Synthesis, Characterization, and Self-Assembly of a Tetrathiafulvalene (TTF)–Triglycyl Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Compounds
2.2.1. General Remarks
2.2.2. Synthetic Procedures and Characterization of Compounds
2.3. Preparation and Characterization of Gel Materials
3. Results and Discussion
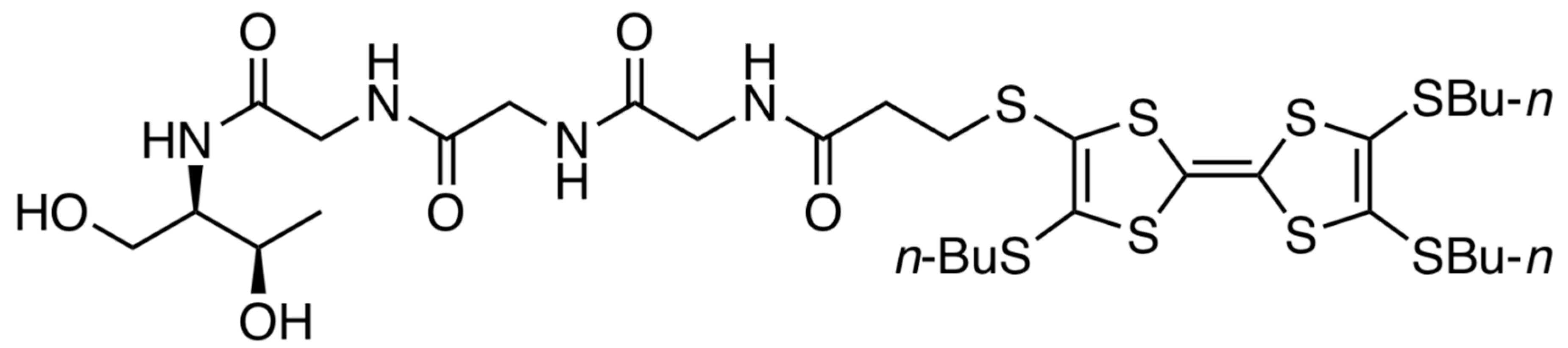
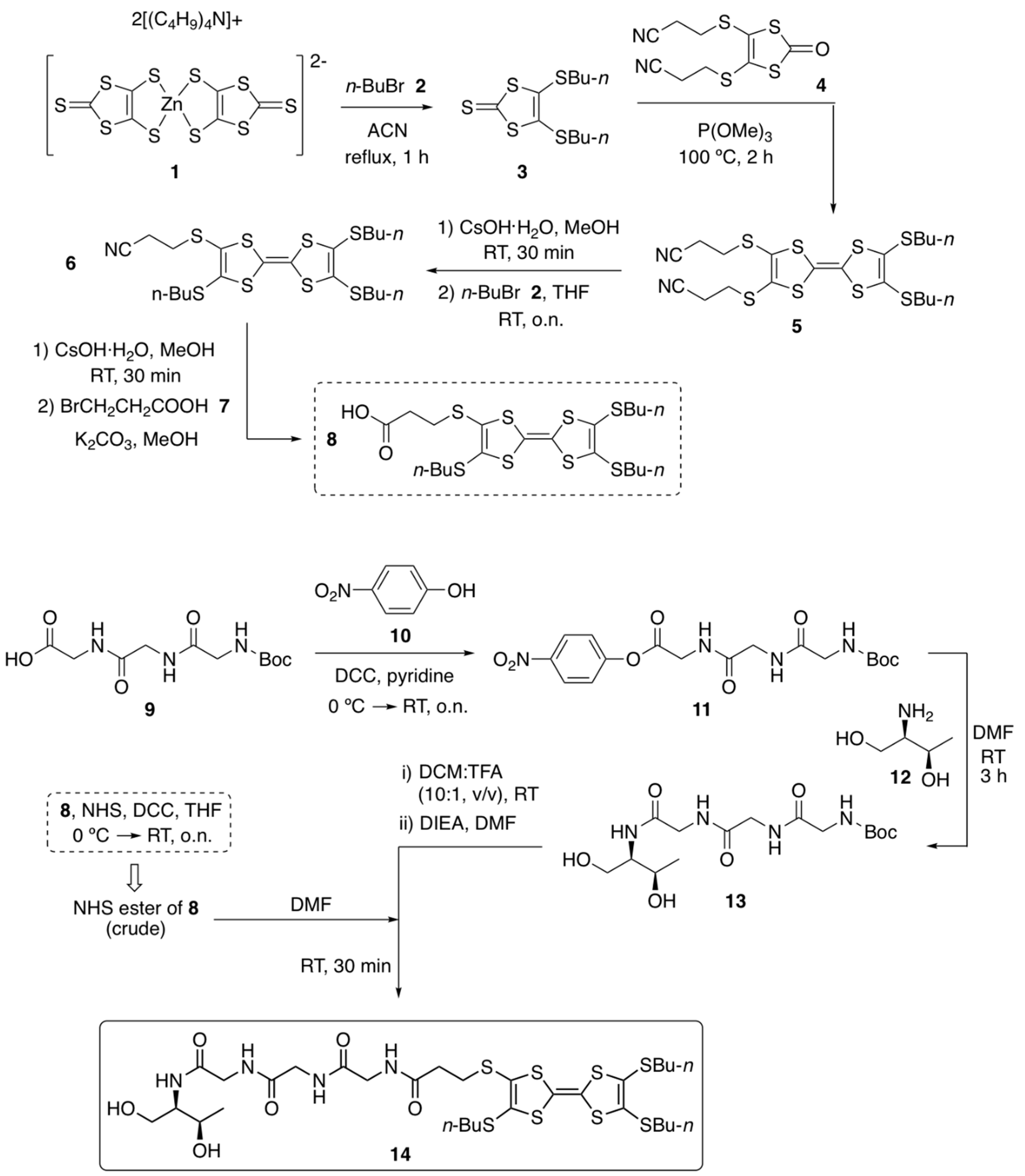
3.1. Synthesis of TTF–Triglycyl Derivative
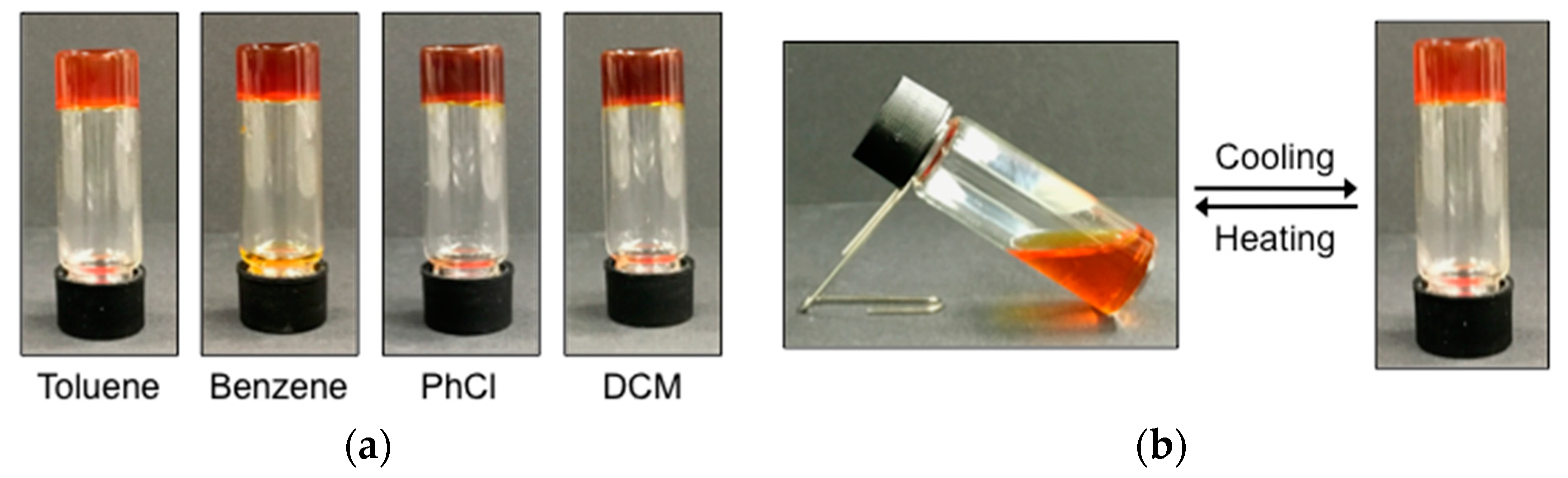
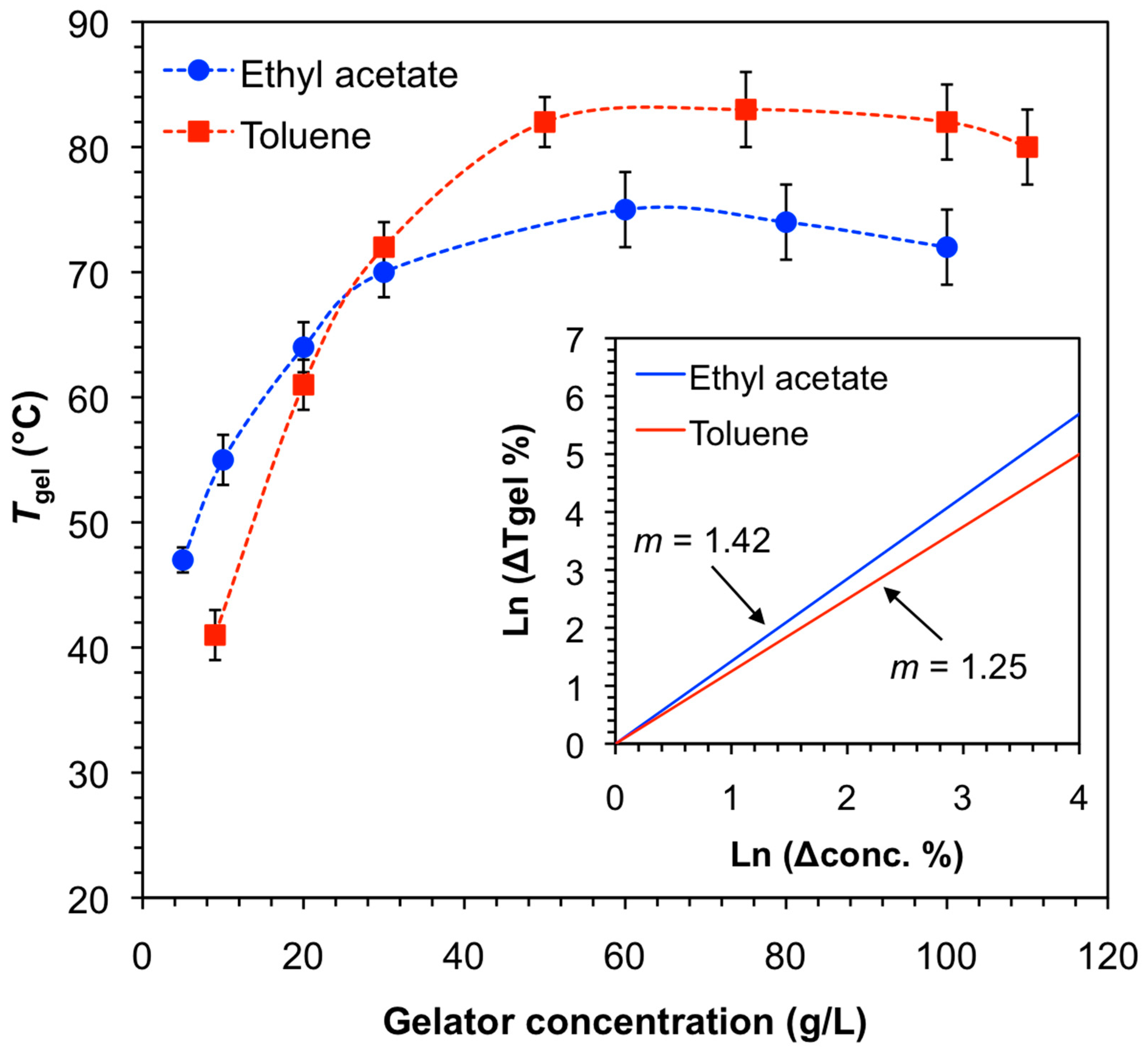
3.2. Gelation Properties
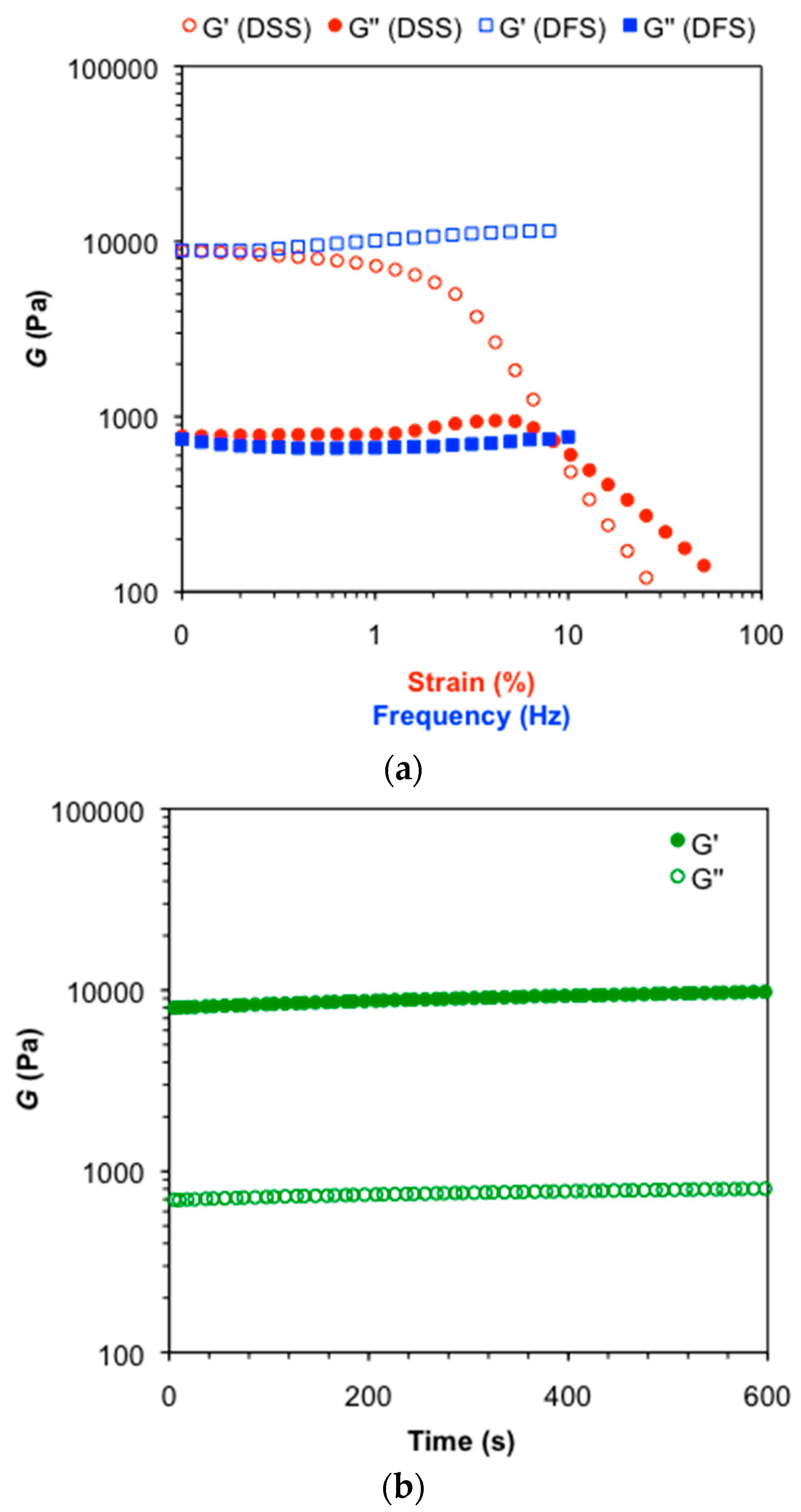
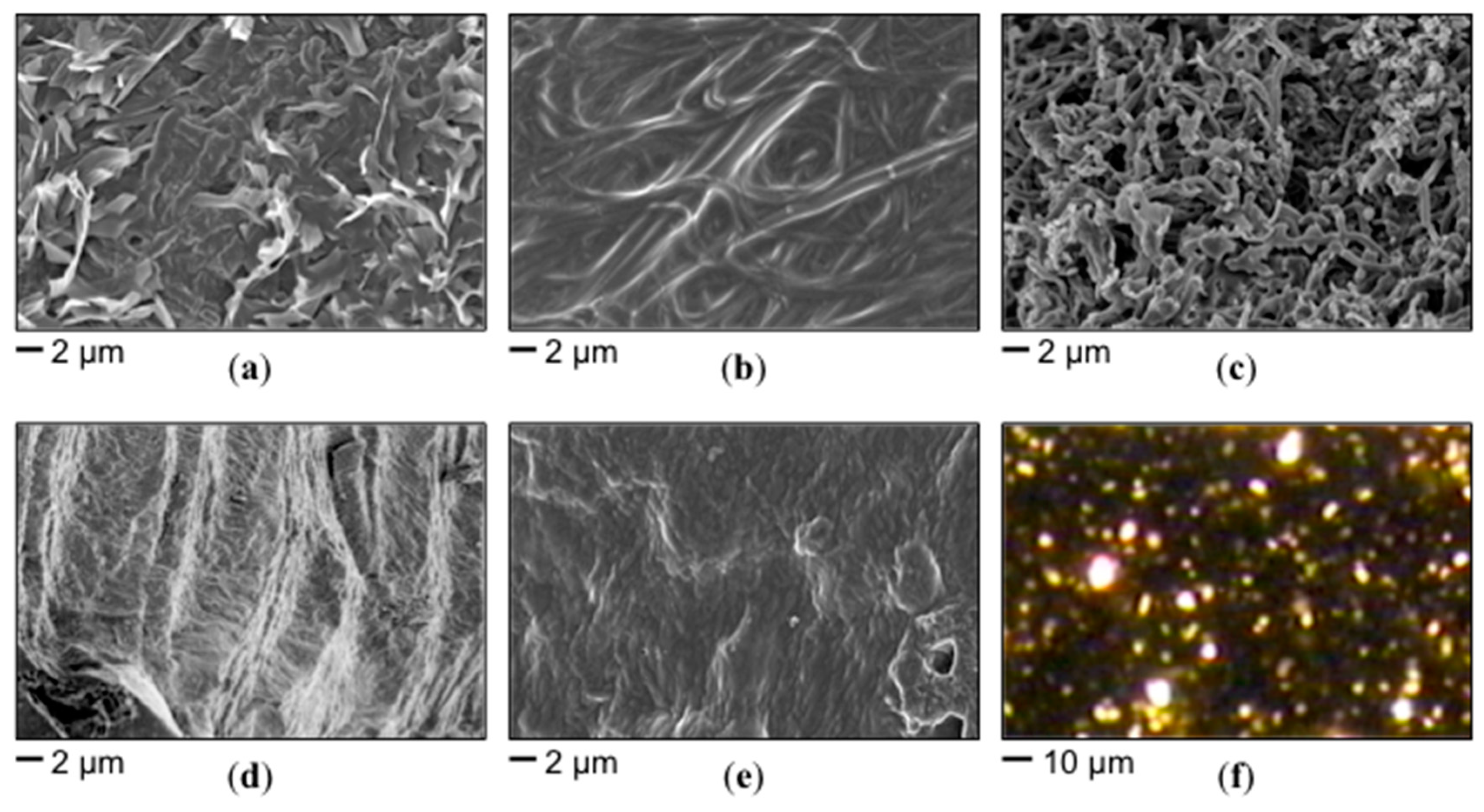
3.3. Characterization of Organogels
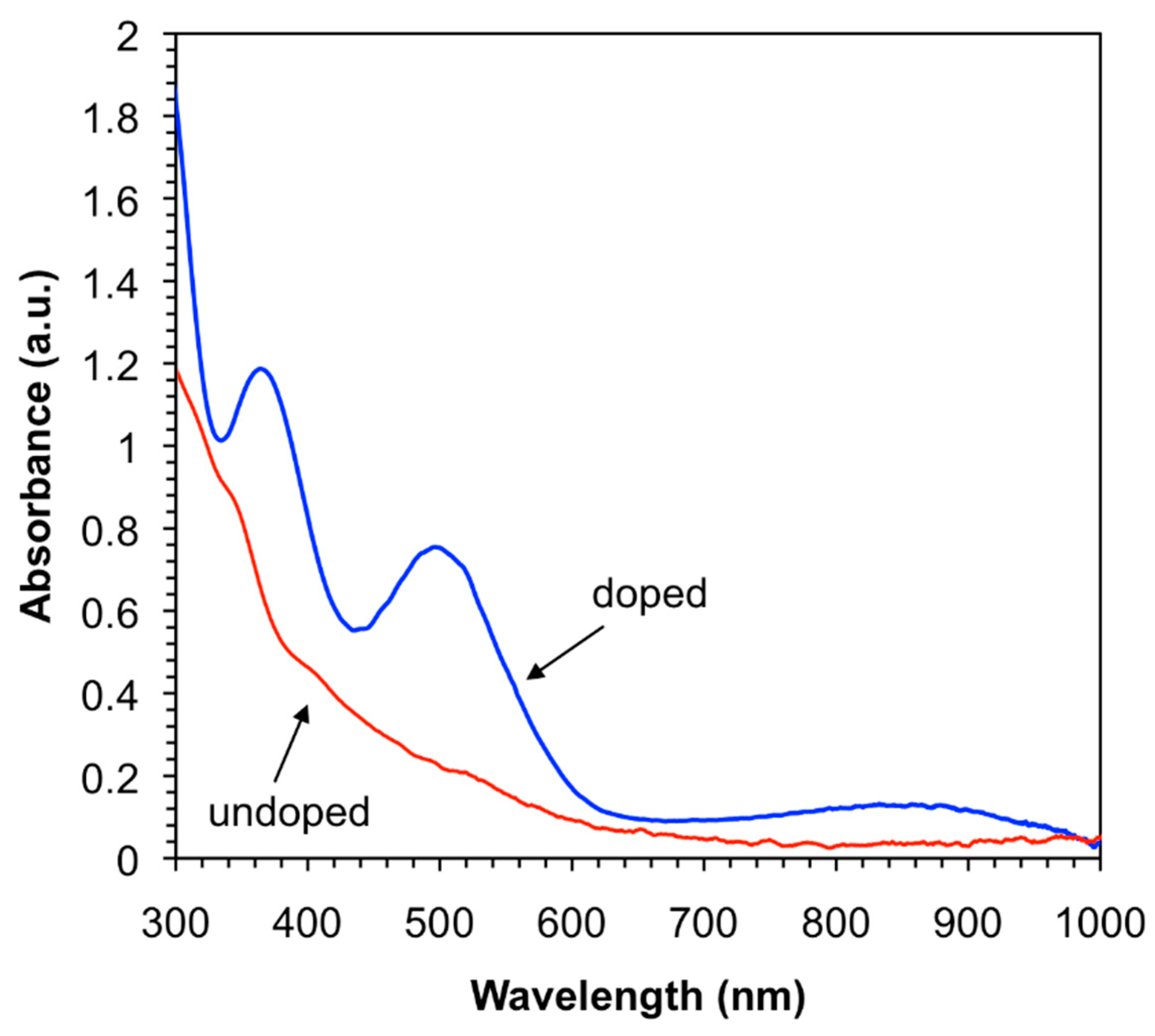
3.4. Effect of Iodine-Doping
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hornum, M.; Kumar, P.; Podsiadly, P.; Nielsen, P. Increasing the stability of DNA:RNA duplexes by introducing stacking phenyl-substituted pyrazole, furan, and triazole moieties in the major groove. J. Org. Chem. 2015, 80, 9592–9602. [Google Scholar] [CrossRef] [PubMed]
- Hrdlicka, P.J.; Karmakar, S. 25 years and still going strong: 2′-O-(pyren-1-yl)methylribonucleotides—Versatile building blocks for applications in molecular biology, diagnostics and materials science. Org. Biomol. Chem. 2017, 15, 9760–9774. [Google Scholar] [CrossRef] [PubMed]
- Krasheninina, O.A.; Novopashina, D.S.; Apartsin, E.K.; Venyaminova, A.G. Recent advances in nucleic acid targeting probes and supramolecular constructs based on pyrene-modified oligonucleotides. Molecules 2017, 22, 2108. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Z.; Paulini, R.; Stütz, J.A.R.; Narayanan, S.; Richert, C. 5-Tethered stilbene derivatives as fidelity- and affinity-enhancing modulators of DNA duplex stability. J. Am. Chem. Soc. 2004, 126, 4762–4763. [Google Scholar] [CrossRef] [PubMed]
- Zahn, A.; Leumann, C.J. Recognition properties of donor- and acceptor-modified biphenyl-DNA. Chem. Eur. J. 2008, 14, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Aviñó, A.; Mazzini, S.; Ferreira, R.; Eritja, R. Synthesis and structural properties of oligonucleotides covalently linked to acridine and quindoline derivatives through a threoninol linker. Bioorg. Med. Chem. 2010, 18, 7348–7356. [Google Scholar] [CrossRef] [PubMed]
