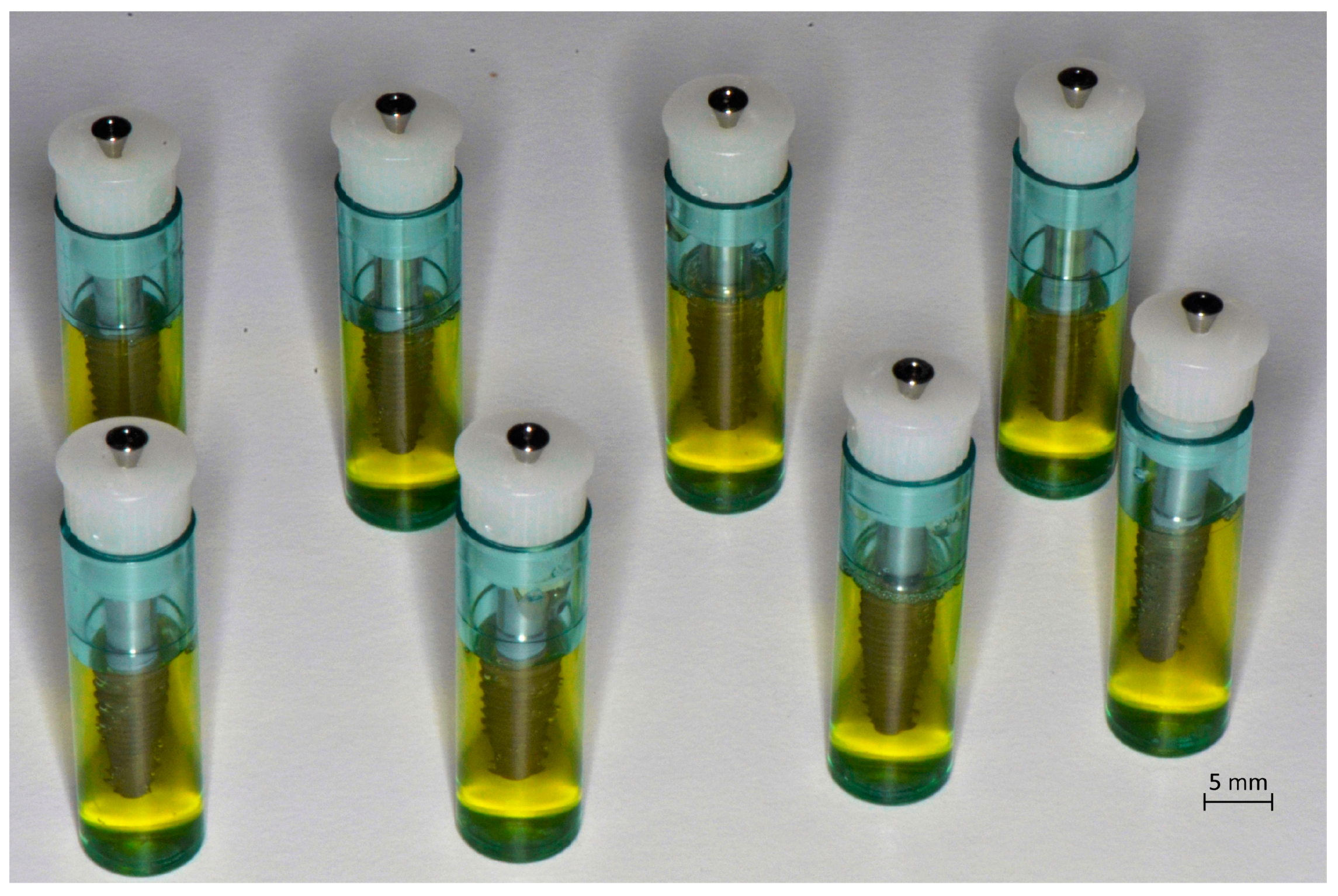
Blood samples (8 mL) were taken by puncture of a vein from five donor patients, non-smokers, and those in good general health. The five donors involved in the study (three men and two women) were aged between 25 and 45 years, with a BMI (Body Mass Index) between 21 and 23 points. The remote and pathological anamnesis were negative. Patients were not on therapy with any type of drug and the blood samples were taken separate from the main meals, on empty stomach. Informed consent was obtained from the patients included in this study. Tubes of blood were processed by a device (Medifuge MF200; Silfradent srl, Forlì, Italy) to obtain CGF, following the manufacturer’s instructions. The resulting CGF was then inserted into dedicated implant ampoules (
Figure 1), so that the coating procedure could be carried out in a closed field; each contained an implant (Immediateload
®, Swiss dental implants, diameter 4 mm and height 8 mm). To incorporate the CGF onto the implant surface, these tubes were inserted into a second device, Round Up (Silfradent srl, Forlì, Italy), and centrifuged for 5 s, following the manufacturer’s instructions (
Figure 2). Evaluation of the quality of the CGF was done on two fractions—the so-called white fraction and the red fraction.
