1. Introduction
Soft tissue elastic properties change in various pathological tissues, such as malignant tumors and inflammatory processes. Strain elastography can be used to quantify this physical feature based on ultrasound imaging. Tissue hardness can be assessed across tissue images describing the Elastic modulus (
E), defined as the relationship between the application of local stress and the resulting strain. This can be expressed as:
Since the stress is not recorded as it travels from the stress source through the tissue as it gradually attenuates, calculating the Elastic modulus from strain data alone is not possible. This phenomenon is sometimes referred to as the “inverse problem” of strain elastography [
1]. Under similar stress, strain in harder tissue is lower than strain in softer tissue [
2,
3]. Thus, a comparison between strain in the reference tissue and lesion produces a ratio that increases above one when the focal lesion is harder than the reference tissue. Strain ratio (
SR) represents the relative difference in tissue hardness [
4]. The definition of
SR is:
Hooke’s law states that for small deformations in elastic media, the strain is linearly proportional with the force (stress) applied. However, this is true for isotropic and homogeneous media with near infinite or free border conditions. These conditions are rarely present in biological tissues, which exhibit non-linear elastic properties of different magnitudes due to differences in tissue structure and function. This may be of importance for the accuracy of strain elastography. By restricting the pre-compression and range of compressions (Δ-stress) to a limited interval, the stress–strain relation of the tissues involved may still be regarded as linear.
Vital tissue contains ducts and veins that act as stress dampers, as well as connective tissue and sliding anatomical surfaces that limit and enhance tissue movement, respectively. Unintended movements may cause strain concentration and reduce the accuracy of SR evaluation. Hence, in vivo conditions often do not meet the preconditions of Hooke´s law for elasticity calculation, and may therefore represent limitations and cause strain-imaging artefacts that increase variability and reduce the reproducibility of SR measurements.
SR expresses a momentarily and relative difference in compressibility in two user-selected areas within selected regions of interest in a strain elastogram. SR is dependent on similar stress applications in the two areas compared, and similar stress attenuation in the tissue between the stress source (probe) and the area of interest. The SR measurement method was first introduced as the “fat-lesion ratio” in breast imaging, where an area of subcutaneous fat was used as the reference to mean strain in the lesion under investigation. Using the subcutaneous fat as reference was perhaps as close to a standardized reference tissue as one can get. However, the same preconditions apply, as the tissue constitution between the probe and target lesion, and the probe distance may influence the strain distribution in reference area fat tissue.
The variations in elasticity of biological tissues is not linear with varying pre-compression or stretch of the tissue. For breast and pancreatic tissues, this has been evaluated by force indentation and deformation studies under increasing strength. This implies that the amount of pre-compression and the range of the stress applied influences the strain result and thereby the elastogram. In one study, the authors recommended a pre-compression level less than 0.2–0.4 kPa for breast imaging [
5].
Another physical condition that complicates the reproducibility of strain ratios is the temporal variability in a live strain cine-loop. The best phases for acquiring strain data is during the compression and decompression phase, since no strain signal is transmitted when the stress is stable between these two phases. Compression and decompression of vital tissue may be caused by applied pressure from the probe or by natural internal movements from arterial pulsation, heart movements, or even breathing. Some strain elastography platforms provide feedback to the examiner about the phase of compression or decompression on which the image is acquired, enabling the use of
SR from similar phases of tissue straining. One study on breast imaging concluded that peak
SR performs better than average
SR in breast tumor characterization [
6].
Several studies reported high accuracy of
SR in determining focal breast lesions as malignant or benign. In a meta-analysis, Sadigh et al. reported a sensitivity and a specificity of 88% and 83%, respectively, for
SR with a receiver operating characteristic-area under curve (ROC-AUC) of 0.92. Strain elastography with
SR has been compared to evaluation using a strain histogram with similar good results [
7].
SR was reported to be better than magnetic resonance imaging (MRI) for breast tumor characterization, but the combination of the two modalities had a better ROC-AUC of 0.914 [
8]. Furthermore, for the evaluation of axillary lymph nodes in breast cancer patients, a combined evaluation of B-mode ultrasound (US) and Real-Time Elastography (RTE) increased specificity [
9]. Also, in the characterization of thyroid nodules as malignant or benign,
SR was reported to have sensitivity of 85–89% with specificity of 80–82% in two meta-analyses [
10,
11]. In trans-rectal applications in prostate and rectal tumors,
SR has been used to improve B-mode identification of malignant tumors with adequate accuracy (ROC-AUC > 90%) [
12,
13].