- Bouquin, N.; Malinovskii, V.L.; Guégano, X.; Liu, S.X.; Decurtins, S.; Häner, R. TTF-modified DNA. Chem. Eur. J. 2008, 14, 5732–5736. [Google Scholar] [CrossRef] [PubMed]
- Schnippering, M.; Zahn, A.; Liu, S.X.; Leumann, C.; Decurtins, S. Synthesis and electrochemical properties of TTF modified oligodeoxynucleotides. Chem. Commun. 2009, 45, 5552–5554. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rentero, S.; Gállego, I.; Somoza, A.; Ferreira, R.; Janoušek, J.; Bělohradský, M.; Stará, I.; Starý, I.; Eritja, R. Interstrand interactions on DNA duplexes modified by TTF units at the 3′ or 5′-ends. RSC Adv. 2012, 2, 4069–4071. [Google Scholar] [CrossRef]
- Pérez-Rentero, S.; Somoza, A.; Grijalvo, S.; Janoušek, J.; Bělohradský, M.; Stará, I.; Starý, I.; Eritja, R. Biophysical and RNA interference inhibitory properties of oligonucleotides carrying tetrathiafulvalene groups at terminal positions. J. Chem. 2013, 2013, 650610. [Google Scholar] [CrossRef]
- Yamada, J.; Akutsu, H. New trends in the synthesis of π-electron donors for molecular conductors and superconductors. Chem. Rev. 2004, 104, 5057–5083. [Google Scholar] [CrossRef] [PubMed]
- Fourmigué, M.; Batail, P. Activation of hydrogen- and halogen-bonding interactions in tetrathiafulvalene-based crystalline molecular conductors. Chem. Rev. 2004, 104, 5379–5418. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.O.; Nielsen, M.B.; Becher, J. Tetrathialfulvalene cyclophanes and cage molecules. Chem. Rev. 2004, 104, 5115–5131. [Google Scholar] [CrossRef] [PubMed]
- Moonen, N.N.P.; Flood, A.H.; Fernández, J.M.; Stoddart, J.F. Towards a racional design of molecular switches and sensors from their basic building blocks. Top. Curr. Chem. 2005, 262, 99–132. [Google Scholar]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef] [PubMed]
- Balzani, V.; Credi, A.; Venturi, M. Light powered molecular machines. Chem. Soc. Rev. 2009, 38, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Olson, M.A.; Benítez, D.; Tkatchouk, E.; Goddard, W.A., III; Stoddart, J.F. Mechanically bonded macromolecules. Chem. Soc. Rev. 2010, 39, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Rovira, C. Bis(ethylenethio)tetrathiafulvalene (BET-TTF) and related dissymmetrical electron donors: From the molecule to functional molecular materials and devices (OFETs). Chem. Rev. 2004, 104, 5289–5317. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Iyoda, M. Conducting supramolecular nanofibers and nanorods. Chem. Soc. Rev. 2010, 39, 2420–2531. [Google Scholar] [CrossRef] [PubMed]
