Volumetric, Radiographic, and Histologic Analyses of Demineralized Dentin Matrix Combined with Recombinant Human Bone Morphogenetic Protein-2 for Ridge Preservation: A Prospective Randomized Controlled Trial in Comparison with Xenograft
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
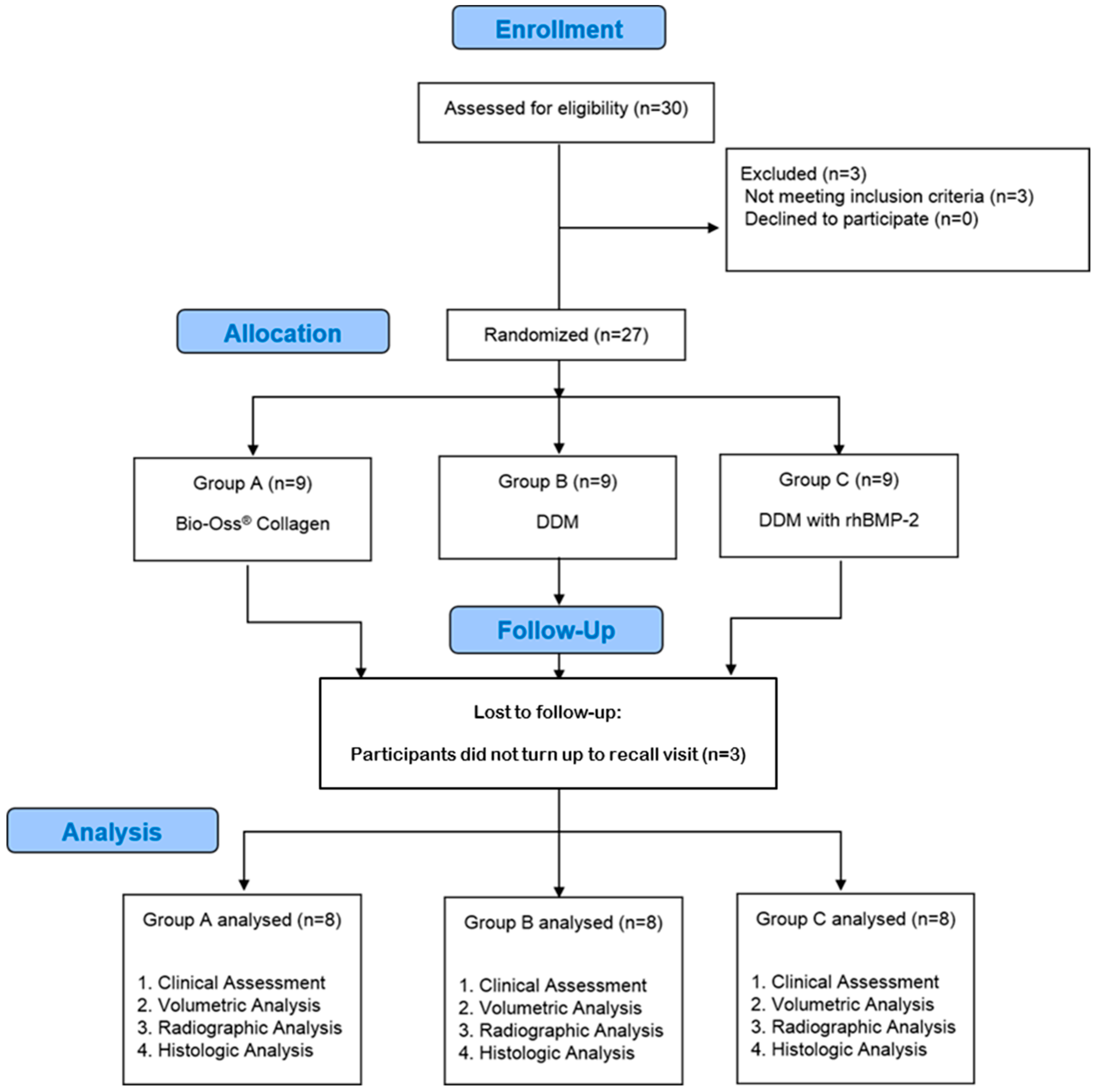
2.1. Enrollment of Patients
- (a)
- Group A: Bio-Oss® Collagen (Geistlich, Wolhusen, Switzerland) graft (n = 10)
- (b)
- Group B: DDM graft (n = 10)
- (c)
- Group C: DDM graft combined with rhBMP-2 (rhBMP-2/DDM) (n = 10)
2.2. Randomization Procedure
2.3. DDM and rhBMP-2 Preparation
2.4. Surgical Protocol
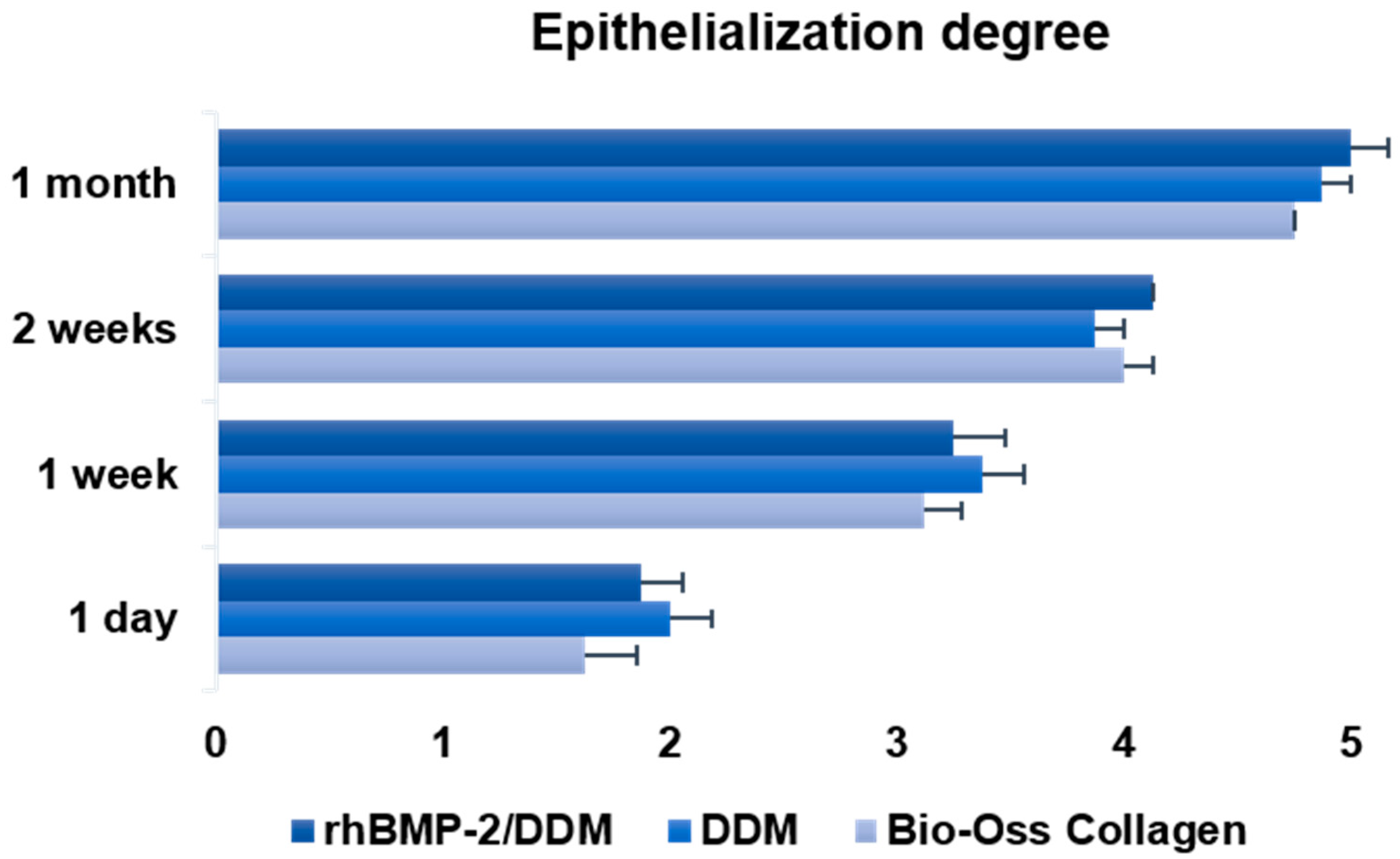
2.5. Clinical Assessment of Epithelialization
- Grade 1: non-existent
- Grade 2: covering less than one-quarter of the wound surface
- Grade 3: covering less than half the wound surface
- Grade 4: covering more than three-quarters of the wound surface
- Grade 5: normal or complete covering of the wound
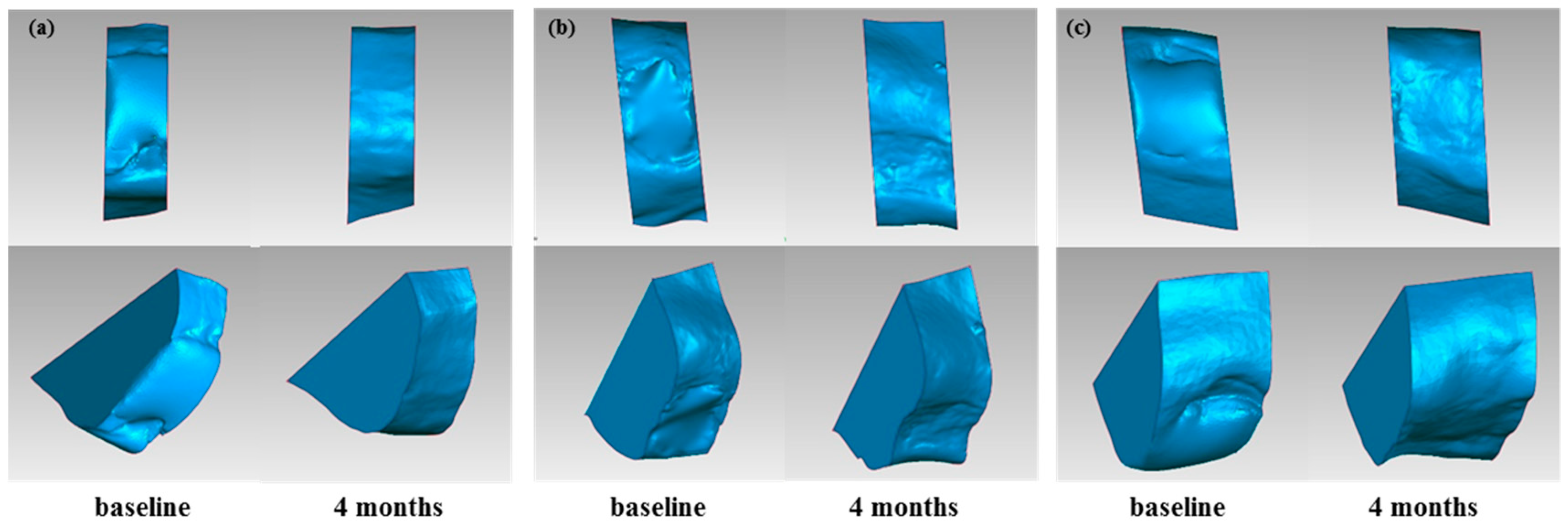
2.6. Volumetric Assessment of Soft and Hard Tissue
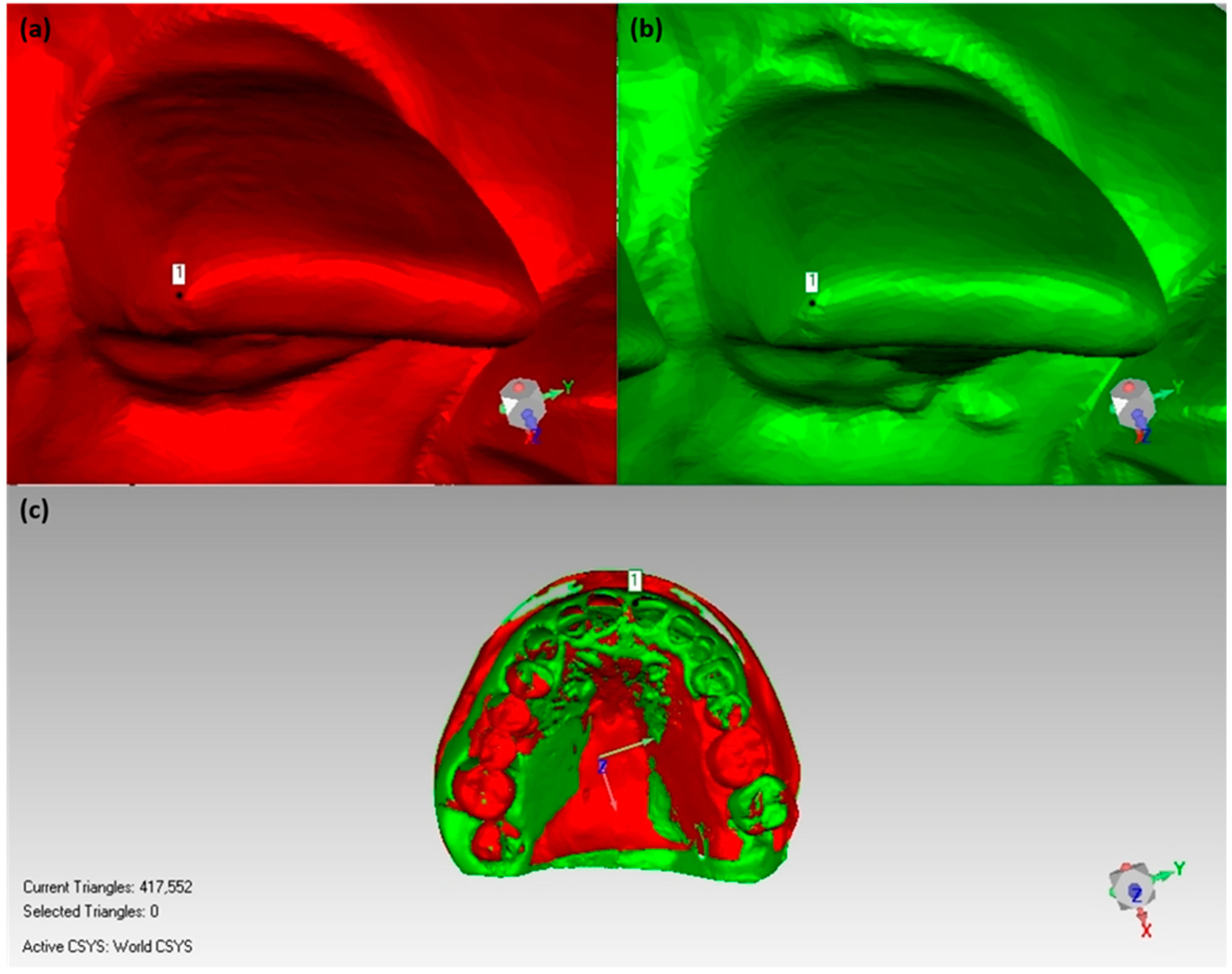
2.6.1. Volume Calculation for Soft Tissue/Stone Cast Measurement
2.6.2. Bone Volume Calculation/Radiographic Assessment
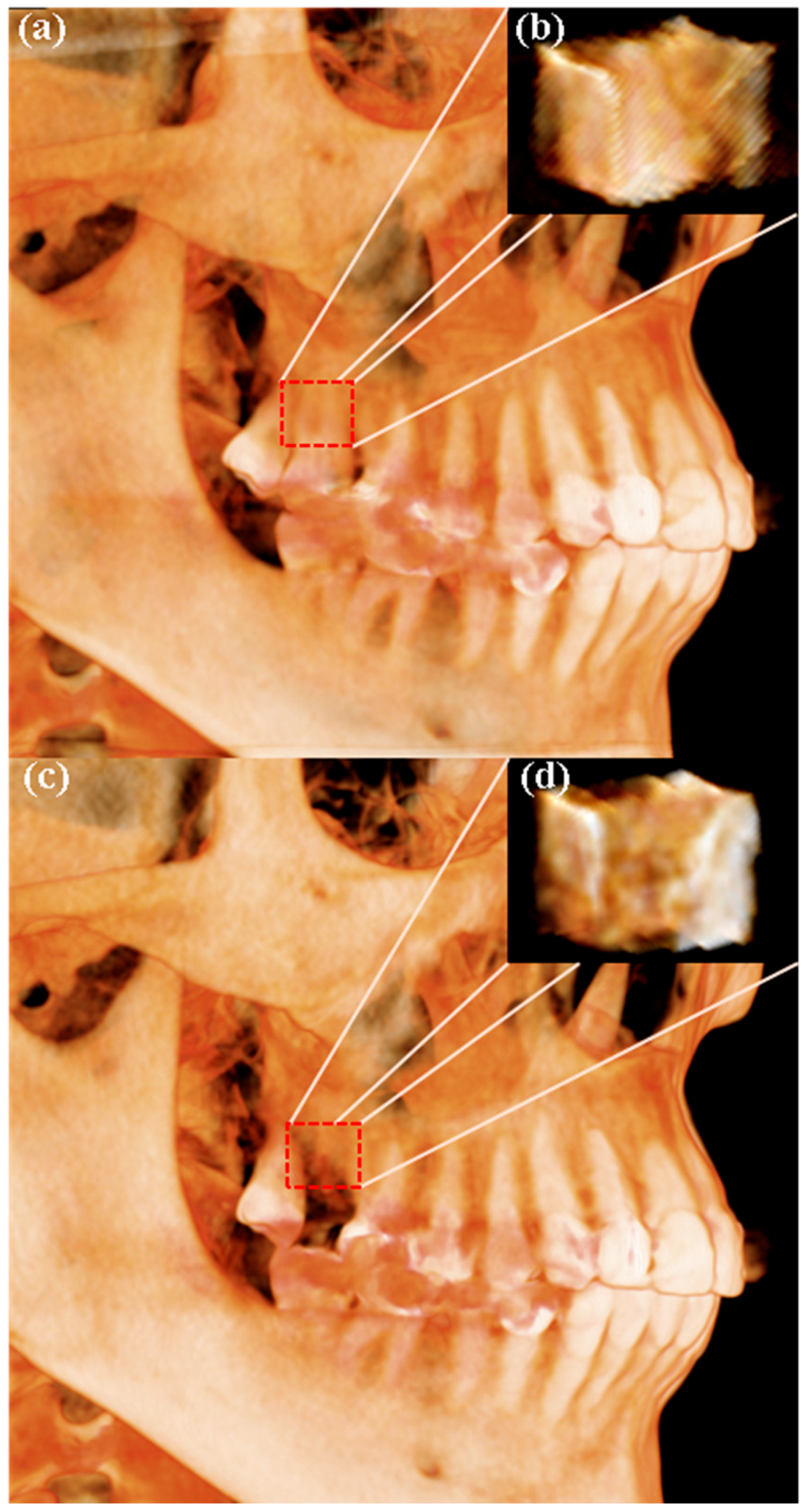
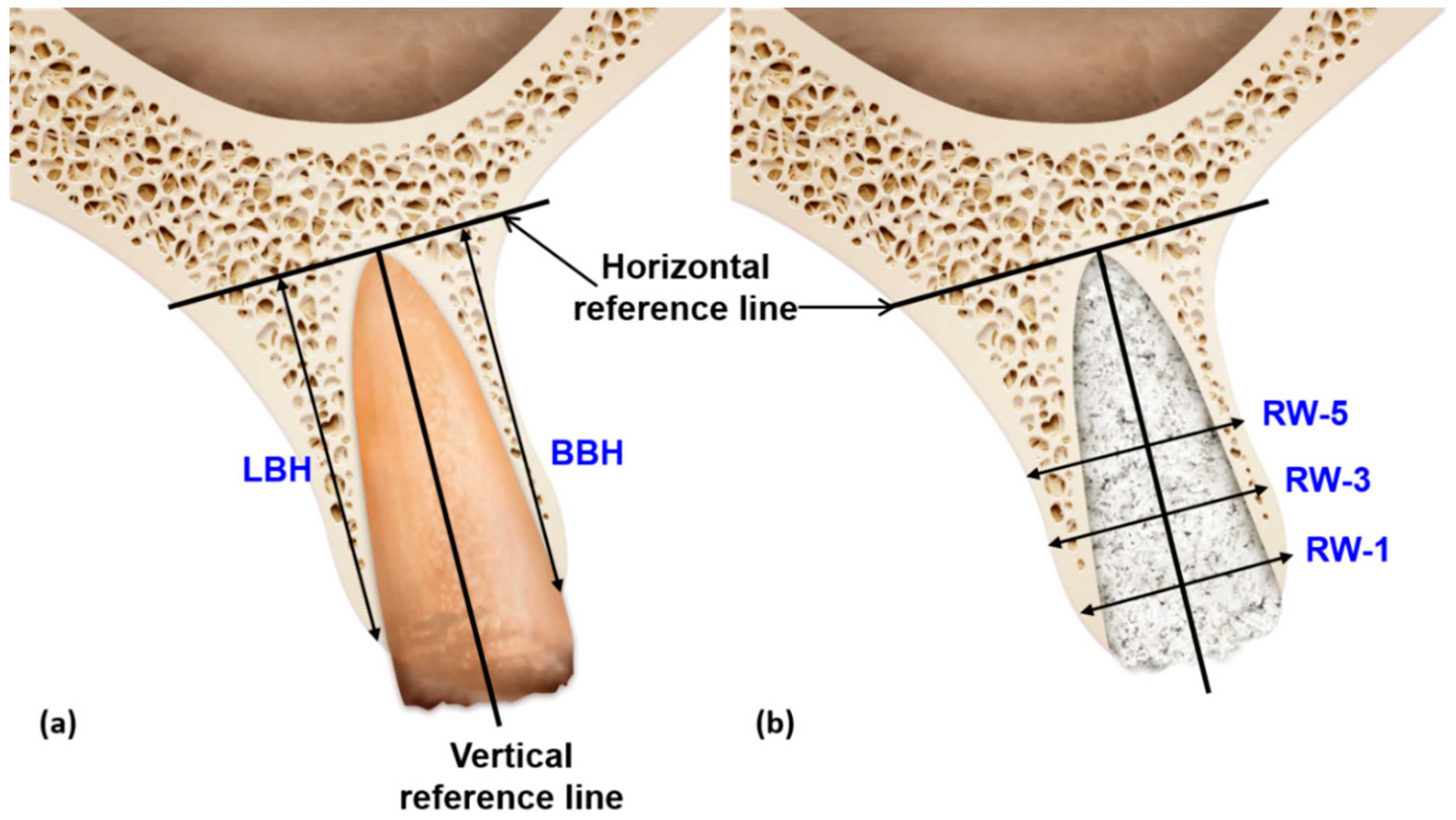
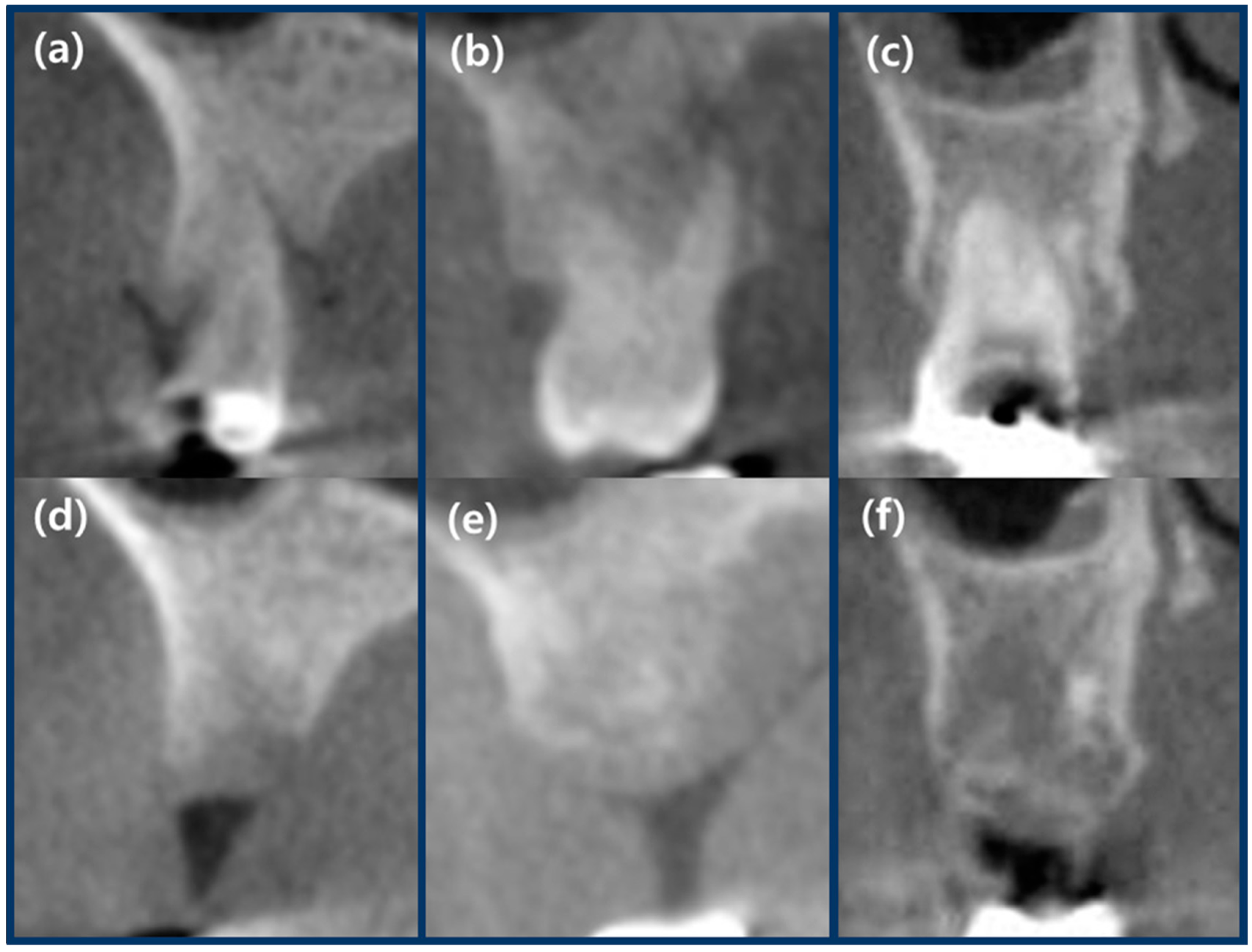
2.7. Radiographic Analysis Using CBCT
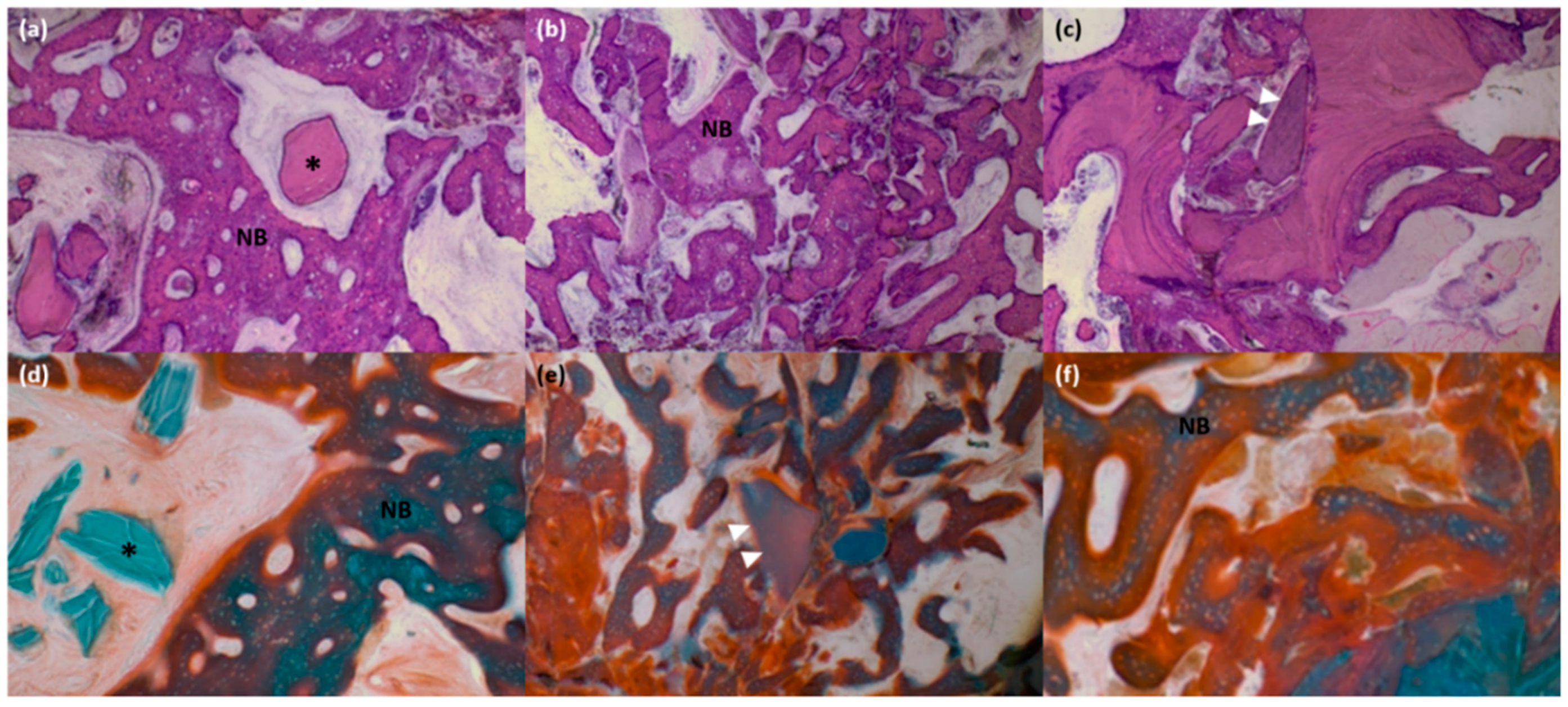
2.8. Histologic and Histomorphometric Evaluation
2.9. Statistical Analyses
3. Results
3.1. Ostoperative Healing Assessment
3.2. Results of Volumetric Analysis
3.3. Results of Radiographic Analysis
3.4. Histologic and Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schropp, L.; Kostopoulos, L.; Wenzel, A. Bone healing following immediate versus delayed placement of titanium implants into extraction sockets: A prospective clinical study. Int. J. Oral Maxillofac. Implants 2003, 18, 189–199. [Google Scholar] [PubMed]
- Tallgren, A.; Lang, B.R.; Walker, G.F.; Ash, M.M., Jr. Roentgen cephalometric analysis of ridge resorption and changes in jaw and occlusal relationships in immediate complete denture wearers. J Oral Rehabil 1980, 7, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent 2003, 23, 313–323. [Google Scholar] [PubMed]