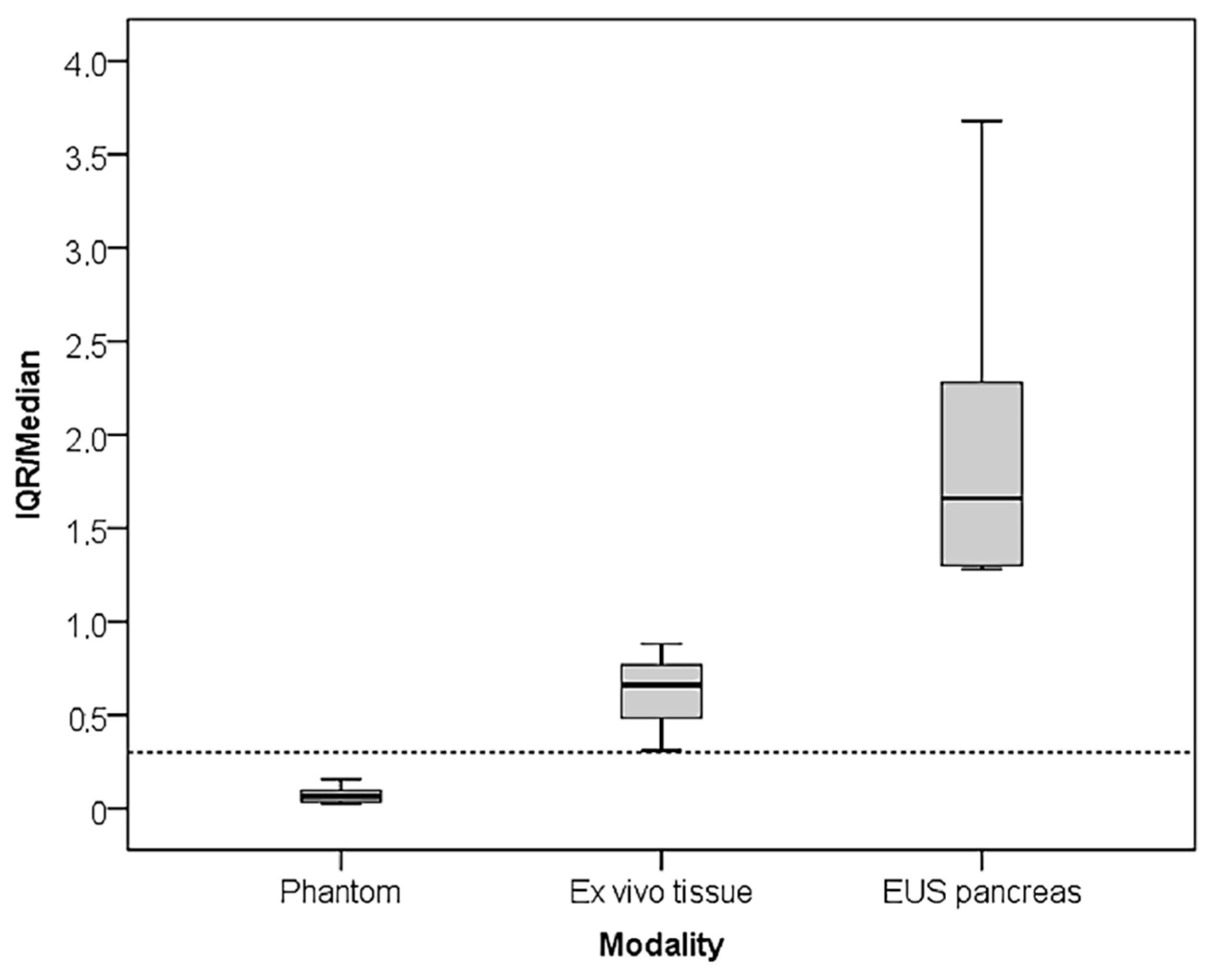
The aim of this study was to retrospectively compare variability in strain-based elastography quantified by SR, recorded in three different applications using Real-Time Elastography in homogeneous tissue-mimicking media, in resected tissue from bowel lesions, and during the endoscopic ultrasound (EUS) of focal pancreatic lesions. We chose to calculate the inter-quartile range (IQR) and median for all applications based on previous studies, since this has become a much-used quality-indicator in transient- and shear-wave elastography platforms. Our hypothesis is that SR measurement variability would increase substantially from phantom scanning to an endoscopic application on pancreatic lesions, which may limit the usefulness of the method in some applications.