The white cup-tubes allowed the obtainment of a fraction, known as LPCGF (liquid phase of CGF), that comprised non-polymerized liquid fibrin. It was isolated by placing the centrifuged blood in a test tube, with a white lid; this was completely smooth within and contained no additives. It produced the material in a liquid state; this would polymerize at room temperature (RT), over the subsequent 15 min.
The red cup-tubes allowed the obtainment of a fraction, known as CGF polymerized fibrin, which was isolated using a test tube with a red lid and textured inner walls, to promote polymerization, through an exclusively physical process. The resulting fraction had a thicker consistency than the fraction obtained by the white cup-tube, a gelatinous appearance, and a higher cell concentration of the non-polymerized fibrin.
2.2. SEM Analysis
The CGF-permeated implant was fixed in 2.5% glutaraldehyde for 2 h at RT. The specimen was fixed with 1% osmium tetroxide for ~2–4 h, and then dehydrated with a graded ethanol series (from 50% to 100%, in steps of about 20%, for 10 min each). After dehydration, the SEM preparation procedure was completed by critically drying the material. The analysis was performed by means of ZEISS EVO 40 (Carl Zeiss, Milano, Italy) in a low vacuum modality and by applying a voltage of 25 kV. The sample was placed on the SEM sample holder, using double-sided adhesive tape, and was observed without any further manipulation, at a lower and higher magnification (50× and 1000×) [
14].
2.3. ELISA Assay
LPCGF (white fraction) and CGF (red fraction), or implants plus CGF/LPCGF, immediately after the preparation, were transferred to the wells containing phosphate buffer saline (PBS, Sigma Aldrich, Milan, Italy). The supernatants were collected at time 0, and after 1, 2, and 3 days. VEGF concentrations in the media were determined by using ELISA and following the manufacturer’s protocols (R&D Systems, Minneapolis, MN, USA). In brief, 100 μL of the culture supernatant was added to each ELISA well, pre-coated with anti-human VEGF polyclonal antibody. After 2 h of incubation at room temperature, the plate was washed and 100 μL of human VEGF conjugate was added to each well. The plate was incubated at RT for 1 h, washed again, and 100 μL of the substrate solution was added to each well. The plate was then incubated at RT, in the dark, for color development. After 30 min, 100 μL of stop solution was added to each well. Absorbance in each well was measured by using a microplate reader at 450 nm. The concentration of VEGF in the culture supernatant was determined through interpolation from the standard curve.
2.4. Statistical Analysis
Data were expressed as mean ±SD. Statistical analysis was determined by paired Student’s t-test. In all comparisons, p < 0.05 was considered as statistically significant.
3. Results and Discussion
CGF is constituted by a fibrin network that includes many cellular components, such as stem cells and growth factors [
8,
15]. The CGF exerted its effects through the degranulation of the platelet granules, which contained various growth factors that are considered important in the initial phase of wound healing. This resulted in an increase in cell proliferation and differentiation, matrix formation, osteoid tissue production, connective tissue formation, angiogenesis, and collagen synthesis. The degranulation process began immediately after platelet aggregation and lasted about 7–8 days. This affected the macrophage cells that continued the repair process. The wound healing rate was directly proportional to the quality of platelet concentration in the clot, inside the graft.