- Iyoda, M.; Hasegawa, M. Star-shaped tetrathiafulvalene oligomers towards the construction of conducting supramolecular assembly. Beilstein J. Org. Chem. 2015, 11, 1596–1613. [Google Scholar] [CrossRef] [PubMed]
- Uji, H.; Kim, H.; Imai, T.; Mitani, S.; Sugiyama, J.; Kimura, S. Electronic properties of tetrathiafulvalene-modified cyclic-β-peptide nanotube. Biopolymers 2016, 106, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rentero, S.; Grijalvo, S.; Peñuelas, G.; Fàbrega, C.; Eritja, R. Thioctic acid derivatives as building blocks to incorporate DNA oligonucleotides onto gold nanoparticles. Molecules 2014, 19, 10495–10523. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y. Gelation with small molecules: From formation mechanism to nanostructure architecture. Top. Curr. Chem. 2005, 256, 1–37. [Google Scholar] [PubMed]
- George, M.; Weiss, R.G. Molecular organogels. Soft matter comprised of low-molecular-mass organic gelators and organic liquids. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G.; Terech, P. Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Weiss, R.G., Terech, P., Eds.; Springer: Dordrecht, Netherlands, 2006. [Google Scholar]
- Smith, D.K. Dendritic supermolecules—Towards controllable nanomaterials. Chem. Commun. 2006, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zaccarelli, E. Colloidal gels: Equilibrium and non-equilibrium routes. J. Phys. Condens. Matter 2007, 19, 323101. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Praveen, V.K.; Vijayakumar, C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 2008, 37, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Das, R.K.; Maitra, U. Supramolecular gels ‘in action’. J. Mater. Chem. 2009, 19, 6649–6687. [Google Scholar] [CrossRef]