- Mayer, Y.; Zigdon-Giladi, H.; Machtei, E.E. Ridge preservation using composite alloplastic materials: A randomized control clinical and histological study in humans. Clin. Implant Dent. Realt. Res. 2016, 18, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Irinakis, T. Rationale for socket preservation after extraction of a single-rooted tooth when planning for future implant placement. J. Can. Dent. Assoc. 2006, 72, 917–922. [Google Scholar] [PubMed]
- Artzi, Z.; Tal, H.; Dayan, D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: Histomorphometric evaluations at 9 months. J. Periodontol. 2000, 71, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Linder, E.; Wennstrom, J.; Lindhe, J. The influence of bio-oss collagen on healing of an extraction socket: An experimental study in the dog. Int. J. Periodontics Restorative Dent. 2008, 28, 123–135. [Google Scholar] [PubMed]
- Carmagnola, D.; Adriaens, P.; Berglundh, T. Healing of human extraction sockets filled with bio-oss. Clin. Oral Implants Res. 2003, 14, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Pelegrine, A.A.; da Costa, C.E.; Correa, M.E.; Marques, J.F., Jr. Clinical and histomorphometric evaluation of extraction sockets treated with an autologous bone marrow graft. Clin. Oral Implants Res. 2010, 21, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Brownfield, L.A.; Weltman, R.L. Ridge preservation with or without an osteoinductive allograft: A clinical, radiographic, micro-computed tomography, and histologic study evaluating dimensional changes and new bone formation of the alveolar ridge. J. Periodontol. 2012, 83, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Thalmair, T.; Fickl, S.; Schneider, D.; Hinze, M.; Wachtel, H. Dimensional alterations of extraction sites after different alveolar ridge preservation techniques—A volumetric study. J. Clin. Periodontol. 