Nanomaterials-Based Electrochemical Sensors for In Vitro and In Vivo Analyses of Neurotransmitters
Abstract
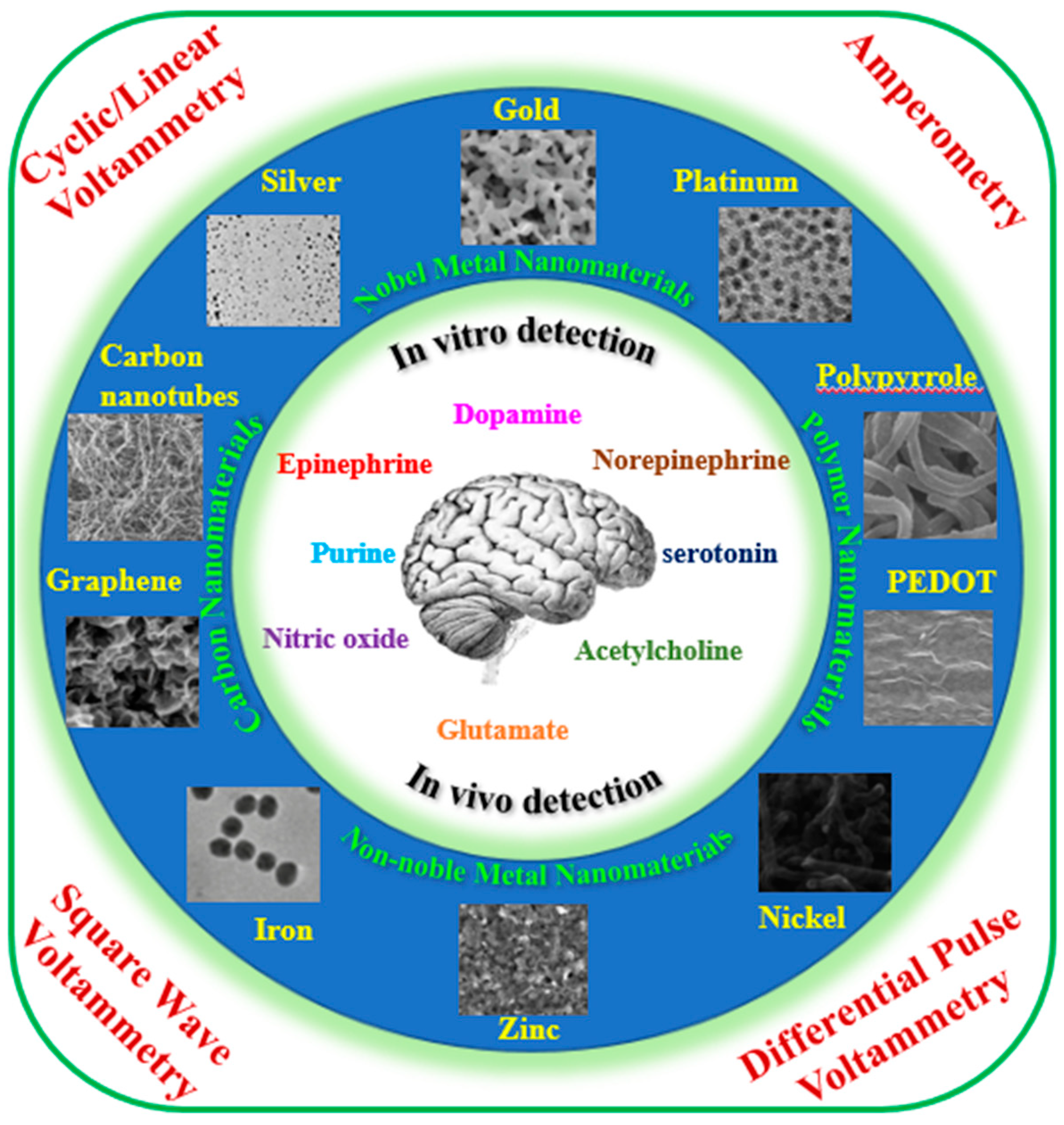
1. Introduction
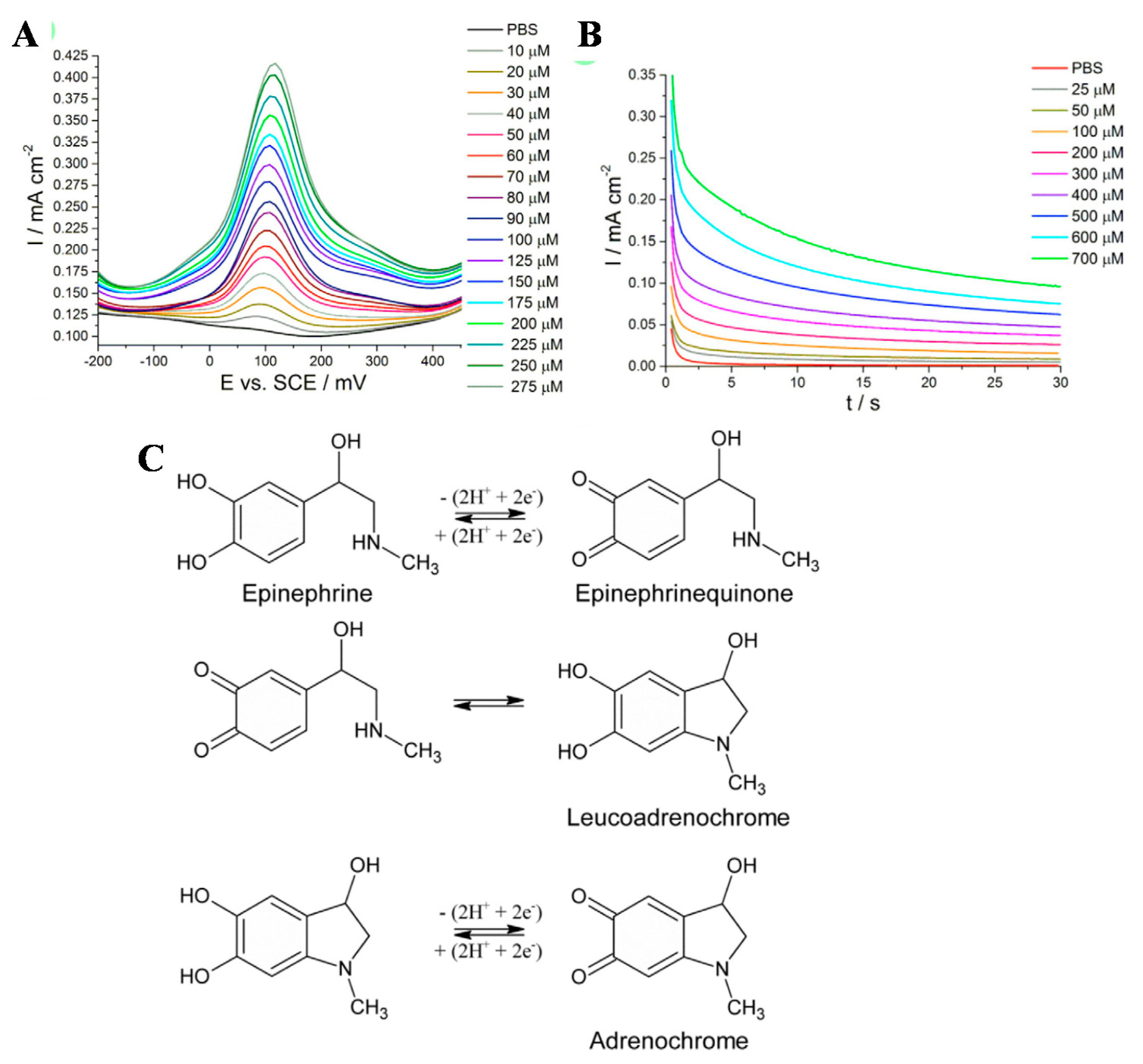
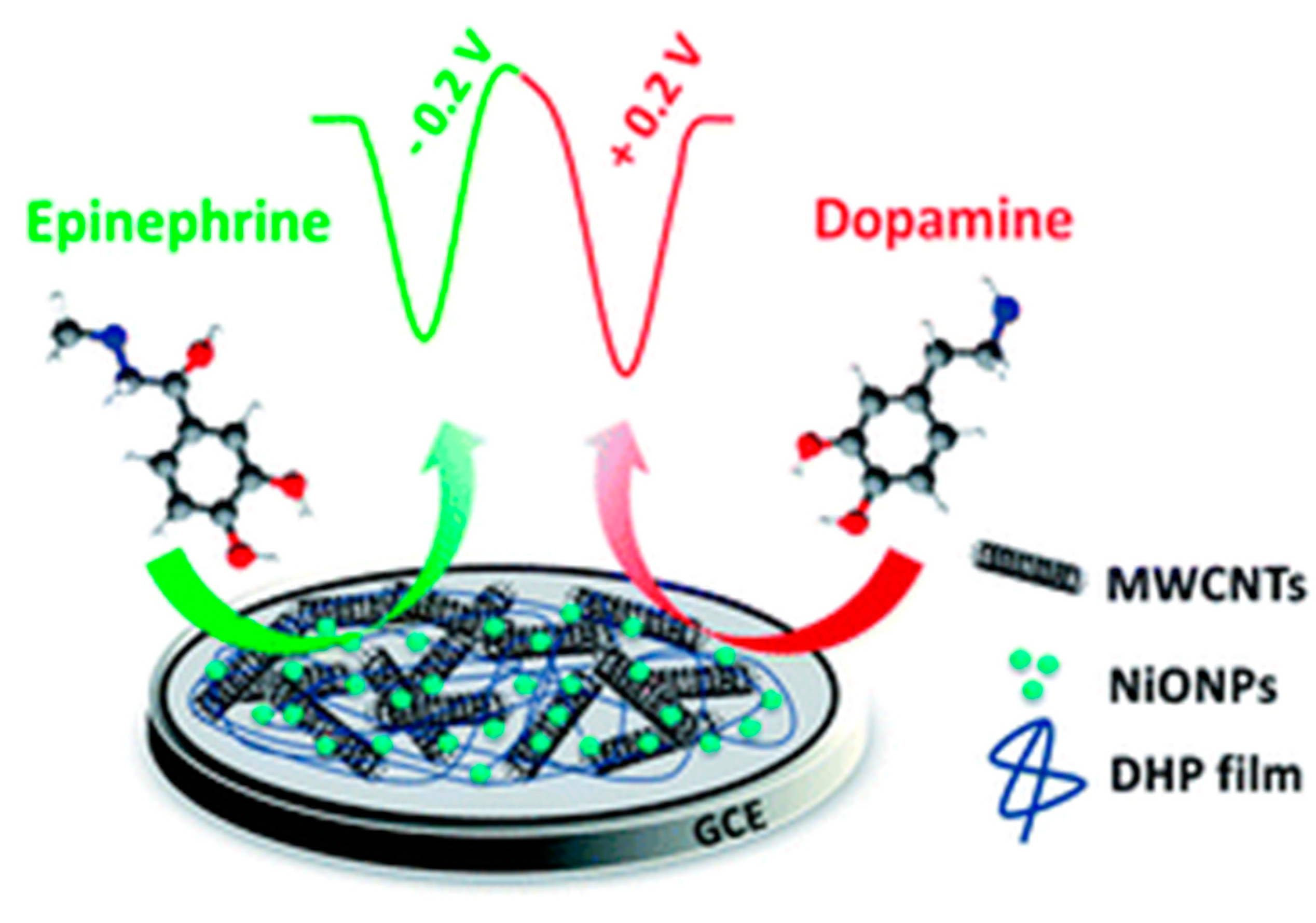
2. In Vitro Detection of Typical Neurotransmitters
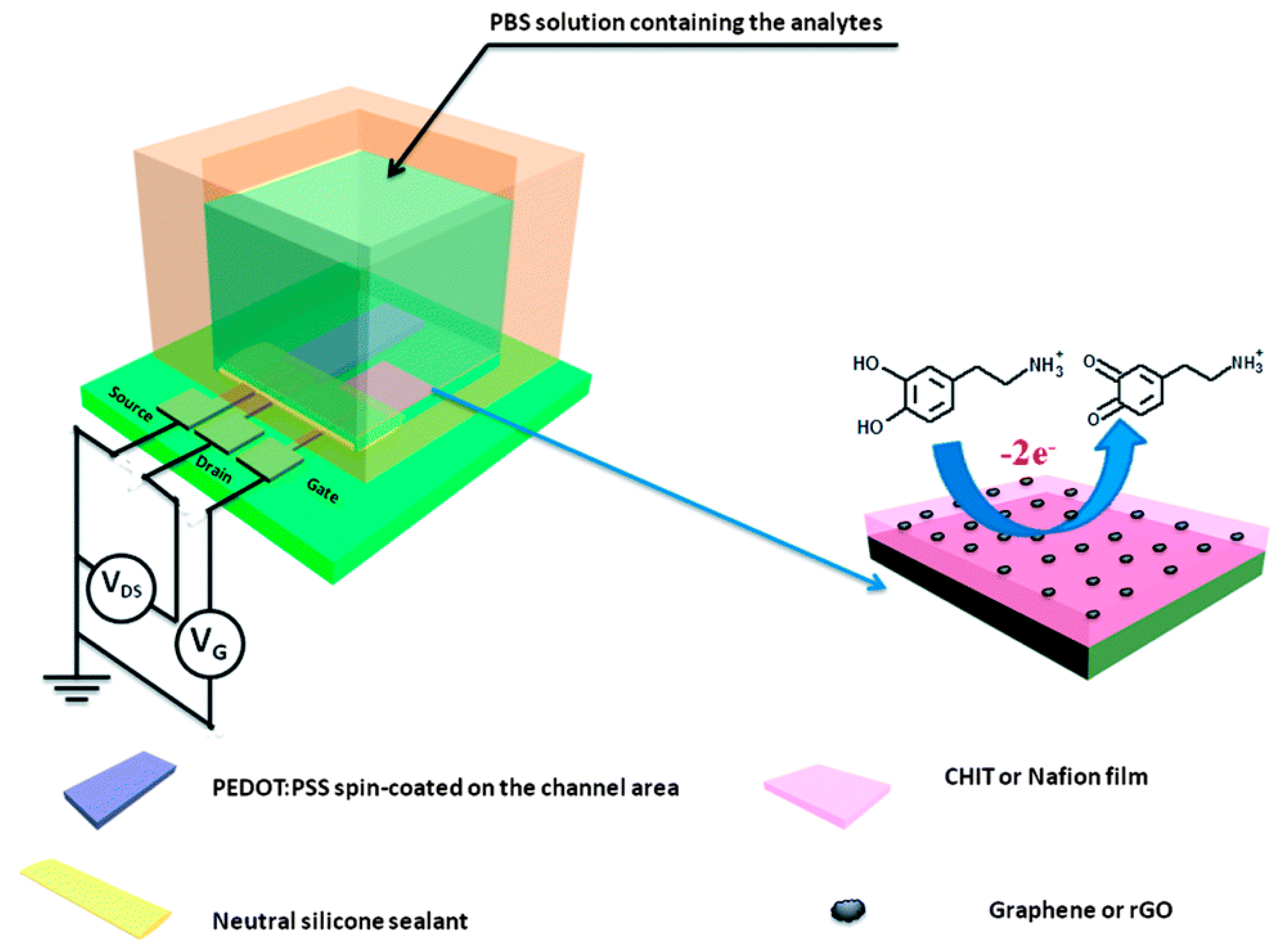
3. In Vivo Analysis of Neurotransmitters
4. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Chiara, G.D. The principles of nerve cell communication. Alcohol Health Res. World 1997, 21, 106–108. [Google Scholar]
- Pearson, R.A. Neurotransmitters and neurotrophins. In Retinal Development; Sernagor, E., Eglen, S., Harris, B., Wong, R., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 99–125. ISBN 9780511541629. [Google Scholar]
- Soleymani, J. Advanced materials for optical sensing and biosensing of neurotransmitters. Trends Anal. Chem. 2015, 72, 27–44. [Google Scholar] [CrossRef]
- Si, B.; Song, E. Recent advances in the detection of neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar] [CrossRef]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta 2009, 653, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.W.; Leblanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Shah, B. Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods 2013, 5, 2158–2173. [Google Scholar] [CrossRef]
- Sáenz, H.S.C.; Hernández-Saravia, L.P.; Selva, J.S.G.; Sukeri, A.; Espinoza-Montero, P.J.; Bertotti, M. Electrochemical dopamine sensor using a nanoporous gold microelectrode: A proof-of-concept study for the detection of dopamine release by scanning electrochemical microscopy. Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A. Application of single-electron transistor to biomolecule and ion sensors. Appl. Sci. 2016, 6, 94. [Google Scholar] [CrossRef]
- Turkevich, J.; Cooper Stevenson, P. A study of the nucleation and growth processes in the synthesis of collodial gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y. “Plugging into enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Yuan, R.; Chai, Y.; Tang, D.; Zhang, Y.; Wang, N.; Li, X.; Zhu, Q. A reagentless amperometric immunosensor based on gold nanoparticles/thionine/Nafion-membrane-modified gold electrode for determination of α-1-fetoprotein. Electrochem. Commun. 2005, 7, 355–360. [Google Scholar] [CrossRef]
- Pan, M.; Gu, Y.; Yun, Y.; Li, M.; Jin, X.; Wang, S. Nanomaterials for electrochemical immunosensing. Sensors 2017, 17, 1041. [Google Scholar] [CrossRef]
- Rajakumar, G.; Gomathi, T.; Rahuman, A.A.; Thiruvengadam, M.; Mydhili, G.; Kim, S.; Lee, T. Biosynthesis and biomedical applications of gold nanoparticles using Eclipta prostrata leaf extract. Appl. Sci. 2016, 6, 222. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Zhong, W.; Hao, N.; Xu, D.; Chen, H. Arrays based on silver nanoparticle aggregates. Anal. Chem. 2010, 82, 5477–5483. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, O.N.; Iost, R.M.; Siqueira, J.R.; Crespilho, F.N.; Caseli, L. Nanomaterials for Diagnosis: Challenges and applications in smart devices based on molecular recognition. ACS Appl. Mater. Interfaces 2014, 6, 14745–14766. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.R.; Govindhan, M.; Chen, A. Carbon nanomaterials based electrochemical sensors/biosensors for the sensitive detection of pharmaceutical and biological compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chen, A. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Wildöer, J.W.G.; Venema, L.C.; Rinzler, A.G.; Smalley, R.E.; Dekker, C. Electronic structure of atomically resolved carbon nanotubes. Nature 1998, 391, 59–62. [Google Scholar] [CrossRef]
- Govindhan, M.; Liu, Z.; Chen, A. Design and electrochemical study of platinum-based nanomaterials for sensitive detection of nitric oxide in biomedical applications. Nanomaterials 2016, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Wen, J.; Chen, A. Electrochemical determination of methylglyoxal as a biomarker in human plasma. Biosens. Bioelectron. 2013, 42, 349–354. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Li, J.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. A single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Sidhureddy, B.; Thiruppathi, A.R.; Chen, A. From graphite to interconnected reduced graphene oxide: One-pot synthesis and supercapacitor application. Chem. Commun. 2017, 53, 7828–7831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ciacchi, L.C.; Wei, G. Recent advances in the synthesis of graphene-based nanomaterials for controlled drug delivery. Appl. Sci. 2017, 7, 1175. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research progress of gas sensor based on graphene and its derivatives: A Review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef]