- Adams, D.J. Dipeptide and tripeptide conjugates as low-molecular-weight hydrogelators. Macromol. Biosci. 2011, 11, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Tamaru, S.; Shinkai, S. What kind of “soft materials” can we design from molecular gels? Chem. Asian J. 2011, 6, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2012, 22, 38–50. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-molecular-weight gels: The state of the art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Vintiloui, A.; Leroux, J.-C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrock, M.O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and anion-binding supramolecular gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; John, V.T.; Irvin, G.C.; Bachakonda, S.H.; McPherson, G.L.; O’Connor, C.J. Synthesis and magnetic properties of a novel ferrite organogel. J. Appl. Phys. 1999, 85, 5965–5967. [Google Scholar] [CrossRef]
- Kato, T. Self-assembly of phase-segregated liquid crystal structures. Science 2002, 295, 2414–2418. [Google Scholar] [CrossRef] [PubMed]
- Kubo, W.; Kambe, S.; Nakade, S.; Kitamura, T.; Hanabusa, K.; Wada, Y.; Yanagida, S. Photocurrent-determining processes in quasi-solid-state dye-sensitized solar cells using ionic gel electrolytes. J. Phys. Chem. B 2003, 107, 4374–4381. [Google Scholar] [CrossRef]
- Sanchez, C.; Llusar, M. Inorganic and hybrid nanofibrous materials templated with organogelators. Chem. Mater. 2008, 20, 782–820. [Google Scholar]
- Díaz, D.D.; Kühbeck, D.; Koopmans, R.J. Stimuli-responsive nanostructured gels as reaction vessels and reusable catalysts. Chem. Soc. Rev. 2011, 40, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.J.; Weiss, R.G. Organogels and low molecular mass organic gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- Tanaka, T. Gels. Sci. Am. 1981, 244, 110–123. [Google Scholar] [CrossRef]
- DeRossi, D.; Kajiwara, Y.; Osada, Y.; Yamauchi, A. Polymer Gels: Fundamentals and Biomedical Applications; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Ahn, S.-K.; Kasi, R.M.; Kim, S.-C.; Sharma, N.; Zhou, Y. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Zubarev, E.R.; Pralle, M.U.; Sone, E.D.; Stupp, S.I. Scaffolding of polymers by supramolecular nanoribbons. Adv. Mater. 2002, 14, 198–203. [Google Scholar] [CrossRef]