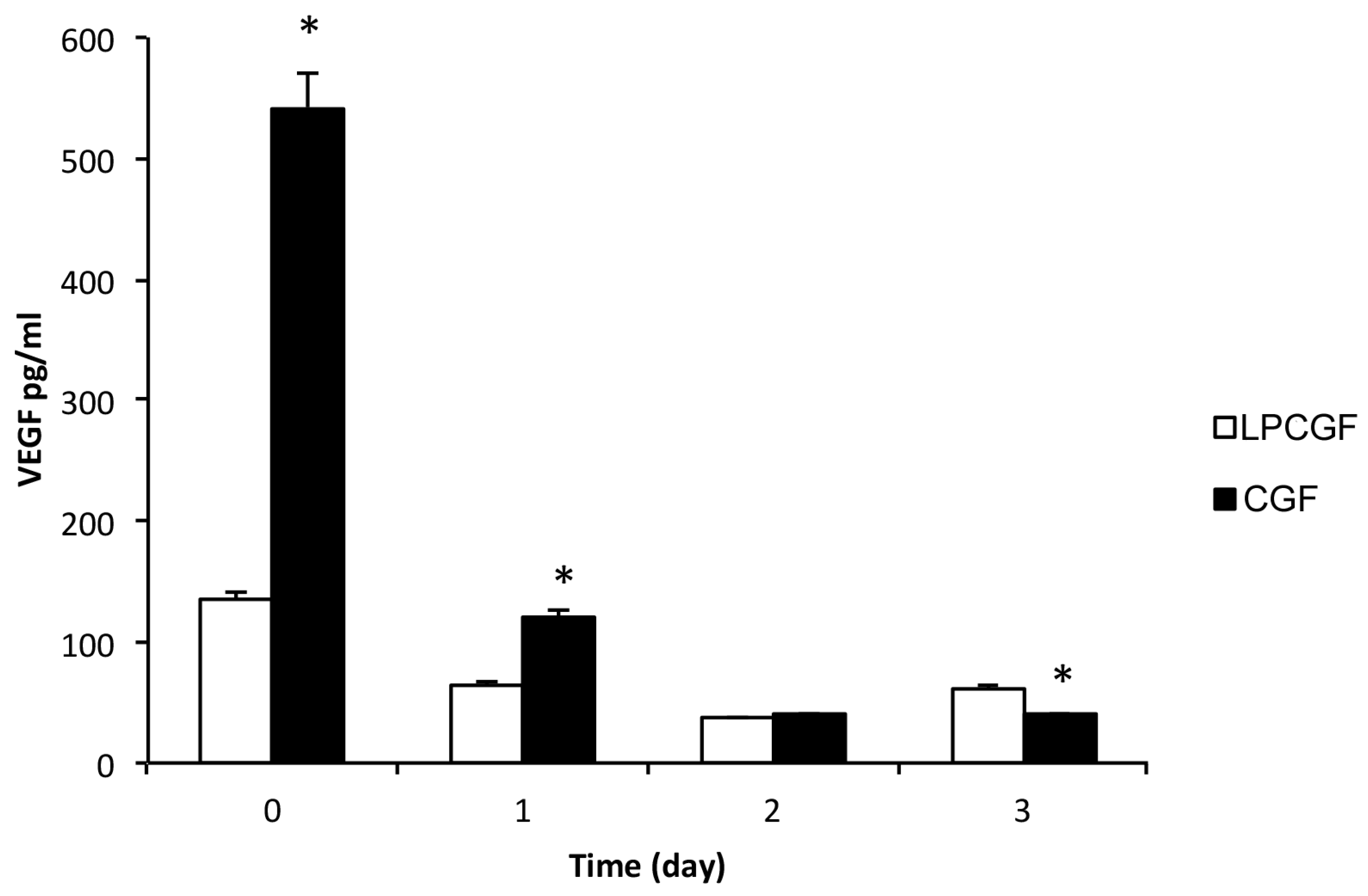
In the present study, we obtained two different concentrated growth factors, named CGF and LPCGF, and we analyzed the release of the growth factor VEGF, from both preparations incubated in PBS, for up to three days, as shown in
Figure 3.