- Garnayak, S.; Patel, S. Oxidation of epinephrine to adrenochrome by cetyltrimethylammonium dichromate: A mechanistic study. Ind. Eng. Chem. Res. 2014, 53, 12249–12256. [Google Scholar] [CrossRef]
- van der Valk, J.P.M.; Berends, I.; Gerth van Wijk, R.; Arends, N.J.T.; van Maaren, M.S.; de Groot, H.; Wichers, H.J.; Emons, J.A.M.; Dubois, A.E.J.; de Jong, N.W. Small percentage of anaphylactic reactions treated with epinephrine during food challenges in Dutch children. Ann. Allergy Asthma Immunol. 2017, 120, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ren, Y.; Bai, Z.; Jiao, J.; Chen, Y.; Han, B.; Chen, Q. Synthesis of tetrahexahedral Au-Pd core–shell nanocrystals and reduction of graphene oxide for the electrochemical detection of epinephrine. J. Colloid Interface Sci. 2018, 512, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, E.; Sulka, G.D. Nanoporous spongelike Au-Ag films for electrochemical epinephrine sensing. J. Electroanal. Chem. 2016, 762, 43–50. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Sulka, G.D. Fabrication of highly ordered nanoporous thin Au films and their application for electrochemical determination of epinephrine. Sens. Actuators B Chem. 2016, 222, 270–279. [Google Scholar] [CrossRef]
- Beitollahi, H.; Karimi-Maleh, H.; Khabazzadeh, H. Nanomolar and selective determination of epinephrine in the presence of norepinephrine using carbon paste electrode modified with carbon nanotubes and novel 2-(4-oxo-3-phenyl-3,4-dihydro-quinazolinyl)-N′-phenyl-hydrazinecarbothioamide. Anal. Chem. 2008, 80, 9848–9851. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Wang, H.-H.; Li, W.-T.; Fang, X.-X.; Guo, X.-C.; Zhou, W.-H.; Cao, X.; Kou, D.-X.; Zhou, Z.-J.; Wu, S.-X. A novel electrochemical sensor for epinephrine based on three dimensional molecularly imprinted polymer arrays. Sens. Actuators B Chem. 2016, 222, 1127–1133. [Google Scholar] [CrossRef]
- Urdaneta, I.; Keller, A.; Atabek, O.; Palma, J.L.; Finkelstein-Shapiro, D.; Tarakeshwar, P.; Mujica, V.; Calatayud, M. Dopamine adsorption on TiO2 anatase surfaces. J. Phys. Chem. C 2014, 118, 20688–20693. [Google Scholar] [CrossRef]
- Larkin, B.A.J.; El-Sayed, M.; Brownson, D.A.C.; Banks, C.E. Crime scene investigation III: Exploring the effects of drugs of abuse and neurotransmitters on bloodstain pattern analysis. Anal. Methods 2012, 4, 721–729. [Google Scholar] [CrossRef]
- Khan, A.F.; Brownson, D.A.C.; Randviir, E.P.; Smith, G.C.; Banks, C.E. 2D hexagonal boron nitride (2D-hBN) explored for the electrochemical sensing of dopamine. Anal. Chem. 2016, 88, 9729–9737. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.M.; van Grinsven, B.; Foster, C.W.; Cleij, T.J.; Banks, C.E. Introducing thermal wave transport analysis (TWTA): A thermal technique for dopamine detection by screen-printed electrodes functionalized with molecularly imprinted polymer (MIP) particles. Molecules 2016, 21, 552. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.A.; Dunn, A.R.; Lohr, K.M.; Alter, S.P.; Cliburn, R.A.; Guillot, T.S.; Miller, G.W. Selective enhancement of dopamine release in the ventral pallidum of methamphetamine-sensitized mice. ACS Chem. Neurosci. 2016, 7, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Savitt, J.; Dawson, V.; Dawson, T. Diagnosis and treatment of Parkinson disease: Molecules to medicine. J. Clin. Investig. 2006, 116, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Chen, Y.; Xu, J.; Yang, X.; Tian, D.-B. Dopamine sensor based on molecularly imprinted electrosynthesized polymers. J. Solid State Electrochem. 2010, 14, 1909–1914. [Google Scholar] [CrossRef]
- Lacher, N.A.; Lunte, S.M.; Martin, R.S. Development of a microfabricated palladium decoupler/electrochemical detector for microchip capillary electrophoresis using a hybrid glass/poly(dimethylsiloxane) device. Anal. Chem. 2004, 76, 2482–2491. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Postetter, D.; Saragnese, D.L.; Randall, C.L.; Mirski, M.A.; Gracias, D.H. Patternable nanowire sensors for electrochemical recording of dopamine. Anal. Chem. 2009, 81, 9979–9984. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, G.; Estrany, F.; Casas, M.T.; Alemán, C.; Armelin, E. Detection of dopamine using chemically synthesized multilayered hollow microspheres. J. Phys. Chem. B 2014, 118, 4702–4709. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Organic electrochemical transistors with graphene-modified gate electrodes for highly sensitive and selective dopamine sensors. J. Mater. Chem. B 2014, 2, 191–200. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Demuru, S.; Nela, L.; Marchack, N.; Holmes, S.J.; Farmer, D.B.; Tulevski, G.S.; Lin, Q.; Deligianni, H. Scalable Nanostructured Carbon Electrode Arrays for Enhanced Dopamine Detection. ACS Sens. 2018, 3, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chao, M.; Wang, Z. Electrochemical detection of dopamine in the presence of epinephrine, uric acid and ascorbic acid using a graphene-modified electrode. Anal. Methods 2012, 4, 1687–1692. [Google Scholar] [CrossRef]