2. Materials and Methods
The
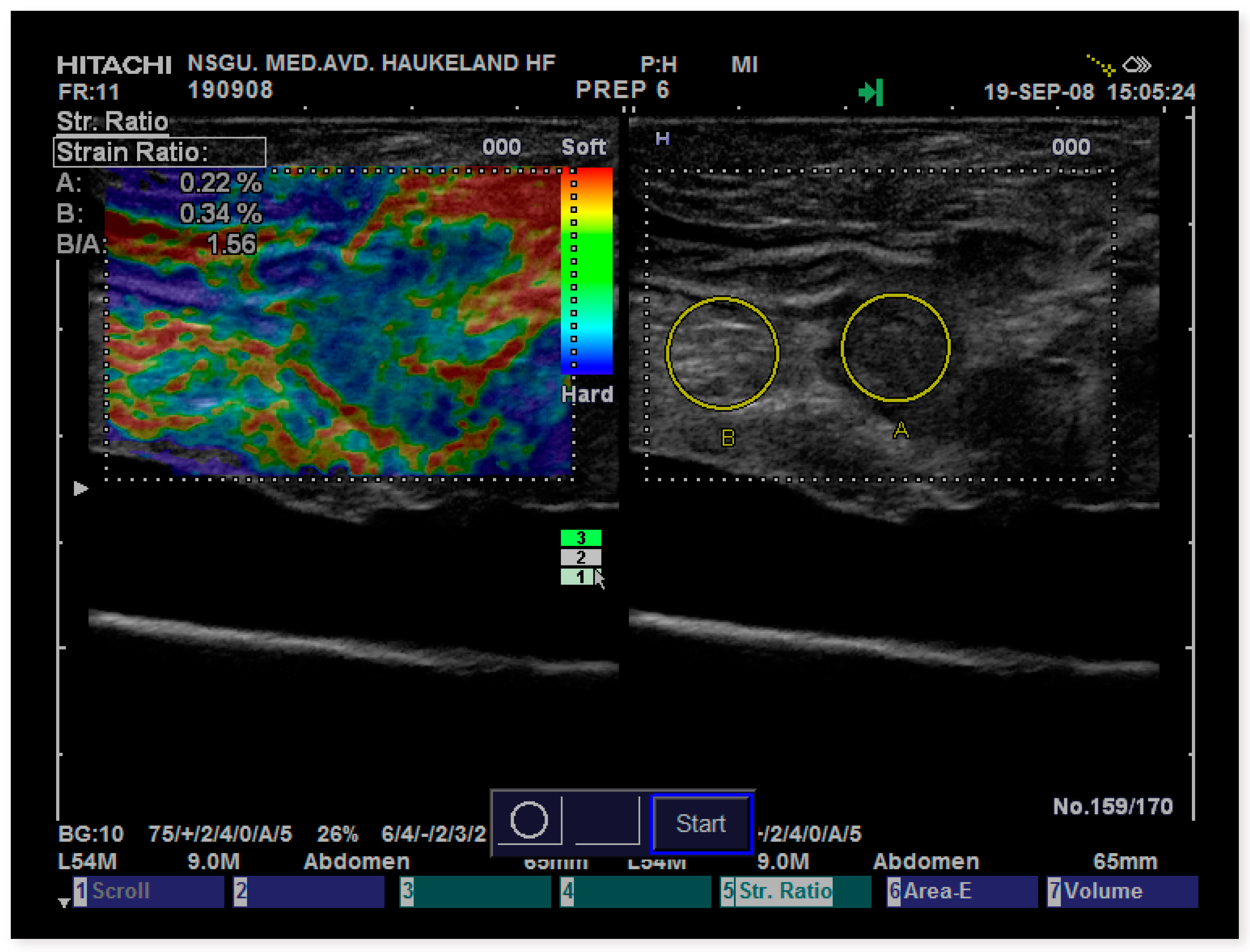
SR data in this study were recorded on the Hitachi (Hitachi Medical Corporation, Tokyo, Japan) Extended Combined Autocorrelation Method (ECAM) also known as Real-Time Elastography (RTE) operated on Hitachi HV-900 and Ascendus platforms (version: V16-04 STEP 2). US data were acquired using external linear probes (L54, 9–13 MHz). The phantom used was a standard model made of Zerdine
® (US pat no. 5196343) embedded in a firm box including eight spherical inclusions with elasticities of 8, 14, 45, and 80 kPa in a background of 25 kPa (CIRS, model 49, Norfolk, Virginia, USA) (
Figure 1).