- Huang, X.; Terech, P.; Raghavan, S.R.; Weiss, R.G. Kinetics of 5α-cholestan-3β-yl N-(2-naphthyl)carbamate/n-alkane organogel formation and its influence on the fibrillar networks. J. Am. Chem. Soc. 2005, 127, 4336–4344. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Takafuji, M.; Sakurai, T. Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific: California, CA, USA, 2004; Volume 9, pp. 473–495. [Google Scholar]
- Shin, J.H.; Gardel, M.L.; Mahadeva, L.; Matsudaira, P.D.; Weitz, A. Relating microstructure to rheology of a bundled and cross-linked F-actin network in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 9636–9641. [Google Scholar] [CrossRef] [PubMed]
- Fages, F. Low Molecular Mass Gelators. In Topics Current Chemistry; Fages, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 256. [Google Scholar]
- Jørgensen, M.; Bechgaard, K. Synthesis and structural characterization of a bis-arborol-tetrathiafulvalene gel: Toward a self-assembling “molecular” wire. J. Org. Chem. 1994, 59, 5877–5882. [Google Scholar] [CrossRef]
- Le Gall, T.; Pearson, C.; Bryce, M.R.; Petty, M.C.; Dahlgaard, H.; Becher, J. Arborol-functionalised tetrathiafulvalene derivatives: Synthesis and thin-film formation. Eur. J. Org. Chem. 2003, 2003, 3562–3568. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, D.; Zhang, G.; Zhu, D. Tetrathiafulvalene (TTF)-based gelators: Stimuli responsive gels and conducting nanostructures. Sci. China Chem. 2011, 54, 596–602. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Zhu, D. A Low-molecular-mass gelator with an electroactive tetrathiafulvalene group: Tuning the gel formation by charge-transfer interaction and oxidation. J. Am. Chem. Soc. 2005, 127, 16372–16373. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Nakaso, S.; Mizoshita, N.; Tochigi, Y.; Shimomura, T.; Moriyama, M.; Ito, K.; Kato, T. Electroactive supramolecular self-assembled fibers comprised of doped tetrathiafulvalene-based gelators. J. Am. Chem. Soc. 2005, 127, 14769–14775. [Google Scholar] [CrossRef] [PubMed]
- Puigmartí-Luis, J.; Laukhin, V.; del Pino, P.A.; Vidal-Gancedo, J.; Rovira, C.; Laukhina, E.; Amabilino, D.B. Supramolecular conducting nanowires from organogels. Angew. Chem. Int. Ed. 2007, 46, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Xing, L.-B.; Cao, W.-N.; Li, X.-B.