- Oleinick, A.; Zhu, F.; Yan, J.; Mao, B.; Svir, I.; Amatore, C. Theoretical investigation of generator-collector microwell arrays for improving electroanalytical selectivity: Application to selective dopamine detection in the presence of ascorbic acid. ChemPhysChem 2013, 14, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.K.; Moshkalev, S.A.; Rout, C.S. Highly sensitive and selective electrochemical dopamine sensing properties of multilayer graphene nanobelts. Nanotechnology 2016, 27, 075504. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-filho, L.C.S.; Silva, T.A.; Vicentini, F.C.; Fatibello-filho, O. Simultaneous voltammetric determination of dopamine and epinephrine in human body fluid samples using a glassy carbon electrode modified with nickel oxide nanoparticles and carbon nanotubes within a dihexadecylphosphate film. Analyst 2014, 139, 2842–2849. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Ultrasensitive and Selective Organic FET-type Nonenzymatic Dopamine Sensor Based on Platinum Nanoparticles-Decorated Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2017, 9, 39526–39533. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martos, I.; Ferapontova, E.E. Electrochemical label-free aptasensor for specific analysis of dopamine in serum in the presence of structurally related neurotransmitters. Anal. Chem. 2016, 88, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Demuru, S.; Deligianni, H. Surface PEDOT:Nafion coatings for enhanced dopamine, serotonin and adenosine sensing. J. Electrochem. Soc. 2017, 164, G129–G138. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, N.; Tomar, V.; Chandra, R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens. Bioelectron. 2018, 106, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Cesarino, I.; Galesco, H.V.; Machado, S.A.S. Determination of serotonin on platinum electrode modified with carbon nanotubes/polypyrrole/silver nanoparticles nanohybrid. Mater. Sci. Eng. C 2014, 40, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tertiș, M.; Cernat, A.; Lacatiș, D.; Florea, A.; Bogdan, D.; Suciu, M.; Săndulescu, R.; Cristea, C. Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture. Electrochem. Commun. 2017, 75, 43–47. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X.; Xia, Y. Electrochemical detection of serotonin based on a poly(bromocresol green) film and Fe3O4 nanoparticles in a chitosan matrix. RSC Adv. 2017, 7, 1847–1851. [Google Scholar] [CrossRef]
- Zestos, A.G.; Jacobs, C.B.; Trikantzopoulos, E.; Ross, A.E.; Venton, B.J. Polyethylenimine carbon nanotube fiber electrodes for enhanced detection of neurotransmitters. Anal. Chem. 2014, 86, 8568–8575. [Google Scholar] [CrossRef] [PubMed]
- Ostock, C.Y.; Bhide, N.; Goldenberg, A.A.; George, J.A.; Bishop, C. Striatal norepinephrine efflux in l-DOPA-induced dyskinesia. Neurochem. Int. 2018, 114, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.D.; Wilken, G.H.; Mitchell, K.K.; Martin, R.S.; Macarthur, H. Simultaneous analysis of vascular norepinephrine and ATP release using an integrated microfluidic system. J. Neurosci. Methods 2016, 266, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, O.; Jozwiak, M.; Geffriaud, T.; Sztrymf, B.; Prat, D.; Jacobs, F.; Monnet, X.; Trouiller, P.; Richard, C.; Teboul, J.L. Norepinephrine exerts an inotropic effect during the early phase of human septic shock. Br. J. Anaesth. 2018, 120, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.M.; Plonka, C.M.; Feingold, B. Norepinephrine levels in children with single ventricle circulation. Prog. Pediatr. Cardiol. 2017, 47, 58–63. [Google Scholar] [CrossRef]
- Samdani, K.J.; Joh, D.W.; Rath, M.K.; Lee, K.T. Electrochemical mediatorless detection of norepinephrine based on MoO3 nanowires. Electrochim. Acta 2017, 252, 268–274. [Google Scholar] [CrossRef]
