Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Adsorbents
2.2. Characterizations
2.3. CO2 Adsorption
3. Results and Discussion
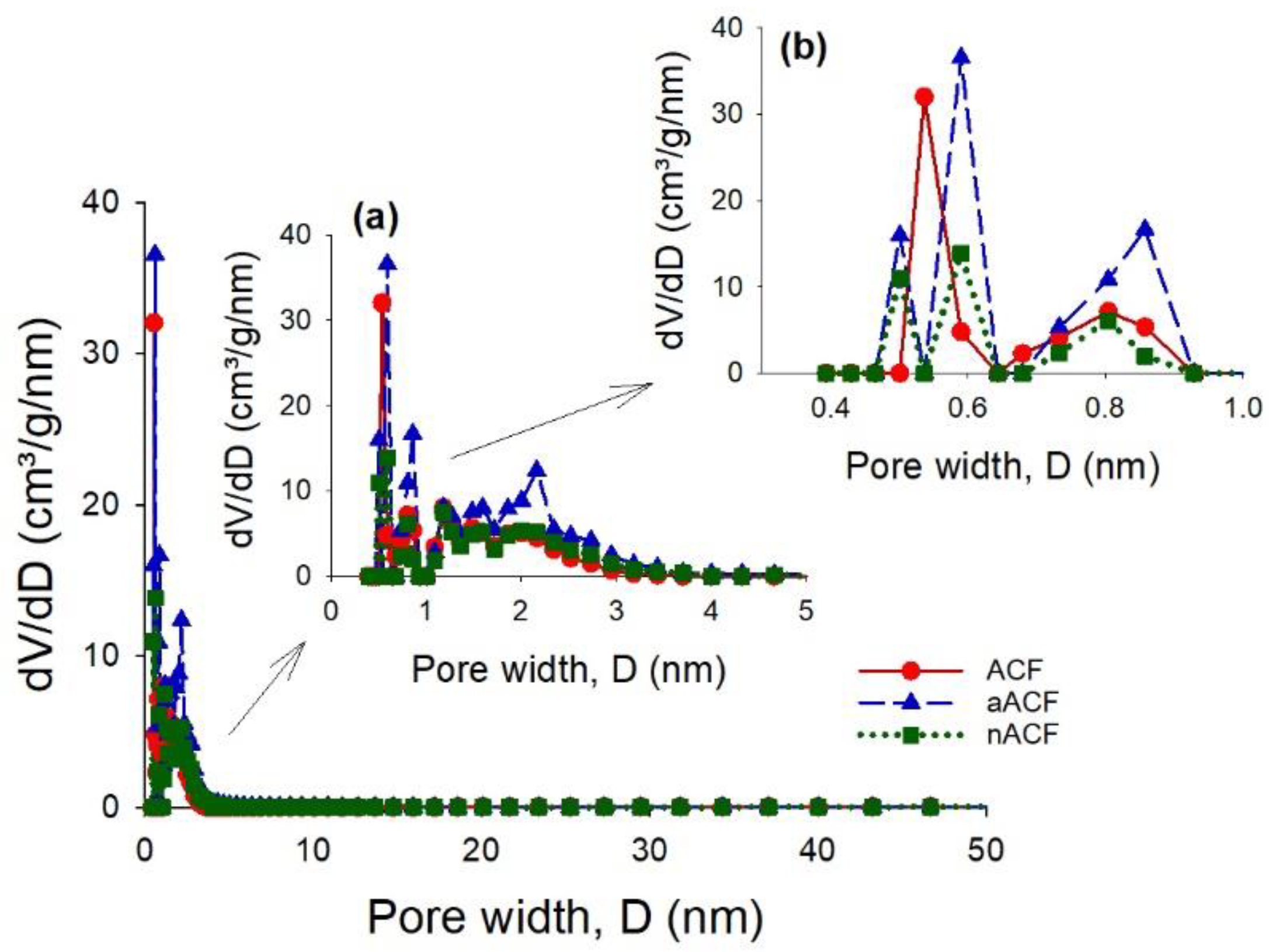
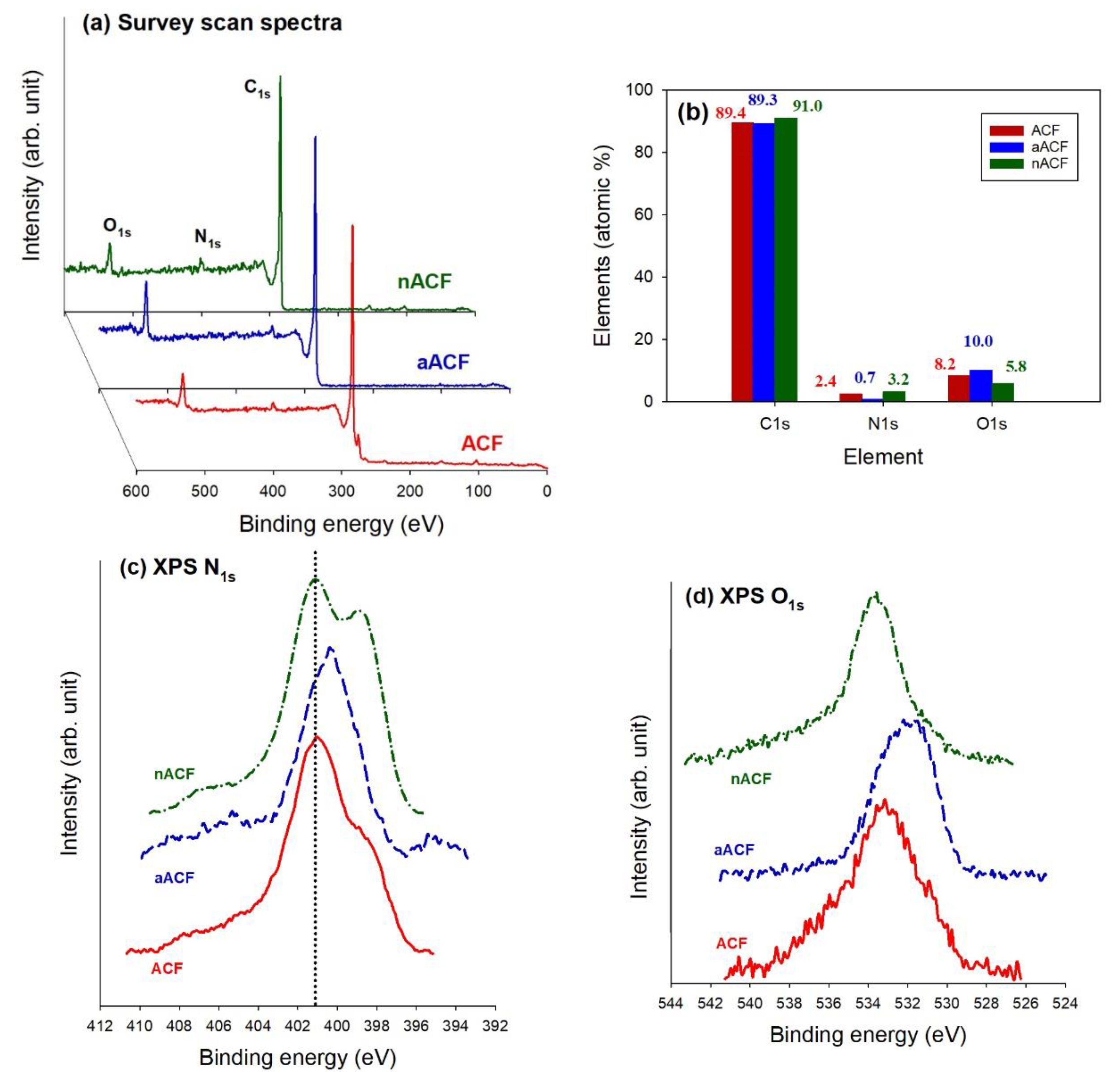
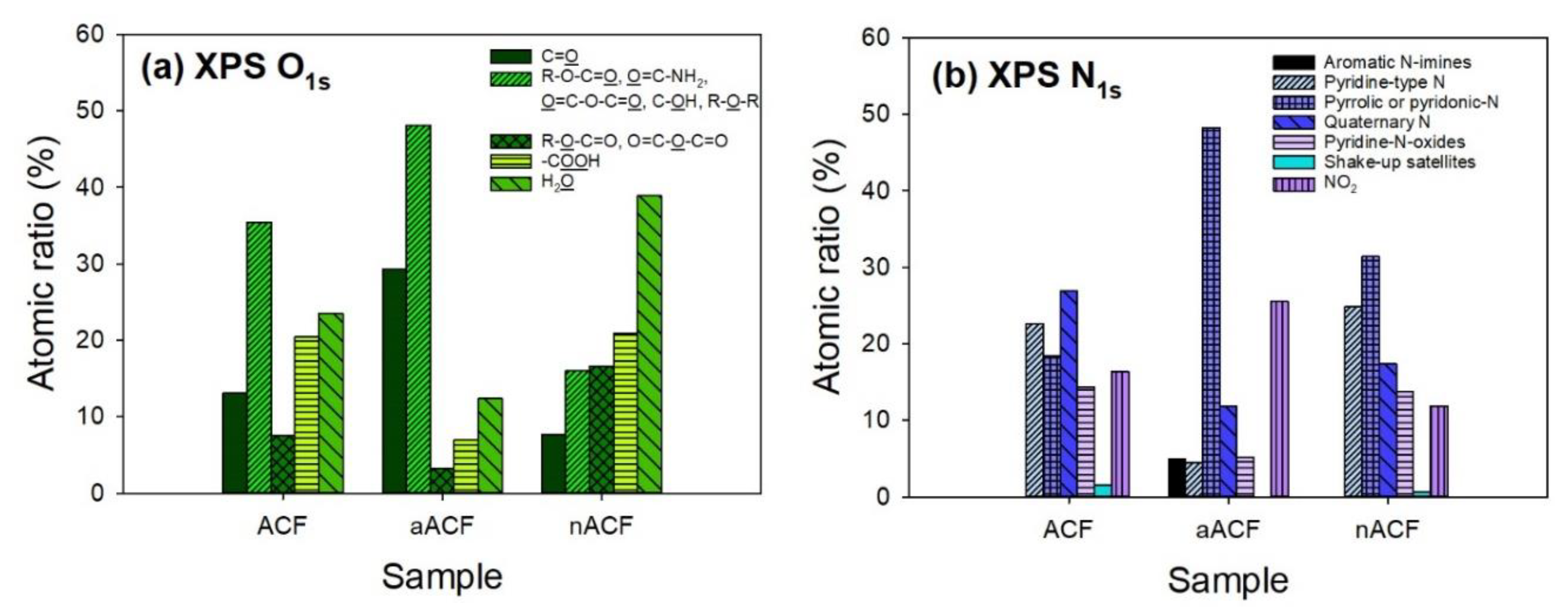
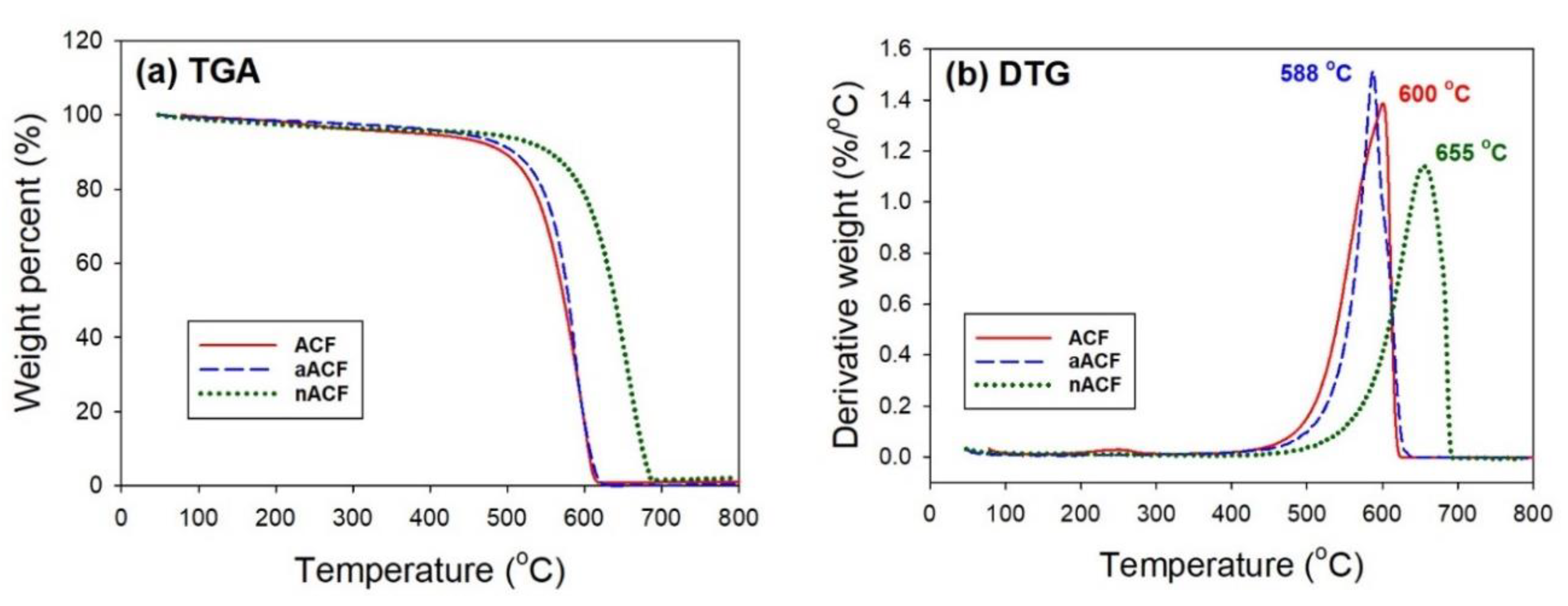
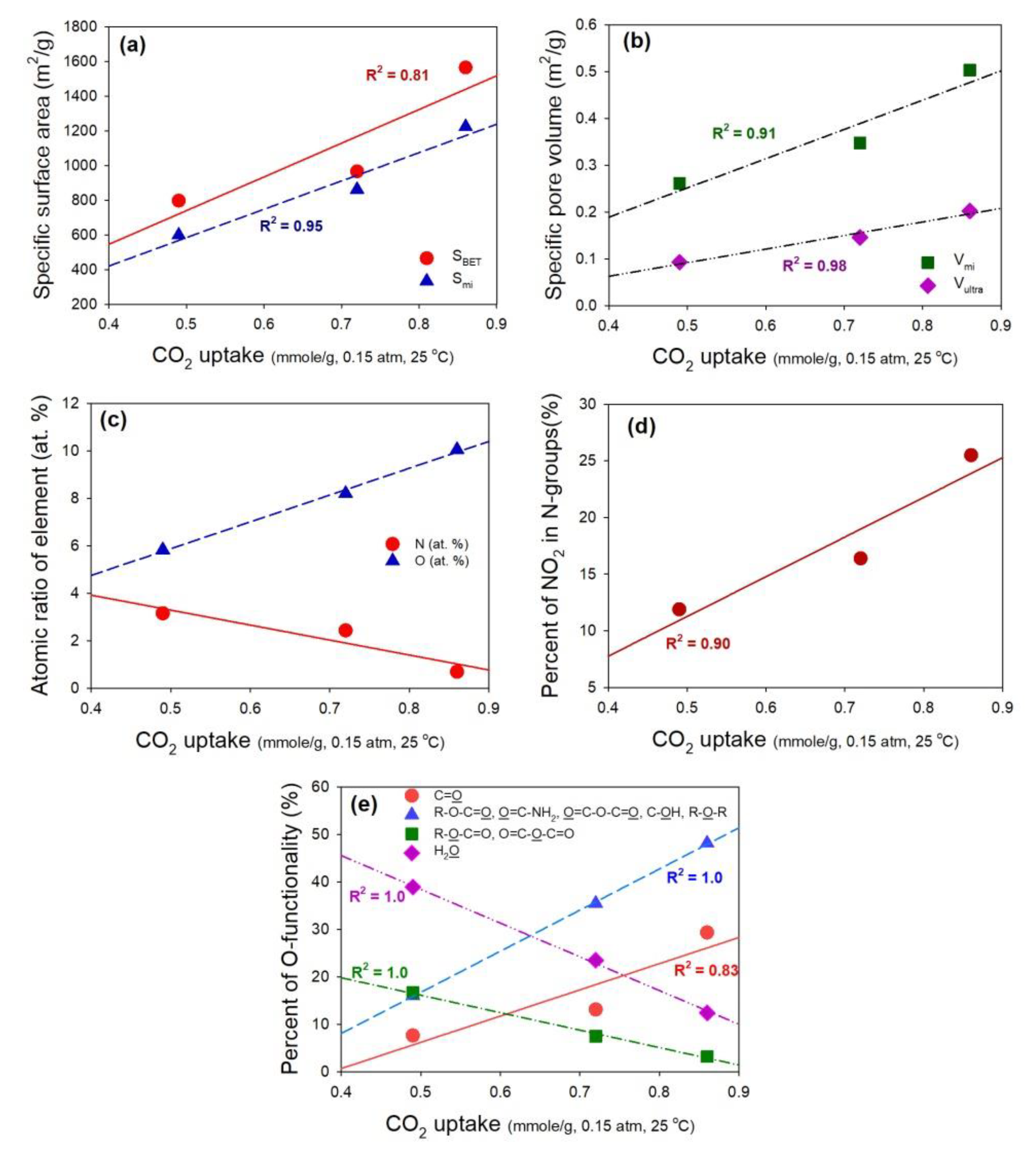
3.1. Textural and Chemical Characterization of the Adsorbents
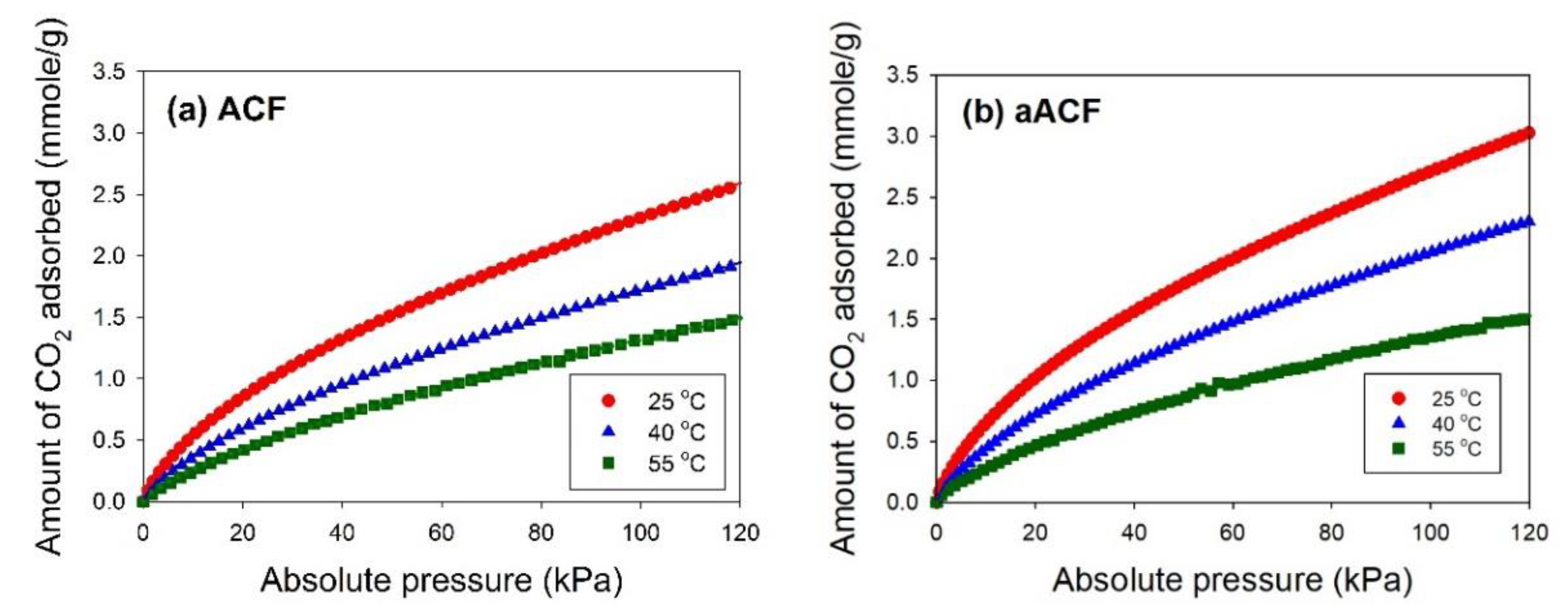
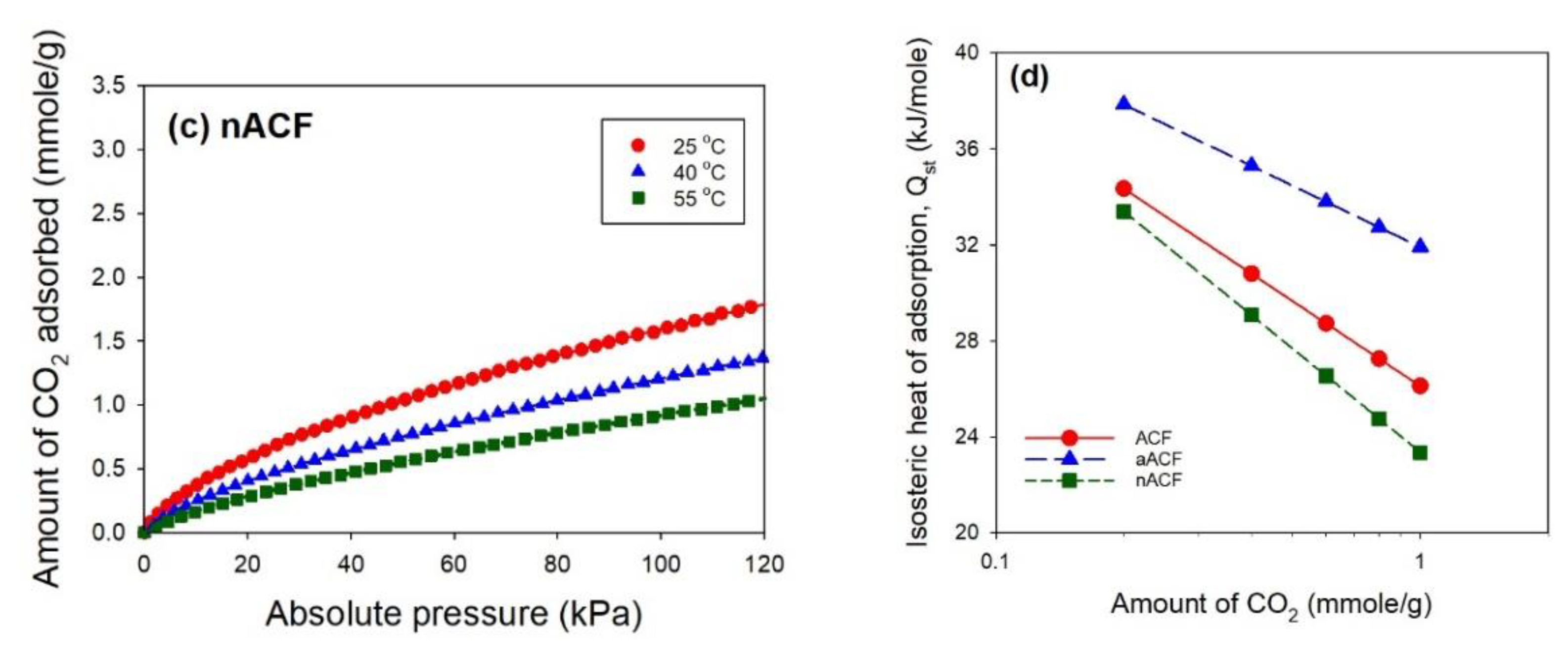
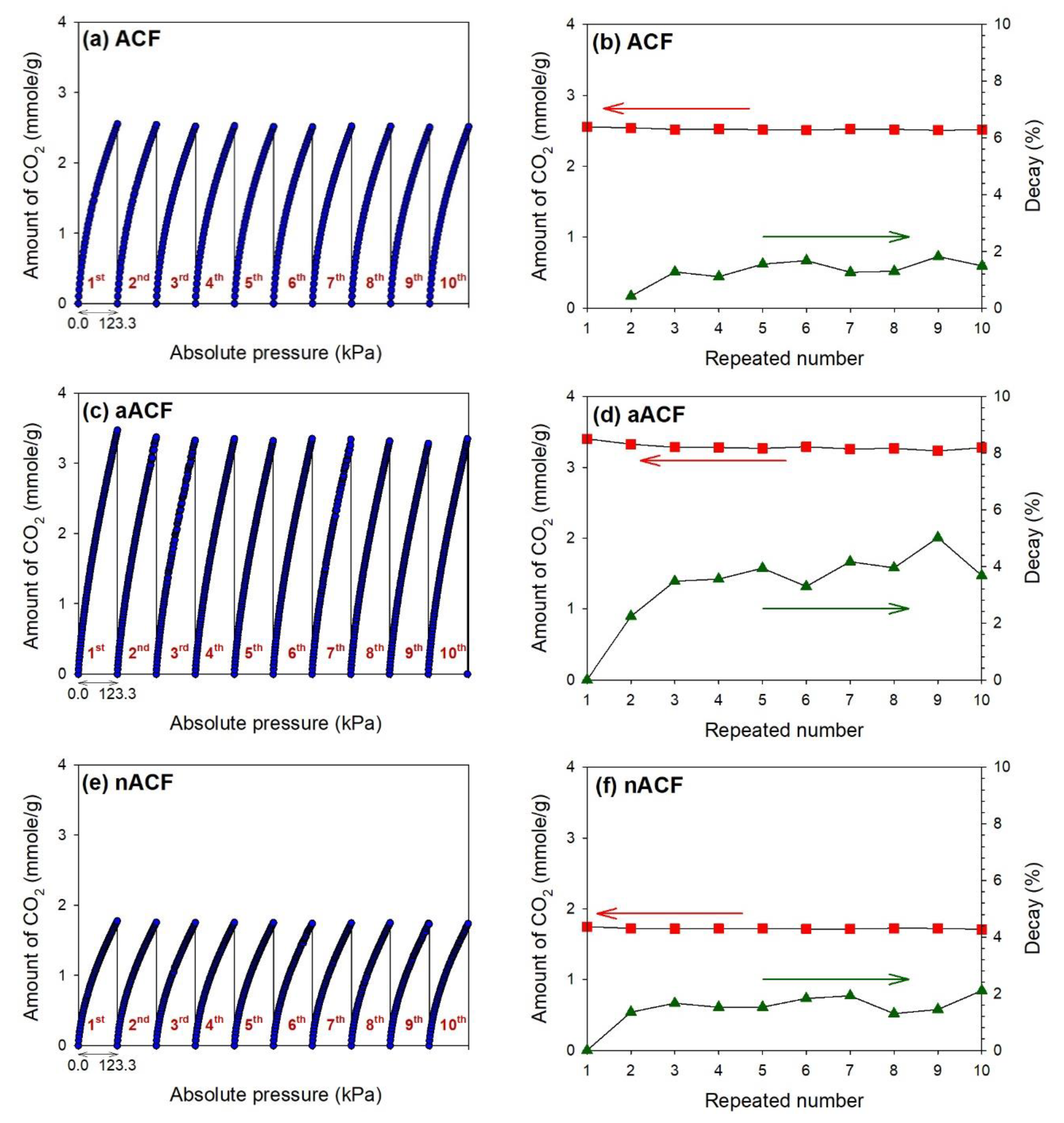
3.2. CO2 Adsorption Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Colloid Interface Sci. 2013, 389, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.D.; Chatti, R.V.; Biniwale, R.B.; Labhsetwar, N.K.; Devotta, S.; Rayalu, S.S. Monoethanol amine modified zeolite 13× for CO2 adsorption at different temperatures. Energy Fuels 2007, 21, 3555–3559. [Google Scholar] [CrossRef]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Pennline, H.; Baltrus, J.P.; Stevens, R.W., Jr. Improved immobilized carbon dioxide capture sorbents. Fuel Process. Technol. 2005, 86, 1449–1455. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Chen, W.; Bai, H.; Hwang, J.F. Capture of CO2 from flue gas via multiwalled carbon nanotubes. Sci. Total Environ. 2009, 407, 3017–3023. [Google Scholar] [CrossRef]
