Abstract
Collagen-based membranes (CBMs) have similar permissive bone formation capabilities when compared to non-absorbable membranes. CBMs have been classified as non-cross-linked membranes (NCLMs) and cross-linked membranes (CLMs) depending on whether the cross-linking between the collagen fibers was artificially increased. The purpose of this study is to evaluate the bone maintenance capacity between NCLMs and CLMs by comparing resorption of regenerated bone. The inclusion criteria consisted of: (1) The use of a CBM, either being an NCLM or CLM for coverage of grafted bone; (2) follow-up for more than one year; (3) the presence of the patient's orthopantomographic X-ray (OPTG) immediately following the installation of implants and one year after. The bone resorption observed in the OPTG was determined by measuring the number of exposed threads of the implant. The number of thread exposures in the implant was compared according to CBM types. OPTGs taken immediately following the installation of implants and at one year after installation were compared. The subject of the measurement was always the implant in which the greatest number of exposed threads were present in each patient. A total of 56 subjects and 97 implants were used in this study. There was no significant difference between NCLM and CLM groups (p > 0.05). However, there was a statistically significant difference (p = 0.02) between the groups when a bone graft was applied to both the maxilla and the mandible. The average number of thread exposures was less than 1.5. In this study, no comparison was made between commonly known causes of bone loss and membrane types.
1. Introduction
The alveolar bone changes dynamically within one year of tooth extraction [1]. Alveolar bone volume decreases by 40 to 60%, on average, within three years [1,2]. It is also reported that the width and height of alveolar bone decreased 23–63% and 11–22%, respectively, within six months after extraction [3]. The width and height of the alveolar bone are historically regarded as amongst the most important factors that allow implant-borne prosthesis to achieve such a highly predictable prognosis [4]. Therefore, augmentation of the alveolar bone is a necessary treatment procedure for the ideal prognosis of the implant prosthesis. It is also important to recover the height of the missing alveolar bone for esthetic results of the implant prosthesis [5]. If an implant was to be placed into a bed with an insufficient alveolar bone volume or quality, complications such as implant thread exposure, peri-implantitis, and premature fixture failure may arise [6,7]. In order to provide the ideal environment for augmentation of the alveolar bone, bone grafts such as autograft or xenograft can be used in guided bone regeneration techniques (GBR). Bone grafting with a block-shaped autogenous bone graft has been recommended for alveolar bone augmentation of greater than 2 mm [8]. An absorbable collagen-based membrane is most widely used in bone graft procedures for blocking out undesired fibrous and epithelial ingrowth. Delgado et al., published in 2014, showed another function of the membrane-covering graft materials. According to their research, minimizing the movement of grafted material results in maximal bone formation. The membrane should be applied to fix the grafted material [9]. In the case of bone graft for the preservation of the extraction socket, they suggested that applying the appropriate pressure (200 g), together with the membrane, may cause the graft material to condense and form a better quality of bone [10].
Collagen-based membranes (CBMs) have similar permissive bone formation capabilities when compared to non-absorbable membranes [11]. CBMs have been classified as non-cross-linked membranes (NCLMs) and cross-linked membranes (CLMs) depending on whether the cross-linking between the collagen fibers was artificially increased. The basic principle of cross-linking is the formation of covalent bonds between collagen fibers. The use of aldehyde and other chemical materials for covalent bonding has been referred to as chemical cross-linking. Carbohydrates such as ribose might also be used in cross-linking. The natural behavior of the collagen membrane depends on the type of material used for cross-linking [12].
CLMs are less likely to cause an inflammatory reaction than NCLMs and are known to have a favorable effect on bone formation due to the delayed absorption of the membrane. However, the use of CLM does not guarantee better quality bone formation. Additionally, the delayed absorption may also allow the membrane to become exposed in the oral cavity and cause infection [13]. The amount and quality of the bone formed are similar regardless of the type of membrane, but the exposure of the grafted bone to the oral environment during the healing period is known to be an important negative factor [14].