- Samdani, K.J.; Samdani, J.S.; Kim, N.H.; Lee, J.H. FeMoO4 based, enzyme-free electrochemical biosensor for ultrasensitive detection of norepinephrine. Biosens. Bioelectron. 2016, 81, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Mohammadi, S. Selective voltammetric determination of norepinephrine in the presence of acetaminophen and tryptophan on the surface of a modified carbon nanotube paste electrode. Mater. Sci. Eng. C 2013, 33, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; Chen, S.M.; Hung, Y.T.; Ali, M.A.; Al-Hemaid, F.M.A. Electrochemical oxidation and determination of norepinephrine in the presence of acetaminophen using MnO2 nanoparticle decorated reduced graphene oxide sheets. Anal. Methods 2014, 6, 6504–6513. [Google Scholar] [CrossRef]
- Dai, M.; Haselwood, B.; Vogt, B.D.; La Belle, J.T. Amperometric sensing of norepinephrine at picomolar concentrations using screen printed, high surface area mesoporous carbon. Anal. Chim. Acta 2013, 788, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Galal, A.; Azab, S.M. Electrochemical determination of neurotransmitters using gold nanoparticles on nafion/carbon paste modified electrode. J. Electrochem. Soc. 2012, 159, H765–H771. [Google Scholar] [CrossRef]
- Atta, N.F.; Ali, S.M.; El-Ads, E.H.; Galal, A. The electrochemistry and determination of some neurotransmitters at SrPdO3 modified graphite electrode. J. Electrochem. Soc. 2013, 160, G3144–G3151. [Google Scholar] [CrossRef]
- Suárez-Pozos, E.; Martínez-Lozada, Z.; Méndez-Flores, O.G.; Guillem, A.M.; Hernández-Kelly, L.C.; Castelán, F.; Olivares-Bañuelos, T.N.; Chi-Castañeda, D.; Najimi, M.; Ortega, A. Characterization of the cystine/glutamate antiporter in cultured Bergmann glia cells. Neurochem. Int. 2017, 106, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Wall, B.A.; Wangari-Talbot, J.; Chen, S. Metabotropic glutamate receptors in cancer. Neuropharmacology 2017, 115, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Li, P.; Ning, Y.-L.; Peng, Y.; Xiong, R.-P.; Yang, N.; Chen, X.; Zhou, Y.-G. Adenosine A 2A receptor inhibition restores the normal transport of endothelial glutamate transporters in the brain. Biochem. Biophys. Res. Commun. 2018, 498, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Sirca, D.; Vardeu, A.; Pinna, M.; Diana, M.; Enrico, P. A robust, state-of-the-art amperometric microbiosensor for glutamate detection. Biosens. Bioelectron. 2014, 61, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.J.; Gerhardt, G.A. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of l-glutamate and other analytes. Anal. Chem. 2001, 73, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Im, H.S.; Lee, D.H.; Kim, H.S.; Choi, Y.K. Ferrocene functionalized single-walled carbon nanotube bundles. Hybrid interdigitated construction film for l-glutamate detection. J. Phys. Chem. C 2007, 106, 1200–1206. [Google Scholar] [CrossRef]
- Dorozhko, E.V.; Korotkova, E.I.; Shabaeva, A.A.; Mosolkov, A.Y. Electrochemical determination of l-glutamate on a carbon-containing electrode modified with gold by voltammetry. Procedia Chem. 2015, 15, 365–370. [Google Scholar] [CrossRef]
- Batra, B.; Pundir, C.S. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, F.; Alttoa, A.; Reif, A. Neuronal nitric oxide synthase (NOS1) and its adaptor, NOS1AP, as a genetic risk factors for psychiatric disorders. Genes Brain Behav. 2015, 14, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.P.; Vizi, E.S. Nitric oxide: A novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001, 24, 211–215. [Google Scholar] [CrossRef]
- Balamurugan, M.; Madasamy, T.; Pandiaraj, M.; Bhargava, K.; Sethy, N.K.; Karunakaran, C. Electrochemical assay for the determination of nitric oxide metabolites using copper(II) chlorophyllin modified screen printed electrodes. Anal. Biochem. 2015, 478, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Govindhan, M.; Chen, A. Enhanced electrochemical sensing of nitric oxide using a nanocomposite consisting of platinum-tungsten nanoparticles, reduced graphene oxide and an ionic liquid. Microchim. Acta 2016, 183, 2879–2887. [Google Scholar] [CrossRef]
- Liu, Z.; Forsyth, H.; Khaper, N.; Chen, A. Sensitive electrochemical detection of nitric oxide based on AuPt and reduced graphene oxide nanocomposites. Analyst 2016, 141, 4074–4083. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nemec-Bakk, A.; Khaper, N.; Chen, A. Sensitive electrochemical detection of nitric oxide release from cardiac and cancer cells via a hierarchical nanoporous gold microelectrode. Anal. Chem. 2017, 89, 8036–8043. [Google Scholar] [CrossRef] [PubMed]