To study surgical bowel specimens, we collected the specimens in the operation room, washed them, and proceeded directly to scanning in a designated scanning box with the bottom covered with 1 cm of agar. Bowel specimens were then fixated in formalin when attached to the bottom with colored pins, marking the scan-planes. A Hitachi HV-900 scanner was used with a L54 M linear probe, 9–13 MHz. We included 9 specimens from patients with Crohn’s disease (16 sections scanned), 16 patients with adenocarcinomas (18 tumor sections scanned), and 3 patients with adenomas (4 lesion sections scanned). One patient had both an adenocarcinoma and an adenoma. Altogether, 38 sections of separate lesions were included. Histology was the reference standard. For further details on the patients and method, please refer to the original publication [
14].
To examine pancreatic lesions, the data were recorded prospectively over a three-year period. We used Hitachi HV-900 with software version V16-04 STEP2. The echoendoscope was a Pentax EG-3870 UTK (Pentax Medical, Hamburg, Germany). We included 48 lesions from 39 patients: 11 adenocarcinomas, 7 malignant neuroendocrine tumors (NETs), 11 benign/indeterminate NET, 8 focal lesions in pancreatitis, 2 microcystic adenomas, and 9 other benign lesions. The reference standard was histology, EUS fine-needle-aspiration (FNA), or follow-up for at least 6 months. For further details and the diagnostic accuracy, please refer to the original publication [
15].
Statistical Methods
We used the mean of the median SR values for each class of lesion, the range of values (max–min) and the interquartile range (IQR) for different objects or lesions. The IQR is a measure of the variability based on the two central quartiles from the 25th–75th percentile. The remaining 50% in the eccentric quartiles are not part of the IQR, but are accounted for by the range, representing the gap between the highest and lowest measured value. Kolmogorov–Smirnov’s test was used to determine the distribution of data, and one-way Analysis of variance (ANOVA) or non-parametric tests were used accordingly. We then used the Kruskal–Wallis test for individual samples to compare the median SR, the IQR, and the IQR/median for the three applications of Real-Time Elastography with SR. We also analyzed the difference in median SR, IQR and IQR/median between observer A and B in the phantom and for benign or malignant pancreatic lesions by EUS elastography using one-way ANOVA and t-test. A difference with a p-value < 0.05 was considered statistically significant. We also used the intraclass correlation coefficient (ICC) to calculate inter observer agreement when possible.
All patients had signed a consent form to participate in the two studies that provided
SR data for comparison of variability. For statistical analysis, we used SPSS, version 24 (SPSS, IBM, New York, NY, USA). Study protocols as well as patient information and consent forms were approved by the institutional committee for Research in Medicine and Biology. The studies were conducted according to the Helsinki Declaration for Research in Medicine and Biology. A excel file (Microsoft Corporation, Redmond, WA, USA) with the pooled data for phantom inclusions, surgical specimens and pancreatic lesions by entity is included as a
Supplementary Materials file.
4. Discussion
In this paper, we presented SR measurement in a tissue-mimicking phantom and in resected tissue, where the stress was applied by pushing gently with the probe itself, focusing on the measurement variability. We also presented data from EUS elastography of focal pancreatic lesions where arterial pulsations, particularly from the aorta, acted as an internal stress source. We showed that the IQR increased with application to more complex anatomical structures and inability to control the stress source to a level that surpasses the median SR value. The mean value of the IQR/median increased 8.6 times from the phantom (0.07) to resected tissue (0.62) and 28.3 times with endosonographic application (2.04).