Previous literature studied the effects of CBMs on the width and height of the generated bone immediately following augmentation [13,14,15,16]. In this current study, radiographic images were used to evaluate the bone resorption profile one year following the installation of implants. Before implant installation, alveolar bone augmentation using an autogenous mandibular block bone and CBMs was performed. The purpose of this study was to evaluate bone maintenance between NCLMs and CLMs by comparing bone resorption around the implants.
2. Materials and Methods
2.1. Patients
This study was performed as a retrospective analysis. Patients who underwent a mandibular block bone graft by the same single operator at the Department of Oral and Maxillofacial Surgery, Pusan National University Dental Hospital, Korea. The inclusion criteria consisted of:
(1) The use of a CBM either being a NCLM: Bio-guideTM (SK Bioland, Seoul, Korea) or a CLM: Ossix plusTM (Regedent AG, Zurich, Switzerland) for coverage of grafted autogenous bone.
(2) Follow-up for more than one year after bone grafting and installation of implants.
(3) Orthopantomographic X-ray (OPTG) of the patients immediately following implant fixture installation of implants and one year after. The underlying disease of individual patients was not considered. The exclusive criteria comprised:
(1) The use of membranes other than Bio-guideTM and Ossix plusTM.
(2) Insufficient imaging data. The total number of patients who passed the inclusion criteria of the present study was 56, including 30 males and 26 females, who were aged 18 to 72 years.
2.2. Bone Augmentation Surgical Protocol
Figure 1 illustrates the surgical protocol by showing a bone graft using a mandibular block bone. Titanium miniscrews of 6 to 9 mm are used to fix the block bone, and particulated mandibular bone is filled around the block bone to occupy the dead space and to create a smooth curvature of the grafted site, see Figure 1A. After the application of fibrin glue to stabilize the particulated bone, CBM is used for coverage and protection of the graft materials, see Figure 1B.
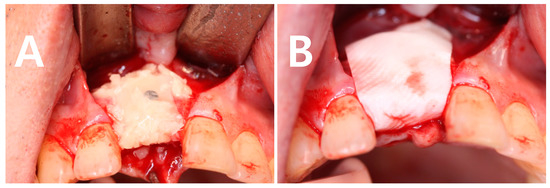
Figure 1.
Clinical photographs of the procedure of a mandibular block bone graft for augmentation of an atrophic alveolar bone. (A) Fibrin glue was applied to the grafted bone, the block bone was secured with titanium miniscrew and the particulated bone was filled around the block bone. (B) collagen-based membranes (CBM) was used on the graft site after trimming of the grafted bone.
2.3. Implantation Protocol
After bone augmentation, patients had an average bone regeneration period of 4.982 ± 0.904 months. After that, the patient received an implantation procedure. Implantation was performed in one or two stages by a single surgeon. We used both the submerged type and non-submerged type implant designs, but this study did not compare the results according to the design difference. The taper and surface treatment method of the implant fixture were not considered. When a two-stage implantation was performed, the patient had an osseointegration time which was typical when compared to those reported previously.
Alveolar bone augmentation increases the width of the alveolar bone to allow the bone to be, at least, 1 mm thick around the implant. It also increases the height of the alveolar bone so that the ratio of crown to root of the final prosthesis is not more than 1:1.
2.4. Radiographic Examination
OPTGs taken immediately, four months, and at the one year following implant placement were used to evaluate the stability of the grafted bone which was covered with CBM and the success of the installed implants, see Figure 2. The number of exposed threads of the implants was measured and evaluated. This was considered as an indication of the postoperative maintenance of the bone grafts and the success of implants. The subject of the measurement was always the implant in which the greatest number of exposed threads was present for each patient. Other variables were recorded, such as the type of membrane, sex, age, site of implant placement (maxilla/mandible, anterior/posterior, horizontally/vertically grafted, external/internal implants type, and one- or two-step installation of the implant fixtures.
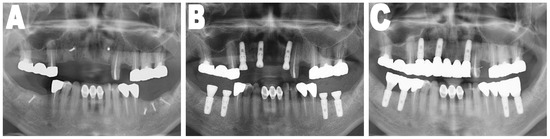
Figure 2.