- König, M.; Thinnes, A.; Klein, J. Microdialysis and its use in behavioural studies: Focus on acetylcholine. J. Neurosci. Methods 2018, 300, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Walker, M.; Grace, J.; Perry, R. Acetylcholine in mind: A neurotransmitter correlate of consciousness? Trends Neurosci. 1999, 22, 273–280. [Google Scholar] [CrossRef]
- Akhtar, M.H.; Hussain, K.K.; Gurudatt, N.G.; Shim, Y.B. Detection of Ca2+-induced acetylcholine released from leukemic T-cells using an amperometric microfluidic sensor. Biosens. Bioelectron. 2017, 98, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ye, S.; Shi, X.; Jing, L.; Liang, C.; Xian, Y. An electrochemical platform for acetylcholinesterase activity assay and inhibitors screening based on Michael addition reaction between thiocholine and catechol-terminated SAMs. Analyst 2011, 136, 5084–5090. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Pundir, C.S. Amperometric determination of acetylcholine—A neurotransmitter, by chitosan/gold-coated ferric oxide nanoparticles modified gold electrode. Biosens. Bioelectron. 2014, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bolat, E.Ö.; Tığ, G.A.; Pekyardımcı, Ş. Fabrication of an amperometric acetylcholine esterase-choline oxidase biosensor based on MWCNTs-Fe3O4 NPs-CS nanocomposite for determination of acetylcholine. J. Electroanal. Chem. 2017, 785, 241–248. [Google Scholar] [CrossRef]
- Aynaci, E.; Yaşar, A.; Arslan, F. An amperometric biosensor for acetylcholine determination prepared from acetylcholinesterase-choline oxidase immobilized in polypyrrole-polyvinylsulpfonate film. Sens. Actuators B Chem. 2014, 202, 1028–1036. [Google Scholar] [CrossRef]
- Ralevic, V. Purines as neurotransmitters and neuromodulators in blood vessels. Curr. Vasc. Pharmacol. 2009, 7, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Song, S.; Zhang, J.; Pan, D.; Wang, L.; Fan, C. A target-responsive electrochemical aptamer switch (TREAS) for reagentless detection of nanomolar ATP. J. Am. Chem. Soc. 2007, 129, 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, L.; Xu, X.; Liu, S. Quantitative detection of potassium ions and adenosine triphosphate via a nanochannel-based electrochemical platform coupled with G-quadruplex aptamers. Anal. Chem. 2014, 86, 10641–10648. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cheng, Z.; Chen, Q.; Qu, L.; Miao, X.; Feng, Q. Construction of a paper-based electrochemical biosensing platform for rapid and accurate detection of adenosine triphosphate (ATP). Sens. Actuators B Chem. 2018, 256, 931–937. [Google Scholar] [CrossRef]
- Tsele, T.P.; Adekunle, A.S.; Fayemi, O.E.; Ebenso, E.E. Electrochemical detection of epinephrine using polyaniline nanocomposite films doped with TiO2 and RuO2 nanoparticles on multi-walled carbon nanotube. Electrochim. Acta 2017, 243, 331–348. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, H.; Jiang, W.; Liang, J.; Sun, Y.; Zhang, Y.; Wu, Q.; Zhang, G.; Song, X.M. Poly(ionic liquid) functionalized polypyrrole nanotubes supported gold nanoparticles: An efficient electrochemical sensor to detect epinephrine. Mater. Sci. Eng. C 2017, 75, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhou, Y.; Liang, X.; Zou, H.; Wang, Z.; Wang, M.; Ma, J. An electrochemical sensor based on graphene / poly (brilliant cresyl blue) nanocomposite for determination of epinephrine. J. Electroanal. Chem. 2016, 763, 25–31. [Google Scholar] [CrossRef]
- Mphuthi, N.G.; Adekunle, A.S.; Ebenso, E.E. Electrocatalytic oxidation of epinephrine and norepinephrine at metal oxide doped phthalocyanine/MWCNT composite sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.N.; Kumara Swamy, B.E.; Gururaj, K.J.; Cheng, C. Simultaneous determination of epinephrine, ascorbic acid and folic acid using TX-100 modified carbon paste electrode: A cyclic voltammetric study. J. Mol. Liq. 2017, 231, 379–385. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, S.; Zhang, H.; Chen, J.; He, Y.; Li, F.; Weng, W.; Ni, J.; Bao, X.; Lin, Y. Carbon dots and chitosan composite film based biosensor for the sensitive and selective determination of dopamine. Analyst 2013, 138, 5417–5423. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhang, Z.; Wang, K.; Yuan, Q.; Wang, X. One-pot synthesis of dendritic Pt3Ni nanoalloys as nonenzymatic electrochemical biosensors with high sensitivity and selectivity for dopamine detection. Nanoscale 2017, 9, 10998–11003. [Google Scholar] [CrossRef] [PubMed]