When applying strain elastography in an endoscopic application, the strain is dependent on endogenic stress sources, such as aortic pulsation and respiratory movements. These sources may vary considerably between patients and can hardly be standardized. Also, the availability of stable reference tissue that should be subject to similar stress as the lesion, may be hard to find in this application. Moreover, further differences may be caused by variable levels of pre-compression and applied stress caused by the endoscope. Since soft tissues have non-linear elastic properties, the tissue will appear harder with more applied stress or increased pre-compression [
5,
16]. An endoscopic application is also challenging because the probe is inserted into the gastrointestinal cavity and cannot be controlled directly by the observer’s hands, as is the case with external strain imaging ultrasonography. Lu et al. performed a meta-analysis of EUS elastography in pancreatic lesions evaluated qualitatively with a visual score, strain histograms,
SR, contrast enhanced EUS, and EUS FNA. They identified a large range in
SR cut-off values based on previous published cut-off values or the ROC curves (3.05 to 24.82). This caused large heterogeneity for the specificity, and three-eighths of the studies were identified as outliers. After removing the outliers, the evaluation for identification of malignant lesions based on qualitative visual scores and strain histograms outperformed the
SR-based evaluation (sensitivity: 0.94, specificity: 0.54, diagnostic odds ratio (OR): 29.42 [
17]. However, strain elastography and
SR-based assessment of rectal tumors using a dedicated radial rectal probe, which allows induction of strain by rapid water-inflation of a balloon around the US probe, was used to improve patient selection for organ-sparing treatment compared to standard multidisciplinary assessment [
18].
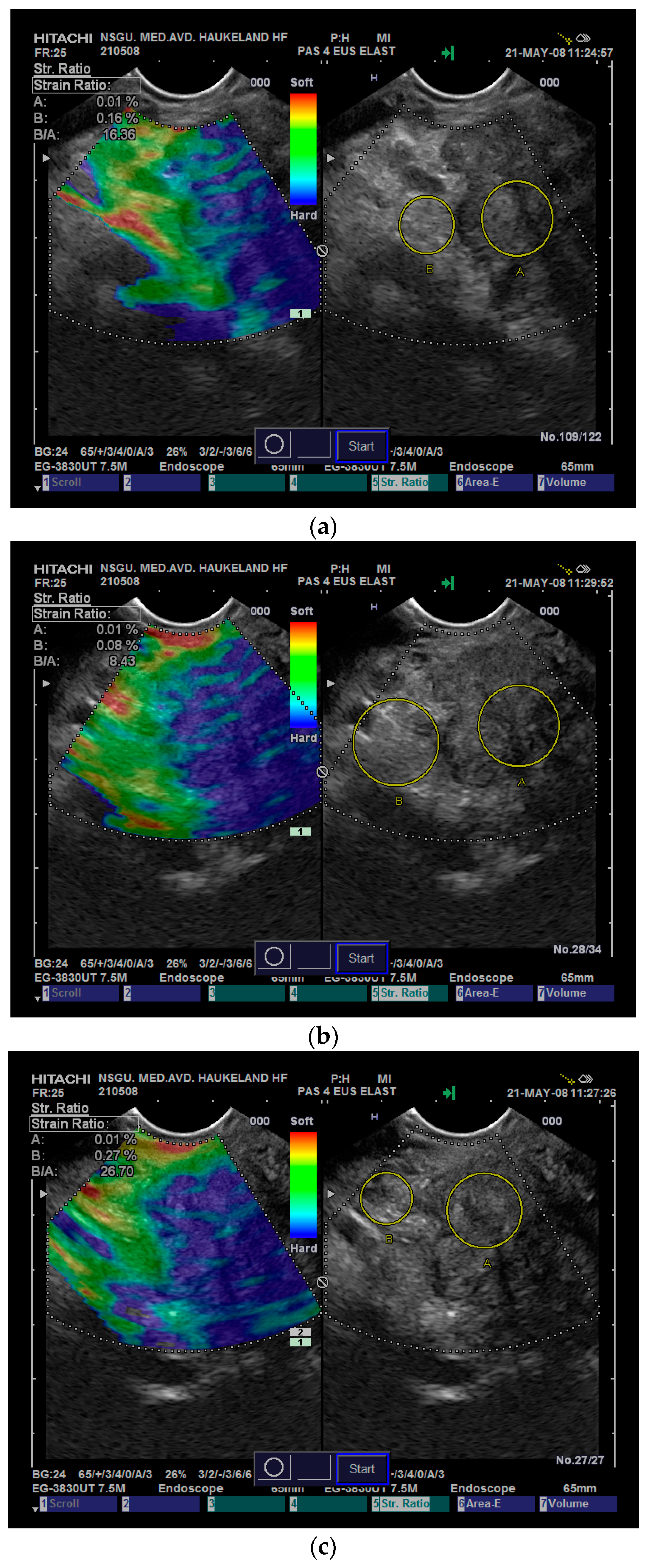
In our experience, the variability in
SR measurements increases when we transitioned from application with good access to relevant reference tissue, which allows for hand-eye coordination of inflicted stress, made possible by the real-time strain-feedback to the examiner on the screen. Selection of the reference area for comparison with a focal lesion also introduces variability that may influence the resulting
SR more than the strain variability of the lesion itself, as illustrated in
Figure 5. To avoid the variability induced by the reference area, some researchers recorded the strain values in the lesion of interest using the Strain Histogram (SH) function and found that it performed equally as well as the
SR in some applications. However, this is dependent on a near standardized application of stress to the lesions of interest. The SH function displays the distribution of the different colors representing different strain intervals in a 256 scale, from no strain (0) to maximum strain (256), as set by the scanner software. From a histogram, quantifying the distribution of recorded strains it is also possible to evaluate if the lesion and reference tissue is homogeneous or heterogeneous by using kurtosis and range. Carlsen et al. showed that the SH (cut-off: 189) performed equally well as
SR (cut-off: 1.44) in differentiating malignant from benign breast lesions, but the modality could not improve diagnostics when compared with radiological Breast Imaging-Reporting and Data System in a limited material [
7]. The median value of the histogram represents the median hardness of the lesion. Opacic et al. suggested using the median histogram value of the reference tissue divided by the median value of the lesion histogram to create a histogram ratio. In a study of pancreatic lesions, this did not perform better than