Orthopantomographic images. (A) On the day of the mandibular block bone graft; (B) Immediately after installation of implants; (C) One year after installation of the implants. Analysis between (B,C) orthopantomographic X-ray (OPTG) images were performed to check the number of exposed threads.
2.5. Statistical Analysis
A one-way analysis of variance (ANOVA) was used for comparative analysis (p < 0.05) and posthoc-tests were performed using Duncan’s new multiple range test. All statistical analyses were performed using the R language program.
3. Results
A total of 56 subjects took part in this study. This included 26 women and 30 men. All adults were at least 18 years of age and ranged up to an age of 72 years. The total number of implants placed were 97. The number of patients in whom NCLMs were used was 52, and the number of patients with CLMs was 4. There were 32 patients who underwent bone grafting and implantation in the maxilla, 13 patients in the mandible, and 11 patients in the maxilla and mandible. In addition, 21 patients were treated in the anterior region, and 35 patients were treated in the posterior region. There were 23 patients with horizontally-augmenting bone grafts, 21 patients with vertically-augmenting grafts, and 12 patients with bi-directional grafts. There were 43 patients with external-type implants and 13 with internal-type implants. A total of 28 patients had a one-stage implantation, and 28 underwent a two-stage implantation. These results are summarized in Table 1, which also shows the number of exposed implant threads observed in each factor.

Table 1.
The numbers of exposed implant threads in each factor. (Abbreviations; Maxilla: Mx., Mandible: Mn, Non-cross-linking membrane: NCLM, Cross-linking membrane: CLM, * p < 0.05)
There was no significant difference between the groups (p > 0.05). However, there was a statistically significant difference (p = 0.02) between the groups when a bone graft was applied to both the maxilla and the mandible. The average number of thread exposures was less than 1.5. However, the number of thread exposures was more than two in the 30-years age group and in the case of multiple implantations in both jaws. Figure 3 visualizes the table.
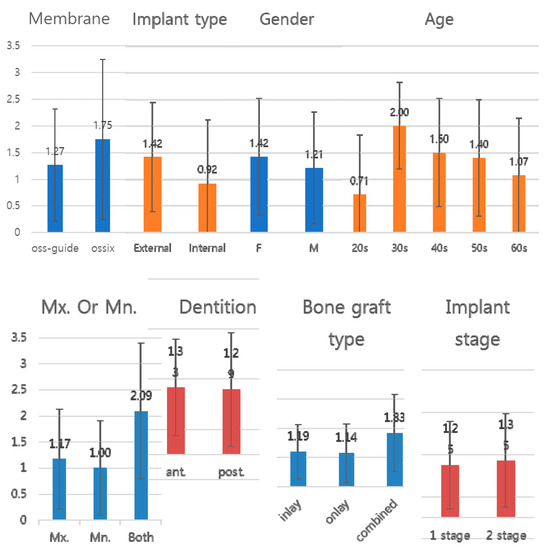
Figure 3.
Graph of Table 1. The y-axis represents the number of implant threads exposed. The values of the y-axis are constant, and the numerical values of each factor can be easily compared. As a result of comparing with constant y-axis values, it can be seen that the number of implant thread exposures caused by various factors is not significantly different. Factors that cause a noticeable number of thread exposures are not observed.
4. Discussion
Collagen-based membranes are commonly used for alveolar bone augmentation in clinical practice. CBMs provide a shielding film that protects the underlying grafted bone from the external environment. Because CBMs are composed of collagen, which occupies most of the normal tissue extracellular matrix, host immune cells, fibroblasts, and endothelial cells migrate through it and settle in and around it easily. Mild, stable host reactions are observed. The quality and quantity of the formed bone are also more predictable [17]. NCLMs completely disintegrate when present in the body for 16 weeks [18]. NCLMs weakly attract bacteria in the mouth [16]. This is a particular disadvantage for bone regeneration using NCLMs.