- Rand, E.; Periyakaruppan, A.; Tanaka, Z.; Zhang, D.; Marsh, M.P.; Andrews, R.J.; Lee, K.H.; Chen, B.; Meyyappan, M.; Koehne, J.E. A carbon nanofiber based biosensor for simultaneous detection of dopamine and serotonin in the presence of ascorbic acid. Biosens. Bioelectron. 2012, 42, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Anithaa, A.C.; Asokan, K.; Sekar, C. Highly sensitive and selective serotonin sensor based on gamma ray irradiated tungsten trioxide nanoparticles. Sens. Actuators B Chem. 2017, 238, 667–675. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Tao, L.; Min, Q.; Xiang, J.; Wang, Q.; Xie, J.; Yue, Y.; Wu, S.; Li, X.; et al. A disposable electrochemical sensor for simultaneous determination of norepinephrine and serotonin in rat cerebrospinal fluid based on MWNTs-ZnO/chitosan composites modified screen-printed electrode. Biosens. Bioelectron. 2015, 65, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. Electrochemical determination of serotonin in urine samples based on metal oxide nanoparticles/MWCNT on modified glassy carbon electrode. Sens. Bio-Sens. Res. 2017, 13, 17–27. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C. Electrochemical sensor for simultaneous determination of epinephrine and norepinephrine based on cetyltrimethylammonium bromide assisted SnO2 nanoparticles. J. Electroanal. Chem. 2017, 801, 503–510. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Zeng, Y.; Tang, H.; Li, L. A novel composite of molecularly imprinted polymer-coated PdNPs for electrochemical sensing norepinephrine. Biosens. Bioelectron. 2015, 65, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Dalkiran, B.; Erden, P.E.; Kilic, E. Graphene and tricobalt tetraoxide nanoparticles based biosensor for electrochemical glutamate sensing. Artif. Cells Nanomed. Biotechnol. 2017, 45, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Kumari, S.; Pundir, C.S. Enzyme and microbial technology construction of glutamate biosensor based on covalent immobilization of glutmate oxidase on polypyrrole nanoparticles/polyaniline modified gold electrode. Enzyme Microb. Technol. 2014, 57, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, V.M.; Wassum, K.M.; Maidment, N.T.; Monbouquette, H.G. Electrochemically deposited iridium oxide reference electrode integrated with an electroenzymatic glutamate sensor on a multi-electrode array microprobe. Biosens. Bioelectron. 2013, 42, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.M.; Xu, G.Q.; Ang, S.G. Amperometric nitric oxide sensor based on nanoporous platinum phthalocyanine modified electrodes. Anal. Chem. 2013, 85, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Z.; Zhao, G.C.; Wei, X.W. Electrodeposited platinum nanoparticles on the multi-walled carbon nanotubes and its electrocatalytic for nitric oxide. Int. J. Electrochem. Sci. 2008, 3, 746–754. [Google Scholar]
- Schuvailo, O.N.; Dzyadevych, S.V.; El’skaya, A.V.; Gauthier-Sauvigne, S.; Csoregi, E.; Cespeglio, R.; Soldatkin, A.P. Carbon fibre-based microbiosensors for in vivo measurements of acetylcholine and choline. Biosens. Bioelectron. 2005, 21, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Heli, H.; Vais, R.D. An electrochemical acetylcholine sensor based on lichen-like nickel oxide nanostructure. Biosens. Bioelectron. 2013, 48, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.T.C.; Sale, M.G.F.; Di Lorenzo, M. Towards timely Alzheimer diagnosis: A self-powered amperometric biosensor for the neurotransmitter acetylcholine. Biosens. Bioelectron. 2017, 87, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Xia, Y.; Xiao, C.; Nie, Z.; Yang, M.; Si, S. Electrochemical oxidation of purine and pyrimidines bases based on the boron-doped nanotube modified electrode. Biosens. Bioelectron. 2012, 31, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhang, Y.; Yu, Z.; Zhang, S. Label-free electrochemical detection of ATP based on amino-functionalized metal-organic framework. Sci. Rep. 2017, 7, 6500. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, H.; Yu, P.; Su, L.; Zhang, D.; Mao, L. Electrochemical sensing of ATP with synthetic cyclophane as recognition element. Sci. China Ser. B Chem. 2009, 52, 741–745. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Sitaula, S.; Griep, M.H.; Karna, S.P.; Ali, M.F.; Swami, N.S. Real-time electrochemical monitoring of adenosine triphosphate in the picomolar to micromolar range using graphene-modified electrodes. Anal. Chem. 2013, 85, 8158–8165. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Su, J.; Huang, Y.; Li, X.; Tao, Y.; Lu, C.; Zhu, J.; Bai, Z.; Meng, J.; Lu, X.; et al. An ATP aptasensor based on the peroxidase-like activity of hemin/graphene oxide nanosheets. Anal. Sci. 2016, 32, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Revilla, R.; Diez-Alarcia, R.; Mostany, R.; Perez, C.C.; Fernandez-Lopez, A. Norepinephrine, epinephrine and MHPG levels in chick brain development. Neuropharmacology 2001, 41, 480–485. [Google Scholar] [CrossRef]