2013, 40, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Froum, S.; Cho, S.C.; Rosenberg, E.; Rohrer, M.; Tarnow, D. Histological comparison of healing extraction sockets implanted with bioactive glass or demineralized freeze-dried bone allograft: A pilot study. J. Periodontol. 2002, 73, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yun, P.Y.; Um, I.W.; Lee, H.J.; Yi, Y.J.; Bae, J.H.; Lee, J. Alveolar ridge preservation of an extraction socket using autogenous tooth bone graft material for implant site development: Prospective case series. J. Adv. Prosthodontics 2014, 6, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.M.; Um, I.W.; Kim, Y.K.; Woo, J.M.; Kim, S.M.; Lee, J.H. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin. Oral Implants Res. 2017, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.P.; D’Lima, C.B.; Samat, U.C.; Karde, P.A.; Patil, A.G.; Dani, N.H. Comparative alveolar ridge preservation using allogenous tooth graft versus free-dried bone allograft: A randomized, controlled, prospective, clinical pilot study. Contemp. Clin. Dent. 2017, 8, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Howell, T.H.; Cochran, D.; Malmquist, J.; Lilly, L.C.; Spagnoli, D.; Toljanic, J.; Jones, A.; Nevins, M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J. Periodontol. 2005, 76, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Gaydarov, N.; Badoud, I.; Vazquez, L.; Bernard, J.P.; Ammann, P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: A prospective randomized controlled study. Implant Dent. 2013, 22, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Min, B.M. Oral Biochemistry; Daehan Narae Publ.: Seoul, Korea, 2007; pp. 8–73. ISBN 978-897-089-146-0. [Google Scholar]
- Kim, Y.K.; Kim, S.G.; Byeon, J.H.; Lee, H.J.; Um, I.U.; Lim, S.C.; Kim, S.Y. Development of a novel bone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, J.; Kim, Y.K. Comparative analysis of guided bone regeneration using autogenous tooth bone graft material with and without resorbable membrane. J. Dent. Sci. 2013, 8, 281–286. [Google Scholar] [CrossRef]