Cross-linked membranes (CLMs) with increased cross-linking between collagen fibers have been introduced to compensate for the drawbacks of NCLMs. Cross-linking is formed by a chemical or dehydrothermal mechanism. Many currently known CLMs are chemically cross-linked membranes. Ossix plusTM, used in this study, also uses the carbohydrate ribose to form cross-linking. The properties of CLMs vary depending on the chemical material used. In general, temperature stability, physical strength, and decomposition resistance are increased in CLMs [15]. Figure 4 illustrates the results of bone grafting using each membrane while a flap was lifted to facilitate implantation. It can be confirmed that there is no difference in the height or width of the formed bone. With Ossix, it takes a long time for the membrane to resorb, so it may still be present in the body at the time when the flap is opened.
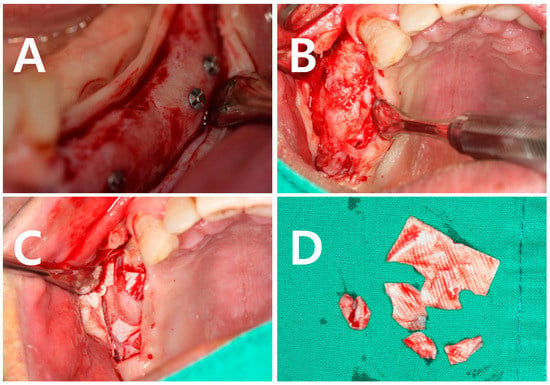
Figure 4.
A clinical picture of augmented alveolar bone taken after a bone augmentation using a mandibular body block bone. After 4.982 ± 0.904 months, a flap was elevated for implant placement. (A) A clinical photo of the augmented bone with an elevated flap. (B) Using an NCLM (Bio-Guide), membrane no residue was observed in soft tissue after 4.982 ± 0.904 months. (C) Using CLM (Ossix), it is possible to observe the membrane that is not absorbed in the body but remains in the patient. (D) Remnants of separated CLM.
The cross-linking rate is proportional to bio-resistance. Many studies have shown that this prolonged duration may contribute to bone mineralization [19]. On the other hand, it can be argued that the delayed absorption not only increases the probability of exposure of the membrane to the oral environment but may also reduce the quality of the bone by causing an inflammatory reaction [20].
The purpose of this study was to compare the efficacy of using controversial collagen-based membranes. However, unlike previous studies, the authors looked for a correlation between early implant bone loss and collagen-based membrane type with evaluation after a period of one year. This intended to show whether the membrane type affects the ability to retain the formed bone. In fact, analysis showed that the type of membrane did not affect bone loss significantly. Other factors such as gender, age, the location of implants in the jaw, implant type, bone graft type, and implantation stage were analyzed to determine whether they correlated with early bone loss. As shown in Figure 3, there is no difference in the significance of each factor. However, when multiple implants were placed in the maxilla and mandible, statistically significant correlations were observed. It is presumed that this is related to factors causing the loss of many teeth, such as general systemic factors, oral normal flora, and occlusal loading.
Another feature of bone-grafted patients with maxillary and mandibular sites was that the implants placed in a patient showed different thread exposure numbers depending on their position and the shape of the upper prosthesis. More thread exposures were observed when multiple prostheses were connected, when the crown to root ratio was not physiologic, or when non-submerged implantation was performed. These results suggest that early implant bone loss is more likely to occur as a result of the already established causes such as surgical trauma, occlusal overload, and microgap, regardless of the type of membrane used [21,22].
In this study, there is no comparison between the commonly known causes of bone loss and membrane types. Therefore, it is still necessary to investigate the effects of the quality and quantity of grafted bone by comparing the known common causes that lead to reduced implant success and the membrane types. The experience with CLMs is quite limited in this study as four patients had CLMs placed while 52 patients received NCLMs. This is also worthy of future study.