- Rees, H.R.; Anderson, S.E.; Privman, E.; Bau, H.H.; Venton, B.J. Carbon nanopipette electrodes for dopamine detection in Drosophila. Anal. Chem. 2015, 87, 3849–3855. [Google Scholar] [CrossRef] [PubMed]
- Crespi, F. Wireless in vivo voltammetric measurements of neurotransmitters in freely behaving rats. Biosens. Bioelectron. 2010, 25, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.S.; Wang, L.; Wang, M.X.; Xu, S.W.; Yu, W.D.; Cai, X.X. A wearable wireless electrochemical instrument used for in-vivo neurotransmitter detection. Chin. J. Anal. Chem. 2015, 43, 93–97. [Google Scholar] [CrossRef]
- Vreeland, R.F.; Atcherley, C.W.; Russell, W.S.; Xie, J.Y.; Lu, D.; Laude, N.D.; Porreca, F.; Heien, M.L. Biocompatible PEDOT:Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Anal. Chem. 2015, 87, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, Z.; Wu, W.; Fu, B.; Wu, H.; Zhang, Z. Recognition unit-free and self-cleaning photoelectrochemical sensing platform on TiO2 nanotube photonic crystals for sensitive and selective detection of dopamine release from mouse brain. Biosens. Bioelectron. 2017, 87, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Pyakurel, P.; Shin, M.; Venton, B.J. Nicotinic acetylcholine receptor (nAChR) mediated dopamine release in larval Drosophila melanogaster. Neurochem. Int. 2018, 114, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Njagi, J.; Ball, M.; Best, M.; Wallace, K.N.; Andreescu, S. Electrochemical quantification of serotonin in the live embryonic zebrafish intestine. Anal. Chem. 2010, 82, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Swamy, B.E.K.; Venton, B.J. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst 2007, 132, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.; Atcherley, C.W.; Pathirathna, P.; Samaranayake, S.; Qiang, B.; Peña, E.; Morgan, S.L.; Heien, M.L.; Hashemi, P. In Vivo ambient serotonin measurements at carbon-fiber microelectrodes. Anal. Chem. 2017, 89, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-T.; Tang, L.-N.; Ning, Y.; Shu, Q.; Liang, F.-X.; Wang, H.; Zhang, G.-J. In vivo monitoring of serotonin by nanomaterial functionalized acupuncture needle. Sci. Rep. 2016, 6, 28018–28024. [Google Scholar] [CrossRef] [PubMed]
- Day, B.K.; Pomerleau, F.; Burmeister, J.J.; Huettl, P.; Gerhardt, G.A. Microelectrode array studies of basal and potassium-evoked release of l-glutamate in the anesthetized rat brain. J. Neurochem. 2006, 96, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Kulagina, N.V.; Shankar, L.; Michael, A.C. Monitoring glutamate and ascorbate in the extracellular space of brain tissue with electrochemical microsensors. Anal. Chem. 1999, 71, 5093–5100. [Google Scholar] [CrossRef] [PubMed]
- Hascup, E.R.; Hascup, K.N. Electrochemical techniques for subsecond neurotransmitter detection in live rodents. Comp. Med. 2014, 64, 249–255. [Google Scholar] [PubMed]
- Yu, Y.; Sun, Q.; Zhou, T.; Zhu, M.; Jin, L.; Shi, G. On-line microdialysis system with poly(amidoamine)-encapsulated Pt nanoparticles biosensor for glutamate sensing in vivo. Bioelectrochemistry 2011, 81, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Wahono, N.; Qin, S.; Oomen, P.; Cremers, T.I.F.; de Vries, M.G.; Westerink, B.H.C. Evaluation of permselective membranes for optimization of intracerebral amperometric glutamate biosensors. Biosens. Bioelectron. 2012, 33, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Griveau, S.; Dumezy, C.; Seguin, J.; Chabot, G.G.; Scherman, D.; Bedioui, F. In vivo electrochemical detection of nitric oxide in tumor-bearing mice. Anal. Chem. 2007, 79, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Rodrigues, M.S. Biomimetic sensor based on hemin/carbon nanotubes/chitosan modified microelectrode for nitric oxide measurement in the brain. Biosens. Bioelectron. 2013, 44, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, E.; Wallace, K.N.; Andreescu, S. Real time electrochemical investigation of the release, distribution and modulation of nitric oxide in the intestine of individual zebrafish embryos. Nitric Oxide Biol. Chem. 2018, 74, 32–38. [Google Scholar] [CrossRef] [PubMed]
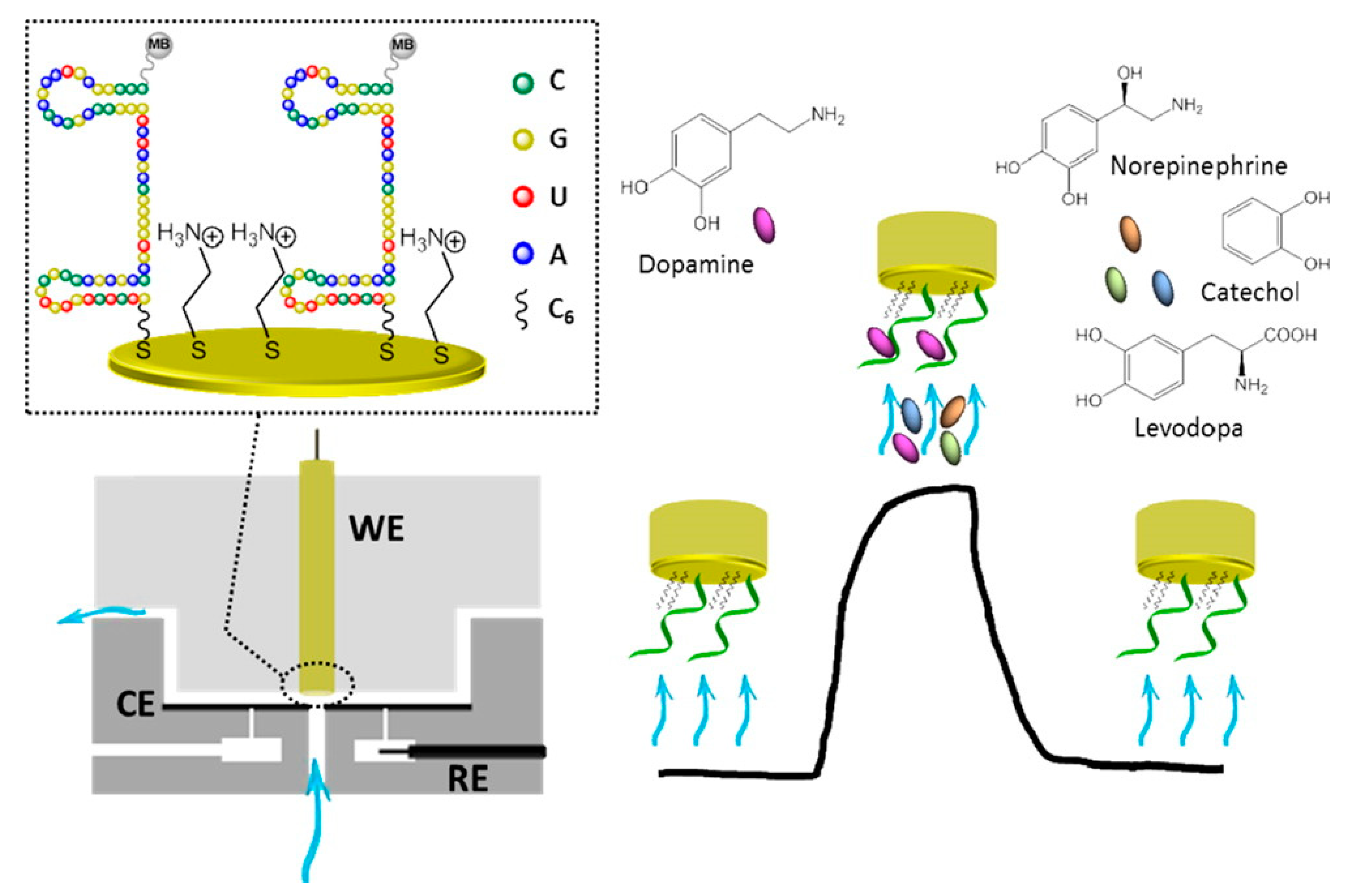
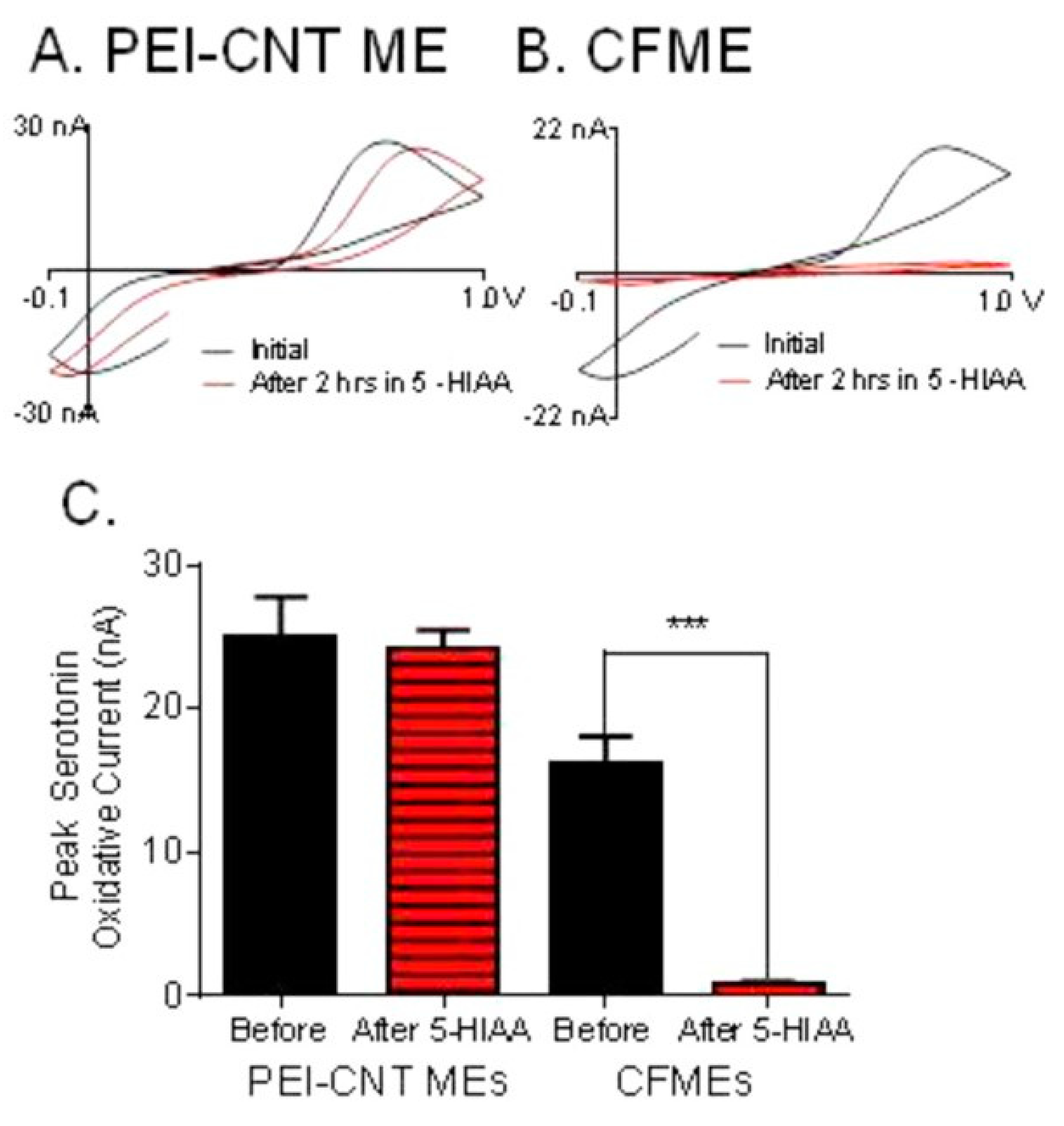
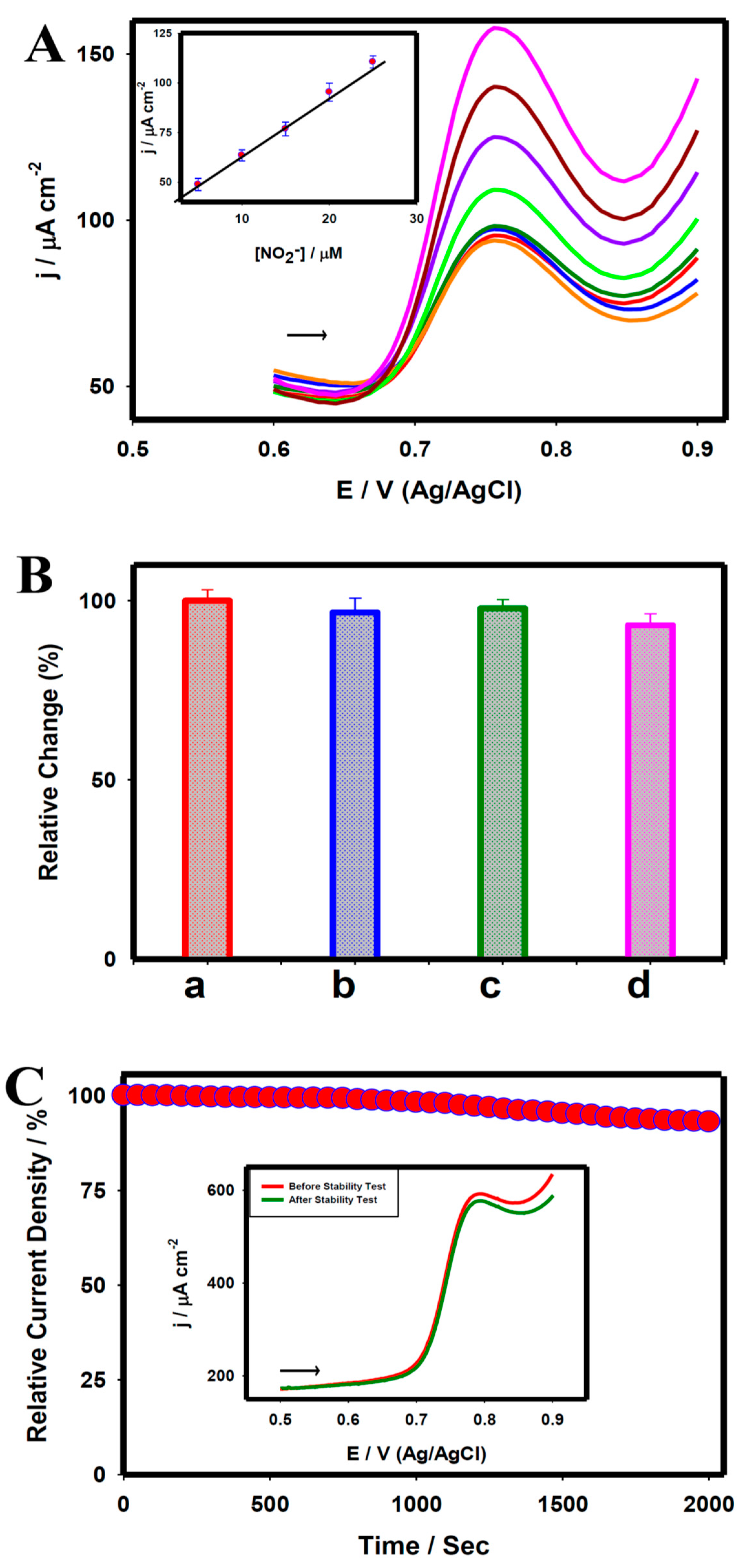