- Jeong, K.I.; Kim, S.G.; Kim, Y.K.; Oh, J.S.; Jeong, M.A.; Park, J.J. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011, 20, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Pohl, V.; Schuh, C.; Fischer, M.B.; Haas, R. A new method using autogenous impacted third molars for sinus augmentation to enhance implant treatment: Case series with preliminary results of an open, prospective longitudinal study. Int. J. Oral Max Implants 2016, 31, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Bone engineering using human demineralized dentin matrix and recombinant human bmp-2. J. Hard Tissue Biol. 2005, 14, 80–81. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kitoh, H.; Sugiura, F.; Ishiguro, N. The effect of recombinant human bone morphogenetic protein-2 on the osteogenic potential of rat mesenchymal stem cells after several passages. Acta Orthop. 2007, 78, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Barber, H.; Frost, D.; Zeitler, D.; Stoelinga, P.; Catone, G. Reconstructive Preprosthetic Oral and Maxillofacial Surgery; Osseous Reconstruction; Saunders WB: Philadelphia, PA, USA, 1995; pp. 821–852. [Google Scholar]
- Herford, A.S.; Lu, M.; Akin, L.; Cicciu, M. Evaluation of a porcine matrix with and without platelet-derived growth factor for bone graft coverage in pigs. Int. J. Oral Max Implants 2012, 27, 1351–1358. [Google Scholar]
- Herford, A.S.; Cicciu, M.; Eftimie, L.F.; Miller, M.; Signorino, F.; Fama, F.; Cervino, G.; Lo Giudice, G.; Bramanti, E.; Lauritano, F.; et al. Rhbmp-2 applied as support of distraction osteogenesis: A split-mouth histological study over nonhuman primates mandibles. Int. J. Clin. Exp. Med. 2016, 9, 17187–17194. [Google Scholar]
- Cicciu, M.; Herford, A.S.; Cicciu, D.; Tandon, R.; Maiorana, C. Recombinant human bone morphogenetic protein-2 promote and stabilize hard and soft tissue healing for large mandibular new bone reconstruction defects. J. Craniofac. Surg. 2014, 25, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Philipp, A.; Annen, B.M.; Signorelli, L.; Thoma, D.S.; Hammerle, C.H.; Attin, T.; Schmidlin, P. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. J. Clin. Periodontol. 2013, 40, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Um, I.W.; An, H.J.; Kim, K.W.; Hong, K.S.; Murata, M. Effects of demineralized dentin matrix used as an rhbmp-2 carrier for bone regeneration. J. Hard Tissue Biol. 2014, 23, 415–422. [Google Scholar] [CrossRef]