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Organogelators based on TTF supramolecular assemblies: Synthesis, characterization, and conductive property. Langmuir 2011, 27, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Jin, L.; Yin, B. T-shaped monopyridazinotetrathiafulvalene-amino acid diad based chiral organogels with aggregation-induced fluorescence emission. Soft Matter 2016, 12, 6373–6384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, Y.; Yin, B. Gel properties of T-shaped tetrathiafulvalene–pyridazine conjugates and F4TCNQ-induced morphological transformation. New J. Chem. 2016, 40, 464–474. [Google Scholar] [CrossRef]
- Kong, D.; Xia, Y.; Li, D.; Hou, R. Super organogelator based on tetrathiafulvalene with four amide derivatives and its F TCNQ chargetransfer complex. Supramol. Chem. 2017, 29, 102–110. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Sun, F.; Zhang, D.; Zhang, G.; Huang, Y.; Zhao, R.; Zhu, D. Multistimuli responsive organogels based on a new gelator featuring tetrathiafulvalene and azobenzene groups: Reversible tuning of the gel-sol transition by redox reactions and light irradiation. J. Am. Chem. Soc. 2010, 132, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, F.; Zhang, D.; Zhang, G.; Daoben, Z. Cholesterol-substituted tetrathiafulvalene (TTF) compound: Formation of organogel and supramolecular chirality. Chin. J. Chem. 2010, 28, 622–626. [Google Scholar] [CrossRef]
- Canevet, D.; del Pino, A.P.; Amabilino, D.B.; Sallé, M. Varied nanostructures from a single multifunctional molecular material. J. Mater. Chem. 2011, 21, 1428–1437. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Z.; Jin, L.; Yin, B. Monopyrrolotetrathiafulvalene-based supramolecular organogels: Multi-stimuli responsiveness and formation of charge-transfer salt gels. Dyes Pigments 2017, 140, 500–511. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, Y.; Beak, S.; Ishimoto, S.; Enozawa, H.; Isomura, E.; Hasegawa, M.; Iyoda, M.; Park, Y. Synthesis and electrical conductivity of perchlorate-doped TTF–diamide nanofibers with double and triple helix structures. J. Mater. Chem. 2010, 20, 10817–10823. [Google Scholar] [CrossRef]