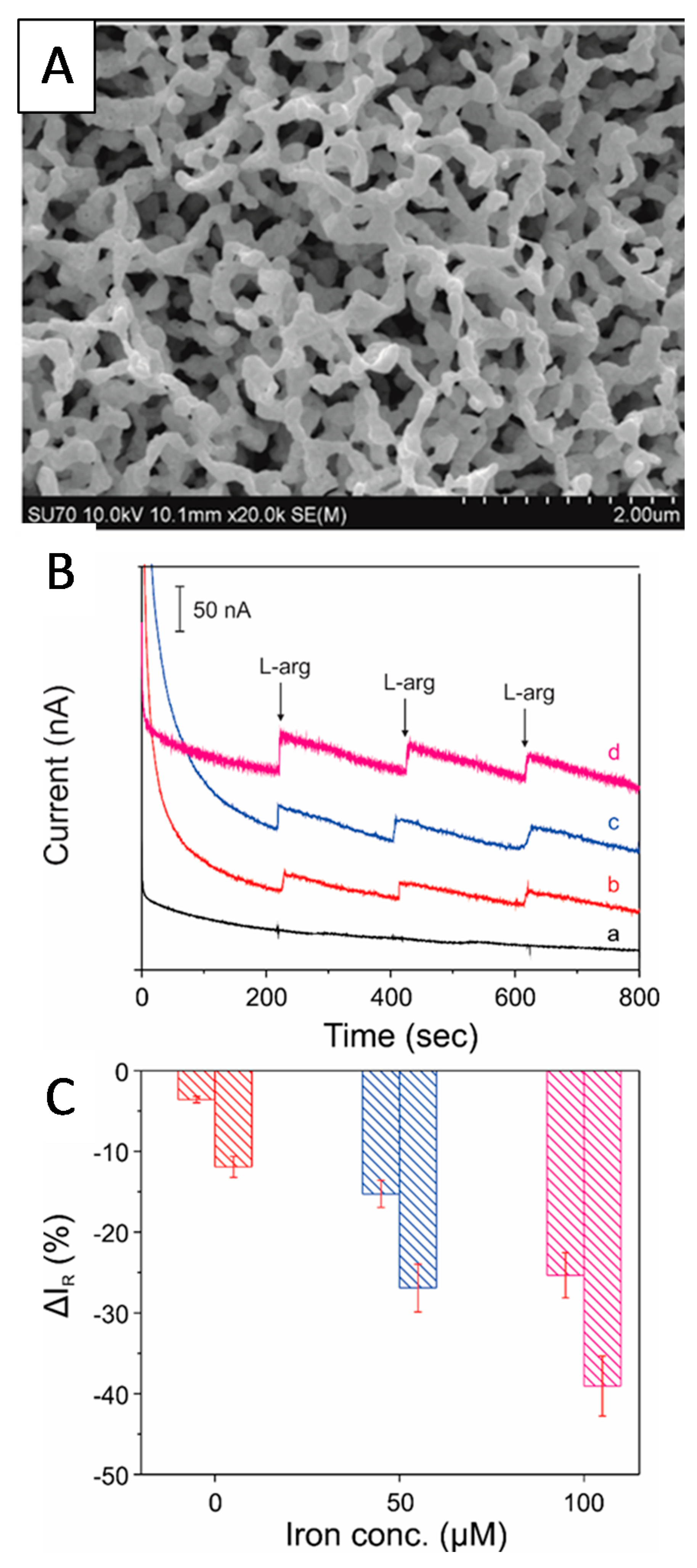

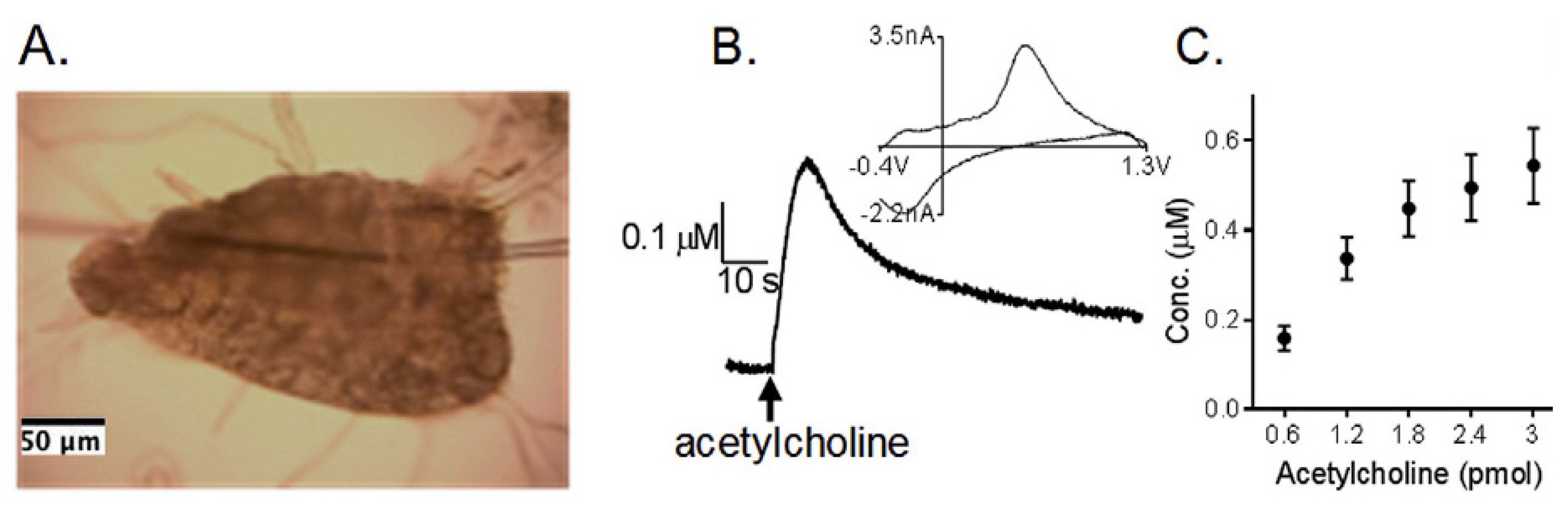
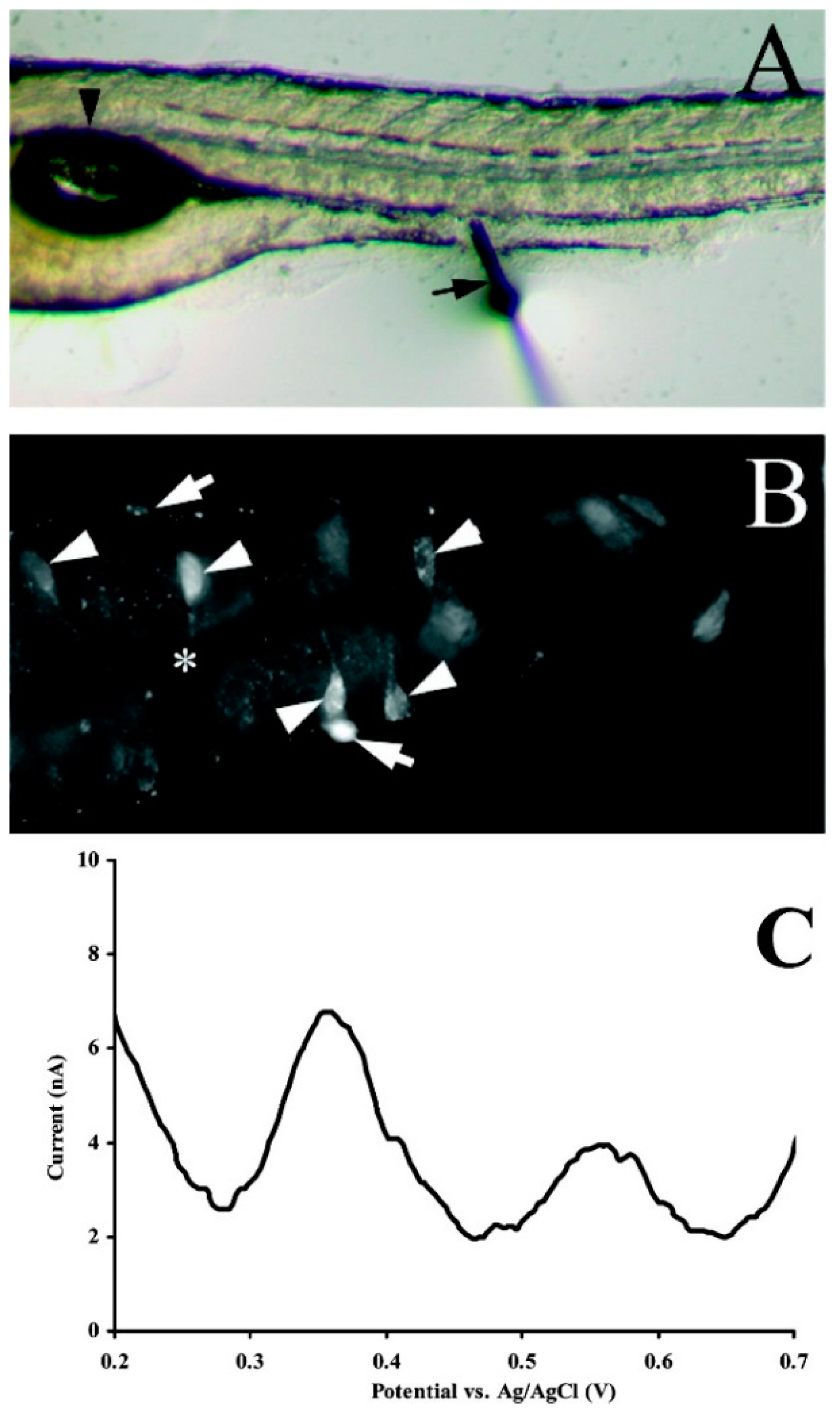
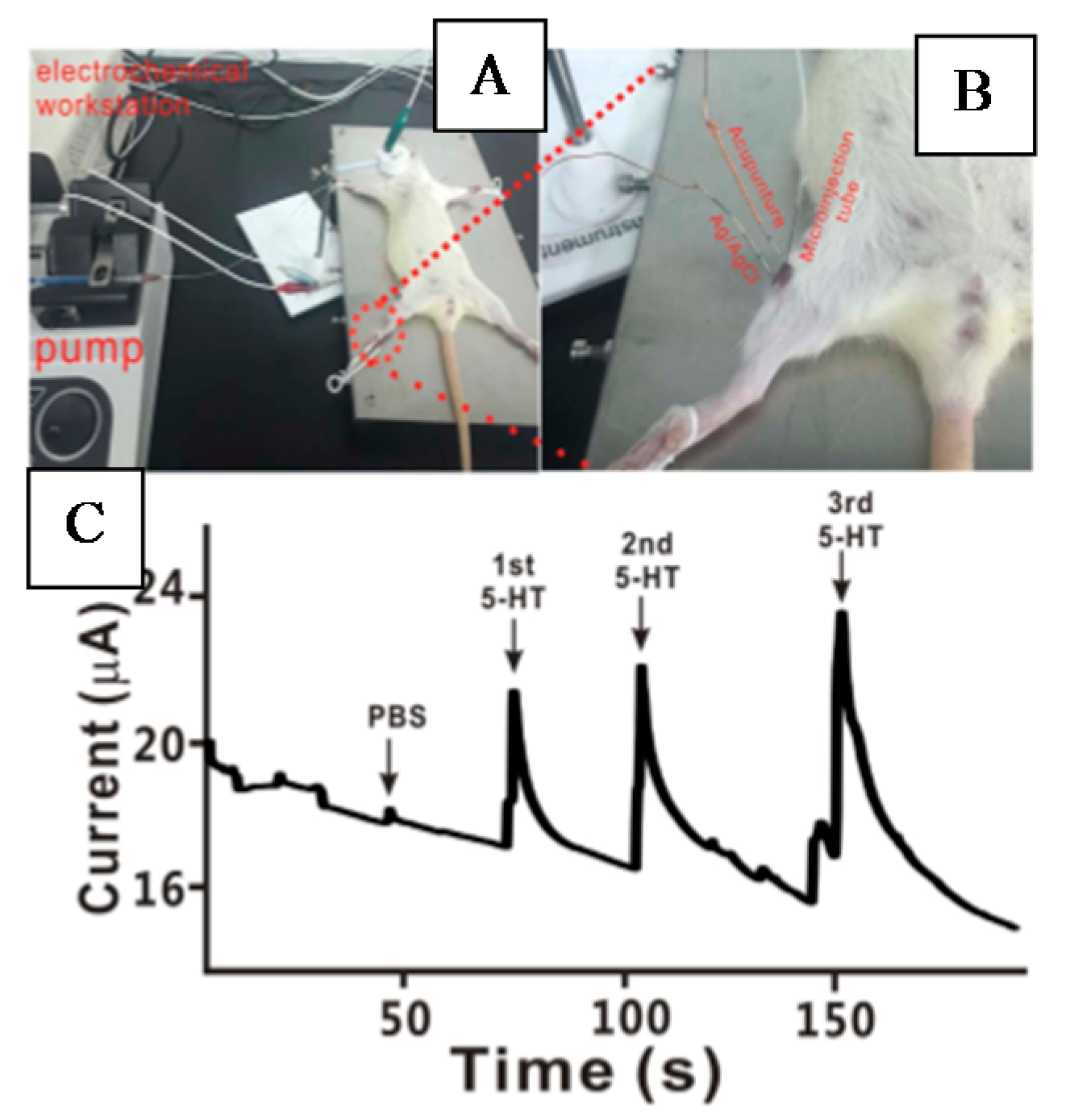
| Neuro-Transmitters | EC Method | Nanomaterials | E/(V vs. RHE) | Interferent | Linear Range (µM) | LOD (µM) | Ref |
|---|---|---|---|---|---|---|---|
| EP | DPV | MWCNT-PANI-TiO2 | 0.821 | 5-HT and AA | 4.9–76.9 | 0.160 | [101] |
| DPV | Polypyrrole nanotubes/Gold nanoparticles | 0.914 | Glucose, D-fructose, citric acid, UA, AA, DA | 35–960 | 0.298 | [102] | |
| CV | Graphene | 0.655 * | AA and UA | 1–1000 | 0.24 | [103] | |
| DPV | MWCNT | 0.782 | AA | 7.5–48 | 4.6 | [104] | |
| CV | TX 100-modified carbon paste electrode | 0.810 | AA and FA | 10–50 | 1.0 | [105] | |
| DA | DPV | 2D-hexagonol boron nitride | 0.862 | AA and UA | 3–75 | 0.65 | [42] |
| DPV | Carbon dots | 0.885 | AA and UA | 0.01–30 | 0.01 | [106] | |
| AP | Pt3Ni nanoalloys | 0.922 | Glucose, AA, and UA | 0.5–250 | 0.01 | [107] | |
| AP | Multilayer graphene nanobelts | 0.81 | Hydrazine, UA, AA, lactic acid, glucose | 2–20 | 0.58 | [55] | |
| CV | Multilayered hollow sphere | 0.922 | None | 0.5–2 | 0.5 | [49] | |
| 5-HT | DPV | Carbon nanofiber | 0.910 | AA | 0.001–1000 | 0.001 | [108] |
| DPV | WO3 nanoparticles | 0.985 | AA, EP, DA, UA, FA, Glucose | 0.01–600 | 0.001 | [109] | |
| SWV | MWCNT/ZnO | 0.920 | AA and UA | 0.05–1 | 0.01 | [110] | |
| DPV | Poly (bromocresol green)/Fe3O4 | 0.985 | AA, UA, and DA | 0.2–100 | 0.060 | [63] | |
| SWV | GCE-MWCNT-NiO | 0.980 | DA and AA | 5.98 × 10−3–62.8 | 0.118 | [111] | |
| NE | AP | FeMoO4 | 0.952 | UA and AA | 0.05–200 | 0.003 | [70] |
| SWV | CTAB-SnO2 | 0.822 | EP | 0.1–300 | 0.006 | [112] | |
| SWV | MWCNT/ZnO | 0.704 | AA and UA | 0.5–30 | 0.2 | [104] | |
| CV | Pd nanoparticles | 0.862 | AA, UA, and FA | 0.5–80 | 0.1 | [113] | |
| AP | MoO3 Nanowires | 1.01 | AA, UA, l-Glu, NH4+, K+, DA, Na+, Fe3+, Cl- | 0.1–200 | 0.11 | [69] | |
| l-Glu | CV | GCE/gold | −0.061 | None | 320–2500 | 0.40 | [82] |
| AP | Co3O4/graphene/chitosan/GluOx | 1.31 | None | 4–600 | 2.0 | [114] | |
| CV | PpyNPs/PANI/AuE | 0.440 | Citric acid, AA, cysteine, methionine, lysine, aspartic acid, glucose, NaCl, glycine | 0.02–400 | 0.1 × 10−3 | [115] | |
| AP | IrOx | −0.078 | DA and AA | 5–300 | 0.32 | [116] | |
| CV | GluOx/cMWCNT/AuNP/CHIT | 0.769 | AA, bilirubin, urea, UA, triglycerides, glucose | 5–500 | 1.60 | [83] | |
| NO | AP | PtW/rGO-IL | 1.40 | None | 0.002–1200 | 0.13 | [87] |
| AP | Nanoporous Pt | 1.37 | None | 0.0–8.75 | 0.001 | [117] | |
| AP | Pt/MWCNT | 0.190 * | None | 0.40–0.10 | 0.10 | [118] | |
| DPV | Au–Pt/rGO | 1.35 | NO2−, NO3−, cysteine, AA, and UA | 0.02–10.0 | 0.003 | [88] | |
| AP | Gold nanoporous microelectrode | 1.42 | AA, UA, cysteine, arginine | 0.005–200 | 0.001 | [89] | |
| ACh | AP | AChE-ChO/PPy-PVS | 1.03 | AA, UA, EtOH, MeOH, paracetamol, cystine, cysteine, glucose | 0.01–0.1 | 0.005 | [96] |
| CV | Chitosan/gold-coated Fe3O4 | 0.86 | None | 0.005–400 | 0.005 | [94] | |
| AP | Carbon fiber/AChE | 0.584 | None | 0.3–100 | 1 | [119] | |
| AP | Nickel oxide | 1.11 | None | 0.25–5.88 | 26.7 | [120] | |
| SWV | AChE/Hpg/Pt | 0.910 | None | 240–1900 | 10 | [121] | |
| Purines | DPV | B-doped CNTs | 1.57 ** | None | 0.5–8 | 0.03 | [122] |
| EIS | Amino-functionalized MOF | OCP | AMP, GTP, ADP, IgP | 0.001–1000 | 0.005 | [123] | |
| CV | SWNTs | 0.260 | None | 10–120 | 10 | [124] | |
| CV | Graphene/AuNPs | 0.210 | None | 1.14 × 10−4–31 | 2.01 × 10−5 | [125] | |
| AP | Hemin/GO nanosheets | 0.636 | CTP, UTP, GTP, ADP, AMP | 0.5 × 10−3–0.1 | 0.08 × 10−3 | [126] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durairaj, S.; Sidhureddy, B.; Cirone, J.; Chen, A. Nanomaterials-Based Electrochemical Sensors for In Vitro and In Vivo Analyses of Neurotransmitters. Appl. Sci. 2018, 8, 1504. https://doi.org/10.3390/app8091504
Durairaj S, Sidhureddy B, Cirone J, Chen A. Nanomaterials-Based Electrochemical Sensors for In Vitro and In Vivo Analyses of Neurotransmitters. Applied Sciences. 2018; 8(9):1504. https://doi.org/10.3390/app8091504
Chicago/Turabian StyleDurairaj, Sharmila, Boopathi Sidhureddy, Joseph Cirone, and Aicheng Chen. 2018. "Nanomaterials-Based Electrochemical Sensors for In Vitro and In Vivo Analyses of Neurotransmitters" Applied Sciences 8, no. 9: 1504. https://doi.org/10.3390/app8091504
APA StyleDurairaj, S., Sidhureddy, B., Cirone, J., & Chen, A. (2018). Nanomaterials-Based Electrochemical Sensors for In Vitro and In Vivo Analyses of Neurotransmitters. Applied Sciences, 8(9), 1504. https://doi.org/10.3390/app8091504