- Um, I.W.; Hwang, S.H.; Kim, Y.K.; Kim, M.Y.; Jun, S.H.; Ryu, J.J.; Jang, H.S. Demineralized dentin matrix combined with recombinant human bone morphogenetic protein-2 in rabbit calvarial defects. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.R.; Jimenez, E.; Martinez, C.; Polanco, R.T.; Hirata, R.; Mousa, R.; Coelho, P.G.; Bonfante, E.A.; Tovar, N. Clinical and histological evaluation of socket grafting using different types of bone substitute in adult patients. Implant Dent. 2014, 23, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, J.Y.; Kim, J.E.; Park, J.C.; Shin, S.W.; Cho, K.S. Ridge preservation using demineralized bone matrix gel with recombinant human bone morphogenetic protein-2 after tooth extraction: A randomized controlled clinical trial. J. Oral Maxilofac. Surg. 2014, 72, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Levi, I.; Halperin-Sternfeld, M.; Horwitz, J.; Zigdon-Giladi, H.; Machtei, E.E. Dimensional changes of the maxillary sinus following tooth extraction in the posterior maxilla with and without socket preservation. Clin. Implant Dent. Relat. Res. 2017, 19, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Ten Heggeler, J.M.; Slot, D.E.; Van der Weijden, G.A. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin. Oral Implants Res. 2011, 22, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implants Res. 2012, 23 (Suppl. 5), 22–38. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.; Holtzclaw, D.; Rosen, P.S. A review on alveolar ridge preservation following tooth extraction. J. Evid. Based Dent. Pract. 2012, 12, 149–160. [Google Scholar] [CrossRef]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implants Res. 2012, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.G.; Donos, N. Alveolar ridge preservation. A systematic review. Clin. Oral Investig. 2013, 17, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jung, J.S.; Im, G.I.; Kim, B.S.; Cho, K.S.; Kim, C.S. Ridge regeneration of damaged extraction sockets using rhbmp-2: An experimental study in canine. J. Clin. Periodontol. 2015, 42, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Laney, W.R.; Broggini, N.; Cirelli, J. Glossary of Oral and Maxillofacial Implants; Quintessenz Publ.: Berlin, Germany, 2007; ISBN 978-393-894-700-5. [Google Scholar]