- Sevilla, M.; Valle-Vigón, P.; Fuertes, A.B. N-doped polypyrrole-based porous carbons for CO2 capture. Adv. Funct. Mater. 2011, 21, 2781–2787. [Google Scholar] [CrossRef]
- Xiao, P.W.; Guo, D.; Zhao, L.; Han, B.H. Soft templating synthesis of nitrogen-doped porous hydrothermal carbons and their applications in carbon dioxide and hydrogen adsorption. Microporous Mesoporous Mater. 2016, 220, 129–135. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Usefulness of CO2 adsorption at 273 K for the characterization of porous carbons. Carbon 2004, 42, 1233–1242. [Google Scholar] [CrossRef]
- Yang, R.; Liu, G.; Li, M.; Zhang, J.; Hao, X. Preparation and N2, CO2 and H2 adsorption of super activated carbon derived from biomass source hemp (Cannabis sativa L.) stem. Microporous Mesoporous Mater. 2012, 158, 108–116. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Tang, Z.; Zhang, Y. CO2 capture by activated and impregnated anthracites. Fuel Process. Technol. 2005, 86, 1487–1502. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, B.; Bao, Z.; Deng, S. Adsorption of carbon dioxide, methane and nitrogen on an ultramicroporous copper metal–organic framework. J. Colloid Interface Sci. 2014, 430, 78–84. [Google Scholar] [CrossRef]