The results showed that both preparations of CGF and LPCGF released VEGF, the concentration of VEGF was higher in CGF than in LPCGF, by about five times, at time 0 (
Figure 3). However, the VEGF release from the CGF drastically decreased by about 78% and 93%, after the first and second day, respectively, compared with time 0 (
Figure 3). The VEGF release was also reduced in LPCGF by about 43% after the first day, and was further lowered on the second day, reaching levels comparable to the VEGF release from the CGF (
Figure 3). Our results expand on the previous findings regarding the release of VEGF by CGF [
15].
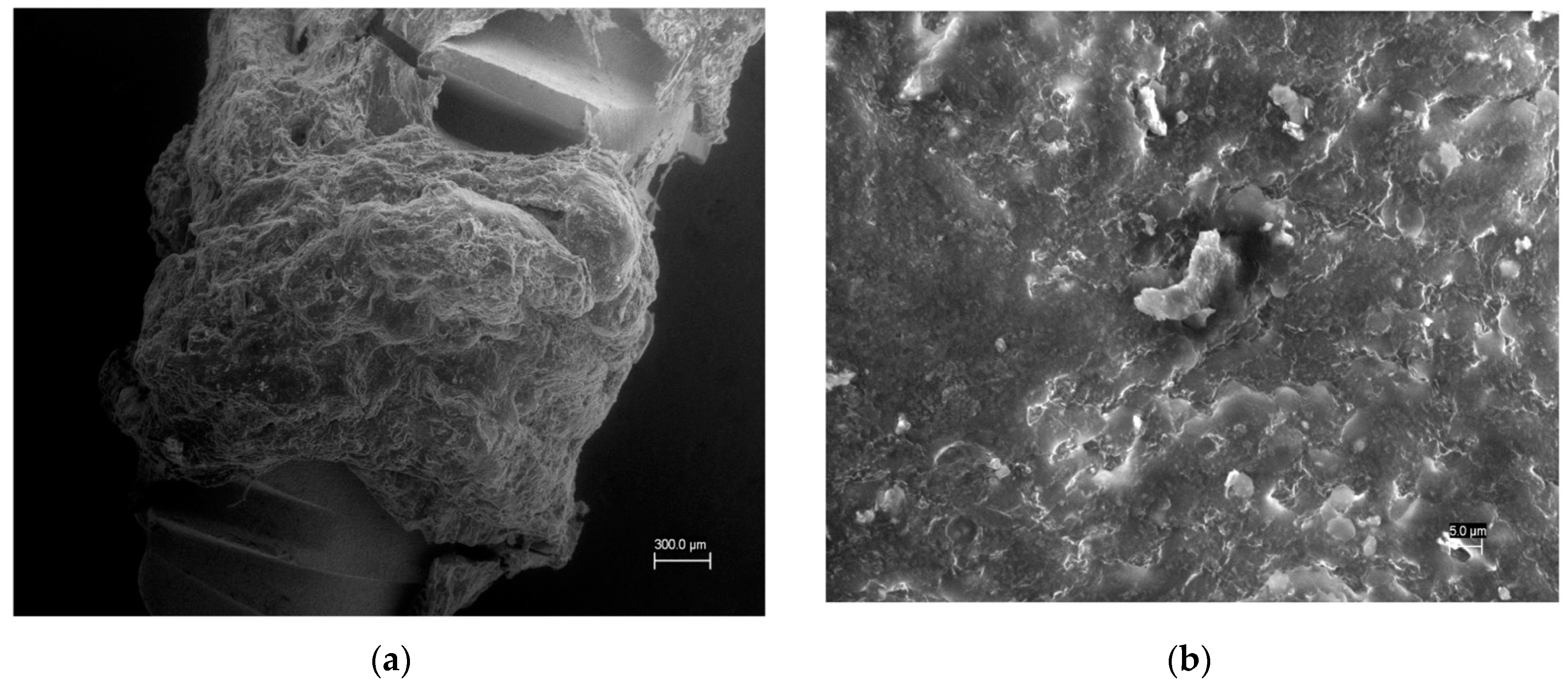
By using an innovative device, we evaluated the potential incorporation of CGF on the surface of the titanium implant. We verified the interaction between the CGF and the titanium implant surface, by SEM analysis, revealing that the CGF actually permeated the surface of the implant (
Figure 4). Having shown that, in our experimental conditions the CGF was able to adhere to the implanted surface forming a fibrin network, we evaluated whether the CGF-permeated implant also allowed the release of growth factors, in particular VEGF, from the implant towards the medium—PBS.