- Vieira, A.E.; Repeke, C.E.; Ferreira Junior Sde, B.; Colavite, P.M.; Biguetti, C.C.; Oliveira, R.C.; Assis, G.F.; Taga, R.; Trombone, A.P.; Garlet, G.P. Intramembranous bone healing process subsequent to tooth extraction in mice: Micro-computed tomography, histomorphometric and molecular characterization. PLoS ONE 2015, 10, e0128021. [Google Scholar] [CrossRef] [PubMed]
- Um, I.W.; Kim, Y.K.; Mitsugi, M. Demineralized dentin matrix scaffolds for alveolar bone engineering. J. Indian Prosthodontics Soc. 2017, 17, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Sato, D.; Hino, J.; Akazawa, T.; Tazaki, J.; Ito, K.; Arisue, M. Acid-insoluble human dentin as carrier material for recombinant human bmp-2. J. Biomed. Mater. Res. A 2012, 100, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Coomes, A.M.; Mealey, B.L.; Huynh-Ba, G.; Barboza-Arguello, C.; Moore, W.S.; Cochran, D.L. Buccal bone formation after flapless extraction: A randomized, controlled clinical trial comparing recombinant human bone morphogenetic protein 2/absorbable collagen carrier and collagen sponge alone. J. Periodontol. 2014, 85, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.B.; Lee, H.J.; Jang, J.W.; Kim, M.J.; Yun, P.Y.; Kim, S.H.; Choi, K.H.; Kim, Y.K.; Cho, K.S.; Shin, S.W. Randomized clinical trial on the efficacy of escherichia coli-derived rhbmp-2 with beta-tcp/ha in extraction socket. J. Adv. Prosthodontics 2011, 3, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Alkan, E.A.; Parlar, A.; Yildirim, B.; Senguven, B. Histological comparison of healing following tooth extraction with ridge preservation using enamel matrix derivatives versus bio-oss collagen: A pilot study. Int. J. Oral Maxillofac. Surg. 2013, 42, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
| Patient Information | Group A | Group B | Group C |
|---|---|---|---|
| Number | 8 | 8 | 8 |