- De Souza, K.C.; Wickramaratne, N.P.; Ello, A.S.; Costa, M.J.F.; da Costa, C.E.F.; Jaroniec, M. Enhancement of CO2 adsorption on phenolic resin-based mesoporous carbons by KOH activation. Carbon 2013, 65, 334–340. [Google Scholar] [CrossRef]
- Vorokhta, M.; Morávková, J.; Řimnáčová, D.; Pilař, R.; Zhigunov, A.; Švábová, M.; Sazama, P. CO2 capture using three-dimensionally ordered micromesoporous carbon. J. CO₂ Util. 2019, 31, 124–134. [Google Scholar] [CrossRef]
- Im, J.S.; Park, S.J.; Kim, T.J.; Kim, Y.H.; Lee, Y.S. The study of controlling pore size on electrospun carbon nanofibers for hydrogen adsorption. J. Colloid Interface Sci. 2008, 318, 42–49. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Pevida, C.; Drage, T.C.; Snape, C.E. Silica-templated melamine–formaldehyde resin derived adsorbents for CO2 capture. Carbon 2008, 46, 1464–1474. [Google Scholar] [CrossRef]
- Sevilla, M.; Falco, C.; Titirici, M.M.; Fuertes, A.B. High-performance CO2 sorbents from algae. RSC Adv. 2012, 2, 12792–12797. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arenillas, A.; Rubiera, F.; Pis, J.J. CO2 capture by adsorption with nitrogen enriched carbons. Fuel 2007, 86, 2204–2212. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, Y.; Tsang, S.C.E. Nitrogen-enriched carbonaceous materials with hierarchical micro-mesopore structures for efficient CO2 capture. Chem. Eng. J. 2012, 185, 374–379. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Seredych, M.; Rodríguez-Castellón, E.; Cheng, Y.; Daemen, L.L.; Ramírez-Cuesta, A.J. Evidence for CO2 reactive adsorption on nanoporous S- and N-doped carbon at ambient conditions. Carbon 2016, 96, 856–863. [Google Scholar] [CrossRef]
- Suzuki, M. Activated carbon fiber: Fundamentals and applications. Carbon 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, C.; Qiao, W.; Ling, L. Fabrication of hierarchical carbon nanosheet-based networks for physical and chemical adsorption of CO2. J. Colloid Interface Sci. 2019, 534, 72–80. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling ofadsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Otowa, T.; Tanibata, R.; Ltoh, M. Production and adsorption characteristics of MAXSORB: High-surface-area active carbon. Gas Sep. Purif. 1993, 7, 241–245. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-doped porous carbon derived from KOH-activated waste wool: A promising material for selective adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Lin, W.H.; Chang, Y.C. The influence of treatment duration on multi-walled carbon nanotubes functionalized by H2SO4/HNO3 oxidation. Appl. Surf. Sci. 2011, 257, 2401–2410. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Lee, C.Y.; Lee, H.C. Surface chemistry of polyacrylonitrile- and rayon-based activated carbon fibers after post-heat treatment. Mater. Chem. Phys. 2007, 101, 199–210. [Google Scholar] [CrossRef]
- Pels, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Marek, A.W.; Pels, J.R.; Moulijn, J.A. The fate of nitrogen functionalities in coal during pyrolysis and combustion. Fuel 1995, 74, 507–516. [Google Scholar]
- Díez, N.; Álvarez, P.; Granda, M.; Blanco, C.; Santamaría, R.; Menéndez, R. CO2 adsorption capacity and kinetics in nitrogen-enriched activated carbon fibers prepared by different methods. Chem. Eng. J. 2015, 281, 704–712. [Google Scholar] [CrossRef]
- Toth, J. Isotherm equations for monolayer adsorption of gases on heterogeneous solid surfaces. In Fundamentals of Adsorption; Myers, A., Belfort, G., Eds.; Engineering Foundation: New York, NY, USA, 1984; pp. 657–665. [Google