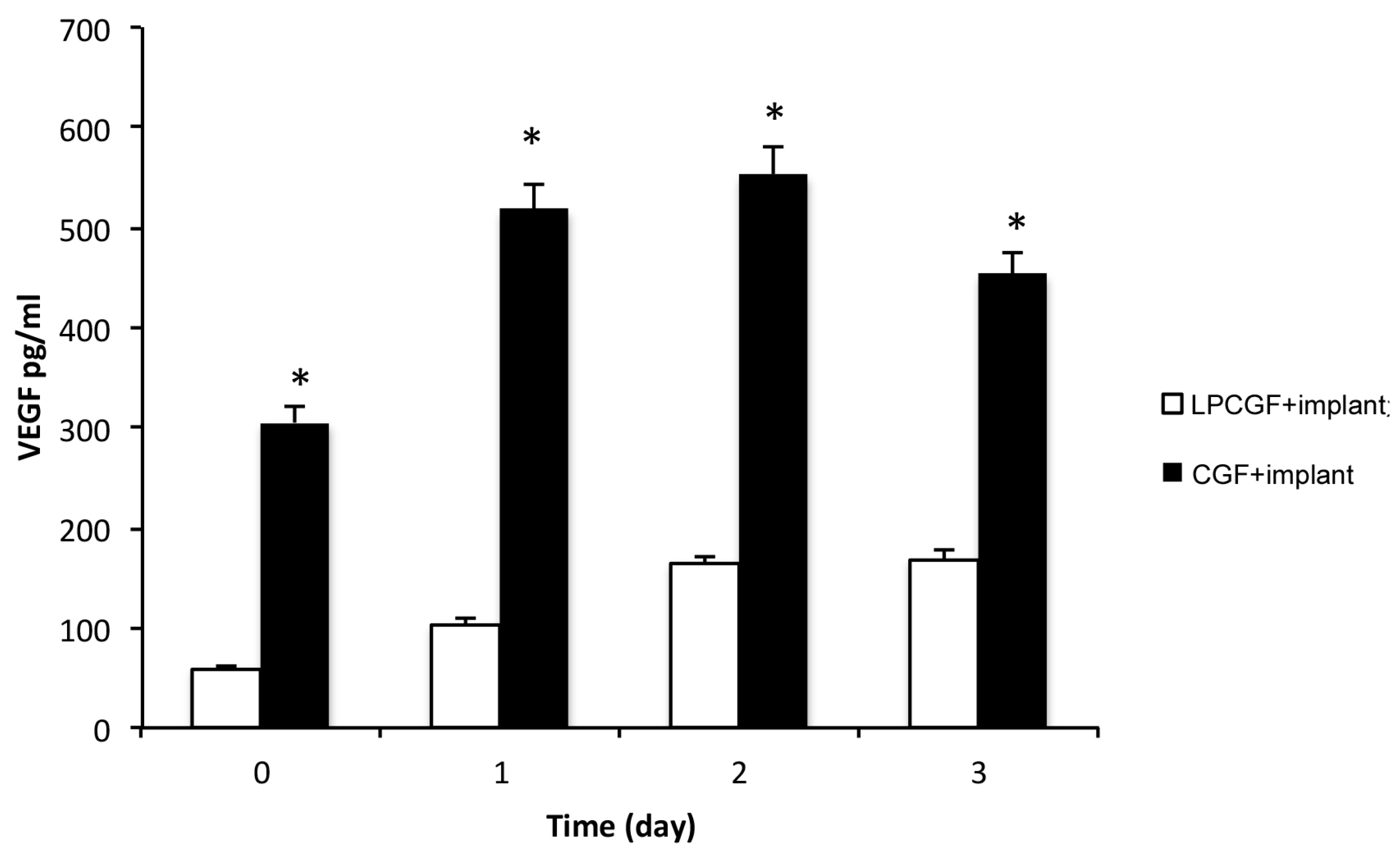
As shown in
Figure 5, the implants permeated with CGF or LPCGF were able to release VEGF in PBS medium. At time 0, in the permeated implant, as well as in the system without implants, the concentration of VEGF was five times higher in CGF than in LPCGF. However, contrary to the CGF or LPCGF without implants, VEGF released from the CGF or the LPCGF-permeated implant, increased on time. In particular, the VEGF levels from the LPCGF-permeated implant increased in a time-dependent manner, until the second day and then remained constant.
The time course in
Figure 5 also shows that the VEGF released from the CGF-permeated implant was significantly raised on the first day, by about 70%, remained almost constant on the second day, and was then lowered by about 15% on the third day (
Figure 5). To the best of our knowledge, our study provided, for the first time, results concerning the incorporation of autologous growth factors on dental implants, and the associated release of VEGF over time.
It is important to emphasize that the use of specific implants was crucial for a better implant micro-surface that enabled the growth factors to settle on the inner surface. The surface of the Immediateload implant was specifically designed to be coated by the patient’s CGF, while presenting excellent characteristics of osseointegration, in the absence of CGF.
From a practical standpoint, a coating with LPCGF could be achieved through a closed system created for the direct addition of LPCGF, within the implant tube, using needles. The procedure is easily reproducible in an outpatient setting, thanks to the dedicated implant tubes. However, when using CGF, although its concentration of VEGF at time zero was much higher than that in LPCGF, it entailed the opening of the tube for inserting the CGF, and its closure, before centrifugation. However, this last procedure (the opening of the tube and the addition of the CGF) took place in an open system, but with a contiguity of time and space that did not limit its clinical application.