SR with a ROC-AUC of 0.843 and a cut-off selection yielding a specificity of 98%, a sensitivity of only 50%, and an accuracy of 69% [
19]. Unfortunately, we did not record the strain histograms in our studies of surgical specimens and pancreatic lesions as this function was not available at the time.
Visual evaluation of the elastogram is direct and intuitive and requires no post-processing. Several scoring systems for categorical scoring of strain images have been proposed. For breast imaging, the five-point Tsukuba scale was proposed by Itoh et al. [
20]. In two meta-analyses,
SR measurements were found to differentiate malignant from benign breast lesions better than using the visual scoring range of one to five [
21,
22]. Amended versions of this visual score have been proposed for other organs, such as for the pancreas and lymph nodes by EUS elastography. Both the continuous visual analog scale (VAS) score and categorical visual scores for endoscopic assessment of rectal tumors has shown comparable results with
SR measurements [
23]. In a comparative study of S
R and the five-point visual elastography breast scale,
SR was more accurate. However, the direct visual impression of an experienced examiner using elastography imaging may contain more information than a five-step visual score can comprehend, which is useful in many cases for lesions in doubt. With training, different aspects of the elastogram, such as strain concentration and artefacts caused by veins and natural sliding surfaces, can be recognized. These findings can hardly be recognized by
SR, histograms, or other formal quantification methods. One group investigated the use of automated pattern and color recognition of elastography of focal pancreatic lesions in 258 patients. They used an artificial neural network image analysis and information about the histological diagnosis from endoscopic elastography as the input. This improved the accuracy, with a ROC-AUC of 0.94, significantly better compared to using only lesion strain histograms that had a ROC-AUC of 0.85. This evaluation was performed using collected data in a multicenter study [
24] but required substantial post-processing. In the future, automated tissue recognition or a material of standardized hardness, serving as a reference within the field-of-view, may be ways to improve
SR in endoscopic applications. Also, averaging strain values over several frames or filtering noisy strain areas in the elastograms may improve
SR measurements. We may possibly also soon see shear-wave elastography for flexible endoscopes.
Limitations
This study was based on previously recorded strain ratios from a tissue mimicking phantom and real tissues, both in vitro and in vivo. The data are based on different numbers of lesions for each application. For the resected tissue and the EUS elastography of pancreatic focal lesions, all recordings were performed by the same observer, whereas the phantom inclusions were performed by two observers. Because the data on surgical specimens and pancreatic lesions in this study were collected from individual lesions and patients, whereas the phantom inclusions represent only four different cases, this also limits the variability in the phantom SR measurements. The same strain elastography system was used for all scans (Hitachi, Real-Time Elastography), but both scanners and software were upgraded between the scanning of surgical specimens, EUS, and the phantom scanning. The ECAM algorithm was applied throughout all scans, but since elastograms are based on B-mode data, improvements in B-mode images through scanner software upgrades may have influenced the results. The scanning of the phantom was completed with the latest version of the scanner software.