Author Contributions
N.-R.C. carried out the analysis of data and prepared the manuscript. G.K.S. conceived of the study, participated in its design and coordination, and helped to draft the manuscript. Y.-D.K. designed the study and drafted the manuscript.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2018R1A5A2023879) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C0708).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tallgren, A. The continuing reduction of the residual alveolar ridges in complete denture wearers: A mixed-longitudinal study covering 25 years. J. Prosthet. Dent. 2003, 89, 427–435. [Google Scholar] [CrossRef]
- Carlsson, G.E.; Thilander, H.; Hedegard, B. Histologic changes in the upper alveolar process after extractions with or without insertion of an immediate full denture. Acta Odontol. Scand. 1967, 25, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implants Res. 2012, 23 (Suppl. 5), 1–21. [Google Scholar] [CrossRef]
- Leong, D.J.; Oh, T.J.; Benavides, E.; Al-Hezaimi, K.; Misch, C.E.; Wang, H.L. Comparison between sandwich bone augmentation and allogenic block graft for vertical ridge augmentation in the posterior mandible. Implant Dent. 2015, 24, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P.; Magner, A.W.; Fletcher, P. The effect of the distance from the contact point to the crest of bone on the presence or absence of the interproximal dental papilla. J. Periodontol. 1992, 63, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Ostman, P.O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontology 2000 2017, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Aloy-Prosper, A.; Penarrocha-Oltra, D.; Penarrocha-Diago, M.; Penarrocha-Diago, M. The outcome of intraoral onlay block bone grafts on alveolar ridge augmentations: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e251–e258. [Google Scholar] [CrossRef] [PubMed]
- Milinkovic, I.; Cordaro, L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Abboud, M.; Ramirez-Fernández, M.P.; Maté-Sánchez, J.E.; Negri, B.; Won, A.; Romanos, G. Porous titanium granules in critical size defects of rabbit tibia with or without membranes. Int. J. Oral Sci. 2014, 6, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.; Romanos, G.E.; Alexandre Gerhke, S.; Gomez-Moreno, G.; Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L. Biological effects of compressive forces exerted on particulate bone grafts during socket preservation: Animal study. Clin. Oral Implants Res. 2018, 29, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Herten, M.; Sager, M.; Becker, J. Angiogenesis pattern of native and cross-linked collagen membranes: An immunohistochemical study in the rat. Clin. Oral Implants Res. 2006, 17, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Al-Nawas, B.; Klein, M.O.; Schliephake, H.; Terheyden, H.; Schwarz, F. Use of a new cross-linked collagen membrane for the treatment of dehiscence-type defects at titanium implants: A prospective, randomized-controlled double-blinded clinical multicenter study. Clin. Oral Implants Res. 2009, 20, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, A.; Gissel, K.; Soudan, M.; Kleber, B.M.; Pitaru, S.; Dietrich, T. Randomized controlled trial on lateral augmentation using two collagen membranes: Morphometric results on mineralized tissue compound. J. Clin. Periodontol. 2011, 38, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Charulatha, V.; Rajaram, A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003, 24, 759–767. [Google Scholar] [CrossRef]
- Jung, R.E.; Fenner, N.; Hammerle, C.H.; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Implants Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Owens, K.W.; Yukna, R.A. Collagen membrane resorption in dogs: A comparative study. Implant Dent. 2001, 10, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Kohavi, D.; Krausz, E.; Steinberg, D.; Rosen, G. Enzymatic degradation of collagen-guided tissue regeneration membranes by periodontal bacteria. Clin. Oral Implants Res. 2003, 14, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Zubery, Y.; Goldlust, A.; Alves, A.; Nir, E. Ossification of a novel cross-linked porcine collagen barrier in guided bone regeneration in dogs. J. Periodontol. 2007, 78, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Zubery, Y.; Nir, E.; Goldlust, A. Ossification of a collagen membrane cross-linked by sugar: A human case series. J. Periodontol. 2008, 79, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.G.K.; Oh, J.H. Recent advances in dental implants. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 33. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Kim, Y.H.; Choi, H.S.; Oh, J.S.; Shin, S.H.; Kim, Y.D. The rate and stability of mandibular block bone graft in recent 5 years. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 21. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).