- Ji, S.-F.; Sun, Y.-G.; Huo, P.; Shen, W.-C.; Huang, Y.-D.; Zhu, Q.-Y.; Dai, J. Effect of metal coordination on photocurrent response properties of a tetrathiafulvalene organogel film. Inorg. Chem. 2014, 53, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Amacher, A.M.; Puigmartí-Luis, J.; Geng, Y.; Lebedev, V.; Laukhin, V.; Krämer, K.; Hauser, J.; Amabilino, D.B.; Decurtins, S.; Liu, S.-X. Coordination-directed self-assembly of a simple benzothiadiazole-fused tetrathiafulvalene to low-bandgap metallogels. Chem. Commun. 2015, 51, 15063–15066. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, X.; Lai, G.; Li, Z.; Ma, Y.; Yuan, X.; Shen, Y.; Wang, C. Urethane tetrathiafulvalene derivatives: Synthesis, selfassembly and electrochemical properities. Beilstein J. Org. Chem. 2015, 11, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Ouyang, J. Electronically and ionically conductive gels of ionic liquids and charge-transfer tetrathiafulvalene-tetracyanoquinodimethane. Langmuir 2011, 27, 10953–10961. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, S.K.M.; Shivarova, N.; Kanibolotsky, A.L.; Zelzer, M.; Gupta, S.; Frederix, P.W.J.M.; Skabara, P.J.; Gleskova, H.; Ulijn, R.V. Conducting nanofibers and organogels derived from the self-assembly of tetrathiafulvalene-appended dipeptides. Langmuir 2014, 30, 12429–12437. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Jin, L.; Chen, T.; Yin, B. MPTTF-containing tripeptide-based organogels: Receptor for 2, 4, 6-trinitrophenol and multiple stimuli-responsive properties. Soft Matter 2016, 12, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Cocker, T.M.; Bachman, R.E.; Weiss, R.G. Gelation of organic liquids by some 5α-cholestan-3β-yl N-(2-aryl)carbamates and 3β-cholesteryl 4-(2-anthrylamino)butanoates. How important are H-bonding interactions in the gel and neat assemblies of aza aromatic-linker-steroid gelators? Langmuir 2000, 16, 20–34. [Google Scholar] [CrossRef]
- Schiller, J.; Alegre-Requena, J.V.; Marqués-López, E.; Herrera, R.P.; Casanovas, J.; Alemán, C.; Díaz, D.D. Self-assembled fibrillar networks of a multifaceted chiral squaramide: Supramolecular multistimuli-responsive alcogels. Soft Matter 2016, 12, 4361–4374. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, K.B.; Svenstrup, N.; Lau, J.; Simonsen, O.; Mork, P.; Kristensen, G.J.; Becher, J. Sequential functionalization of bis-protected tetrathiafulvalene-dithiolates. Synthesis 1996, 1996, 407–418. [Google Scholar] [CrossRef]