Scholar]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, S.; He, Y.; Li, J.; Fan, W. Hierarchical porous polyacrylonitrile-based activated carbon fibers for CO2 capture. J. Mater. Chem. 2011, 21, 14036–14040. [Google Scholar] [CrossRef]
- Burchell, T.D.; Judkins, R.R. Passive CO2 removal using a carbon fiber composite molecular sieve. Energy Convers. Manag. 1996, 37, 947–954. [Google Scholar] [CrossRef]
- An, H.; Feng, B.; Su, S. CO2 capture capacities of activated carbon fibre-phenolic resin composites. Carbon 2009, 47, 2396–2405. [Google Scholar] [CrossRef]
- Moon, S.H.; Shim, J.W. A novel process for CO2/CH4 gas separation on activated carbon fibers—electric swing adsorption. J. Colloid Interface Sci. 2006, 298, 523–528. [Google Scholar] [CrossRef]
- Li, Q.; Yang, J.; Feng, D.; Wu, Z.; Wu, Q.; Park, S.S.; Ha, C.S.; Zhao, D. Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2 capture. Nano Res. 2010, 3, 632–642. [Google Scholar] [CrossRef]
- Lee, M.S.; Park, S.J. Silica-coated multi-walled carbon nanotubes impregnated with polyethyleneimine for carbon dioxide capture under the flue gas condition. J. Solid State Chem. 2015, 226, 17–23. [Google Scholar] [CrossRef]