| Age (years) | 49.75 ± 17.21 | 46.63 ± 18.12 | 45.38 ± 15.62 |
| Men/women | 5/3 | 4/4 | 5/3 |
| Biotype (thin/thick) | 4/4 | 2/6 | 5/3 |
| Grafted site (premolar/molar) | 3/5 | 0/7 | 1/6 |
| Group A (Bio-Oss Collagen) | Group B (DDM) | Group C (rhBMP-2/DDM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 Months | Ratio | Baseline | 4 Months | Ratio | Baseline | 4 Months | Ratio | |
| Soft Tissue Volume (mm3) | |||||||||
| Mean ± SD | 1343.68 ± 328.12 | 1117.53 ± 271.5 | 83.39 ± 5.52 | 1430.45 ± 201.42 | 1162.90 ± 198.8 | 80.99 ± 4.34 | 1430.94 ± 242.67 | 1171.64 ± 04.88 | 82.09 ± 7.91 |
| Median | 1500.55 | 1210.51 | 84.60 | 1421.00 | 1202.67 | 80.61 | 1383.36 | 1163.68 | 83.69 |
| Min | 739.98 | 624.91 | 75.11 | 1004.39 | 757.39 | 75.40 | 1182.92 | 815.08 | 68.70 |
| Max | 1655.29 | 1402.85 | 91.18 | 1629.35 | 1383.86 | 86.98 | 1743.84 | 1443.54 | 91.10 |
| p < 0.05 * | p < 0.05 * | p < 0.05 * | |||||||
| Hard Tissue Volume (mm3) | |||||||||
| Mean ± SD | 848.38 ± 426.26 | 637.25 ± 316.11 | 75.55 ± 9.79 | 1179.75 ± 84.32 | 892.75 ± 367.17 | 86.12 ± 7.22 | 780.13 ± 417.88 | 617.88 ± 370.63 | 78.35 ± 11.01 |
| Median | 712.00 | 567.50 | 78.51 | 1314.50 | 973.50 | 87.18 | 672.00 | 463.00 | 78.34 |
| Min | 402.00 | 302.00 | 63.31 | 626.00 | 491.00 | 76.51 | 283.00 | 214.00 | 65.90 |
| Max | 1439.00 | 1088.00 | 91.79 | 1413.00 | 1371.00 | 97.03 | 1312.00 | 1254.00 | 95.58 |
| p < 0.05 * | p < 0.05 * | p < 0.05 * | |||||||
| Group A | Group B | Group C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (mm) | Baseline | 4 Months | Δ | Baseline | 4 Months | Δ | Baseline | 4 Months | Δ |
| BBH | 8.34 ± 2.50 | 7.21 ± 2.05 | 1.14 ± 0.81 | 9.18 ± 2.14 | 8.21 ± 1.88 | 0.97 ± 0.39 | 8.78 ± 1.71 | 7.95 ± 1.73 | 0.82 ± 0.36 |
| LBH | 8.34 ± 2.54 | 7.70 ± 2.52 | 0.65 ± 0.37 | 9.26 ± 2.09 | 8.50± 2.06 | 0.76 ± 0.29 | 8.61 ± 1.65 | 8.12 ± 1.78 | 0.50 ± 0.22 |
| RW-1 | 10.68 ± 2.41 | 9.00 ± 2.76 | 1.68 ±1.11 | 12.52 ± 1.68 | 11.74 ± 1.57 | 0.78 ± 0.41 | 12.14 ± 1.42 | 10.60 ± 1.90 | 1.54 ± 0.74 |
| RW-3 | 11.84 ± 2.28 | 10.60 ± 2.61 | 1.24 ± 0.65 | 14.29 ± 1.84 | 13.80 ± 1.80 | 0.49 ± 0.47 | 12.67 ± 1.99 | 11.87 ± 2.03 | 0.79± 0.54 |