- Zhao, B.T.; Blesa, M.J.; Le Derf, F.; Canevet, D.; Benhaoua, C.; Mazari, M.; Allain, M.; Sallé, M. Carboxylic acid derivatives of tetrathiafulvalene: Key intermediates for the synthesis of redox-active calixarene-based anion receptors. Tetrahedron 2007, 63, 10768–10777. [Google Scholar] [CrossRef][Green Version]
- Toniolo, C.; Palumbo, M. Solid-state infrared absorption spectra and chain arrangement in some synthetic homooligopeptides in the intermolecularly hydrogen-bonded pleated-sheet β-conformation. Biopolymers 1977, 16, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.E.; Tadic-Galeb, B.; Yoder, P.R. Optical System Design, 2nd ed.; McGraw Hill: New York, NY, USA, 2008. [Google Scholar]
| Solvent 2 | CGC (g/L) | Gelation Time (min) | Tgel (°C) |
|---|---|---|---|
| Acetone | 40 ± 10 | 60 ± 10 | 36 ± 3 4 |
| Benzene | 6 ± 1 | 78 ± 5 | 37 ± 2 |
| Benzonitrile | 35 ± 10 | 55 ± 5 | 42 ± 2 |
| Chlorobenzene | 9 ± 1 | 78 ± 5 | 38 ± 2 |
| Chloroform | 18 ± 2 | 10 ± 2 | 50 ± 3 |
| Dichloromethane | 29 ± 2 | 10 ± 2 | 49 ± 2 |
| 1,4-Dioxane | 50 ± 10 | 60 ± 10 | 51 ± 3 |
| Ethyl acetate | 5 ± 1 | 10 ± 2 | 47 ± 2 |
| Toluene | 9 ± 1 | 90 ± 10 | 41 ± 2 |
| Xylene 3 | 8 ± 1 | 90 ± 10 | 46 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rentero, S.; Eritja, R.; Häring, M.; Saldías, C.; Díaz, D.D. Synthesis, Characterization, and Self-Assembly of a Tetrathiafulvalene (TTF)–Triglycyl Derivative. Appl. Sci. 2018, 8, 671. https://doi.org/10.3390/app8050671
Pérez-Rentero S, Eritja R, Häring M, Saldías C, Díaz DD. Synthesis, Characterization, and Self-Assembly of a Tetrathiafulvalene (TTF)–Triglycyl Derivative. Applied Sciences. 2018; 8(5):671. https://doi.org/10.3390/app8050671
Chicago/Turabian StylePérez-Rentero, Sónia, Ramon Eritja, Marleen Häring, César Saldías, and David Díaz Díaz. 2018. "Synthesis, Characterization, and Self-Assembly of a Tetrathiafulvalene (TTF)–Triglycyl Derivative" Applied Sciences 8, no. 5: 671. https://doi.org/10.3390/app8050671
APA StylePérez-Rentero, S., Eritja, R., Häring, M., Saldías, C., & Díaz, D. D. (2018). Synthesis, Characterization, and Self-Assembly of a Tetrathiafulvalene (TTF)–Triglycyl Derivative. Applied Sciences, 8(5), 671. https://doi.org/10.3390/app8050671