| Sample | SBET (m2/g) | Smi α (m2/g) | Vt β (cm3/g) | Vme α (cm3/g) | Vmi α (cm3/g) | Vultra α (cm3/g) | Smi/SBET | Vmi/Vt | Vultra/Vt |
|---|---|---|---|---|---|---|---|---|---|
| ACF | 966 ± 16 | 862 | 0.4635 | 0.0427 | 0.3472 | 0.1456 | 0.892 | 0.749 | 0.314 |
| aACF | 1565 ± 26 | 1224 | 0.7357 | 0.1135 | 0.5028 | 0.2020 | 0.782 | 0.683 | 0.275 |
| nACF | 797 ± 10 | 599 | 0.3848 | 0.0612 | 0.2610 | 0.0923 | 0.752 | 0.678 | 0.240 |
| Sample | Temperature (°C) | KF (mmole/g/kPa1/n) | n | R2 | ARE (%) |
|---|---|---|---|---|---|
| ACF | 25 | 0.134 | 1.61 | 0.9997 | 2.26 |
| 40 | 0.084 | 1.52 | 0.9996 | 2.37 | |
| 55 | 0.052 | 1.43 | 0.9995 | 2.38 | |
| aACF | 25 | 0.164 | 1.64 | 0.9998 | 1.89 |
| 40 | 0.102 | 1.54 | 0.9998 | 1.76 | |
| 55 | 0.063 | 1.50 | 0.9993 | 1.54 | |
| nACF | 25 | 0.089 | 1.59 | 0.9998 | 1.48 |
| 40 | 0.052 | 1.47 | 0.9999 | 1.06 | |
| 55 | 0.031 | 1.36 | 0.9999 | 1.23 |
| Support Materials | Surface Modification | SBET (m2/g) | Concentration of CO2 | Adsorption Temp. (°c) | CO2 Uptake (mmole/g) | Reference |
|---|---|---|---|---|---|---|
| ACFs | KOH | 1565 | 1 atm | 25 | 2.74 | This study |
| ACFs | KOH | 527~1720 | 1 bar | 25 | 1.9~2.6 | [34] |
| ACFs | KOH | 780 | 1 atm | 25 | ~1.0 | [37] |
| ACFs | 1461 | 1 atm | 25 | 2.80 | [38] | |