From a clinical point of view, it would be very important and interesting to evaluate if a slow and gradual release of VEGF by LPCG, over time, would be more effective, than a quick release of VEGF by CGF. In fact, the presence of VEGF on the implant surface was crucial, since this growth factor could improve the osseointegration of the dental implant [
16].
Incorporation of the CGF/ LPCGF on the surface of the titanium implant could be carried out in private practice, but always according to the national laws of a country, which might be different from one country to another. The organization should first obtain all necessary authorizations for medical and surgical practice.
4. Conclusions
The results reported here showed that a titanium dental implant surface, permeated with CGF or LPCGF, contained fibrin, which is fundamental to accommodate the cellular network. The permeated dental implant surface was found to slowly release VEGF, a growth factor indispensable in creating intercellular communication and neo-angiogenesis, during bone regeneration and healing [
17,
18,
19].
The devices used in this study could be employed to produce the first biologically active implant surface, permeated with both fibrin (which is essential to accommodate the cellular network) and growth factors (which are essential to create intercellular communication and neo-angiogenesis). By using this procedure, the osseointegration process becomes bilateral, operating both from the bone towards the implant, and from the implant towards the bone. This could reduce healing time and potentiate the physiological response.
It will, thus, become possible to expand the application of this type of surface in other fields of medicine, including orthopedics, maxillofacial surgery, and plastic surgery. Further studies are needed to investigate the use of biologically active surfaces, in greater depth, and to further improve the implant micro-surfaces, making them increasingly permeated by the autologous growth factors.