| RW-5 | 12.71 ± 2.27 | 12.20 ± 2.24 | 0.50 ± 0.19* | 15.94 ± 1.88 * | 15.74 ± 1.89 * | 0.20 ± 0.15 | 13.38 ± 1.80 † | 13.06 ± 1.73† | 0.32 ± 0.21 |
| Ridge Preservation Sites | Number | Group A | Group B | Group C |
|---|---|---|---|---|
| Grafted area (%) | n = 6 | 13.20 ± 9.79 | 10.72 ± 9.83 | 11.02 ± 12.72 |
| New bone area (%) | n = 6 | 22.00 ± 11.01 | 32.88 ± 14.48 | 39.09 ± 15.30 |
| Soft tissue area (%) | n = 6 | 64.80 ± 10.11 | 56.40 ± 8.58 | 49.88 ± 11.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, G.-U.; Jeon, T.-H.; Kang, M.-H.; Um, I.-W.; Song, I.-S.; Ryu, J.-J.; Jun, S.-H. Volumetric, Radiographic, and Histologic Analyses of Demineralized Dentin Matrix Combined with Recombinant Human Bone Morphogenetic Protein-2 for Ridge Preservation: A Prospective Randomized Controlled Trial in Comparison with Xenograft. Appl. Sci. 2018, 8, 1288. https://doi.org/10.3390/app8081288
Jung G-U, Jeon T-H, Kang M-H, Um I-W, Song I-S, Ryu J-J, Jun S-H. Volumetric, Radiographic, and Histologic Analyses of Demineralized Dentin Matrix Combined with Recombinant Human Bone Morphogenetic Protein-2 for Ridge Preservation: A Prospective Randomized Controlled Trial in Comparison with Xenograft. Applied Sciences. 2018; 8(8):1288. https://doi.org/10.3390/app8081288
Chicago/Turabian StyleJung, Gyu-Un, Tae-Hyun Jeon, Mong-Hun Kang, In-Woong Um, In-Seok Song, Jae-Jun Ryu, and Sang-Ho Jun. 2018. "Volumetric, Radiographic, and Histologic Analyses of Demineralized Dentin Matrix Combined with Recombinant Human Bone Morphogenetic Protein-2 for Ridge Preservation: A Prospective Randomized Controlled Trial in Comparison with Xenograft" Applied Sciences 8, no. 8: 1288. https://doi.org/10.3390/app8081288
APA StyleJung, G.-U., Jeon, T.-H., Kang, M.-H., Um, I.-W., Song, I.-S., Ryu, J.-J., & Jun, S.-H. (2018). Volumetric, Radiographic, and Histologic Analyses of Demineralized Dentin Matrix Combined with Recombinant Human Bone Morphogenetic Protein-2 for Ridge Preservation: A Prospective Randomized Controlled Trial in Comparison with Xenograft. Applied Sciences, 8(8), 1288. https://doi.org/10.3390/app8081288