| ACFs | 140~925 | 1 atm | 20 | 0.55–2.28 | [39] | |
| ACFs | 907~1914 | 1 bar | 25 | 0.6~0.7 | [40] | |
| Carbon | 1343 | 1 bar | 25 | 1.50 | [14] | |
| Hierarchical carbon nanosheet | KOH | 1556 | 1 bar | 25 | 3.10 | [25] |
| Carbon sphere | Ethylenediamine | 550 | 1 atm | 25 | 2.90 | [41] |
| Carbon | SOCl2, TEPA | 823 | 1 bar | 25 | 3.2 | [20] |
| Carbon | KOH, urea | 862 | 1 bar | 25 | 2.91 | [29] |
| Silica-coated MWCNTs | Polyethylene-imine | 375 | 1 bar | 25 | 1.41 | [42] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.-C.; Yeh, C.-Y.; Weng, C.-H. Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers. Appl. Sci. 2019, 9, 1977. https://doi.org/10.3390/app9101977
Chiang Y-C, Yeh C-Y, Weng C-H. Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers. Applied Sciences. 2019; 9(10):1977. https://doi.org/10.3390/app9101977
Chicago/Turabian StyleChiang, Yu-Chun, Cheng-Yu Yeh, and Chih-Hsien Weng. 2019. "Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers" Applied Sciences 9, no. 10: 1977. https://doi.org/10.3390/app9101977
APA StyleChiang, Y.-C., Yeh, C.-Y., & Weng, C.-H. (2019). Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers. Applied Sciences, 9(10), 1977. https://doi.org/10.3390/app9101977




